Abstract
Despite the rapid rise in diversity and quantities of engineered nanomaterials produced, the impacts of these emerging contaminants on the structure and function of ecosystems have received little attention from ecologists. Moreover, little is known on how manufactured nanomaterials may interact with nutrient pollution in altering ecosystem productivity, despite the recognition that eutrophication is the primary water quality issue in freshwater ecosystems worldwide. In this study, we asked two main questions: (1) To what extent do manufactured nanoparticles affect the biomass and productivity of primary producers in wetland ecosystems? (2) How are these impacts mediated by nutrient pollution? To address these questions, we examined the impacts of a citrate-coated gold nanoparticle (AuNPs) and of a commercial pesticide containing Cu(OH)2 nanoparticles (CuNPs) on aquatic primary producers under both ambient and enriched nutrient conditions. Wetland mesocosms were exposed repeatedly with low concentrations of nanoparticles and nutrients over the course of a 9-month experiment, in an effort to replicate realistic field exposure scenarios.
In the absence of nutrient enrichment, there were no persistent effects of AuNPs or CuNPs on primary producers or ecosystem productivity. However, when combined with nutrient enrichment, both NPs intensified eutrophication. When either of these NPs were added in combination with nutrients, algal blooms persisted for >50 days longer than in the nutrient only treatment. In the AuNP treatment, this shift from clear waters to turbid waters led to large declines in both macrophyte growth and rates of ecosystem gross primary productivity (average reduction of 52 ± 6% and 92 ± 5%, respectively) during the summer. Our results suggest that nutrient status greatly influences the ecosystem-scale impact of two emerging contaminants, and that synthetic chemicals may be playing an under-appreciated role in the global trends of increasing eutrophication. We provide evidence here that chronic exposure to Au and Cu(OH)2 nanoparticles at low concentrations can intensify eutrophication of wetlands and promote the occurrence of algal blooms.
Keywords: Nutrients, Multiple stressors, Gold nanoparticles, Copper nanoparticles, Egeria, Nanomaterial, Aquatic ecosystems, Algae, Macrophytes, Algal bloom, eutrophication
INTRODUCTION
The rising concentrations and diversity of synthetic chemicals in the environment is an important marker of the Anthropocene (Lewis and Maslin 2015). However, our understanding of the ecological consequences of emerging contaminants lags far behind that of other global change drivers, such as elevated atmospheric CO2, habitat loss or climate change (Bernhardt et al. 2017). Although many emerging contaminants, like metal nanomaterials, are becoming globally ubiquitous (Jambeck et al. 2015, Stehle and Schulz 2015, González-Alonso et al. 2017), they are found at both the highest concentrations and in the greatest diversity in wastewaters and agricultural runoff, where they co-occur with high nutrient loads and historically problematic contaminants (King et al. 2016). The nutrient pollution side of this issue is well studied. Eutrophication resulting from excess phosphorus (P) and nitrogen (N) inputs to water bodies stimulates algal blooms and low oxygen conditions that often result in a loss of biodiversity (Smith et al. 1999). Though eutrophication is recognized as the primary water quality issue in freshwater ecosystems worldwide (Smith and Schindler 2009), little attention has been paid to how emerging synthetic chemicals, like nanomaterials may interact with nutrient pollution and alter ecosystem productivity and biodiversity.
There is growing evidence that our failure to examine the interactions between nutrient and other synthetic chemical pollutants may be compromising our understanding of impaired aquatic ecosystems. For instance, two studies found that phosphorus availability modulated the response of phytoplankton and periphyton communities to silver nanoparticles in lakes, leading to a reduction of the contaminant toxicity for pelagic primary producers and an increased toxicity for the benthic communities (Das et al. 2014, Norman et al. 2015). The deleterious effects of synthetic chemicals may be amplified under high nutrient loads due to increased uptake rates and faster transfer of the contaminants through exposed food webs (Berglund 2003). Or on the contrary, the uptake of toxic compounds in freshwater food webs has been shown to decrease in presence of high algal biomass, especially during algal blooms in eutrophic systems (Pickhardt et al. 2002). Because eutrophication often shifts ecosystem pH and alters organic matter composition and concentrations, nutrient enrichment can thus alter the fate, transport and reactivity of pollutants (Skei et al. 2000, Valenti et al. 2011, Kong et al. 2017). While the ecological consequences of both nutrient pollution and synthetic chemical exposures are often mediated through complex top-down or bottom-up effects, such complicated trophic interactions are still rarely included in ecological or ecotoxicological studies (Rohr et al. 2006, Gessner and Tlili 2016), at the exception of some well-studied conventional pesticides (Van den Brink et al. 2000, 2009, Lin et al. 2012, Yin et al. 2018).
One important class of emerging contaminants that represents a rapidly growing sector of the synthetic chemical industry are engineered nanoparticles (NPs) that are being produced in increasing quantity to perform a wide range of applications in medicine, food, cosmetics, electronics or as agrochemicals (Kah 2015, Mitrano et al. 2015, Servin et al. 2015, Sun et al. 2015). Nanomaterials are now recognized as emerging contaminants of terrestrial and aquatic ecosystems where they join other emerging and established contaminants (e.g., nutrient pollution, metals, pharmaceuticals). While many studies have examined the effects of engineered nanomaterials on organisms, they have typically examined them in isolation from other likely co-occurring contaminants that may modulate their ecological effects (Simonin and Richaume 2015, Bundschuh et al. 2016).
Hence, in this study, we asked two main questions: To what extent do engineered nanomaterials affect the biomass and productivity of primary producers in wetland ecosystems? How are these impacts modulated by nutrient enrichment? To answer these questions, we assessed the impact of repeated exposures of wetland ecosystems to two different nanomaterials: citrate-coated gold nanoparticles and a nanopesticide containing Cu(OH)2 nanoparticles (later called AuNPs and CuNPs, respectively). With the growing use of nanomaterials in agrochemical products and the application of biosolids containing high concentrations of NPs, wetlands receiving waters from agriculture fields will likely be a major environmental sink for these emerging contaminants (Dale et al. 2015, Kah 2015). We added CuNPs in the form of a commercial pesticide (Kocide® 3000), whereas the AuNPs were custom synthesized. Our intention in the AuNP enrichment was to use these particles as a tracer for nanoparticle behavior because AuNPs typically have low aqueous solubility and in contrast with Cu, there are low natural background concentrations of Au in the environment (Ferry et al. 2009, Keller et al. 2017). We conducted our experiment in large wetland mesocosms that were assigned to one of two nutrient conditions (Ambient vs. Nutrient Enriched). Over the course of a nine-month experiment, we examined the interactive effects of continuous, low dose additions of AuNPs or CuNPs (low μg/L range) with nutrient enrichment (inorganic N and P additions) on aquatic primary producers within a set of outdoor replicated wetland mesocosms. The submerged portion of our mesocosms was dominated by the aquatic macrophyte Egeria densa and supported complex aquatic food webs including numerous macroinvertebrate taxa and large populations of Gambusia holbrookii. In this paper, we specifically assessed the individual and interactive effects of nanoparticles and nutrient enrichment treatments on water chemistry, biomass, metal accumulation, and activity of aquatic primary producers.
We hypothesized that CuNPs would have significant ecological impacts on primary producers due to the well-described toxicity and antimicrobial activity of this fungicide, even in the μg/L range in aquatic ecosystems (Wang et al. 2011b, Thwala et al. 2016, Keller et al. 2017). The direction of this effect was difficult to predict a priori, as CuNP fungicides could directly affect algal communities and the photosynthetic potential of macrophytes (Regier et al. 2015), or they might indirectly benefit autotrophs because of their toxicity to competing microbes. We expected to observe significant effects of AuNP on wetland autotrophs, as AuNPs have been found to induce toxicity and to be bioavailable for different model organisms (Barrena et al. 2009, Tsyusko et al. 2012, Sabo-Attwood et al. 2012, Glenn and Klaine 2013). We anticipated significant treatment interactions between nutrient enrichment and both NPs, as previous studies have reported interactive effects of nutrient enrichment and pollutants on algal and periphyton stoichiometry and on macrophyte growth (Fulton et al. 2009, Das et al. 2014, Norman et al. 2015). However, the direction of this interaction was difficult to predict as nutrient availability may either compensate for or enhance the impact of metal pollution on aquatic primary producers (Gessner and Tlili 2016).
MATERIALS AND METHODS
1. Wetland mesocosm setup and experimental design
Our outdoor wetland mesocosm facility is located in the Duke Forest, a research forest adjacent to Duke University in Durham County, NC, USA. Each mesocosm is a large box built from weather-treated lumber (dimensions: 3.66 × 1.22 × 0.81 m) that was partially filled with sand. This sand was graded to create a flat, deep segment 0.8 m in length adjacent to a 2.8 m hillslope rising at a 13° slope and then lined with a food grade liner. This setup allowed us to create three different hydrologic zones within each mesocosm: a permanently flooded zone (aquatic zone), a periodically flooded zone (transition zone) and a rarely flooded zone (upland zone). More details about the construction, set-up and monitoring of the mesocosms are further described in Lowry et al. (2012a) and Colman et al. (2014). In early June 2015, we filled the mesocosms with a sandy-loam soil (Soils and Sand, Durham, NC) with a texture of 56.1% sand, 24.3% loam, 19.6% clay, total organic matter content of 3.05% and a pH of 6.3. Initially, 250 L of groundwater from the experimental site in the Duke Forest was used to fill the mesocosms. The organisms were introduced sequentially in the system between June and September 2015. In the aquatic zone, we added: the macrophyte Egeria densa; the aquatic snails Physella acuta and Lymnaea sp.; and the fish Gambusia holbrookii (eastern mosquitofish). Algae and zooplankton inoculum was added in 250 mL of water from a local wetland biweekly to avoid strong divergences between mesocosms over time because of limited dispersal (Hall et al. 2004). To homogenize the water chemistry and algal composition between mesocosms, the water was circulated between all the mesocosms throughout July 2015 using submersible pond pumps. In the transition zone, the following plant species were planted: Juncus effusus, Carex lurida and Lobelia cardinalis. In the upland zone, Panicum virgatum and Chasmanthium latifolium were planted, Lolium multiflorum and Andropogon gerardii were seeded. These mesocosms were open to colonization by other organisms and populations of insects and spiders were also monitored during the experiment.
