Abstract
Objective This study aimed to determine the correlation between the vascular mast cells and inflammatory changes in the potentially malignant disorders, compared to the oral squamous cell carcinoma (OSCC) in varying degrees of dysplasia.
Materials and Methods Thirty samples were selected: 10 of OSCC, 10 of oral leukoplakia, and 10 of actinic keratosis. The toluidine blue technique was used on the mast cells, and hematoxylin and eosin were used for analyzing the lymphocytes, mitosis, and vessels. The quantification was performed using the ImageJ software after obtaining the images by light microscopy with a × 40 objective. Analysis of variance with p < 0.05 was considered for statistical significance.
Results Mast cells ( p < 0.0158), vessels ( p < 0.9431), lymphocytes ( p < 0.0001), and mitoses ( p < 0.0009) were found in OSCC. In potentially malignant disorders, a lower density of these structures and a higher concentration of mitosis in the actinic keratosis were observed.
Conclusion The results showed evidence of a positive correlation between mast cells and vascularization in the OSCC, suggesting the aggression of the disease. Intense inflammatory infiltrate indicates that other molecular events are involved in the carcinogenesis process, and further studies are necessary for a better understanding of it.
Keywords: carcinogenesis, lymphocytes, mast cells, mitosis, vascularization
Introduction
Precancerous condition is defined as a biological condition that does not necessarily alter the local tissue clinical appearance, but is associated with a significantly increased risk of cancer. Oral squamous cell carcinoma is the most prevalent malignancy in the oral region worldwide. 1 Recent studies have investigated the role of mast cells in the infiltrate of these disorders contributing to their progression or to inhibit tumor growth. 2 This study aims to verify the inflammatory behavior of potentially malignant oral disorders such as oral leukoplakia (OL) and actinic keratosis (AK) as compared with oral squamous cell carcinoma (OSCC) in order to detect when these lesions may evolve in a malignant way, and to consequently identify early oral cancer.
Materials and Methods
The study was submitted to and approved by the Research Ethics Committee (2.253.859).
The developed search was an experimental comparative analysis, based on the material analysis of cases diagnosed in the pathology service of the authors’ educational institution.
Thirty cases were analyzed, including 10 OSCC cases (group 1), 10 cases of OL (group 2), and 10 cases of AK (group 3). For inclusion in this research, the diagnosis of the lesions had to be conclusive, and the report had to contain the microscopic variables and local findings of the lesions, regardless of age and gender. In addition, specimens associated with other lesions or with insufficient amounts of tissue for analysis were excluded from the study. For microscopic analysis, biopsied slides with 4-μm sections that were previously prepared and stained with the hematoxylin were used. Each sample stained with HE was analyzed in 3 microscopic fields, and for mast cells quantification, the slides were stained with toluidine blue (0.1%) and analyzed in 10 microscopic fields, as shown in Fig. 1 . Images of the histological samples were obtained by means of light microscopy (BIOVAL) with a × 40 objective and optovar of × 1.6, recorded by a Zeiss Axiocam 503 color camera, × 10 objective, resulting in a total increase of × 640. The images were saved on a computer and weighted with ImageJ Java software version 1.48 v [ImageJ software developer Java (open source), NIHUSA] through the Cell Counter plugin. The microscopic variables studied included the following:
Fig. 1.
Histopathological image showing mast cells in connective tissue (blue staining toluidine, ×400).
Dysplastic alterations
Typical and atypical mitosis count
Inflammatory infiltrate intensity
Blood vessel count
Mast cell count in the areas with higher concentrations
Dysplastic alterations were observed according to the cellular modifications, such as increased nucleoli, nuclear hyperchromatism, nuclear pleomorphism, increased nucleus/cytoplasm ratio, increased mitotic activity, and cellular pleomorphism. In addition, epithelial changes were observed, such as basal layer hyperplasia, hypercellularity, and abnormal stratification of the epithelium. The intensity of the inflammatory infiltrate was classified quantitatively and considered intense when the total cell count was above 121 and moderate with a count between 71 and 120, with discrete counts between 10 and 701. 3
The analysis of variance was performed with the software StatCalc version 8.2.2 of AcaStat (AcaStat Software developer, Poinciana, Florida) and assigned p < 0.05 for statistical significance.
