Abstract
Objective The aim of this study was to evaluate the effect of neutralizing agents on the shear bond strength of hydrofluoric (HF)–etched porcelain in nonaging and aging conditions.
Subjects and Methods One hundred and twenty feldspathic porcelain specimens were prepared and divided into six groups to undergo different surface conditioning methods—group 1: control; group 2: HF; group 3: HF + calcium hydroxide; group 4: HF + calcium carbonate; group 5: HF + calcium gluconate; and group 6: HF + ultrasonic. All samples were immersed in 37°C distilled water for 24 h. Half of the samples were thermocycled in water for 5,000 cycles. The shear bond strength test was performed using a universal testing machine at a crosshead speed of 0.5 mm/min. Data were statistically analyzed by two-way ANOVA and Tukey's multiple comparison test at a 95% confidence level. The surface micromorphology and surface elements were analyzed using scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDX), respectively.
Results The shear bond strengths of groups 2–6 were significantly higher than the control group in both aging and nonaging conditions ( p < 0.05). There were no significant differences among all of the HF-etched porcelain groups ( p > 0.05). SEM images of groups 2–6 illustrated similar patterns of irregularity on the specimen surfaces. Elemental analysis of EDX demonstrated identical elements on surfaces of specimens of groups 2–6.
Conclusion Within the limitations of this study, shear bond strength values between HF-etched porcelain, HF-etching followed by application of neutralizing agents, and HF-etching followed by ultrasonic cleaning were not significantly different in both nonaging and aging conditions.
Keywords: dental porcelain, hydrofluoric acid, neutralizing agents, restoration repair, surface treatment
Introduction
Dental porcelain offers key advantages in its extremely pleasing esthetic appearance as it can mimic the various colors and shades of natural teeth and its superior biocompatibility. 1 2 However, as dental porcelains are brittle, potential technical problems include the chipping or fracture of the veneering ceramic. 3 Furthermore, it cannot be directly repaired by the same material, due to the high temperatures involved with the sintering process. The available options for repair of such fractures range from bonding of a new veneer over the fractured porcelain to the most commonly used method of bonding composite filling materials to the fractured surface. 4 5 6
The repair process involves hydrofluoric (HF) acid etching of the porcelain surface followed by the application of silane. These procedures are well known and recommended for the improved attachment of composite resin to ceramic. 5 7 8 9 10 11 HF acid etching results in changes in porcelain surface morphology that enhances micromechanical retention, but HF acid is also known to have hazardous effects in vivo, as it was found to be a harmful and irritating compound to human soft tissues. 12 13
HF acid is an inorganic acid of hydrogen fluoride in water, known to be hazardous to human tissues. Unlike other acids, the dissociated fluoride ion produces severe toxicity. Primary care for patients exposed to HF consists of removal of any contaminated clothing, thorough flushing of the exposed area with a large amount of water, and topical or systemic application of calcium gluconate—depending on the severity. The goal of primary care is to neutralize, precipitate, and eliminate the fluoride ion and to prevent progressive tissue destruction. 12 13 14
Accordingly, many questions have been raised as to the risks and possible toxicity of using HF acid in the oral cavities. Panah et al 15 recommended the use of rubber dams and neutralizing agents, such as sodium bicarbonate and calcium carbonate, to protect the tooth surface and oral tissues. However, some studies reported that using neutralizing agents after HF treatment on ceramic might reduce bond strength between the adhesive cement/ceramic interface. As a result, precipitation of HF acid and neutralizing agents could prevent penetration of resin material to obtain mechanical interlocking on ceramic-etched surface. 16 17 18
Interestingly, there have been no studies regarding the use of calcium gluconate and its effects on bond strength, but very few studies on the effects of other neutralizing agents were noted. To date, no study has determined the composition of the residual resulting in products of neutralizing agents and HF acid after surface cleaning.
The aim of this study was divided into two parts: (1) to evaluate the effect of neutralizing agents such as calcium hydroxide, calcium carbonate, and calcium gluconate on the shear bond strength of HF-etched dental porcelain, immediately post application and after simulated aging, and (2) to analyze the surface morphology and determine the elements present on the porcelain surface after surface treatment using scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDX), respectively. The null hypotheses for this experiment were (1) the neutralizing agents would affect the shear bond strength of HF-etched porcelain, immediately post application and after simulated aging, and (2) the porcelain surface treated with neutralizing agents would demonstrate difference in surface morphology and surface elements.