Mesocosms were designated as either Nutrient Enriched or Ambient Nutrient, with nutrient additions to the Nutrient Enriched mesocosms beginning on September 28, 2015, over three months before the beginning of the nanomaterial additions. The Ambient Nutrient mesocosms received 1 liter of mesocosm water each week without any nutrients added to mimic dosing disturbance, while the Nutrient Enriched mesocosms received 1 liter of mesocosm water each week that had been supplemented with 88 mg of N as KNO3 and 35 mg of P as KH2PO4. While our addition rate had an N:P molar ratio of 5.5, the measured nutrient N:P ratios in the water column averaged at 16.8 ± 1.6 in the Nutrient Enriched mesocosms (23 ± 3.0 in the Ambient Nutrient mesocosms). Our goal was to push the mesocosms towards eutrophic conditions without attaining hypereutrophic conditions. We achieved this with an annual addition rate of 2.5 g N m−2 of and 1.0 g P m−2, which falls between thresholds for eutrophication in wetland ecosystems receiving water from agroecosystems (Verhoeven et al. 2006).
On January 18, 2016, we randomly assigned the NP treatments to cross the nutrient treatments, resulting in a full-factorial experiment with three replicate mesocosms for each of six treatment/nutrient-status combinations: Control-Ambient Nutrient, Control-Nutrient Enriched, AuNP-Ambient Nutrient, AuNP-Nutrient Enriched, CuNP-Ambient Nutrient, CuNP-Nutrient Enriched. Nanoparticle additions were done weekly over the 270-day (9 month) experiment, with NP additions (described below) mixed with 1 liter of mesocosm water which was then distributed throughout the entire aquatic zone of each mesocosm directly below surface water. Control treatments received the same volume of mesocosm water without any NPs.
The citrate-stabilized AuNPs were synthesized by the Center for the Environmental Implications of Nanotechnology (CEINT) at Duke University and had an average primary particle diameter of 11.9 ± 1.2 nm (TEM measurement) in the stock suspension and an average hydrodynamic diameter of 10.9 ±1.4 nm and an apparent zeta potential of −13± 6 mV in mesocosm water without nutrient addition at pH 7.5 (Dynamic Light Scattering measurement based on particle number, Zetasizer Nano ZS, Malvern, UK). In the nutrient enriched mesocosm water, the hydrodynamic diameter was 12.0 ± 0.2 nm and the zeta potential was −14 ± 1 mV, indicating overall a high stability of the AuNPs in mesocosm water. The mesocosms exposed to AuNPs received a weekly dose of 19 mg Au uniformly applied to the aquatic zone resulting in a total dose of 750 mg Au added over the 9 months of the experiment.
The copper hydroxide NPs (CuNPs) were a commercial nanopesticide (DuPont, KOCIDE® 3000). As a commercial product, this pesticide does not contain only Cu(OH)2 NPs and present some trace amounts of other elements including a polymer matrix that could influence its toxicity and bioavailability (Keller et al. 2017). The CuNPs had an average primary particle diameter of 38.7 ± 8.2 nm in the stock powder and an average hydrodynamic diameter of 120 ± 30 nm and zeta potential of −32.0 ± 5.5 mV in the mesocosm water without nutrient addition at pH 7.5. In the nutrient enriched mesocosm water, the hydrodynamic diameter was 106 ± 16 nm and the zeta potential was −32.8 ± 1.6 mV. The mesocosms exposed to CuNPs received a weekly dose of 35 mg of Kocide, except for the first week of the treatment receiving an initial pulse of 347 mg that resulted in a total dose of 1.664 g Kocide per mesocosm over the 9 months of the study. The measured Cu content in the Kocide product is 27% (dry wt.). Thus, the total dose of Cu-based NPs added to the mesocosms was then 450 mg of Cu over the course of the experiment. The dissolution rates of the CuNPs were assessed in situ using Float-ALyzer G2 membrane dialysis devices (Spectrum Laboratories, Rancho Dominguez, CA) with a molecular weight cutoff of 8–10 kDa. It was observed that after 48 h, 60 ± 8% of the CuNPs were dissolved in absence of nutrient addition and 70 ± 3% in nutrient enriched conditions (Vencalek et al. 2016).
The concentrations of CuNP and AuNP applied to the mesocosms were different because they were added for different goals. The concentration for CuNP was designed to simulate field rates. We assumed a conservative ratio between contributing land surface to wetland area of 10:1 for the aquatic compartment of our wetland (1.83 m2 at initiation of treatments). Based on the application rates from the label of Kocide 3000®, we assumed an application rate of 20 kg per hectare (intermediate value between mean rates for tree crops and field crops). We then used a literature value measured for a Kocide Cu(OH)2 fungicide for the rate of loss of agrochemicals from surface soils of 6% (Rice et al. 2001). When scaled to the 9-month window of our experiment, this gave an addition rate of 450 mg of Cu per mesocosm. AuNPs were used as a particle tracer (low dissolution) that would behave more like a particle than CuNPs (high dissolution). As such, we needed to ensure that it would be readily detectable across time and in various compartments of the mesocosms. Based on past experiments, we chose 750 mg of Au addition per mesocosm. As a whole, the amount of both CuNPs and AuNPs was designed to yield water column concentrations in the low μg/L range.
Au and Cu concentrations in unfiltered surface water were measured before and after dosing every week. Samples were acid digested (HNO3 and HCl, 3: 1 ratio) and measured using ICP-MS (Agilent 7900, Santa Clara, CA, USA). On average, the Au concentration in surface water 2 hours after dosing was 74.1 ± 47.9 μg/L and 7 days after dosing (right before the new weekly dosing) was 4.4 ± 3.7 μg/L. On average, Cu concentration in water after dosing was 50.8 ±30.0 μg/L and 7 days after dosing was 13.1 ± 10.9 μg/L. In the control mesocosms, the Au and Cu concentrations were below the method detection limit (0.15 μg/L) for most of the measurements.
2. Water chemistry and ecosystem metabolism rates
Over the course of the experiment, we extensively monitored the mesocosm water volume, temperature, turbidity, pH, conductivity (Eureka Manta 2, Austin TX), anion concentrations: NO3−, SO4−, Cl−, Br− (Dionex ICS 2000 Ion Chromatograph) and ortho-PO4 (Beckman DU-64 Spectrophotometer) on a daily or weekly basis. The weather conditions were also monitored continuously during the experiment for air temperature, precipitations, wind, relative humidity and barometric pressure (Campbell Scientific, Logan UT). Some selected parameters are presented in Appendix S1.
On a monthly basis, the concentrations of total N and dissolved organic carbon (DOC) were measured on a Shimadzu TOC-VCPH Analyzer with a TNM-1 module and total P on a Beckman DU-64 Spectrophotometer.
Dissolved oxygen (DO) concentrations (at 15 cm deep) were measured weekly before dawn using a YSI D0200 or YSI 556. Continuous DO measurements (15-min intervals) for 1 to 4 consecutive days were conducted monthly using a EXO1 data sondes probe (YSI Incorporated, Yellow Springs, OH, USA) in order to calculate the ecosystem metabolism rates: ecosystem gross primary productivity (GPP) and ecosystem respiration (ER) that were estimated from the day-night dynamics of DO in surface waters. We performed metabolism rate calculations using the LakeMetabolizer package in R (Winslow et al. 2016) with the k.vachon function to estimate the coefficient of gas exchange across the air–water interface (k) and the metab.ols function using the ordinary least squares model to estimate the GPP and ER rates.
3. Algae
Knowing that our treatments could cause modifications in the abundance of planktonic and epiphytic algae, we collected both quantitative data on phytoplankton and qualitative data on epiphytic algae. For phytoplankton, chlorophyll a (Chl-a) concentrations were measured weekly in surface waters at 15 cm deep (Turner Designs, San Jose CA and CR1000 data logger, Campbell Scientific). Epiphytic algal abundance was recorded in weekly photographs of each mesocosm. Based on published correlations between planktonic and epiphytic algal abundance under low to moderate nutrient enrichment (Sand-Jensen and Søndergaard 1981, Sand-Jensen and Borum 1991), an algal bloom threshold was established based on Chl-a concentrations in the water column which was consistent with the literature for phytoplankton (Carlson 1977, Pace et al. 2017), and consistent with evidence of the existence and persistence of a mat of epiphytic algae as documented in mesocosm photographs. The algal bloom threshold was estimated at 3.37 μg/L Chl-a in this experimental system.
