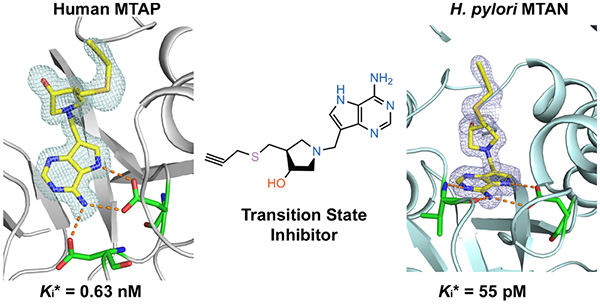
Abstract
Bacterial 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN) hydrolyzes adenine from its substrates to form S-methyl-5-thioribose and S-ribosyl-l-homocysteine. MTANs are involved in quorum sensing, menaquinone synthesis, and 5′-methylthioadenosine recycling to S-adenosylmethionine. Helicobacter pylori uses MTAN in its unusual menaquinone pathway, making H. pylori MTAN a target for antibiotic development. Human 5′-methylthioadenosine phosphorylase (MTAP), a reported anticancer target, catalyzes phosphorolysis of 5′-methylthioadenosine to salvage S-adenosylmethionine. Transition-state analogues designed for HpMTAN and MTAP show significant overlap in specificity. Fifteen unique transition-state analogues are described here and are used to explore inhibitor specificity. Several analogues of HpMTAN bind in the picomolar range while inhibiting human MTAP with orders of magnitude weaker affinity. Structural analysis of HpMTAN shows inhibitors extending through a hydrophobic channel to the protein surface. The more enclosed catalytic sites of human MTAP require the inhibitors to adopt a folded structure, displacing the phosphate nucleophile from the catalytic site.
Graphical Abstract
INTRODUCTION
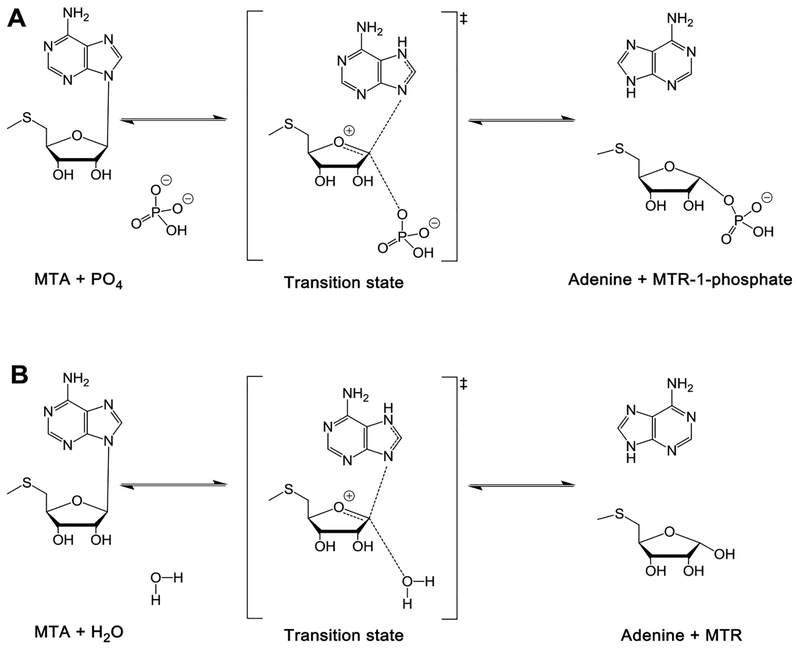
S-Adenosylmethionine (SAM) is involved in biological methylation reactions, in polyamine biosynthesis, and as a precursor of glutathionine.1,2 Two molecules of 5′-methylthioadenosine (MTA) are formed from SAM in the synthesis of each spermine molecule (Scheme 1). In humans, MTA is metabolized only by methylthioadenosine phosphorylase (MTAP) to form 5-methylthio-α-d-ribose 1-phosphate (MTR) and adenine. These are precursors for methionine and ATP, which can be recycled to SAM (Figure 1).3,4 Inhibition of MTAP in mammals causes elevated MTA and decreased recycling of MTA to SAM.5,6 Synthetic lethal genetic analysis of MTAP-deleted cancer cell lines indicates the sensitivity of these cell lines to pathways related to SAM-related methyl transfer.7–10 It has also been proposed that inhibitors of MTAP may have anticancer applications alone or in drug combinations, as they demonstrate anticancer properties in mouse xenograft models.5,6
Scheme 1.
Figure 1.
Transition states and reactions catalyzed by MTAP and HpMTAN. (A) MTAP catalyzes the reaction via a ribocationic transition state and phosphate as the nucleophile. Adenine and methylthio-α-d-ribose 1-phosphate are the products. Bonds to the leaving group and the attacking nucleophile are weak, less than 0.1 Pauling bond order, making the reaction more SN1 than SN2 in character. (B) HpMTAN also catalyzes its reaction via a ribocationic transition state. Water acts as the nucleophile. Adenine and methylthio-D-ribose are the products.
Most bacteria express methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN) instead of MTAP. The enzyme hydrolyzes MTA and S-adenosylhomocysteine (SAH) to MTR or S-ribosylhomocysteine (SRH) and adenine, respectively (Scheme 1 and Figure 1). The MTAN reaction product, SRH, is used in the biosynthesis of homoserine lactones to form AI-2 quorum-sensing molecules.11–13 MTAN is not essential in most bacteria, but a few species use the unusual futalosine pathway for the biosynthesis of menaqui-none, where MTAN plays an essential role.14,15 These organisms include the pathogens Helicobacter pylori and Campylobacter jejuni. Inhibition of this pathway is reported to be lethal to H. pylori.16,17 Inhibitors of MTAN are also expected to reduce polyamine biosynthesis and block the production of quorum-sensing autoinducer (AI-2) molecules. For these reasons, human MTAP and bacterial MTAN enzymes are of interest as drug targets.
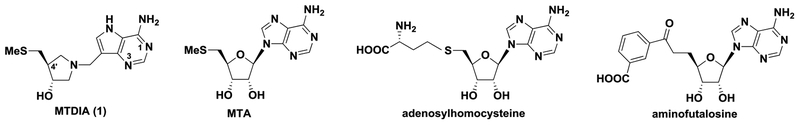
Transition-state analogue enzyme inhibitors have the potential to bind orders of magnitude more tightly than substrates. Studies focused on N-ribosyltransferases have identified transition states with ribocation character.18 MTAP and MTAN share MTA as a substrate and form similar transition states, leading to similar interactions with transition-state analogues (Figure 1).19–21 MTAP inhibitors have shown efficacy in animal models against human tumors, whereas MTAN inhibitors influence bacterial quorum sensing and are antibiotics in organisms using the futalosine pathway of menaquinone synthesis, notably, H. pylori. We have reported transition-state analogues as inhibitors of human MTAP and several bacterial MTAN enzymes.16–26 Of several inhibitory chemical scaffolds, the DADMe-immucillin structure exemplified by MTDIA (1) is optimal for these enzymes (Scheme 1). Both enzymes tolerate substituents in the 4′-position of the 3′-hydroxypyrrolidine ring—in particular, for the bacterial MTAN enzymes. Here, we report on new analogues to explore the structure—activity relationships for human MTAP and HpMTAN.
The transition-state structures of MTAN and MTAP enzymes have been solved by kinetic isotope effect measurements and quantum chemical calculations.19,21,27,28 Transition-state analogues for these enzyme.24–26 Two inhibitors, in particular, methylthio-DADMe-immucillin-A (MTDIA) and parachlorophenylthio-DADMe-immucillin-A (pCl-PhT-DADMe-ImmA), were slow-onset inhibitors with dissociation constants (Ki*) of 86 and 10 pM, respectively, for human MTAP and 86 and 570 pM, respectively, for HpMTAN.17,22 Inhibitors reported here provide insight into the 4′-substituent inhibitor specificity for transition-state analogues of HpMTAN and human MTAP.
RESULTS AND DISCUSSION
Synthesis of New Transition-State Analogue Inhibitors.
Modifications in the 9-Deazaadenine Moiety.