Results
An important statistical significance was observed between the groups. Higher concentrations of mast cells, vessels, and lymphocytes were seen in the OSCC group (group 1) with a statistical significance of p < 0.0158, p < 0.9431, and p < 0.0001, respectively, when compared to the OL (group 2) and AK (group 3) lesions. While in the mitotic figure quantification, the AK lesions showed higher counts, with p < 0.0009, as shown in Table 1 and represented in Fig. 2 .
Table 1. Comparison of the means and standard deviation of mast cells, lymphocytes, blood vessels, and mitosis between oral squamous cell carcinoma, oral leukoplakia, and actinic keratosis.
| Groups | Mast Cells | Lymphocytes | Blood Vessels | Mitosis | ||||
|---|---|---|---|---|---|---|---|---|
| Abbreviations: OSCC: Oral squamous cell carcinoma, OL: Oral leukoplakia, AK: Actinic keratosis. Group 1 (OSCC), group 2 (OL), group 3 (AK), P (statistical significance). | ||||||||
| Group 1 | 35.5 | 22.2 | 189.3 | 137.8 | 12.2 | 15.9 | 2.2 | 2.1 |
| Group 2 | 34.4 | 16.9 | 29 | 19.6 | 11.6 | 9.9 | 1.5 | 1.9 |
| Group 3 | 15 | 6.8 | 19.8 | 18.2 | 10.5 | 5.3 | 8.6 | 6.5 |
| ANOVA | p < 0.0158 | p < 0.0001 | p < 0.9431 | p < 0.0009 | ||||
Fig. 2.
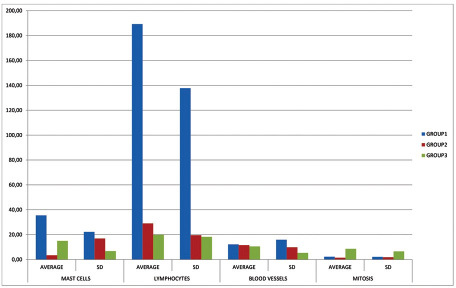
Graphical representation of the averages of mast cells, lymphocytes, blood vessels, and mitosis of group 1 (oral squamous cell carcinoma), group 2 (oral leukoplakia), and group 3 (actinic keratosis); SD: Standard deviation.
Discussion
For many years, mast cells have been considered the main agents of reactions such as allergic reactions through the release of bioactive cytokines, chemokines, proteases, leukotrienes, and polyamines. Emerging roles for mast cells have recently been identified, which highlight their relevance not only in innate immunity but also in adaptive immunity. Morphologically, they are oval with a central nucleus and a large amount of granules in the cytoplasm and are usually located close to the blood vessels. 4
These cells have been associated with resistance to tumor growth–producing molecules such as IL-1, 4, and 6, and tumor necrosis factor alpha, as well as with benefits, by promoting expanded vascular delivery. 5
The potential effects of mast cells on tumor growth according to Maltby et al's study 6 can be categorized as direct effects on tumor cells, such as mast cell–mediated cytotoxicity, or indirect effects, such as mast cell–directed angiogenesis, tissue remodeling of the surrounding environment, and recruitment of immune cells. In vitro studies have shown that the addition of whole mast cells may lead to neovascularization, which requires granular components of mast cells to perform, whereas the presence of mast cell membranes alone is insufficient. In addition, stem cell factor (SCF) produced by excess tumor cells has been implicated in the accumulation of mast cells in the periphery of developing tumors and in the increased production of VEGF.
Neto et al 7 observed that mast cell deficiency in the inflammatory infiltrate increased the incidence of tumors, suggesting a very important positive role of these cells in immunological defense against tumors.