4. Macrophytes
To measure stem-level rates of photosynthesis and respiration and the accumulation of the NPs in new plant growth, 3 stems from the macrophyte Egeria densa were collected (7 cm clippings shoots) at the water surface every 3 months (Day 90, 193 and 269). These stems were thoroughly rinsed to remove periphyton before measuring photosynthesis and respiration rates in a bottle assay. The stems were incubated in 40 mL vials in filter-sterilized mesocosm water (from where the stem originated) for 3–4 hours under light and for 3–4 hours under dark conditions in a growth chamber (25°C, Photosynthetically Active Radiation (PAR): 250–450 μmol m−2 s−1 ). The CO2 concentration was measured in the headspace using a LI-COR LI 6200 and 6250 and the slope of accumulation or decline of CO2 over time was used to calculate the photosynthesis and respiration rates. To express the photosynthesis and respiration rates per unit of surface area, the leaf area was measured by scanning each individual leaf and using the WinFolia software (Regent Instruments Incorporated, Quebec Canada). The dried Egeria densa stems were later digested using either HNO3:H2O2:HCl (6:3:22) for control or Au exposed tissues, or HNO3:H2O2 (2:1) for Cu exposed tissues. Total metal concentration was then measured using ICP-MS (Agilent 7700, Santa Clara, CA, USA). The absence of drift during the analysis was checked by measuring two standards (5ppb and 10ppb, Cole-Parmer) every ten samples. The calculated method detection limit was 0.5 μg of Au or Cu per g of dry plants.
At the same three month intervals, Egeria densa growth rates were measured by harvesting all the plant biomass that had colonized mesh columns (Tenax Cintoflex-M Aquaculture and Hatchery Netting, 2 cm mesh size, column diameter: 20 cm, height: 50 cm) located in the aquatic zone of the mesocosms. Three mesh columns per mesocosm were positioned in the aquatic zone along a depth gradient. At each harvest, the biomass collected in the three mesh columns was combined and dried at 60°C for at least 72 hours and the water volume inside each column was measured to determine the Egeria densa growth rates per unit volume.
5. Statistical analyses
Analysis of Chl-a, dissolved oxygen, GPP, ER, Egeria densa photosynthesis, respiration rates, growth rates, metal concentrations, total N, total P and DOC concentrations were conducted using generalized linear mixed-effects modeling to determine the effects of NP exposure (Control, AuNPs, CuNPs), nutrient status (Ambient Nutrient, Nutrient Enriched) and their interactions. In model selection and post hoc tests, we chose P < 0.05 to discriminate significant effects. Main effects and interactions were nested by day and mesocosm was treated as a random effect in the models to account for serial correlation among observations from the same mesocosms over time (Zuur et al. 2009). The models were fit following a framework similar to the one described in King et al. (2016) using the glmer function of the lme4 package in R 3.2.3 (R Core Team 2015). For non-repeated measurements, such as the number of algal bloom days, a generalized linear model was used to test the effects of nanoparticle exposure, nutrient enrichment, and their interaction by using the glm function and a similar framework to fit the model to the data. The most appropriate probability distribution (family) for each analyte was initially constrained by the type of response variable (e.g., for counts: Poisson or negative binomial; for continuous: Gaussian or Gamma; for proportions: binomial). Model fitting was then done using a tiered approach. First, to test and correct for disparities between the assumed and actual distribution of analytes, multiple models were compared using different link functions (e.g., identity, inverse, log) with residual plots and Aikake Information Criterion (AIC) values used to select the most parsimonious model (Zuur et al. 2009). Second, to test for and correct for the presence or absence of interactions, the full model with all interactions was compared with models with nonsignificant interactions being removed and residual plots and AIC values again being used to select the most parsimonious model. To determine at what levels the differences arose due to treatment, nutrient status, and/or time, post hoc comparisons were performed using the lsmeans function in the lsmeans package in R. Results were graphed using the ggplot2 package in R.
RESULTS
1. Exacerbated eutrophication in nutrient enriched AuNP and CuNP mesocosms
The water column total N concentration was more than 30% higher in both AuNP-Nutrient Enriched and CuNP-Nutrient Enriched treatments compared with the Control-Nutrient Enriched mesocosms on Days 27 and 56 (Figs. 1a and 1b; NP effect, p = 0.004). Similarly, DOC concentrations were more than 30% higher than controls in the AuNP-Nutrient Enriched on days 56 and 90 (Fig. 1c) and more than 49% higher in CuNP-Nutrient Enriched mesocosms than controls on days 27 and 56 (Fig. 1d, NP effect (p < 0.001), nutrient effect (p = 0.005)). In the AuNP-Ambient Nutrient and CuNP-Ambient Nutrient mesocosms, there was no detectable change in Total N or DOC concentrations compared to Control-Ambient Nutrient mesocosms (data not shown). Unlike total N or DOC, total P concentration were not significantly altered by any experimental treatment on any date (Figs. 1e, 1f).
Fig. 1.
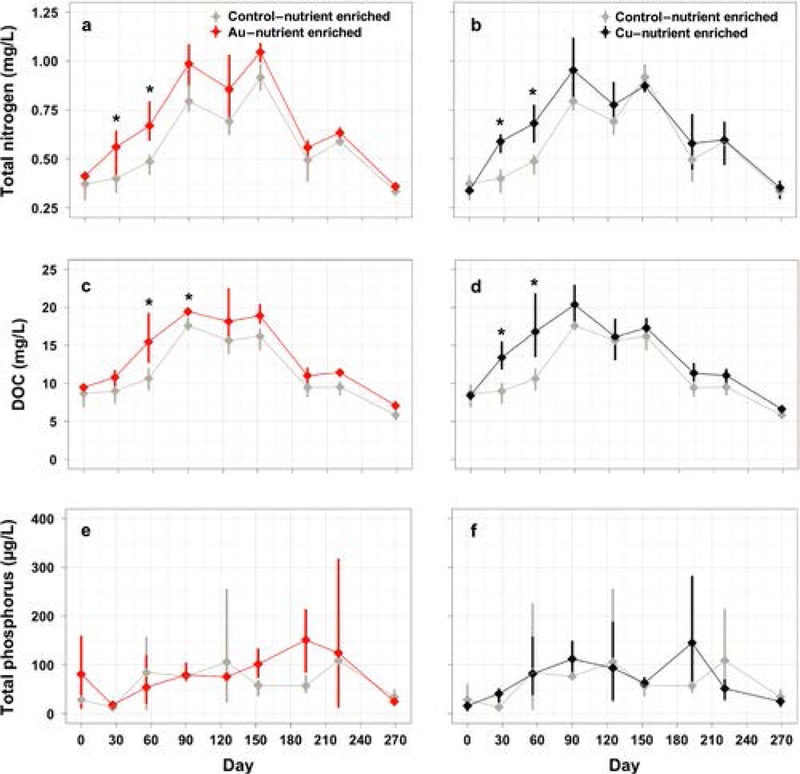
Total nitrogen (a, b), dissolved organic carbon (c, d), total phosphorus (e, f) in the water column in the nutrient enriched treatments only. The AuNP (a, c, e) and CuNP (b, d, f) nutrient enriched treatments are displayed in different panels along with their respective control treatment means and 95% confidence intervals are presented and significant effects are indicated by asterisks.
2. Repeated nanomaterial exposure in nutrient enriched conditions increased algal bloom frequency and magnitude
On multiple dates, phytoplankton biomass (measured as water column Chl-a) was significantly higher in the AuNP-Nutrient Enriched (Fig. 2b) and CuNP-Nutrient Enriched treatments (Fig. 2d) compared to the Control-Nutrient Enriched treatment. In contrast, we never observed a significant Chl-a response to nutrients added without NPs (significant NP × nutrient interaction, p = 0.006). In the absence of nutrient enrichment, the Chl-a concentration was significantly higher or lower compared to the Control-Ambient Nutrient treatment at several dates in both CuNP (higher at 2 dates, lower at 1 date, Fig. 2c) and AuNP (higher at 3 dates, lower at 3 dates, Fig. 2a) (overall NP effect, p < 0.001). The highest Chl-a concentrations recorded in this experiment were observed in the AuNP-Nutrient Enriched treatment (>30 μg/L) during major algal bloom events (between Day 150 and 210, summer season). Over the course of the experiment, the number of days when a large algal bloom was observed was 3.5 times higher in the Nutrient Enriched AuNPs and CuNPs exposed mesocosms (77 to 79 days) than in the Control-Nutrient enriched mesocosms (23 days, Fig. 3). In the absence of nutrient enrichment, the number of algal bloom days was not significantly different in the mesocosms exposed to NPs and Control conditions (Fig. 3).
Fig. 2.