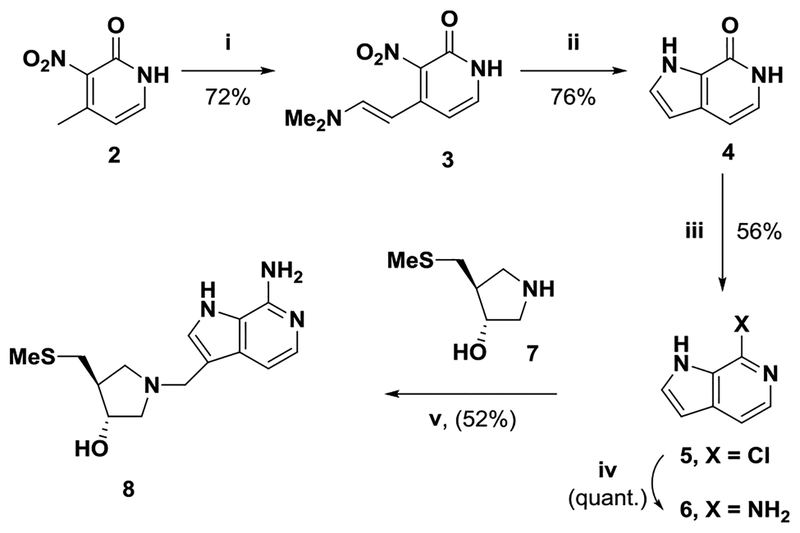
The N3 of 1 makes no catalytic site contacts in MTAP or MTAN enzymes, motivating the synthesis of a 3-deaza-analogue of 1.17,23,29 Treatment of 2-hydroxy-4-methyl-3-nitropyridine (2) with Brederick′s reagent afforded enamine 3 (Scheme 2), which with zinc in acetic acid gave 3,9-dideazahypoxanthine (4). This material was converted to 6-chloro-derivative 5, which on treatment with catalytic copper(I) chloride in aqueous ammonia afforded 3,9-dideazaadenine (6). Mannich reaction of 6 and pyrrolidine 7 gave the desired 3-deazaMTDIA 8.22
Scheme 2.
Reagents: (i) tBuOCH(NMe2)2, DMF, 100 °C;(ii) Zn, HOAc; (iii) POCl3 100 °C; (iv) aq NH3, CuCl, 120 °C; and (v) HCHO, aq EtOH, 80−100 °C
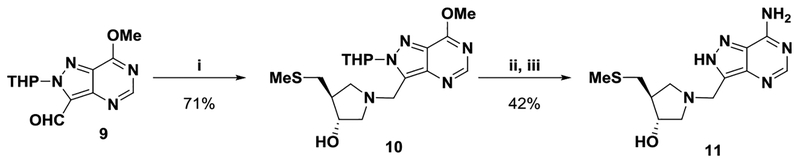
During transition-state analogue design work for purine nucleoside phosphorylase, 8-aza-immucillins were found to be powerful inhibitors.26,30,31 The 8-aza-analogue of 1 was targeted for synthesis. Treatment of aldehyde 932 with pyrrolidine 7 and 2-methylpyridine borane complex gave 10 (Scheme 3). Ammonolysis followed by deprotection afforded 8-aza-MTDIA 11.
Scheme 3.
Reagents: (i) Picoline Borane, 7, MeOH; (ii) 7 N NH3/MeOH, 120 °C; and (iii) aq HCl, MeOH
Modifications in the 5′-Thio Substituent.
Both the human MTAP and HpMTAN enzymes tolerate diversity in the 5′-thio substituents—particularly for the MTANs.24 With this in mind, we prepared 5′-substituted thio-MTDIA analogues by coupling 5′-alkynyl derivatives with some azides using click chemistry.
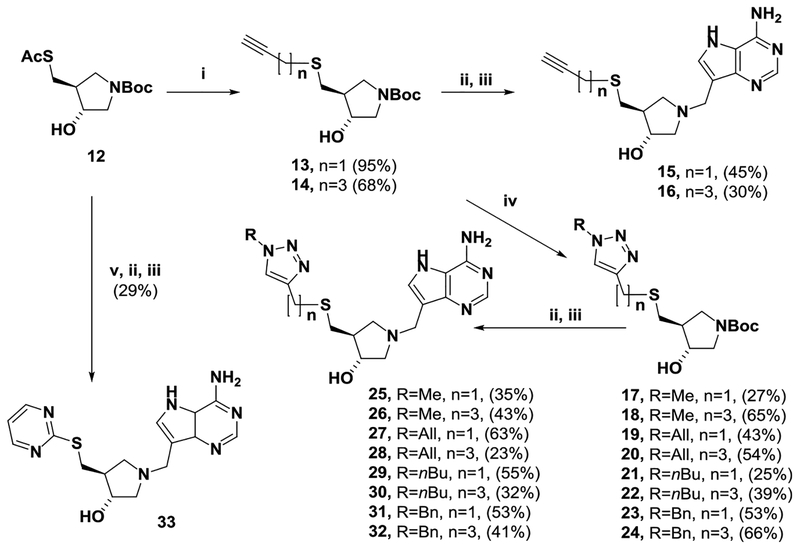
Thioacetate 1217 was treated with sodium methoxide/methanol followed by either propargyl bromide or 5-mesyloxy-pent-1-yne to give the alkynylthio-substituted compounds 13 and 14 (Scheme 4). These compounds, after acidic treatment to remove the Boc group followed by a Mannich reaction with 9-deazaadenine, then afforded 15 and 16. Acetylenes 13 and 14 were individually treated with methyl iodide, allyl bromide, n-butyl bromide, or benzyl bromide along with sodium azide and catalytic copper(I) iodide affording the triazole “click” products 17–24.33 Deprotection followed by a Mannich reaction with 9-deazaadenine34 then provided the 5′-substituted thio-analogues 25–32. 2-Pyrimidinethio analogue 33 was prepared by steps v, ii, and iii (Scheme 4).
Scheme 4.
HCl (conc.) 3:1 v/v; (iii) 9-Deazaadenine, Formaldehyde, EtOH/Water, 70–100 °C (Microwave), 2–6 h; (iv) MeI, Allyl Bromide, nBuBr or BnBr, NaN3, CuI, MeOH; and (v) NaOMe, MeOH, and then 2-Chloropyrimidine
Inhibition of MTAP and HpMTAN.
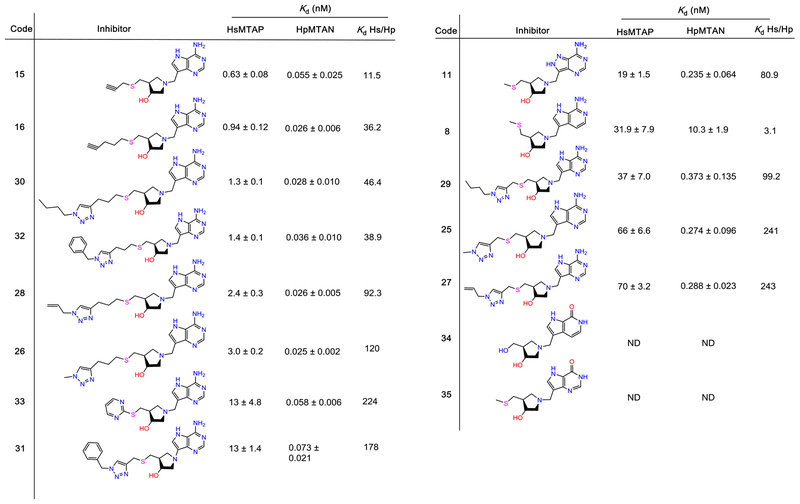
Most of the compounds described here are structurally related to methylthio-DADMeimmucillin-A (MTDIA), a transition-state analogue of MTAP and MTANs. Here, the varied 5′-alkylthio groups yielded strong inhibitors of MTAP and HpMTAN (Figure 2) with dissociation constants (Kd values) varying by over 2 orders of magnitude. Tight binding of these transition-state analogues depends on the ribocation mimic of the transition state provided by the cationic hydroxypyrrolidine and protonation of N7 in the 9-deazaadenine, a second important feature of the transition-state structure. The 6-amino group is essential, as its loss prevents binding (Figure 2).
Figure 2.
Transition-state analogue inhibitors of MTAP and HpMTAN. Inhibitors were ranked based on their Kd value for MTAP. ND means inhibition not detected at a 5 μM inhibitor concentration. The specificity ratio (Kd Hs/Hp) is the affinity for human MTAP relative to HpMTAN.
Crystal Structures of Analogues with MTAP and HpMTAN.
Structural analysis of inhibitors 15, 16, 30, and 32 bound to human MTAP and HpMTAN correlated the catalytic site interactions with the binding affinity to these enzymes (Figure 2 and Table 1). Human MTAP and HpMTAN were cocrystallized with the inhibitors and crystal structures solved by molecular replacement using PHASER at high resolutions (Table 1).35 Structural analysis using MolProbity indicated that none of the amino acid residues are outliers in the Ramachandran plots (Table 1).36
Table 1.