In the present study, we found higher densities of mast cells, blood vessels, and intense inflammatory infiltrate and a relatively small number of mitoses in OSCC than in AK and OL. Recent studies have shown similar results. Ingaleshwar et al 8 carried out a study by analyzing vessel and mast cell density in OSCC compared to the healthy mucosa and found a linear increase of vessels as the density of mast cells increased in malignant lesions, suggesting a positive correlation. Another similar study by Iamaroon et al 9 compared the correlation between the mast cells and microvessel quantity in OSCC, cancerous dysplasia, hyperkeratotic lesions, and clinically healthy mucosa and revealed increased densities of these histopathological structures based on the progression of the disease, that is, the count was higher in carcinoma when compared to the other lesions and the normal oral mucosa. This is due to the production of several mediators. In addition, heparin, which is the dominant proteoglycan in mast cells, has many properties, one of which is mitogenic power on endothelial cells to stimulate the migration of endothelial cells. 8 A study carried out by Pittoni et al 10 supported the idea that the high density of the inflammatory infiltrate found in this research may also contribute to tumor growth through the release of MMP-9, a proteinase secreted by inflammatory cells, including mast cells that cleave the SCF receptor on the tumor cell membrane, which increases the attraction between these cells through the interaction between this receptor and the c-kit found in mast cells. Another study by Lopamudra das Roy et al 11 confirmed the above results by showing the increased recruitment of mast cells within tumors from mice with arthritis, in which metastasis in the lungs and bones was facilitated.
Other studies have shown divergent results. StaSikOwSka-Kanicka et al 12 also associated the density of infiltrative cells and microvessels in OSCC and observed a significantly lower number of mast cells in lesions with unfavorable prognoses when compared to those with favorable prognoses and proposed that the deficiency of these cells in malignant lesions is associated with poor prognosis.
Sathyakumar et al 13 observed a direct relationship between the density of mast cells and blood vessels when comparing normal mucosa to leukoplakia with and without dysplasia, showing progressive increases in density, being lowering in normal oral mucosa and higher in lesions with dysplasia. Despite the fact that there is a positive correlation between these histological components, Ali Tahir et al 14 believed that it is not direct since it is not only the mast cells that release proinflammatory enzymes. Similar results were observed by Michailidou et al 15 and Rojas et al 16 The first suggested a positive association between mast cell concentration and vascularization in the normal buccal mucosa, leukoplakia with and without dysplasia, and OSCC, with a linear increase in concentrations, that is, a lower concentration in the normal oral mucosa and higher concentrations in leukoplakias with dysplasia and carcinoma. Rojas et al evaluated clinically healthy lips and AK and observed an increase in mast cells in keratosis, especially in the areas close to solar elastosis, compared to the normal oral mucosa.
The reduced number of mast cells observed in the lesions of OL by Neto et al 7 is equivalent to the results found in this study, but diverges regarding reduced mast cell count in OSCC. Lachter et al 17 found similar results when observing fewer mast cells in cancerous alterations and malignant neoplasms in colorectal lesions when compared with normal colorectal tissue.
Thus, in this research, significantly high mast cell density, lymphocyte vascularization, and a low mitosis count were observed in the carcinoma, whereas decreases in these factors were observed in OL and AK, with the exception of AK, which showed a significant increase of mitosis.
The decrease in the number of mast cells in these lesions may be related to the migration failure of these cells, reflecting an important modification in the microenvironment during the initiation and progression of a tumor. Furthermore, the lesion may be in the second phase of the carcinogenesis process, characterized by self-regulation of tumor cell growth. 18 Other studies have shown that there is research that further reveals that the reduction of the expression of E-cad, tumor suppressor gene that is expressed in the epithelial tissues, may increase tumor progression. 19
Conclusion
In this study, mast cell and microvessel densities were significantly increased in OSCC lesions, suggesting tumor progression and aggression through the positive regulation of angiogenesis by these cells. However, mast cells are only part of this complex process of carcinogenesis, together with other factors secreted in the tumor environment that can modulate angiogenesis. Further studies should be conducted to contribute to a better understanding of these events.
Financial Support and Sponsorship
None.
Footnotes
Conflicts of Interest None.