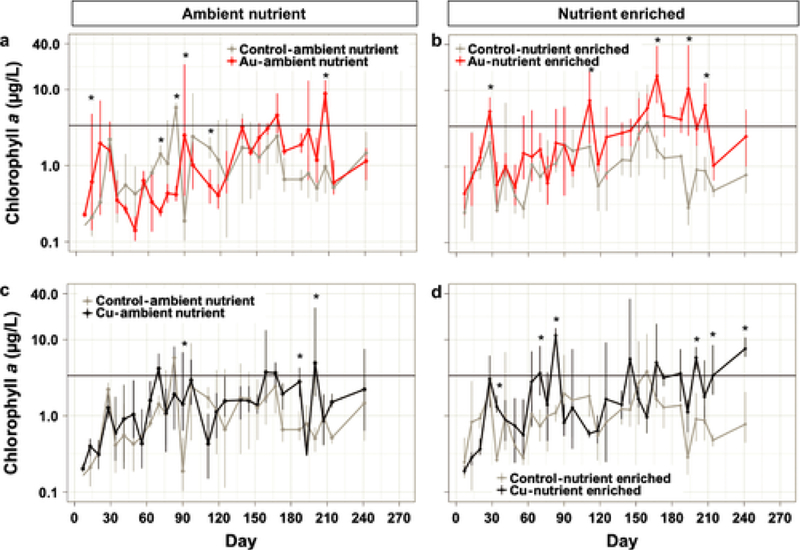
Chlorophyll a concentration on a log-scale in the surface water over the 9 months of the experiment. The different treatments are displayed in different panels along with their respective control treatment: (a) AuNP-Ambient Nutrient, (b) AuNP-Nutrient Enriched, (c) CuNP-Ambient Nutrient, (d) CuNP-Nutrient Enriched. Significant effects are indicated by an asterisk. The horizontal line represents the threshold at which a major algal bloom event was observed.
Fig. 3.
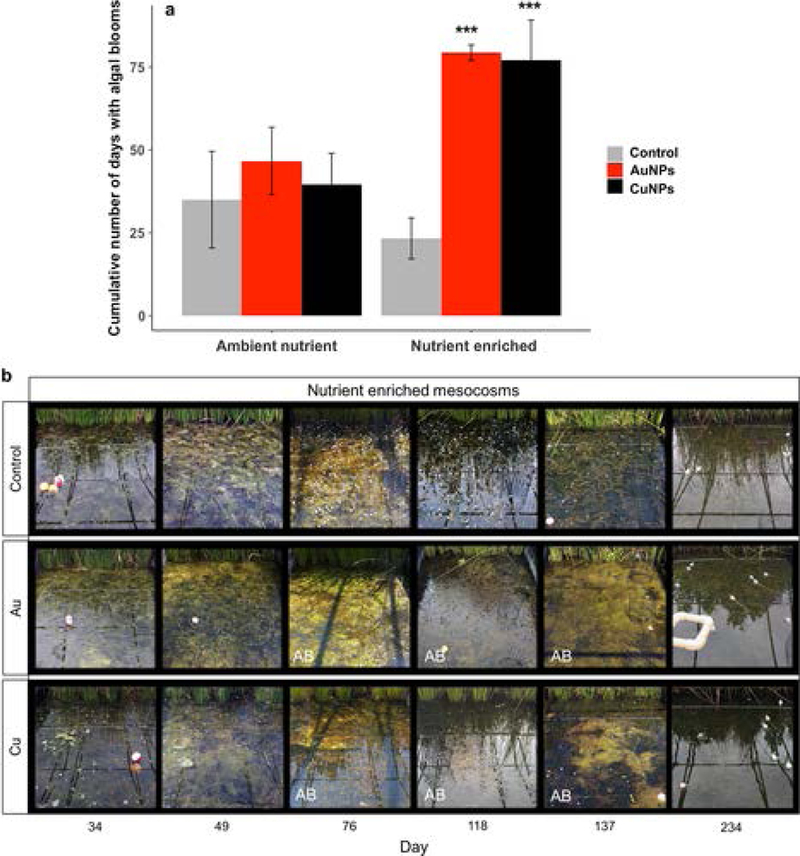
a) Cumulative number of algal bloom days in each treatment. Asterisks denote significant (P ≤ 0.001) difference between each NP treatment and the control at the same nutrient level. b) Photographs of the aquatic zone of three representative mesocosms in the Nutrient Enriched Control, AuNP and CuNP treatments at six time points during the experiment. Photographs of mesocosms experiencing a major algal bloom are indicated with the letters “AB”. The small white forms that we can observe on Day 76, 117 and 138 in the control mesocosm are Egeria densa flowers.
3. Consequences to dissolved oxygen and metabolism rates
Under nutrient enriched conditions, pre-dawn dissolved oxygen (DO) levels were consistently lower than nutrient enriched controls in both the AuNP treatment between Day 20 and 116 (Fig. 4b) and in the CuNP treatment between Day 20 and 63 (Fig. 4d). NPs alone had no consistent effect on DO concentrations in the absence of nutrient enrichment (Figs. 4a, 4c). All mesocosms had pre-dawn DO concentrations consistently below 10% throughout the summer (Day 132 and 234) and were predominantly hypoxic.
Fig. 4.
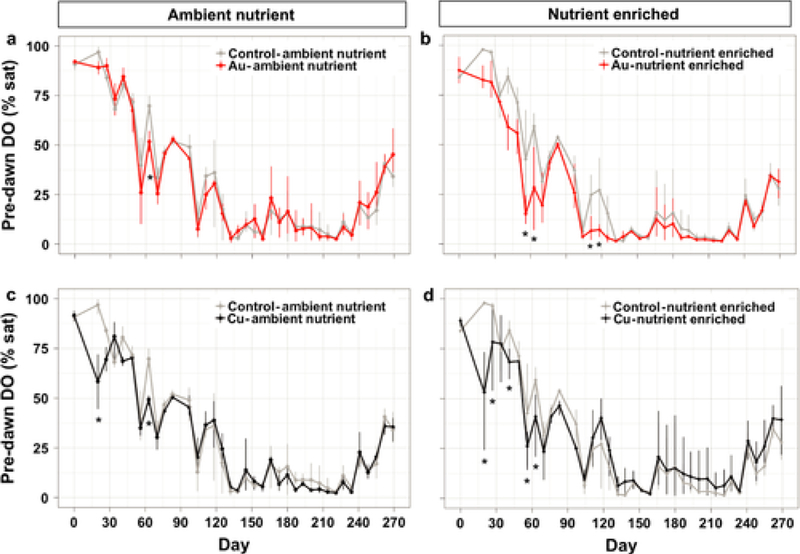
Dissolved oxygen saturation at the surface water before dawn over the 9 months of the experiment. The different treatments are displayed in different panels along with their respective control treatment: (a) in AuNP-Ambient Nutrient, (b) AuNP-Nutrient Enriched, (c) CuNP-Ambient Nutrient, (d) CuNP-Nutrient Enriched. Asterisks denote significant (P ≤ 0.05) difference between NP treatment and control of each sampling date.
Gross primary productivity (GPP) and Ecosystem Respiration (ER) varied widely over the course of the experiment and the highest ecosystem metabolism rates were observed in the spring (Day 86, Fig. 5). The metabolism rates were only significantly affected by the AuNP-Nutrient Enriched treatment (Fig. 5). In this treatment, GPP was suppressed to near zero on 4 measurement dates (Day 155, 156, 191, 192; −91 to −94%), while ER was significantly reduced on one date (Day 155, −94%).
Fig. 5.
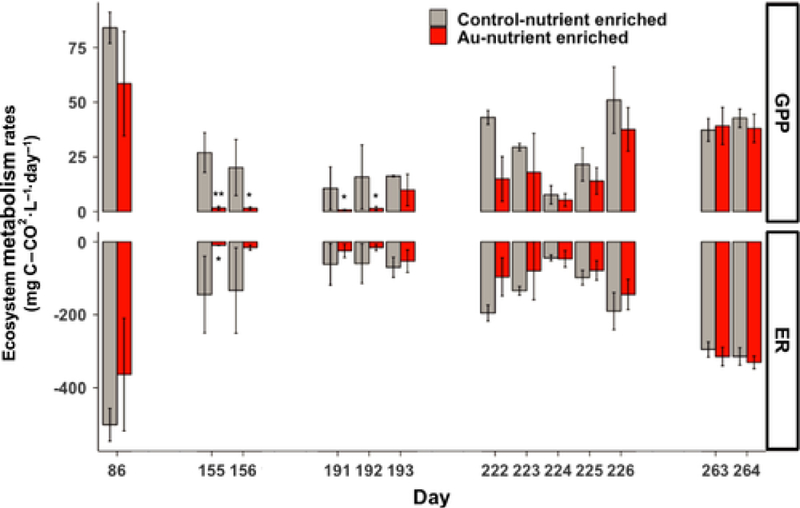
Ecosystem metabolism: Gross Primary Productivity (GPP, top) and Ecosystem Respiration (ER, bottom) in the AuNP and Control-Nutrient Enriched treatments measured at discrete times between Day 86 and the end of the experiment. Asterisks denote significant (* P ≤ 0.05, ** P ≤ 0.01) difference between NP treatment and control of each sampling date.
4. Physiological effects of Au and CuNPs and bioaccumulation in Egeria densa
In the AuNP-Ambient Nutrient treatment, photosynthesis rates (measured on new stems on Days 90, 193, and 269) were significantly reduced at all dates (−22% to −39%, Fig. 6), while in the AuNP-Nutrient Enriched treatment, photosynthesis was decreased only on Day 90 (−42%, Fig. 6). On Day 90, a reduction of the respiration rates was found in the nutrient enriched AuNP mesocosms (−63%, Fig. 6) and on Day 193 in the Ambient Nutrient AuNPs mesocosms (−63%, Fig. 6). In the mesocosms exposed to CuNPs, no effects were observed on Egeria photosynthesis, but respiration rates were significantly decreased on Day 90 (−80%, Fig. 6) in the nutrient enriched conditions.
Fig. 6.