Data Collection and Refinement Statistics of MTAP and HpMTAN Complexes
| H. sapiens MTAP | H. pylori MTAN | |||||||
|---|---|---|---|---|---|---|---|---|
| Dataseta | MTAP + 15 | MTAP + 16 | MTAP + 30 | MTAP + 32 | HpMTAN + 15 | HpMTAN + 16 | HpMTAN + 30 | HpMTAN + 32 |
| Unit Cell Data | ||||||||
| space group | P321 | P321 | C2221 | C2221 | P41212 | P41212 | P212121 | P41212 |
| cell parameters (Å, deg) | a = 122.82 | a = 121.81 | a = 79.36 | a = 79.39, | a = 73.25 | a = 73.48 | a = 72.56 | a = 73.35 |
| b = 122.82 | b = 121.81 | b = 135.13, | b = 135.32 | b = 73.25 | b = 73.48 | b = 74.03 | b = 73.35 | |
| c = 44.62 | c = 44.37 | c = 158.86 | c = 159.27 | c = 176.14 | c = 176.21 | c = 176.89 | c = 176.38 | |
| α, β = 90, γ = 120.0 | α, β = 90, γ = 120 | α, β, γ = 90 | α, β, γ = 90 | α, β, γ = 90 | α, β, γ = 90 | α, β, γ = 90 | α, β, γ = 90 | |
| Vm (Å3/Dalton) | 2.9 | 2.9 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| number of subunits in the asymmetric unit | 1 | 1 | 3 | 3 | 2 | 2 | 4 | 2 |
| Data Collection | ||||||||
| beamline | LRL-CAT | LRL-CAT | LRL-CAT | LRL-CAT | LRL-CAT | LRL-CAT | LRL-CAT | LRL-CAT |
| wavelength (Å) | 0.97931 | 0.97931 | 0.97931 | 0.97931 | 0.97931 | 0.97931 | 0.97931 | 0.97931 |
| temperature (K) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| resolution range (Å) | 53.18–1.62 (1.65–1.62) | 60.91–1.62 (1.65–1.62) | 79.43–1.91 (1.95–1.91) | 79.63–1.99 (2.04–1.99) | 67.64–1.60 (1.63–1.60) | 88.10–1.62 (1.65–1.62) | 176.89–1.61 (1.64–1.61) | 88.19–1.45 (1.47–1.45) |
| total number of observed reflections | 605 199 (28 477) | 600 969 (27711) | 497 405 (33 410) | 438 725 (30 921) | 927 868 (45 362) | 852 192 (25 016) | 912 840 (44 290) | 1242 406 (60 058) |
| number of unique reflections | 48 526 (2329) | 48 221 (2355) | 66 387 (4430) | 59 017 (4097) | 64 250 (3121) | 61 452 (2548) | 123 910 (5998) | 86 142 (4167) |
| Rmerge (%)b | 9.8 (121.8) | 11.0 (133.5) | 9.4 (109.3) | 10.0 (116.2) | 14.6 (152.8) | 11.5 (109.4) | 9.9 (99.1) | 14.9 (175.4) |
| Rpim (%)c | 2.9 (36.0) | 3.2 (40.3) | 3.7 (42.6) | 3.9 (45.0) | 4.0 (41.3) | 3.1 (34.9) | 3.9 (39.0) | 4.1 (47.5) |
| CC1/2 (%) | 99.9 (75.8) | 99.9 (71.4) | 99.8 (70.2) | 99.9 (72.5) | 99.9 (69.8) | 99.8 (69.6) | 99.7 (80.2) | 99.7 (76.0) |
| ⟨I/σ(I)⟩d | 18.3 (2.0) | 14.9 (2.0) | 13.0 (1.9) | 13.4 (1.9) | 13.8 (2.0) | 14.8 (2.0) | 11.4 (2.1) | 11.2 (2.0) |
| completeness (%) | 98.6 (96.3) | 100 (100) | 99.9 (100) | 99.9 (100) | 100 (99.9) | 98.7 (84.0) | 100 (100) | 100 (100) |
| multiplicity | 12.5 (12.2) | 12.5 (11.8) | 7.5 (7.5) | 7.4 (7.5) | 14.4 (14.5) | 13.9 (9.8) | 7.4 (7.4) | 14.4 (14.4) |
| Wilson B-factor (Å2) | 15.5 | 15.5 | 27.2 | 35.2 | 15.1 | 14.7 | 16.7 | 13.2 |
| Refinement | ||||||||
| Rwork (%)e | 16.9 | 15.5 | 19.7 | 20.1 | 16.4 | 16.7 | 18.0 | 17.7 |
| Rfree (%)f | 19.0 | 17.6 | 22.2 | 23.6 | 19.3 | 19.3 | 20.3 | 20.0 |
| no. of atoms | 2444 | 2428 | 6758 | 6615 | 4208 | 4152 | 8152 | 4168 |
| protein atoms | 2137 | 2132 | 6290 | 6199 | 3596 | 3587 | 7104 | 3568 |
| ligand atoms | 22 | 24 | 93 | 102 | 44 | 48 | 124 | 68 |
| solvent atoms | 285 | 272 | 375 | 314 | 568 | 517 | 924 | 532 |
| Model Quality | ||||||||
| RMS Deviation from Ideal Value | ||||||||
| bond length (Å) | 0.011 | 0.011 | 0.009 | 0.01 | 0.01 | 0.01 | 0.01 | 0.009 |
| bond angle (deg) | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 | 1.6 | 1.7 | 1.6 |
| Average B-Factor | ||||||||
| protein atoms (Å2) | 21.6 | 22.3 | 38.3 | 43.8 | 17.7 | 20.6 | 23.3 | 18.8 |
| ligand atoms (Å2) | 16.4 | 17.3 | 41.0 | 46.1 | 13.3 | 15.5 | 23.1 | 21.0 |
| waters (Å2) | 36.1 | 36.5 | 40.1 | 42.1 | 31.5 | 33.3 | 34.5 | 31.5 |
| Ramachandran plotg | ||||||||
| most favored regions (%) | 97.5 | 98.2 | 97.7 | 98.0 | 97.1 | 96.9 | 97.0 | 96.6 |
| allowed regions (%) | 2.5 | 1.8 | 1.7 | 2.0 | 2.9 | 3.1 | 3.0 | 3.4 |
| outlier regions (%) | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PDB ID entry | 6DYZ | 6DZ0 | 6DZ3 | 6DZ2 | 6DYU | 6DYV | 6DYY | 6DYW |
Values in parentheses refer to the highest resolution shell.
Rmerge = (∑hkl∑i|Ii(hkl) − <I(hkl)|>/)∑hkl∑i<Ii(hkl)>, where Ii(hkl) is the intensity of the ith measurement of reflection (hkl) and <I(hkl)> is its mean intensity.
Rpim = (∑hkl[1/(Nhkl − 1)]1/2∑i|Ii(hkl) − <I(hkl)>|)/∑hkl∑i<Ii(hkl)>, where Ii(hkl) is the intensity of the ith measurement of reflection (hkl), <I(hkl)> is its mean intensity, and N is the number of measurements.
I is the integrated intensity and σ(I) is its estimated standard deviation.
Rwork = (∑hkl|Fo − Fc|)/∑hklFo, where Fo and Fc are the observed and calculated structural factors.
Rfree is calculated as for Rwork but from a randomly selected subset of the data (5%), which were excluded from the refinement calculation.
Calculated by MOLPROBITY.