Subjects and Methods
Shear Bond Strength Testing
The materials used in this study are summarized in Table 1 . One hundred and twenty specimens were prepared by mixing feldspathic porcelain powder shade T1 (Noritake, Super Porcelain EX-3, Kuraray Noritake Dental Inc, Aichi, Japan) with forming liquid. The specimens were subsequently formed using a putty silicone index (Elite HD, Zhermack, Badia Polesine, Italy) with a diameter of 8.0 mm and a depth of 6.0 mm. The ceramic slurry was placed into the silicone mold in small incremental portions by a cement spatula and condensed until the entire mold space was full. Excess liquid was removed with soft, absorbent paper. The feldspathic porcelain blocks were removed from the silicone indexes and fired as per the manufacturer's instructions. Sintering shrinkage was around 25%, making the final specimen 6.0 mm in diameter and 4.5 mm in height. Next, retentive grooves were created at the bottom of the porcelain specimens with slow-speed diamond discs (Superflex, Edenta, Switzerland) to promote mechanical retention between the feldspathic porcelain and dental gypsum Type IV. A polyvinyl chloride (PVC) tube with a diameter of 22.0 mm and a height of 15.0 mm was then filled with dental gypsum, and the specimen placed to a depth where its margin was 1.0 mm higher than the edge of the tube. After the gypsum had set, the surface of the ceramic specimens was polished using a 600-grit silicon carbide abrasive paper (3M Wetordry abrasive sheet, 3M, Minnesota, United States) and lubricant in an automatic machine (Nano 2000 grinder-polisher with FEMTO 1000 polishing head, Pace Technologies, Arizona, United States). A force of 2 kg/cm 2 was applied during polishing, and the silicon carbide abrasive papers were set to rotate at a rate of 100 rotations per minute anticlockwise. During the polishing process, the specimens were rotated clockwise. Polishing with 600-grit silicon carbide abrasive paper was carried out for 5 min after which the process was repeated using a 1000-grit silicon carbide abrasive paper. A new abrasive sheet was used for each specimen. After polishing, all specimens were ultrasonically cleaned in distilled water for 5 min to remove any surface residual and then air dried. The specimens were randomly divided into six groups ( n = 10) according to its surface treatments. They were listed as follows:
Table 1. Materials used in this study.
| Materials | Manufacturer | Compositions |
|---|---|---|
| Abbreviations: Bis-GMA, bisphenyl glycidyl methacrylate; HEMA, hydroxyethyl methacrylate. | ||
| Feldspathic porcelain Lot: DNWHX | Noritake, Super Porcelain EX-3, Kuraray Noritake Dental Inc, Aichi, Japan | SiO 2 (65%), Al 2 O 3 (14%), CaO (<1%), MgO (<1%), K 2 O (9%), Na 2 O (9%), Li 2 O (<1%) |
| Hydrofluoric acid Lot: BD68D | Ultradent Products Inc, Ultradent Porcelain Etch, Utah, United States | 9% buffered hydrofluoric acid |
| Adper Scotchbond Multipurpose Adhesive Lot N684362 | 3M ESPE, Dental products, St Paul, Minnesota, United States | Bis-GMA, HEMA, tertiary amines, photoinitiator |
| Calcium hydroxide Lot: K26007147 931 | Merck KGaA, 64271 Darmstadt, Germany | Ca (OH) 2 powder |
| Calcium carbonate B/No.1601210132 | UNIVAR, Taren Point NSW 2229, Australia | CaCO 3 powder |
| Calcium gluconate Lot: 592551 | A.N.B. Laboratories Co, Ltd., Bangkok, Thailand | Calcium gluconate (C 12 H 22 CaO 14 ) 500 mg in water 10 mL |
Group 1: Served as the control group.
Group 2: Etched with 9% HF gel (Ultradent Porcelain Etch, Ultradent Products Inc, South Jordan, Utah, United States) for 90 s, rinsed with deionized water spray for 60 s, and gently air dried.
Group 3: Etched with 9% HF gel for 90 s, application of 0.02 g calcium hydroxide for 60 s, rinsed with distilled water spray for 60 s, and gently air dried.
Group 4: Etched with 9% HF gel for 90 s, application of
0.02 g calcium carbonate for 60 s, rinsed with distilled water spray for 60 s, and gently air dried.