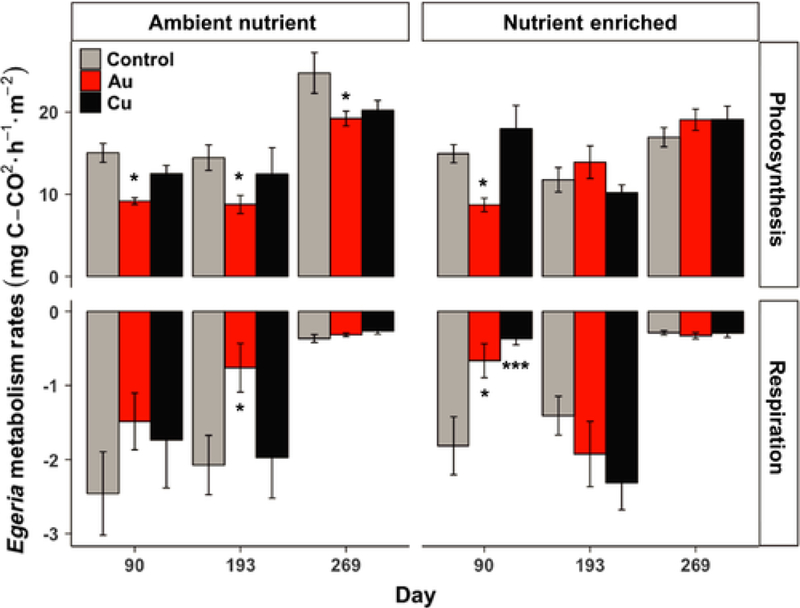
Egeria densa stem photosynthesis (top) and respiration (bottom) rates in the Ambient Nutrient treatments (right) and in the Nutrient Enriched treatments (left). Asterisks denote significant (* P ≤ 0.05, *** P ≤ 0.001) difference between NP treatment and control at the same nutrient level of each sampling date.
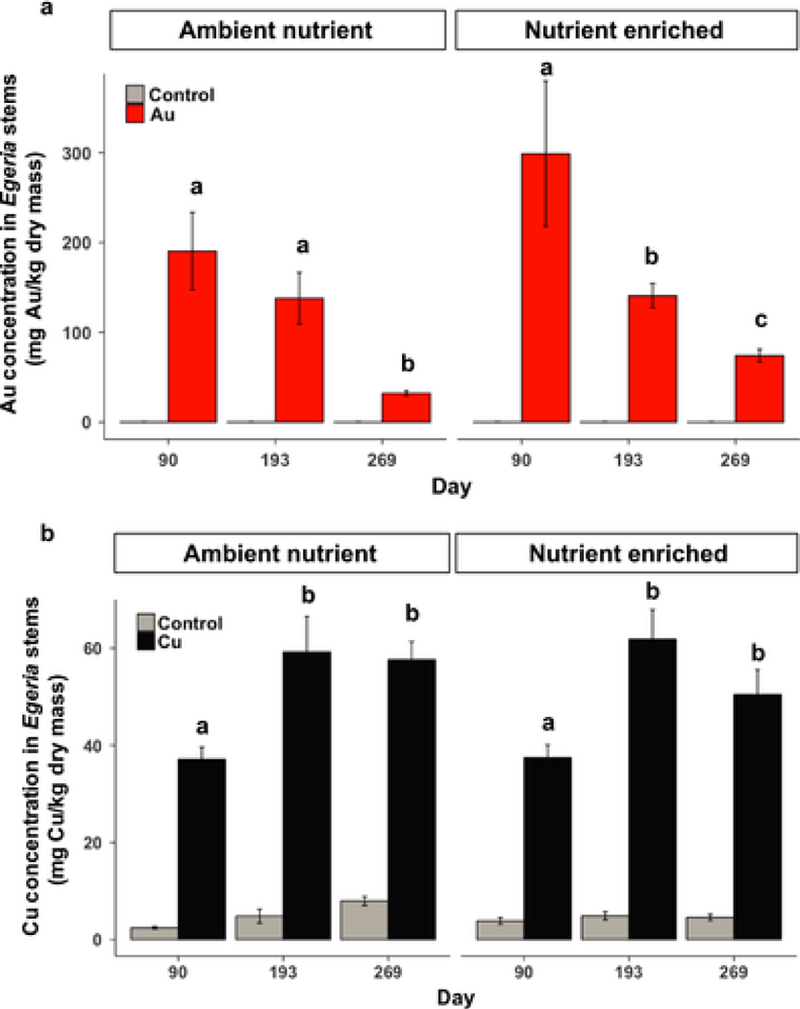
On all destructive sampling dates, Au and Cu concentrations in Egeria stems (new growth) were significantly higher in plants collected from NP treatments than those from controls (p < 0.001, Fig. 7). The highest concentration of Au was observed in Egeria densa stems on Days 90 and 193 with concentrations ranging from 137 to 298 mg/kg on average (Fig. 7a), while the Cu concentrations averaged between 37 and 61 mg/kg during the experiment (Fig. 7b). The Au concentrations decreased significantly over time in Egeria densa stems, especially between day 193 and 269. In both Ambient Nutrient and Nutrient Enriched conditions, Cu concentrations increased between Day 90 and 193 and then remained stable to Day 269.
Fig. 7.
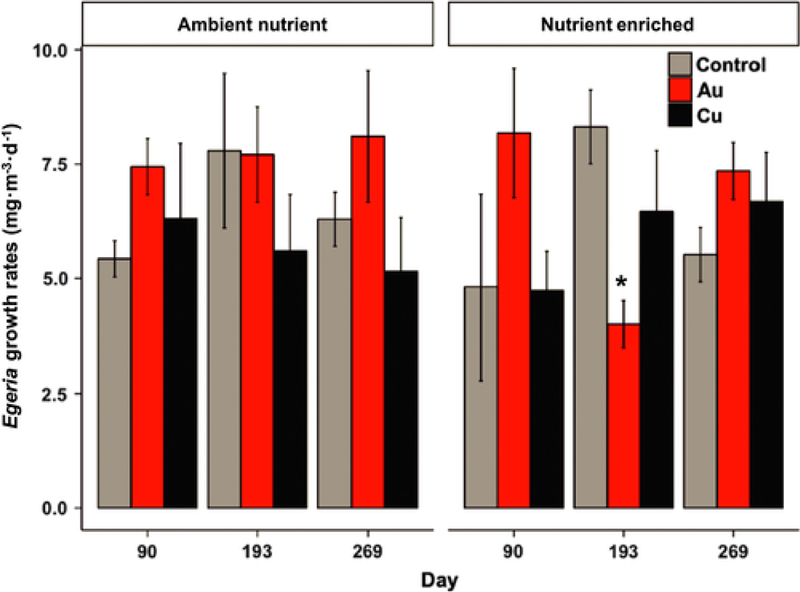
Egeria densa growth rates in the Ambient Nutrient treatments (right) and in the Nutrient Enriched treatments (left). Asterisk denotes significant (P ≤ 0.05) difference between NP treatment and control at the same nutrient level of each sampling date.
During the same months, Egeria densa growth rates were measured in mesh columns placed in the water column and the highest growth rates in the Controls were observed during the summer (Day 193, Fig. 8). The Nutrient Enrichment treatment did not significantly alter Egeria growth rates (p = 0.82). The only significant treatment effect observed was a 52% decline in Egeria growth rates in the AuNP-Nutrient Enriched on Day 193 (Fig. 8).
Fig. 8.
Gold (a) and copper (b) concentrations (mg of metal per kg of plant, dry weight) in Egeria densa stems. Note that the y axis scales are different between the two graphs. Different letters indicate significantly different dates in the same treatment. Within each nutrient level, treatment means of the three sampling dates annotated by the same letters are not discernible at α = 0.05.
DISCUSSION
We found that when added together with nutrients, AuNPs enhanced the frequency and duration of algal blooms and this was accompanied by a reduction in macrophyte growth and photosynthesis, leading to extended periods of water column hypoxia and reduced ecosystem productivity. Adding CuNPs in combination with nutrient enrichment caused similar increases in algal blooms and reductions in dissolved oxygen, but these changes were not accompanied by a decline in ecosystem productivity. In the absence of nutrient enrichment, there were no consistent effects of AuNPs or CuNPs on primary producers and whole ecosystem behavior.
Larger impacts of gold nanoparticles than copper-based nanopesticide
We were surprised to discover that our “nanoparticle-tracers,” AuNPs, caused larger and more frequent ecosystem responses than the Cu-based nanopesticide. The macrophyte response to AuNPs could result from a direct effect of the rapid Au accumulation into Egeria densa in the first 6 months of the experiment, while CuNPs showed both less bioaccumulation and non-significant effects on leaf-level Egeria densa physiology. These patterns in bioaccumulation and impact may be related to the contrasting behavior of CuNPs and AuNPs in the water column. The CuNPs used in this experiment had a larger initial size than the AuNPs, showed evidence of aggregation, but also rapidly dissolved in situ in mesocosm water (t1/2~8h) and dissolved Cu2+ was likely complexed by dissolved organic matter (Vencalek et al. 2016). In contrast, AuNPs were less aggregated in the water column (~10 nm) and are assumed to have low solubility under environmental conditions (Lee and Ranville 2012). The two metal NPs were dosed at different concentrations in the mesocosms, but our results still suggest that under these environmentally realistic conditions, the metal bioaccumulation rate in an aquatic plant was higher when exposed to the small and stable AuNPs than the much more soluble CuNPs.