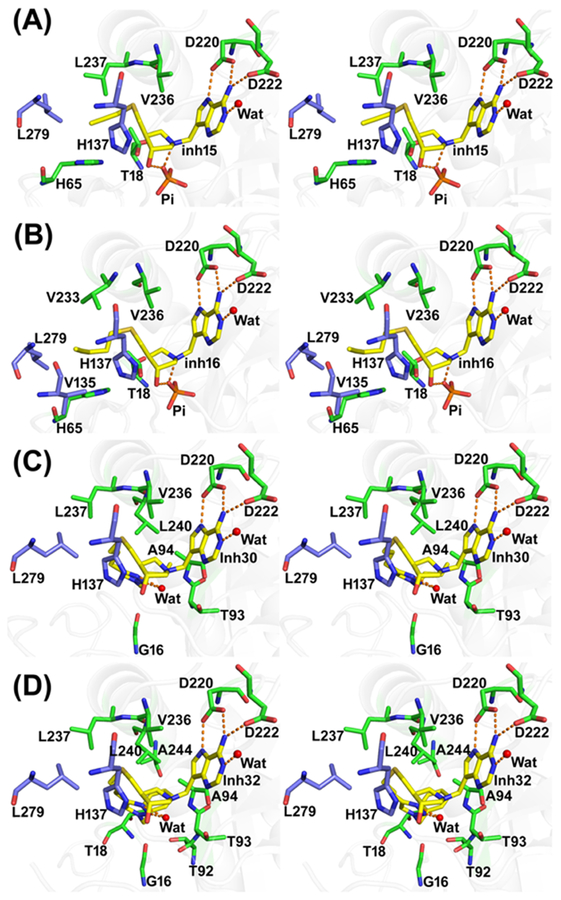
Human MTAP in complex with four inhibitors was solved at1.62−1.99 Å resolutions in diffierent space groups using the apo human MTAP monomer as the initial phasing model in PHASER. Human MTAP catalytic sites are located at the subunit interfaces of the trimer. Asymmetric units of human MTAP crystals contain a monomer with 15 and 16, and a trimer with 30 and 32. The trimer is the physiological state of human MTAP. Solvent accessible surface area of the subunit—subunit interfaces is 1187 Å2. The low RMSD for Cα (0.357–0.710 Å) of the four inhibitor-bound structures indicates only minor structural differences. MTAP monomers contain 10 β-sheets (residues are β1, 11–16; β2, 29–33; β3, 45–50; β4, 54–59; β5, 87–98; β6, 106–110; β7, 112–116; β8, 161–164; β9, 165–172; β10, 210–220) with 6 α-helices (from residues α1, 73–84; α2, 146–159; α3, 180–189; α4, 200–208; α5, 233–259; α6, 264–274; Figure S1). The electron density map of the peptide backbone and amino acid side chains are clearly resolved. Inhibitor binding is also well defined in the catalytic sites (Figure S2). The purine and pyrrolidine rings of the four inhibitors bind in the same conformation but the 5′-alkylthio group of 30 and 32 binds in a conformation different from 15 and 16 (Figure S3). The N1 of the inhibitor forms a hydrogen bond with a structural water molecule, whereas N6 forms hydrogen bonds with the carboxyl oxygens of both Asp220 and Asp222. Asp220 also forms a hydrogen bond interaction with N7, making it bidentate with respect to inhibitor binding. MTAP complexes of 15 and 16 have the hydroxyl groups and N1′ (corresponding to the C1′ of the substrate) of the pyrrolidine ring in hydrogen bond interactions with a phosphate oxygen and with Thr18 (Figure 3). The candidate nucleophilic oxygen (O2, nearest to the reaction center) of phosphate is hydrogen-bonded with Thr93 (OG1), Thr197 (OG1), and a water molecule. The O3 of the phosphate is in hydrogen bond interactions with peptide nitrogen of Thr18 and Ala94. Phosphate O4 forms hydrogen bonds with Arg60 (NH1) and His61 (NE2). These interactions are missing in MTAP complexes with 30 and 32, as phosphate is displaced in these structures. Instead, a chloride ion is bound near the phosphate binding pocket and is coordinated with Thr193 (OG1), Thr197 (OG1), and a water molecule. The 5′-alkylthio group binding mode of 15 and 16 is different from 30 and 32, where the extended chain folds into the phosphate binding site (Figure 3 and Table 2).
Figure 3.
Stereoview of the binding sites of MTAP in complex with transition-state analogue inhibitors. The inhibitor complexes of 15, 16, 30, and 32 are shown in panels A, B, C, and D, respectively. The residues interacting with inhibitors from monomer-A are shown in green and from the neighboring subunit are shown in light blue. Selected hydrogen bond interactions are shown in orange dotted lines.
Table 2.
Amino Acid Residues in Contact with the 5′-Alkylthio Groups of Transition-State Analogue Inhibitorsa
| inhibitors | MTAP | HpMTAN |
|---|---|---|
| 15 | Thr18, His65, Val236, Leu237, His137*, Leu279* | Met10, Ile52, Phe153, Phe208, Phe107*, His109*, Pro115* |
| 16 | Thr18, His65, Val233, Val236, Val135*, His137*, Leu279* | Met10, Ile52, Phe153, Phe208, Leu104*, Phe107* |
| 30 | Gly16, Thr93, Ala94, Val236, Leu237, Leu240, His137*, Leu279* | Ile52, Phe153, Phe208, Leu104*, Phe107*, His109*, Pro115*, Lys132*, Asn136* |
| 32 | Gly16, Thr18, Thr92, Thr93, Ala94, Val236, Leu237, Leu240, Ala244, His137*, Leu279* | Ile52, Phe153, Phe208, Leu104*, Phe107*, His109*, Pro115* |
Amino acids interacting within the 5′-alkylthio pocket (to 3.9 Å) are reported. These residues interacting from the neighboring subunits are highlighted with an asterisk (*).
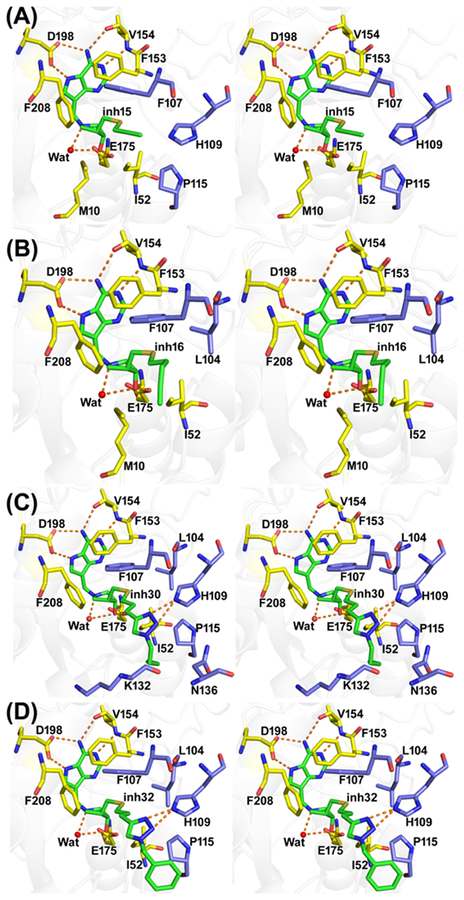
HpMTAN was cocrystallized with the same inhibitors and the structures solved at resolutions of 1.45–1.62 Å (Table 1). Previous and current analysis indicates a functional dimer for HpMTAN. The solvent accessible surface area of the dimer interface is 1654 Å2. HpMTAN contains seven helices including one 310-helix and 10 β-sheets, similar to other MTAN structures.37–39 They are arranged in three αβα-layer structures with central mixed β-sheets (Figure S4). The low RMSD (0.091–0.195 Å) of the inhibitor complexes indicates highly similar Cα chains. Inhibitors were bound in both active sites of HpMTAN with low B-factors and clear electron density maps (Figure S5). Except for the 5′-alkylthio groups, the binding modes of the inhibitors are similar (Figure S3). Inhibitors with extended 5′-alkylthio groups fill the full extent of the 5′-binding pocket, whereas short 5′-alkylthio groups do not. The binding modes of the purine and pyrrolidine rings of the inhibitors are the same. Hydrogen bond interactions to 9-deazaadenine include the Val154 nitrogen with N1, N6 with the carbonyl oxygen of Val154, and N7 with a carboxyl oxygen of Asp198 (OD2). N7 protonation is important to transition-state formation during the MTAN hydrolysis reaction. The structural nucleophilic water oxygen is 2.7 Å from N1′, in hydrogen bond contacts with Glu13 (OE2) and Arg194 (NH1). The 3′-hydroxyl group of the pyrrolidine is hydrogen-bonded with a carboxyl oxygen of Glu175 (Figure 4). Inhibitor 5′-alkylthio groups occupy different parts of the 5′-alkylthio binding pocket, which extends toward the solvent exterior (Table 2 and Figure 4). Inhibitors 15 and 16 fill the 5′-alkylthio group binding pocket to engage most of the interactions with the enzyme.
Figure 4.
Stereoview of the binding sites of HpMTAN in complex with transition-state analogue inhibitors. The inhibitor complexes of 15, 16, 30, and 32 are shown in panels A, B, C, and D, respectively. The residues interacting with inhibitors from monomer-A are shown in yellow and from monomer-B are shown in light blue. Selected hydrogen bond interactions are shown in orange dotted lines.
Structural Comparisons.