References
- 1.Vigneswaran N, Williams M D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(02):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangwar R S, Friedman S, Seaf M, Levi-Schaffer F. Mast cells and eosinophils in allergy: close friends or just neighbors. Eur J Pharmacol. 2016;778:77–83. doi: 10.1016/j.ejphar.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Sharma B, Sriram G, Saraswathi T R, Sivapathasundharam B. Immunohistochemical evaluation of mast cells and angiogenesis in oral squamous cell carcinoma. Indian J Dent Res. 2010;21(02):260–265. doi: 10.4103/0970-9290.66655. [DOI] [PubMed] [Google Scholar]
- 4.Shea-Donohue T, Stiltz J, Zhao A, Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12(05):349–357. doi: 10.1007/s11894-010-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbas A K, Lichtman A H, Pillai S. 8th ed. Philadelphia, PA: Elsevier; 2014. Cellular and Molecular Immunology; p. 544. [Google Scholar]
- 6.Maltby S, Khazaie K, McNagny K M. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796(01):19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira-Neto H H, Leite A F, Costa N L. Decrease in mast cells in oral squamous cell carcinoma: possible failure in the migration of these cells. Oral Oncol. 2007;43(05):484–490. doi: 10.1016/j.oraloncology.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Ingaleshwar P S, Pandit S, Desai D, Redder C P, Shetty A S, Mithun K M. Immunohistochemical analysis of angiogenesis by CD34 and mast cells by toluidine blue in different grades of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2016;20(03):467–473. doi: 10.4103/0973-029X.190950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32(04):195–199. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 10.Pittoni P, Piconese S, Tripodo C, Colombo M P. Tumor-intrinsic and -extrinsic roles of c-Kit: mast cells as the primary off-target of tyrosine kinase inhibitors. Oncogene. 2011;30(07):757–769. doi: 10.1038/onc.2010.494. [DOI] [PubMed] [Google Scholar]
- 11.Das Roy L, Curry J M, Sahraei M. Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-Kit signaling. Breast Cancer Res. 2013;15(02):R32. doi: 10.1186/bcr3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Danilewicz M. Association of infiltrating cells with microvessel density in oral squamous cell carcinoma. Pol J Pathol. 2017;68(01):40–48. doi: 10.5114/pjp.2017.67614. [DOI] [PubMed] [Google Scholar]
- 13.Sathyakumar M, Sriram G, Saraswathi T, Sivapathasundharam B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral precancerous lesion-leukoplakia. J Oral Maxillofac Pathol. 2012;16(03):343–348. doi: 10.4103/0973-029X.102481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir A, Nagi A H, Ullah E, Janjua O S. The role of mast cells and angiogenesis in well-differentiated oral squamous cell carcinoma. J Cancer Res Ther. 2013;9(03):387–391. doi: 10.4103/0973-1482.119311. [DOI] [PubMed] [Google Scholar]
- 15.Michailidou E Z, Markopoulos A K, Antoniades D Z. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;2:126–132. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas I G, Martínez A, Pineda A, Spencer M L, Jiménez M, Rudolph M I. Increased mast cell density and protease content in actinic cheilitis. J Oral Pathol Med. 2004;33(09):567–573. doi: 10.1111/j.1600-0714.2004.00242.x. [DOI] [PubMed] [Google Scholar]
- 17.Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum. 1995;38(03):290–293. doi: 10.1007/BF02055605. [DOI] [PubMed] [Google Scholar]
- 18.Anuradha A, Kiran Kumar Naik B, Vijay Srinivas G, Devi R S, Puneet H K. Incidence of mast cells in oral squamous cell carcinoma: a short study. J Oncol. 2014;2014:614291. doi: 10.1155/2014/614291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridevi U, Jain A, Nagalaxmi V, Kumar U V, Goyal S. Expression of E-cadherin in normal oral mucosa, in oral precancerous lesions and in oral carcinomas. Eur J Dent. 2015;9(03):364–372. doi: 10.4103/1305-7456.163238. [DOI] [PMC free article] [PubMed] [Google Scholar]