We expected Egeria tissue metal concentrations to be similar at each harvest since the stems collected were about the same age (new growth) and were exposed to a similar water column NP concentrations. This expectation was met for CuNPs but not for AuNPs. Tissue Cu concentrations in Egeria was stable across the 3 harvest dates. However, in the AuNP treated mesocosms, the highest Au accumulation was measured on Day 90 and then tissue Au concentration in Egeria densa stems declined over the remaining six months of the experiment. This result raises a number of questions about the environmental variables that mediate AuNP uptake in plants. It is unlikely that these large decreases were explained by higher plant turnover or regulation of Au concentrations in tissue but they may have been controlled by an external factor sensitive to seasonal variations and/or NP dosing. We speculate that changes in the composition and capacity of the periphyton growing on Egeria stems could be responsible for the differences in bioaccumulation over time that we observed. Periphyton can act as a barrier to prevent contaminant uptake by the macrophytes (e.g. NPs strongly adsorbed to the biofilm) or could be involved in the facilitation of the uptake of NPs by mediating their dissolution or modifying NP aggregation and surface properties (Schwab et al. 2016). It is also possible that E. densa exudes a sulfhydryl rich metal binding protein, which could modulate the bioavailability of Au as it has been previously shown for AgNPs (Bone et al. 2012, Unrine et al. 2012).
We cannot resolve whether the higher tissue concentrations or a higher toxicity of AuNPs led to more severe effects of AuNPs than CuNPs in this experiment. In previous lab based assays, metal NPs have been reported to reduce both algal and macrophyte growth and photosynthesis (Hoecke et al. 2013, Thwala et al. 2016). In these studies, researchers typically attribute these negative impacts on increases in oxidative stress or membrane damage caused by the NPs but the literature is scarce on the specific toxicity mechanisms of AuNPs in plants and algae. However, one study in Caenorhabditis elegans demonstrated that citrate coated AuNPs can cause unfolded protein response and endoplasmic reticulum stress by denaturing proteins that they come into contact with (Tsyusko et al., 2012).
Despite the known toxicity of Cu to aquatic organisms, Cu from CuNPs was less bioavailable and had less impact on autotrophs than AuNPs. The study of the toxicity and ecological impacts of AuNP has received less attention than other NPs, such as Ag-NPs and TiO2-NPs, because of their lower industrial production rates. The use of AuNPs in pharmaceuticals is growing (bioimaging, gene/drug delivery, phototherapy, home diagnostics) (Ghosh et al. 2008, Rossi et al. 2016) but widespread environmental exposure to this contaminant is unlikely. The important implications of this study for future ecological research on contaminant impacts are the recognition that lab based toxicity studies can have limited power to predict ecological impact. What is needed is research that uncovers the mechanisms through which AuNPs can disrupt and alter ecosystem processes so that we can prevent environmental exposures of contaminants possessing similar traits.
Metal nanoparticle contaminants can intensify eutrophication in wetland ecosystems
We hypothesized that nutrient enrichment would alter the ecological impact of NPs and we indeed found multiple lines of evidence showing interactive effects between nutrient enrichment and NPs. We observed very limited effects of both AuNPs and CuNPs in the absence of nutrient enrichment, but when added together with nutrients, both NPs exacerbated the adverse effects of eutrophication in wetlands (algal blooms, hypoxia, and decreased productivity). This experiment suggests that nutrient status can greatly influence the ecosystem-scale impact of emerging contaminants, and that metal-based synthetic chemicals may be playing an under-appreciated role in the global trends of increasing eutrophication (Heisler et al. 2008, Smith and Schindler 2009).
We found little evidence that nutrient enrichment directly affect NP properties, with neither NP aggregation nor surface charge being affected by nutrient enrichment. The rate of AuNP and CuNP assimilation into macrophyte tissues was unaffected by nutrient enrichment, suggesting that other unmeasured properties of the NPs (e.g. organic coatings, surficial chemistry) were not significantly altered by the nutrient regime. Together these results suggest that there are limited direct interactions between nutrient status and the bioavailability or surficial reactivity of these NPs. However, our results demonstrate that nutrient enrichment can alter organismal responses to NPs. Photosynthetic rates of individual Egeria plants were more suppressed by AuNPs in the absence of nutrient enrichment than under high nutrient conditions.
Under low nutrient conditions, the energetic costs to take up nutrients might be more important and thus limiting the energy available to be invested to offset the effects of external stressors. This result supports the nutrient rescue hypothesis, that high nutrient availability allows individuals to invest in detoxification responses or compensate for the energetic costs of contaminant stress more easily (Leflaive et al. 2015, Aristi et al. 2016). Despite this finding at the individual plant scale, this ‘rescue’ did not propagate to ecosystem scales where we consistently observed ecological responses to NP exposure only in the nutrient enriched treatments.
Under nutrient enriched conditions, AuNPs and CuNPs were associated with an increased frequency and magnitude of algal blooms in these experimental systems. These algal blooms led to decreases in dissolved oxygen in the water column and lowered primary productivity after 5 and 6 months of AuNP exposures (late spring-summer season). The extended algal bloom in the AuNPs-Nutrient Enriched treatment lead to 91–94% declines in ecosystem GPP. We hypothesize that this pronounced response arises from relatively small disparities in the effects of our contaminant stressor on algae vs. macrophytes (Figure 9). In the presence of the NPs, floating algae were better able to capitalize on the nutrients added in our nutrient enrichment treatments and thus could shade out their benthic competitors. We hypothesize that the 52% of the reduction we observed in macrophyte growth rates in the AuNPs – Enriched Nutrient treatment resulted from altered competitive interactions (in particular shading) rather than from direct AuNP toxicity.
Fig. 9.
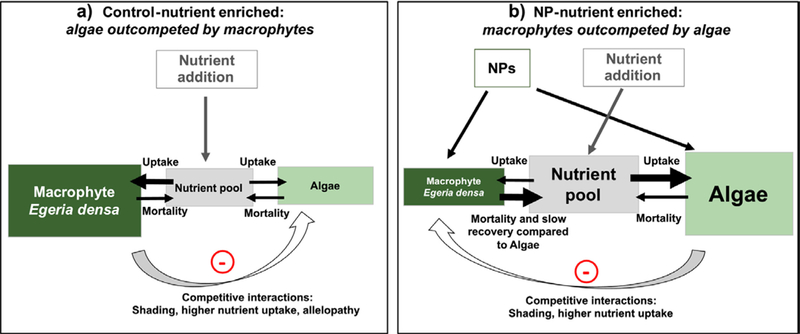
Conceptual diagram representing our hypothesis of how nutrient availability in the water column and the competitive interactions between macrophytes and algae are modified under (A) nutrient enrichment alone compared to (B) nutrient enrichment combined with NPs additions.
There are many direct and indirect mechanisms that may explain how NPs and nutrients together caused the observed increased magnitude and duration of bloom conditions in the summer months of the experiment. In the first three months of the experiment, total N and DOC concentrations increased by 30 to 60% in the water columns of mesocosms in the NPs – Nutrient Enriched treatments compared to the nutrient only controls. These increases could be explained by mortality of the algae and macrophytes immediately after we initiated the contaminant exposure (previously observed by Roussel et al. 2007, Colman et al. 2014) or by an increase in consumer excretion rates in response to contaminant exposure (Taylor et al. 2016). Either mechanism could explain the internal eutrophication we observed in the presence of NPs, and could explain the observed intensification of the effects of our nutrient enrichment treatment. This compounding of internal mineralization with nutrient enrichment likely generated the appropriate conditions for the cyclical algal blooms observed in the NPs-Nutrient Enriched treatments.
An alternative explanation for the observed algal blooms is that the NP exposures selected for tolerant or resistant algal taxa that were then competitively superior to macrophytes under NP exposure scenarios. Since our mesocosms contained only a single macrophyte species, such community adaptation was not possible for these benthic plants. We speculate that the slower growth and recovery rates of macrophytes relative to algae would disadvantage these benthic autotrophs. Adding to the complexity of this competitive interaction, previous studies have documented that increased nutrient availability or NP stress can decrease the production of allelopathic secondary compounds (e.g. phenolics, flavonoids) by macrophytes (Richardson et al. 1999, Gross et al. 2007, Wang et al. 2011a). Such a shift in resource allocation by macrophytes would further advantage their planktonic competitors. Altogether, these results suggest that early in the experiment, mesocosms exposed to nutrient enrichment and AuNPs saw a combination of an initial increase in nutrient availability and modifications in competitive interactions between algae and macrophytes (Fig. 9). This combination of direct and indirect effects of NPs likely contributed to the cascading effects that caused a suppression of ecosystem productivity four to five months later during algal blooms.
Our results provide an example of emerging contaminants having large ecosystem-level impacts on aquatic ecosystems, with both AuNPs and CuNPs causing higher frequency and magnitude of algal blooms. Our experiment also demonstrated that such impacts are highly context dependent, as we only observed these ecosystem impacts when NPs were superimposed with nutrient enrichment. Studying the interactive effects of multiple stressors in realistic biological systems is crucial, as this work again confirms that their consequences on ecosystem structure and function are very difficult to predict. While many questions about the mechanisms responsible for these effects remain unanswered, our findings provide evidence that synthetic chemicals such as metal NPs can exacerbate aquatic ecosystem eutrophication.