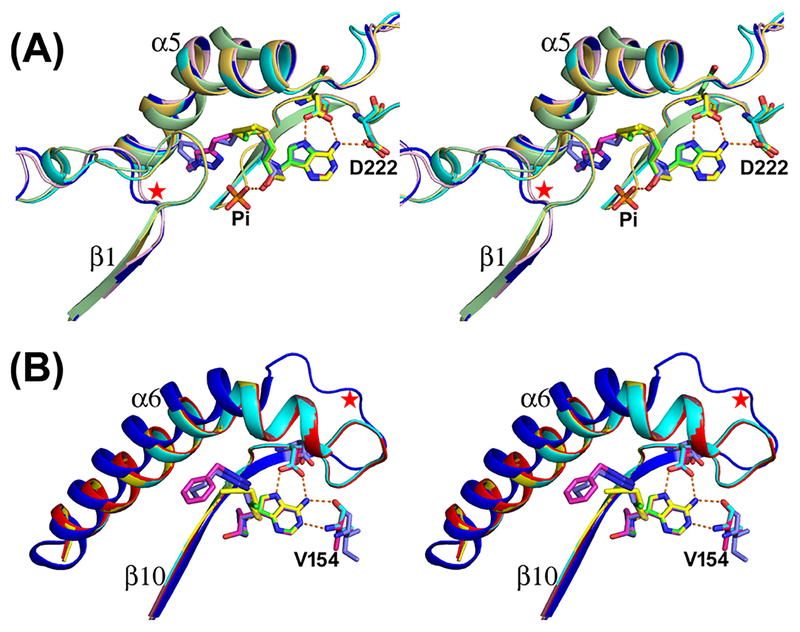
Crystal structures of human MTAP with inhibitors 30 and 32 (large 5′-alkylthio groups) differ from those with 15 and 16 (smaller 5′-alkylthio groups). Binding of 30 and 32 caused rearrangement of the loop between β1 and β2 (residues 17–28) to a more open conformation than with 15 and 16 (Figure 5). In complex with 15 and 16, this loop is closed. The rearrangement of the β1–β2 loop with 30 and 32 also alters the β4–α1 loop conformation and so His61 is displaced slightly and His65 is flipped 180°. The hydroxyl group of the pyrrolidine ring is rotated 18° toward the outside in 30 and 32 complexes. These conformational changes alter the phosphate binding site, which is occupied by the bulky 5′-alkylthio groups. For the structural comparisons, unliganded MTAP (PDB ID: 3OZE) and a complex with MTA and sulfate (PDB ID: 1CG6) were compared.40,41 These structures are similar to those with inhibitors 15 and 16.
Figure 5.
Catalytic site conformations of apo- and ligand-bound MTAP and HpMTAN. (A) Stereoview superposition of unliganded MTAP (PDB ID: 3OZE; green) with four inhibitor-bound structures, including MTAP-15 (PDB ID: 6DYZ; cyan), MTAP-16 (PDB ID: 6DZ0; yellow), MTAP-30 (PDB ID: 6DZ3; blue), and MTAP-32 (PDB ID: 6DZ2; light pink), are overlapped. The β1−β2 loop (highlighted with a red star) is altered substantially on the binding of 30 and 32. (B) Superposition of an unliganded MTAN binding site (E. coli MTAN PDB ID: 1Z5P; blue) with four inhibitor-bound complexes of HpMTAN, including MTAN-15 (PDB ID: 6DYU; brick red), MTAN-16 (PDB ID: 6DYV; cyan), MTAN-30 (6DYY; yellow), and MTAN-32 (6DYW; red). Helix 6 and associated loop of the unliganded MTAN change conformation to elongate helix 6 as a result of inhibitor binding (highlighted with a red star).
HpMTAN structures with the same four inhibitors were solved at high resolution in the P41212 space group (Table 1). In unliganded E. coli MTAN (PDB ID: 1Z5P), both catalytic sites are in an open configuration, but in unliganded HpMTAN (PDB ID: 3NM4), the binding of a Tris buffer molecule induced a closed conformation in monomer-A with monomer-B in an open conformation.39,42,43 The ligand-induced conformation change moves the β10−α6 loop approximately 7 Å closer to the binding site to form the closed state. When the HpMTAN bound to 15, 16, 30, and 32 was compared to p-ClPh-Thio-DADMe-ImmA bound to HpMTAN and resolved with neutron diffraction (PDB ID: 5K1Z), hydrogen bond contacts of the DADMe-ImmA core were found to be intact, including the N7 hydrogen sharing with Asp198.44 All HpMTAN−inhibitor complexes are in closed configurations but differ in the 5′-alkylthio group binding pocket (Table 2; Figures 4 and 5).
Structure−Inhibition Relationship.
Inhibitors 15 and 16 gave Kd values of 0.63 and 0.94 nM, respectively, for human MTAP, slightly better than 30 and 32, with Kd values of 1.3 and 1.4 nM, respectively. 9-Deazaadenine of the inhibitors is colocated in the catalytic site for the four inhibitors, whereas the 3′-hydroxyl group of the pyrrolidine of 30 and 32 is shifted upward relative to 15 and 16 (Figures S1 and S3). The 5′-alkylthio groups of the inhibitors, important for the potency, are bound in different conformations. Inhibitors 15 and 16 occupy the hydrophobic binding site normally occupied by the 5′-methylthiol or 5′-homocysteinyl groups of the natural substrates. In contrast, 30 and 32 induced β1–β2 loop rearrangement by exceeding the size of the 5′-alkylthio binding pocket. Upon 30 and 32 binding, the inhibitor triazoles reposition under the 3′-hydroxypyrrolidine to occupy the phosphate binding site. As 30 and 32 occupy both nucleoside and phosphate binding sites, phosphate is not bound in the 30 and 32 MTAP complexes. Ion pair formation between the cationic 3′-hydroxypyrrolidine ring and anionic phosphate is part of the transition-state ensemble, contributing to tight binding of structurally compatible analogues. Remarkably, most of the binding affinity is retained in 30 and 32 without bound phosphate.
Enzyme inhibition experiments revealed that 15, 16, 30, and 32 are 26–55 picomolar inhibitors of HpMTAN (Figure 2). The 5′-alkylthio binding site accommodates groups approximately 10 Å, extending from the 4′-carbon of the pyrrolidine group, and longer groups are accommodated, as the binding channel opens toward the solvent. The optimal fit of 16 to the site explains its high binding affinity, but even relatively small 5′-alkylthio groups (15) or bulky and hydrophilic triazole groups (30 and 32) are picomolar inhibitors. In the HpMTAN−15 complex, the unfilled space in the 5′-alkylthio binding pocket is occupied by an ethylene glycol molecule interacting with solvent molecules and a carboxyl oxygen Asp209 (OD1). The groups of 30 and 32 are larger, forcing them into the solvent space beyond the organized binding site. The distal atoms of 30 and 32 are disordered, resulting in decreased affinity. Despite these diffierences, the sum of the interactions provides favorable binding energy (Table 2 and Figure 4).
Inhibitor catalytic site contacts in both HpMTAN and MTAP show no interactions with the N3 atom of 9-deazaadenine. In contrast, both enzymes have hydrogen bond interactions with the protonated N7. As protonation of N7 is a feature of the transition state, this is an important characteristic of high-affinity inhibitors. A 3,9-dideazaadenine inhibitor (8) retains the desired protonation at N7 in a scaffold otherwise identical to MTDIA (1). However, (1) is an 86 pM inhibitor for both HpMTAN and MTAP, whereas the dissociation constant for (8) increased by >100-fold to 10 and 32 nM for HpMTAN and MTAP, respectively, indicating weaker N7 to enzyme interactions because of the loss of electron contribution from N3. The N6 exocyclic amino group is doubly hydrogen-bonded at the catalytic sites, and its replacement by O6 eliminates binding to the catalytic sites, even at concentrations of 5 μM (34 and 35).
CONCLUSIONS
Human MTAP is a validated drug target. Its genetic deletion in 15% of all human cancers makes those malignancies more susceptible to inhibitors of PRMT5, MAT2A, or RIOK1.7–10 HpMTAN catalyzes an essential step in menaquinone synthesis in H. pylori but not in common gut bacteria, making it a species-specific target in the treatment of peptic ulcers. New transition-state analogues of HpMTAN and MTAP were synthesized to explore variations of the MT-DADMeimmucillin-A chemical scaffold and the structure-function relationship to these target enzymes. The 4′-position of the 3′-hydroxypyrrolidine ring of DADMe-immucillin-A was varied by the click chemistry addition of varied substituents. Inhibition constants indicated the best compounds (15 and 16) to be picomolar inhibitors against both MTAP and HpMTAN, with 12- to 36-fold preference for the HpMTAN. Bulky 4′-substituents (e.g., 25–29 and 33) showed a 92- to 243-fold preference for HpMTAN. The hydrophobic pocket accepting the 4′-substituent in HpMTAN can accommodate methylthio (in MTA), homocysteine (in SAH), and the bulky side chain of aminofutalosine. It does so in a hydrophobic tunnel that opens toward the solvent. Human MTAP has a more restricted 4′-binding pocket that accepts only the methylthio and homocysteine groups. Larger substituents fold under the 3′-hydroxypyrrolidine ring and occupy the phosphate binding site while retaining other contacts to the 9-deazaadenine and hydroxypyrrolidine that dominate the transition-state binding energy. Four transition-state analogues were cocrystallized with MTAP and HpMTAN. Structural analysis revealed that the binding of 30 and 32 to human MTAP caused a structural rearrangement to displace phosphate and accommodate long and bulky 5′-alkylthio groups. With HpMTAN, inhibitor 16 takes full advantage of the HpMTAN binding pocket and thus binds more tightly than other inhibitors.
MATERIALS AND METHODS
Chemical Synthesis of Transition-State Analogues.
The MTDIA chemical scaffold was explored by synthesizing a new generation of transition-state analogues for HpMTAN and MTAP. The 4′-position of the 3′-hydroxypyrrolidine ring of MTDIA was varied by click chemistry. All reactions were performed under an argon or nitrogen atmosphere unless water was used as solvent or the reaction mixture was heated to above 100 °C. All final compounds gave satisfactory purity (≥95%) by HPLC and by 1H and 13C NMR spectroscopies. Details of the chemical synthesis are provided in the Supporting Information.
Expression and Purification of Human MTAP.
Human MTAP was prepared, as previously described with some modifications.41 In brief, a plasmid containing the coding region for MTAP was transformed into BL21-CodonPlus(DE3)-RIPL E. coli chemically competent cells. Nucleotide sequencing validated the DNA sequence for MTAP. The culture was grown at 37 °C and 200 rpm in LB medium containing 100 μg mL−1 ampicillin. Heterologous protein expression was induced when OD600 reached 0.6–0.8 by the addition of 1 mM IPTG (final concentration). After 8 h induction at 37 °C and 200 rpm, the cells were harvested by centrifugation (5000g for 20 min) and stored at −80 °C. All subsequent steps were carried out at 4 °C unless stated otherwise.
The pellet was suspended in lysis buffer (50 mM HEPES−NaOH, 5 mM imidazole at pH 7.0) (2.5 mL g−1 of cell pellet) with the addition of protease inhibitor cOmplete Mini EDTA-free (one tablet per 20 g of cell pellet; Roche) and homogenized by stirring for 30 min. A spatula tip of lysozyme (Sigma-Aldrich) and DNAse I (Sigma-Aldrich) was added to the mixture and, after 30 min of stirring, cells were disrupted by sonication (15 s on, 15 s off, at 30% amplitude for 30 min) and centrifuged (20 000g for 20 min) to remove cell debris. The recombinant MTAP contains 14 additional amino acids at N-terminus of the native enzyme, including a His6 tag (and a TEV protease cleavage site). The supernatant was incubated with Ni-NTA agarose (1.0 mL of slurry/g of cell pellet; Qiagen) for 45 min with rocking, and the mixture was poured into an empty column and washed with 12 column volumes of cell lysis buffer. The collection of 4 column volume fractions from a 50–500 mM imidazole stepwise elution gradient gave proteins analyzed by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) (200 V and 185 mA for 60 min in MOPS running buffer) analysis, and the fractions containing the target protein with a purity of over 95% (150–500 mM imidazole) were pooled. Purified MTAP contains adenine, which was removed by dialysis against 50 mM HEPES−NaOH at pH 7.0 with 0.2% (m/v) activated charcoal (Sigma-Aldrich) overnight, using 10 kDa dialysis cassettes (Thermo Scientific). Adenine analysis in MTAP involved denaturation with 10% (v/v) perchloric acid. Denatured protein was removed by centrifugation, and the concentration of adenine in the supernatant was tested by spectrophotometry. Adenine-free MTAP was concentrated to approximately 300 μM or 10 mg mL−1 (extinction coefficient is estimated to be 30.94 mM−1 cm−1 at 280 nm), and aliquots were frozen in liquid nitrogen and stored at −80 °C. A total of 21 mg of protein was obtained from 10 L of culture.
Expression and Purification of HpMTAN.
HpMTAN was prepared, as previously described with some modifications.16,17 Briefly, the plasmid-containing His-tag HpMTAN gene was transformed into BL21 (DE3) E. coli chemically competent cells. Nucleotide sequencing validated the DNA sequence for HpMTAN. The culture was grown at 37 °C and 200 rpm in LB medium containing 50 μg mL−1 ampicillin, and heterologous protein expression was induced when OD600 reached 0.6–0.8 by the addition of 0.5 mM IPTG (final concentration). Temperature was lowered to 30 °C upon the addition of IPTG, and the culture was grown for an additional 20 h. Cells were harvested by centrifugation (5000g for 20 min) and stored at −80 °C. All subsequent steps were carried out at 4 °C unless stated otherwise. The purification of HpMTAN was the same as described above for MTAP. A total of 210 mg of protein was obtained from 2 L of culture.
Inhibition Assays of MTAP.
MTAP catalytic activity was measured using the absorbance difference between MTA and adenine.41 A second assay followed the conversion of MTA to 2,8-dihydroxyadenine based on the oxidation of adenine by xanthine oxidase to give an absorbance change at 305 nm (ε305 = 15.5 mM−1 cm−1). Inhibition constants were analyzed by fitting rate data to the Morrison quadratic equation.45 Reactions in a 1 mL cuvette contained 100 mM K2PO4, 1 mM DTT, 800 μM MTA, 3 nM MTAP, 1 unit of xanthine oxidase, and varying concentrations of each inhibitor. Equilibrium dissociation constants were determined from reaction rate inhibition following slow-onset binding (Ki*). The rates of each reaction were taken 40 min after the initiation of the reaction, a time when slow-onset equilibrium had occurred.
Inhibition Assays of HpMTAN.
Inhibition constants were determined as above. Reactions (1 mL) contained 100 mM HEPES pH 7.2, 1 mM DTT, 100 mM NaCl, 1 mM MTA, 0.6 nM HpMTAN, 1 unit of xanthine oxidase, and varying concentrations of each inhibitor (0–100 μM). Reactions were monitored, as described above for MTAP to obtain the inhibition constants following slow-onset binding when appropriate (Ki*). The rates of each reaction were taken 40 min after the initiation of the reaction, to a time when slow-onset equilibrium had occurred.
Cocrystallization with Transition-State Analogues.
Cocrystallization of MTAP and HpMTAN with four tight binding transition-state analogues (15, 16, 30, and 32) used sitting drop vapor diffusion at 22 °C. MTAP or HpMTAN (5 mg mL−1) was mixed with inhibitors in a 1:2 molar ratio and incubated for 2 h on ice. The proteins were screened for crystal-forming conditions with the Microlytic (MCSG1–4) and Hampton (crystal screenHT) kits. Crystallization trials were in 96-well INTELLI plates, using the CRYSTAL-GRYPHON crystallization robot (ART ROBBINS). Crystallization drops contained 0.5 μL of enzyme−inhibitor mixture and 0.5 μL of well solution. The volume of the well solution was 70 μL. Good-quality crystals were obtained in 1 week (Table 1).
Data Collection and Processing.
Diffraction data were collected at the LRL-CAT beamline (Argonne National Laboratory, Argonne,IL) at a wavelength of 0.97931 Å (Table 1). Data were processed using the iMOSFLM program and scaled by the AIMLESS program in the CCP4 suite, using the appropriate space group (Table 1).46,47 Data quality was analyzed using the SFCHECK and XTRIAGE.47,48 Matthews coefficient (Vm) calculations indicated the number of monomers present in the unit cells.
Structure Determination and Refinement.
Crystal structures of MTAP and HpMTAN in complex with transition-state analogue inhibitors were solved by molecular replacement using PHASER.35 Chain-A of wild-type MTAP (PDB ID: 5TC6) and HpMTAN (PDB ID: 4WKP) structures was used as the initial phasing model. The model obtained from PHASER was manually adjusted and completed using the graphics program COOT.49 Structure refinement was performed with REFMAC5, using standard protocols for the NCS refinement.50 Inhibitor molecules were deleted from the models to initiate the refinement. After water was added, inhibitor molecules were fitted into their electron densities (Table 1).
Structure Analysis.