CONCLUSIONS
Perhaps the most fascinating outcome of this experiment was the discovery that the addition of small amounts of a single synthetic nanoparticle could in fact catalyze the transformation of our wetland ecosystems from clear waters to turbid waters with large floating algal mats. The increasing frequency and magnitude of algal blooms is expected to increase with climate change but the role of synthetic chemicals, like metal pollutants or pharmaceuticals in these events is not currently considered (Heisler et al. 2008, O’Neil et al. 2012). The effects observed here might be specific to these metal NP contaminants but we postulate that any chemical contaminant that causes differential stress for algae relative to macrophytes or modifies nutrient availability has the potential to intensify eutrophication (Figure 9). This experiment shows that large declines in the rates of ecosystem productivity and changes in the relative dominance of different autotrophs can result from sub-lethal effects at very low concentrations. We cannot determine from the current experiment whether AuNPs reduced the ability of macrophytes to compete for nutrients and produce allelochemicals or if AuNPs selected for bloom-forming algal species. Further work should explore how each of these competitive interactions may be affected by contaminant exposure.
This study also shows that the ability to detect these ecosystem level effects is highly sensitive to the timing of experimentation and sampling. The most marked treatment responses were observed during the warmest months of the year and dissipated in the subsequent autumn, even as we continued to add both nutrients and NPs to the mesocosms. This suggests that the effect of these materials—and perhaps many conventional contaminants—may be most pronounced and most measurable during periods of intense competition, which in many aquatic ecosystems are characterized by warm temperatures, high rates of grazing pressure, and high productivity by both macrophytes and algae.
Supplementary Material
ACKNOWLEDGEMENTS
We want to thank Ethan Baruch, Joseph Delesantro, Eric Moore, Erin Vanderjeugdt, Samuel Mahanes, Henry Camp, Jennifer Rocca, Bradley Shewmaker, Lizzy Stokes-Cawley, Mathieu Therezien, Nathan Bossa, Sayako Inoue, Jane Cooper, Benjamin Castellon and Meredith Frenchmeyer for their help during the set-up of this experiment and numerous field work days. Matthew T. Ruis was supported by a training grant from NIEHS (T32-ES021432). This work was supported by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, Center for the Environmental Implications of Nanotechnology (CEINT). Additional funds for graduate student summer support was supplied by the Duke Wetland Center Endowment. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred.
Footnotes
DATA AVAILABILITY STATEMENT
The data used in this article can be found with the following DOI: 10.6084/m9.figshare.6118553 and at this link: https://doi.org/10.6084/m9.figshare.6118553
LITERATURE CITATIONS
- Aristi I, Casellas M, Elosegi A, Insa S, Petrovic M, Sabater S, and Acuña V. 2016. Nutrients versus emerging contaminants–Or a dynamic match between subsidy and stress effects on stream biofilms. Environmental Pollution 212:208–215. [DOI] [PubMed] [Google Scholar]
- Barrena R, Casals E, Colón J, Font X, Sánchez A, and Puntes V. 2009. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857. [DOI] [PubMed] [Google Scholar]
- Berglund O 2003. Periphyton density influences organochlorine accumulation in rivers. Limnology and Oceanography 48:2106–2116 [Google Scholar]
- Bernhardt ES, Rosi EJ, and Gessner MO. 2017. Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment 15:84–90. [Google Scholar]
- Bone AJ, Colman BP, Gondikas AP, Newton KM, Harrold KH, Cory RM, Unrine JM, Klaine SJ, Matson CW, and Di Giulio RT. 2012. Biotic and Abiotic Interactions in Aquatic Microcosms Determine Fate and Toxicity of Ag Nanoparticles:Part 2–Toxicity and Ag Speciation. Environmental Science & Technology 46:6925–6933. [DOI] [PubMed] [Google Scholar]
- Bundschuh M, Seitz F, Rosenfeldt RR, and Schulz R. 2016. Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshwater Biology 61:2185–2196. [Google Scholar]
- Carlson RE 1977. A trophic state index for lakes1. Limnology and Oceanography 22:361–369. [Google Scholar]
- Colman BP, Espinasse B, Richardson CJ, Matson CW, Lowry GV, Hunt DE, Wiesner MR, and Bernhardt ES. 2014. Emerging Contaminant or an Old Toxin in Disguise? Silver Nanoparticle Impacts on Ecosystems. Environmental Science & Technology 48:5229–5236. [DOI] [PubMed] [Google Scholar]
- Dale AL, Lowry GV, and Casman EA. 2015. Stream Dynamics and Chemical Transformations Control the Environmental Fate of Silver and Zinc Oxide Nanoparticles in a Watershed-Scale Model. Environmental Science & Technology 49:7285–7293. [DOI] [PubMed] [Google Scholar]
- Das P, Metcalfe CD, and Xenopoulos MA. 2014. Interactive Effects of Silver Nanoparticles and Phosphorus on Phytoplankton Growth in Natural Waters. Environmental Science & Technology 48:4573–4580. [DOI] [PubMed] [Google Scholar]
- Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, Murphy CJ, and Shaw TJ. 2009. Transfer of gold nanoparticles from the water column to the estuarine food web. Nature Nanotechnology 4:441. [DOI] [PubMed] [Google Scholar]
- Fulton BA, Brain RA, Usenko S, Back JA, King RS, and Brooks BW. 2009. Influence of nitrogen and phosphorus concentrations and ratios on Lemna gibba growth responses to triclosan in laboratory and stream mesocosm experiments. Environmental Toxicology and Chemistry 28:2610–2621. [DOI] [PubMed] [Google Scholar]
- Gessner MO, and Tlili A. 2016. Fostering integration of freshwater ecology with ecotoxicology. Freshwater Biology 61:1991–2001. [Google Scholar]
- Ghosh P, Han G, De M, Kim CK, and Rotello VM. 2008. Gold nanoparticles in delivery applications. Advanced Drug Delivery Reviews 60:1307–1315. [DOI] [PubMed] [Google Scholar]
- Glenn JB, and Klaine SJ. 2013. Abiotic and Biotic Factors That Influence the Bioavailability of Gold Nanoparticles to Aquatic Macrophytes. Environmental Science & Technology 47:10223–10230. [DOI] [PubMed] [Google Scholar]
- González-Alonso S, Merino LM, Esteban S, López de Alda M, Barceló D, Durán JJ, López-Martínez J, Aceña J, Pérez S, Mastroianni N, Silva A, Catalá M, and Valcárcel Y. 2017. Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environmental Pollution 229:241–254. [DOI] [PubMed] [Google Scholar]
- Gross EM, nee Körner SH, Lombardo P, and Mulderij G. 2007. Searching for allelopathic effects of submerged macrophytes on phytoplankton—state of the art and open questions. Hydrobiologia 584:77–88. [Google Scholar]
- Hall SR, Leibold MA, Lytle DA, and Smith VH. 2004. Stoichiometry and Planktonic Grazer Composition Over Gradients of Light, Nutrients, and Predation Risk. Ecology 85:2291–2301. [Google Scholar]
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, and Suddleson M. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecke KV, Schamphelaere KACD, Ali Z, Zhang F, Elsaesser A, Rivera-Gil P, Parak WJ, Smagghe G, Howard CV, and Janssen CR. 2013. Ecotoxicity and uptake of polymer coated gold nanoparticles. Nanotoxicology 7:37–47. [DOI] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, and Law KL. 2015. Plastic waste inputs from land into the ocean. Science 347:768–771. [DOI] [PubMed] [Google Scholar]
- Kah M 2015. Nanopesticides and Nanofertilizers: Emerging Contaminants or Opportunities for Risk Mitigation? Frontiers in Chemistry 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, Cherr GN, Hong J, Gardea-Torresdey JL, Godwin HA, Hanna S, Ji Z, Kaweeteerawat C, Lin S, Lenihan HS, Miller RJ, Nel AE, Peralta-Videa JR, Walker SL, Taylor AA, Torres-Duarte C, Zink JI, and Zuverza-Mena N. 2017. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7:28–40. [Google Scholar]
- King RS, Brain RA, Back JA, Becker C, Wright MV, Toteu Djomte V, Scott WC, Virgil SR, Brooks BW, Hosmer AJ, and Chambliss CK. 2016. Effects of pulsed atrazine exposures on autotrophic community structure, biomass, and production in field-based stream mesocosms. Environmental Toxicology and Chemistry 35:660–675. [DOI] [PubMed] [Google Scholar]
- Kong X, He W, Qin N, Liu W, Yang B, Yang C, Xu F, Mooij WM, and Koelmans AA. 2017. Integrated ecological and chemical food web accumulation modeling explains PAH temporal trends during regime shifts in a shallow lake. Water Research 119:73–82. [DOI] [PubMed] [Google Scholar]
- Lee B-T, and Ranville JF. 2012. The effect of hardness on the stability of citrate-stabilized gold nanoparticles and their uptake by Daphnia magna. Journal of Hazardous Materials 213–214:434–439. [DOI] [PubMed] [Google Scholar]
- Leflaive J, Felten V, Ferriol J, Lamy A, Ten-Hage L, Bec A, and Danger M. 2015. Community structure and nutrient level control the tolerance of autotrophic biofilm to silver contamination. Environmental Science and Pollution Research 22:13739–13752. [DOI] [PubMed] [Google Scholar]