Crystal structures of unliganded wild-type MTAP (PDB ID: 3OZE, chain: B), HpMTAN (PDB ID: 3NM4, chain: B), and EcMTAN (PDB ID: 1Z5P, chain: A) were used for structure comparisons. The MTA complex with MTAP (PDB ID: 3T94) and p-ClPh-Thio-DADMe-ImmA complex with HpMTAN (PDB ID: 5K1Z) were also used in the structural comparisons. All structural superimpositions used the SSM protocol of COOT, and the geometry analyses of the final model used MolProbity.36 Additional structure analyses, including the calculation of the B-factor profiles, used BAVERAGE of the CCP4 suite.47 Structural figures were generated with the molecular graphics program PyMOL. For MTAP and HpMTAN structures, subunit-A was used for all of the structural analyses and comparisons.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by research grant GM041916 from the National Institutes of Health and the New Zealand Foundation for Research Science and Technology contract C08X0701. The Albert Einstein Crystallographic Core X-ray diffraction facility is supported by NIH Shared Instrumentation Grant S10 OD020068. Data collection also involved resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. Use of the Lilly Research Laboratories Collaborative Access Team (LRL CAT) beamline at Sector 31 of the Advanced Photon Source was provided by Eli Lilly Company, which operates the facility.
ABBREVIATIONS
- MTDIA
methylthio-DADMe-immucillin-A
- MTA
S-methyl-5′-thioadenosine
- All
allyl
- DQF-COSY
double-quantum filtered correlation spectroscopy
- Q-TOF
quadrupole time-of-flight
- MTAN
5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase
- HpMTAN
Helicobacter pylori MTAN
- MTAP
5′-methylthioadenosine phosphorylase
- MTR
5-methylthio-α-d-ribose 1-phosphate
- SAM
S-adenosylmethio-nine
- SAH
S-adenosylhomocysteine
- SRH
S-ribosylhomocysteine
- NMR
nuclear magnetic resonance
- HPLC
high-performance liquid chromatography
- RT
room temperature
- t-Bu
tert-butyl
- DMF
dimethylformamide
- aq
aqueous
- Et
ethyl
- Me
methyl
- THP
tetrahydropyranyl
- BOC
tert-butyloxycarbonyl
- Ac
acyl
- n-Bu
n-butyl
- Bn
benzyl
- PRMT5
protein arginine methyltransferase 5
- MAT2A
S-adenosylmethionine synthetase 2A
- HSQC
heteronuclear single quantum coherence spectroscopy
- DEPT
distortionless enhancement by polarization transfer
- APT
attached proton test
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01642.
Title page and abbreviations; index to Supporting Information content; Figure S1, human MTAP subunit ribbon structure comparing free and liganded enzymes; Figure S2, electron density omit maps for MTAP− inhibitor complexes of 15, 16, 30, 32; Figure S3, inhibitor geometry at the catalytic sites of human MTAP and HpMTAN; Figure S4, HpMTAN subunit ribbon structures comparing free and liganded MTANs; Figure S5, electron density omit maps for HpMTAN−inhibitor complexes of 15, 16, 30, 32; Table S1, crystallization and crystal handling; details of chemical synthesis; SMILES formula data for all inhibitions indexed by compound number; NMR spectra of all inhibitors (PDF)
Accession Codes
PDB ID CODES: 6DYZ, 6DZ0, 6DZ3, 6DZ2, 6DYU, 6DYV, 6DYY, 6DYW. The authors will release the atomic coordinates and experimental data upon article publication.
The authors declare no competing financial interest.
REFERENCES
- (1).Lieber CS; Packer L S-Adenosylmethionine: Molecular, Biological, and Clinical Aspects–an Introduction. Am. J. Clin. Nutr 2002, 76, 1148S–1150S. [DOI] [PubMed] [Google Scholar]
- (2).Loenen WAM S-Adenosylmethionine: Jack of All Trades and Master of Everything? Biochem. Soc. Trans 2006, 34, 330–333. [DOI] [PubMed] [Google Scholar]
- (3).Battaglia V; DeStefano Shields C; Murray-Stewart T; Casero RA Jr. Polyamine Catabolism in Carcinogenesis: Potential Targets for Chemotherapy and Chemoprevention. Amino Acids 2014, 46, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Tabor CW; Tabor H Polyamines. Annu. Rev. Biochem 1984, 53, 749–790. [DOI] [PubMed] [Google Scholar]
- (5).Basu I; Cordovano G; Das I; Belbin TJ; Guha C; Schramm VL A Transition State Analogue of 5′-Methylthioadeno-sine Phosphorylase Induces Apoptosis in Head and Neck Cancers. J. Biol. Chem 2007, 282, 21477–21486. [DOI] [PubMed] [Google Scholar]
- (6).Basu I; Locker J; Cassera MB; Belbin TJ; Merino EF; Dong X; Hemeon I; Evans GB; Guha C; Schramm VL Growth and Metastases of Human Lung Cancer Are Inhibited in Mouse Xenografts by a Transition State Analogue of 5′-Methylthioadenosine Phosphorylase. J. Biol. Chem 2011, 286, 4902–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Mavrakis KJ; McDonald ER 3rd; Schlabach MR; Billy E; Hoffman GR; deWeck A; Ruddy DA; Venkatesan K; Yu J; McAllister G; Stump M; deBeaumont R; Ho S; Yue Y; Liu Y; Yan-Neale Y; Yang G; Lin F; Yin H; Gao H; Kipp DR; Zhao S; McNamara JT; Sprague ER; Zheng B; Lin Y; Cho YS; Gu J; Crawford K; Ciccone D; Vitari AC; Lai A; Capka V; Hurov K; Porter JA; Tallarico J; Mickanin C; Lees E; Pagliarini R; Keen N; Schmelzle T; Hofmann F; Stegmeier F; Sellers WR Disordered Methionine Metabolism in MTAP/CDKN2A-Deleted Cancers Leads to Dependence on PRMT5. Science 2016, 351, 1208–1213. [DOI] [PubMed] [Google Scholar]
- (8).Kryukov GV; Wilson FH; Ruth JR; Paulk J; Tsherniak A; Marlow SE; Vazquez F; Weir BA; Fitzgerald ME; Tanaka M; Bielski CM; Scott JM; Dennis C; Cowley GS; Boehm JS; Root DE; Golub TR; Clish CB; Bradner JE; Hahn WC; Garraway LA MTAP Deletion Confers Enhanced Dependency on the PRMT5 Arginine Methyltransferase in Cancer Cells. Science 2016, 351, 1214–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pfister SX; Ashworth A Marked for Death: Targeting Epigenetic Changes in Cancer. Nat. Rev. Drug Discovery 2017, 16, 241–263. [DOI] [PubMed] [Google Scholar]
- (10).Marjon K; Cameron MJ; Quang P; Clasquin MF; Mandley E; Kunii K; McVay M; Choe S; Kernytsky A; Gross S; Konteatis Z; Murtie J; Blake ML; Travins J; Dorsch M; Biller SA; Marks KM MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell Rep 2016, 15, 574–587. [DOI] [PubMed] [Google Scholar]
- (11).Parveen N; Cornell KA Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase, a Critical Enzyme for Bacterial Metabolism. Mol. Microbiol 2011, 79, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chen X; Schauder S; Potier N; Van Dorsselaer A; Pelczer I; Bassler BL; Hughson FM Structural Identification of a Bacterial Quorum-Sensing Signal Containing Boron. Nature 2002, 415, 545–549. [DOI] [PubMed] [Google Scholar]
- (13).Xavier KB; Bassler BL LuxS Quorum Sensing: More than Just a Numbers Game. Curr. Opin. Microbiol 2003, 6, 191–197. [DOI] [PubMed] [Google Scholar]
- (14).Dairi T An alternative menaquinone biosynthetic pathway operating in microorganisms: an attractive target for drug discovery to pathogenic Helicobacter and Chlamydia strains. J. Antibiot 2009, 62, 347–352 [DOI] [PubMed] [Google Scholar]
- (15).Li X; Apel D; Gaynor EC; Tanner ME 5-methylthioadenosine nucleosidase is implicated in playing a key role in a modified futalosine pathway for menaquinone biosynthesis in Campylobacter jejuni. J. Biol. Chem 2011, 286, 19392–19398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang S; Haapalainen AM; Yan F; Du Q; Tyler PC; Evans GB; Rinaldo-Matthis A; Brown RL; Norris GE; Almo SC; Schramm VL A Picomolar Transition State Analogue Inhibitor of MTAN as a Specific Antibiotic for Helicobacter pylori. Biochemistry 2012, 51, 6892–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang S; Cameron SA; Clinch K; Evans GB; Wu Z; Schramm VL; Tyler PC New Antibiotic Candidates against Helicobacter pylori. J. Am. Chem. Soc 2015, 137, 14275–14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Schramm VL Enzymatic Transition States, Transition-State Analogs, Dynamics, Thermodynamics, and Lifetimes. Annu. Rev. Biochem 2011, 80, 703–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Singh V; Schramm VL Transition-State Structure of Human 5′-Methylthioadenosine Phosphorylase. J. Am. Chem. Soc 2006, 128, 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gutierrez JA; Luo M; Singh V; Li L; Brown RL; Norris GE; Evans GB; Furneaux RH; Tyler PC; Painter GF; Lenz DH; Schramm VL Picomolar Inhibitors as Transition-State Probes of 5′-Methylthioadenosine Nucleosidases. ACS Chem. Biol 2007, 2, 725–734. [DOI] [PubMed] [Google Scholar]
- (21).Singh V; Lee JE; Nunez S; Howell PL; Schramm VL Transition State Structure of 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase from Escherichia coli and Its Similarity to Transition State Analogues. Biochemistry 2005, 44, 11647–11659. [DOI] [PubMed] [Google Scholar]
- (22).Evans GB; Furneaux RH; Lenz DH; Painter GF; Schramm VL; Singh V; Tyler PC Second Generation Transition State Analogue Inhibitors of Human 5′-Methylthioadenosine Phosphorylase. J. Med. Chem 2005, 48, 4679–4689. [DOI] [PubMed] [Google Scholar]
- (23).Haapalainen AM; Thomas K; Tyler PC; Evans GB; Almo SC; Schramm VL Salmonella enterica MTAN at 1.36 A Resolution: A Structure-Based Design of Groupored Transition State Analogs. Structure 2013, 21, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Longshaw AI; Adanitsch F; Gutierrez JA; Evans GB; Tyler PC; Schramm VL Design and Synthesis of Potent “Sulfur-Free” Transition State Analogue Inhibitors of 5′-Methylthioadenosine Nucleosidase and 5′-Methylthioadenosine Phosphorylase. J. Med. Chem 2010, 53, 6730–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Singh V; Evans GB; Lenz DH; Mason JM; Clinch K; Mee S; Painter GF; Tyler PC; Furneaux RH; Lee JE; Howell PL; Schramm VL Femtomolar Transition State Analogue Inhibitors of 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase from Escherichia coli. J. Biol. Chem 2005, 280, 18265–18273. [DOI] [PubMed] [Google Scholar]
- (26).Evans GB; Furneaux RH; Schramm VL; Singh V; Tyler PC Targeting the Polyamine Pathway with Transition-State Analogue Inhibitors of 5′-Methylthioadenosine Phosphorylase. J. Med. Chem 2004, 47, 3275–3281. [DOI] [PubMed] [Google Scholar]
- (27).Singh V; Schramm VL Transition-State Analysis of S. pneumoniae 5′-Methylthioadenosine Nucleosidase. J. Am. Chem. Soc 2007, 129, 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Namanja-Magliano HA; Stratton CF; Schramm VL Transition State Structure and Inhibition of Rv0091, a 5′-Deoxyadenosine/5′-methylthioadenosine Nucleosidase from Myco-bacterium tuberculosis. ACS Chem. Biol 2016, 11, 1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Singh V; Shi W; Evans GB; Tyler PC; Furneaux RH; Almo SC; Schramm VL Picomolar Transition State Analogue Inhibitors of Human 5′-Methylthioadenosine Phosphorylase and XRay Structure with MT-Immucillin-A. Biochemistry 2004, 43, 9–18. [DOI] [PubMed] [Google Scholar]
- (30).Evans GB; Furneaux RH; Schramm VL; Singh V; Tyler PC Targeting the Polyamine Pathway with Transition-State Analogue Inhibitors of 5′-Methylthioadenosine Phosphorylase. J. Med. Chem 2004, 47, 3275–3281. [DOI] [PubMed] [Google Scholar]
- (31).Kicska GA; Tyler PC; Evans GB; Furneaux RH; Shi W; Fedorov A; Lewandowicz A; Cahill SM; Almo SC; Schramm VL Atomic Dissection of the Hydrogen Bond Network for Transition-State Analogue Binding to Purine Nucleoside Phosphorylase. Biochemistry 2002, 41, 14489–14498. [DOI] [PubMed] [Google Scholar]
- (32).Evans GB; Furneaux RH; Lewandowicz A; Schramm VL; Tyler PC Synthesis of Second-Generation Transition State Analogues of Human Purine Nucleoside Phosphorylase. J. Med. Chem 2003, 46, 5271–5276. [DOI] [PubMed] [Google Scholar]
- (33).Rostovtsev VV; Green LG; Fokin VV; Sharpless KB A Stepwise Huisgen Cycloaddition Process: Copper (I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem 2002, 114, 2708–2711. [DOI] [PubMed] [Google Scholar]
- (34).Evans GB; Cameron SA; Luxenburger A; Guan R; Suarez J; Thomas K; Schramm VL; Tyler PC Tight Binding Enantiomers of Pre-Clinical Drug Candidates. Bioorg. Med. Chem 2015, 23, 5326–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ Phaser Crystallographic Software. J. Appl. Crystallogr 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Chen VB; Arendall WB 3rd; Headd JJ; Keedy DA; Immormino RM; Kapral GJ; Murray LW; Richardson JS; Richardson DC MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kim RQ; Offen WA; Davies GJ; Stubbs KA Structural Enzymology of Helicobacter pylori Methylthioadenosine Nucleosidase in the Futalosine Pathway. Acta Crystallogr., Sect. D: Biol. Crystallogr 2014, 70, 177–185. [DOI] [PubMed] [Google Scholar]
- (38).Mishra V; Ronning DR Crystal Structures of the Helicobacter pylori MTAN Enzyme Reveal Specific Interactions between S-Adenosylhomocysteine and the 5′-Alkylthio Binding Subsite. Biochemistry 2012, 51, 9763–9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ronning DR; Iacopelli NM; Mishra V Enzyme-Ligand Interactions That Drive Active Site Rearrangements in the Helicobacter pylori 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase. Protein Sci 2010, 19, 2498–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Appleby TC; Erion MD; Ealick SE The Structure of Human 5′-Deoxy-5′-Methylthioadenosine Phosphorylase at 1.7 Å″ Resolution Provides Insights into Substrate Binding and Catalysis. Structure 1999, 7, 629–641. [DOI] [PubMed] [Google Scholar]
- (41).Guan R; Ho M-C; Brenowitz M; Tyler PC; Evans GB; Almo SC; Schramm VL Entropy-Driven Binding of Picomolar Transition State Analogue Inhibitors to Human 5′-Methylthioadeno-sine Phosphorylase. Biochemistry 2011, 50, 10408–10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lee JE; Cornell KA; Riscoe MK; Howell PL Structure of E. coli 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase Reveals Similarity to the Purine Nucleoside Phosphorylases. Structure 2001, 9, 941–953. [DOI] [PubMed] [Google Scholar]
- (43).Lee JE; Smith GD; Horvatin C; Huang DJT; Cornell KA; Riscoe MK; Howell PL Structural Snapshots of MTA/AdoHcy Nucleosidase Along the Reaction Coordinate Provide Insights into Enzyme and Nucleoside Flexibility During Catalysis. J. Mol. Biol 2005, 352, 559–574. [DOI] [PubMed] [Google Scholar]
- (44).Banco MT; Mishra V; Ostermann A; Schrader TE; Evans GB; Kovalevsky A; Ronning DR Neutron Structures of the Helicobacter pylori 5′-Methylthioadenosine Nucleosidase Highlight Proton Sharing and Protonation States. Proc. Natl. Acad. Sci. USA 2016, 113, 13756–13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Morrison JF; Walsh CT The Behavior and Significance of Slow-Binding Enzyme Inhibitors. Adv. Enzymol. Relat. Areas Mol. Biol 1988, 61, 201–301. [DOI] [PubMed] [Google Scholar]
- (46).Battye TG; Kontogiannis L; Johnson O; Powell HR; Leslie AG IMOSFLM: A New Graphical Interface for Diffraction-Image Processing with MOSFLM. Acta Crystallogr., Sect. D: Biol. Crystallogr 2011, 67, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Collaborative Computational Project, N. 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr 1994, 50, 760–763. DOI: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- (48).Adams PD; Afonine PV; Bunkoczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung LW; Kapral GJ; Grosse-Kunstleve RW; McCoy AJ; Moriarty NW; Oeffner R; Read RJ; Richardson DC; Richardson JS; Terwilliger TC; Zwart PH PHENIX: A Comprehensive Python-Based System for Macro-molecular Structure Solution. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Emsley P; Lohkamp B; Scott WG; Cowtan K Features and Development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Murshudov GN; Vagin AA; Dodson EJ Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Crystallogr., Sect. D: Biol. Crystallogr 1997, 53, 240–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.