- Lewis SL, and Maslin MA. 2015. Defining the Anthropocene. Nature 519:171–180. [DOI] [PubMed] [Google Scholar]
- Lin R, Buijse L, Dimitrov MR, Dohmen P, Kosol S, Maltby L, Roessink I, Sinkeldam JA, Smidt H, Wijngaarden RPAV, and Brock TCM. 2012. Effects of the fungicide metiram in outdoor freshwater microcosms: responses of invertebrates, primary producers and microbes. Ecotoxicology 21:1550–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry GV, Espinasse BP, Badireddy AR, Richardson CJ, Reinsch BC, Bryant LD, Bone AJ, Deonarine A, Chae S, Therezien M, Colman BP, Hsu-Kim H, Bernhardt ES, Matson CW, and Wiesner MR. 2012. Long-Term Transformation and Fate of Manufactured Ag Nanoparticles in a Simulated Large Scale Freshwater Emergent Wetland. Environmental Science & Technology 46:7027–7036. [DOI] [PubMed] [Google Scholar]
- Mitrano DM, Motellier S, Clavaguera S, and Nowack B. 2015. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environment International 77:132–147. [DOI] [PubMed] [Google Scholar]
- Norman BC, Xenopoulos MA, Braun D, and Frost PC. 2015. Phosphorus Availability Alters the Effects of Silver Nanoparticles on Periphyton Growth and Stoichiometry. PLOS ONE 10:e0129328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JM, Davis TW, Burford MA, and Gobler CJ. 2012. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 14:313–334. [Google Scholar]
- Pace ML, Batt RD, Buelo CD, Carpenter SR, Cole JJ, Kurtzweil JT, and Wilkinson GM. 2017. Reversal of a cyanobacterial bloom in response to early warnings. Proceedings of the National Academy of Sciences 114:352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, and Blum JD. 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proceedings of the National Academy of Sciences 99:4419–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
- Regier N, Cosio C, von Moos N, and Slaveykova VI. 2015. Effects of copper-oxide nanoparticles, dissolved copper and ultraviolet radiation on copper bioaccumulation, photosynthesis and oxidative stress in the aquatic macrophyte Elodea nuttallii. Chemosphere 128:56–61. [DOI] [PubMed] [Google Scholar]
- Rice PJ, McConnell LL, Heighton LP, Sadeghi AM, Isensee AR, Teasdale JR, Abdul-Baki AA, Harman-Fetcho JA, and Hapeman CJ. 2001. Runoff loss of pesticides and soil: a comparison between vegetative mulch and plastic mulch in vegetable production systems. Journal of Environmental Quality 30:1808–1821. [DOI] [PubMed] [Google Scholar]
- Richardson CJ, Ferrell GM, and Vaithiyanathan P. 1999. Nutrient Effects on Stand Structure, Resorption Efficiency, and Secondary Compounds in Everglades Sawgrass. Ecology 80:2182–2192. [Google Scholar]
- Rohr JR, Kerby JL, and Sih A. 2006. Community ecology as a framework for predicting contaminant effects. Trends in Ecology & Evolution 21:606–613. [DOI] [PubMed] [Google Scholar]
- Rossi M, Della Pina C, and Falletta E. 2016. Gold Nanomaterials: From Preparation to Pharmaceutical Design and Application. Current Pharmaceutical Design 22:1485–1493. [DOI] [PubMed] [Google Scholar]
- Roussel H, Ten-Hage L, Joachim S, Le Cohu R, Gauthier L, and Bonzom J-M. 2007. A long-term copper exposure on freshwater ecosystem using lotic mesocosms: Primary producer community responses. Aquatic Toxicology 81:168–182. [DOI] [PubMed] [Google Scholar]
- Sabo-Attwood T, Unrine JM, Stone JW, Murphy CJ, Ghoshroy S, Blom D, Bertsch PM, and Newman LA. 2012. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6:353–360. [DOI] [PubMed] [Google Scholar]
- Sand-Jensen K, and Borum J. 1991. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41:137–175. [Google Scholar]
- Sand-Jensen K, and S0ndergaard M. 1981. Phytoplankton and Epiphyte Development and Their Shading Effect on Submerged Macrophytes in Lakes of Different Nutrient Status. Internationale Revue der gesamten Hydrobiologie und Hydrographie 66:529–552. [Google Scholar]
- Servin A, Elmer W, Mukherjee A, la Torre-Roche RD, Hamdi H, White JC, Bindraban P, and Dimkpa C. 2015. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. Journal of Nanoparticle Research 17:1–21. [Google Scholar]
- Simonin M, and Richaume A. 2015. Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review. Environmental Science and Pollution Research 22: 13710–13723. [DOI] [PubMed] [Google Scholar]
- Skei J, Larsson P, Rosenberg R, Jonsson P, Olsson M, and Broman D. 2000. Eutrophication and Contaminants in Aquatic Ecosystems. AMBIO: A Journal of the Human Environment 29:184–194. [Google Scholar]
- Smith VH, and Schindler DW. 2009. Eutrophication science: where do we go from here? Trends in Ecology & Evolution 24:201–207. [DOI] [PubMed] [Google Scholar]
- Smith VH, Tilman GD, and Nekola JC. 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100:179–196. [DOI] [PubMed] [Google Scholar]
- Stehle S, and Schulz R. 2015. Agricultural insecticides threaten surface waters at the global scale. Proceedings of the National Academy of Sciences 112:5750–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Shijirbaatar A, Fang J, Owens G, Lin D, and Zhang K. 2015. Distinguishable Transport Behavior of Zinc Oxide Nanoparticles in Silica Sand and Soil Columns. Science of The Total Environment 505:189–198. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Back JA, Brooks BW, and King RS. 2016. Consumer-mediated nutrient recycling is influenced by interactions between nutrient enrichment and the antimicrobial agent triclosan. Freshwater Science 35:856–872. [Google Scholar]
- Thwala M, Klaine SJ, and Musee N. 2016. Interactions of metal-based engineered nanoparticles with aquatic higher plants: A review of the state of current knowledge. Environmental Toxicology and Chemistry 35:1677–1694. [DOI] [PubMed] [Google Scholar]
- Tsyusko OV, B PM, Eric B, Greg J, David S, Daniel S, Michael T, and U JM. 2012. Toxicogenomic Responses of the Model Organism Caenorhabditis elegans to Gold Nanoparticles. Environmental Science & Technology. [DOI] [PubMed] [Google Scholar]
- Unrine JM, Colman BP, Bone AJ, Gondikas AP, and Matson CW. 2012. Biotic and Abiotic Interactions in Aquatic Microcosms Determine Fate and Toxicity of Ag Nanoparticles. Part 1. Aggregation and Dissolution. Environmental Science & Technology 46:6915–6924. [DOI] [PubMed] [Google Scholar]
- Valenti TW, Taylor JM, Back JA, King RS, and Brooks BW. 2011. Influence of drought and total phosphorus on diel pH in wadeable streams: Implications for ecological risk assessment of ionizable contaminants. Integrated Environmental Assessment and Management 7:636–647. [DOI] [PubMed] [Google Scholar]
- Van den Brink PJ, Crum SJH, Gylstra R, Bransen F, Cuppen JGM, and Brock TCM. 2009. Effects of a herbicide–insecticide mixture in freshwater microcosms: Risk assessment and ecological effect chain. Environmental Pollution 157:237–249. [DOI] [PubMed] [Google Scholar]
- Van den Brink PJ, Hattink J, Bransen F, Van Donk E, and Brock TCM. 2000. Impact of the fungicide carbendazim in freshwater microcosms. II. Zooplankton, primary producers and final conclusions. Aquatic Toxicology 48:251–264. [DOI] [PubMed] [Google Scholar]
- Vencalek BE, Laughton SN, Spielman-Sun E, Rodrigues SM, Unrine JM, Lowry GV, and Gregory KB. 2016. In Situ Measurement of CuO and Cu(OH)2 Nanoparticle Dissolution Rates in Quiescent Freshwater Mesocosms. Environmental Science & Technology Letters 3:375–380. [Google Scholar]
- Verhoeven JTA, Arheimer B, Yin C, and Hefting MM. 2006. Regional and global concerns over wetlands and water quality. Trends in Ecology & Evolution 21:96–103. [DOI] [PubMed] [Google Scholar]
- Wang C, Lu J, Zhang S, Wang P, Hou J, and Qian J. 2011a. Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans. Ecotoxicology and Environmental Safety 74:1297–1303. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li J, Zhao J, and Xing B. 2011b. Toxicity and Internalization of CuO Nanoparticles to Prokaryotic Alga Microcystis aeruginosa as Affected by Dissolved Organic Matter. Environmental Science & Technology 45:6032–6040. [DOI] [PubMed] [Google Scholar]
- Winslow LA, Zwart JA, Batt RD, Dugan HA, Woolway RI, Corman JR, Hanson PC, and Read JS. 2016. LakeMetabolizer: an R package for estimating lake metabolism from free-water oxygen using diverse statistical models. Inland Waters 6:622–636. [Google Scholar]
- Yin XH, Brock TCM, Barone LE, Belgers JDM, Boerwinkel M-C, Buijse L, van Wijngaarden RPA, Hamer M, and Roessink I. 2018. Exposure and effects of sediment-spiked fludioxonil on macroinvertebrates and zooplankton in outdoor aquatic microcosms. Science of The Total Environment 610–611:1222–1238. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, and Smith GM. 2009. Zero-Truncated and Zero-Inflated Models for Count Data Pages 261–293 Mixed effects models and extensions in ecology with R. Springer, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