Group 5: Etched with 9% HF gel for 90 s, application of
0.02 g calcium gluconate for 60 s, rinsed with distilled water spray for 60 s, and gently air dried.
Group 6: Etched with 9% HF gel for 90 s, rinsed with distilled water for 1 min, followed by ultrasonic cleaning in distilled water for 10 min, and then gently air dried.
One-sided tape (ScotchBlue Painter's Tape, 3M, Minnesota, United States) with a thickness of around 80 μm was cut into a 10 mm × 10 mm size, with a 2.0 mm diameter hole at the center. The tape was placed on the feldspathic porcelain surface. A small brush (Applicator tips, Dentsply DeTrey GmbH, Konstanz, Germany) was used to apply bonding agent (Adper ScotchBond Multi-Purpose Adhesive, 3M ESPE, Dental products, St Paul, Minnesota, United States) on the entirety of the prepared feldspathic porcelain surface. Another brush was used to remove excess bonding at the margins of the tape. Next, a force of approximately 40–50 pounds per square inch was applied to the triple syringe (Mobile Dental Unit, Thai Dental Products, Bangkok, Thailand) to remove any residual solvent droplets and confirm water/oil-free air blow. The solvent of the adhesive was then dried off completely (this was noticed by the absence of moving liquid droplets and the resulting shiny surface of feldspathic porcelain). With the photopolymerizing unit placed perpendicularly and at a distance of 1.0 mm from the feldspathic porcelain specimen, polymerization of the adhesive was performed by light curing for 20 s (Elipar FreeLight 2 LED Curing Light, 3M ESPE, St. Paul, Minnesota, United States) with the intensity of 1000 mW/cm 2 .
A hollow silicone mold with a diameter of 3.0 mm and a depth of 2.0 mm was placed on the top of the treated feldspathic porcelain specimen to encircle the center hole of the tape. Next, resin composite shade A3E (Filtek Z350 XT, 3M ESPE, Dental products, St Paul, Minnesota, United States) was placed until the silicone mold was full; it was light cured for 20 s. Next, the silicone mold and the tape were carefully removed and the resin composite light cured for another 20 s. The specimen was then immersed in 37°C distilled water for 24 h in an incubator (Contherm 160M, Contherm Scientific Ltd, Korokoro, Lower Hutt, New Zealand) according to ISO/TS 11405. The silicone index was cleaned with ethyl alcohol and gently air dried between specimens.
Half of the specimens of each group were submitted to shear bond strength testing by a universal testing machine (EZ-500N, Shimadzu Corporation, Kyoto, Japan). Each specimen was fixed in the testing machine, and the shearing blade was placed parallel to the junction between the feldspathic porcelain and resin composite at a distance of 1.0 mm. The shear load was applied at a 0.5 mm/min crosshead speed until failure. The shear bond strength (MPa) was calculated by dividing the highest shear bond strength by the surface area of the resin composite–feldspathic porcelain interface. The adhesive area of each specimen was measured before testing with a digital caliper (Digital Vernier Caliper Mitutoyo CD-6 CS, Mitutoyo Co, Japan). After shear bond strength testing, the debonded surfaces were viewed under a stereomicroscope (Olympus Stereo Microscopes, SZ61, Japan) at a magnification of ×40 to study the mode of failure. The modes of failure were divided into one of four categories: (1) adhesive failure at the junction of feldspathic porcelain and resin composite with no evident resin composite on the surface of the feldspathic porcelain; (2) cohesive failure within the body of resin composite, where resin composite was seen covering the entire surface of the feldspathic porcelain or fracture of the entire layer of resin composite was seen; (3) cohesive failure within the body of feldspathic porcelain, where feldspathic porcelain was seen covering the entire surface of resin composite or fracture of the entire layer of feldspathic porcelain was seen; and (4) mixed failure or a combination of adhesive and cohesive failure where the feldspathic porcelain surface demonstrated both characteristics mentioned previously.
The other half of the specimens were thermocycled in water for 5000 cycles alternating in intervals of 60 s between 5°C and 55°C, with a transfer time of 15 s. After thermocycling, the specimens’ shear bond strengths were measured following the protocol previously described.
Surface Analysis after Surface Treatment
Six sintered feldspathic specimens of 8.0 mm in diameter and 6.0 mm in height were prepared using the same protocol stated previously; each specimen underwent one of the six surface treatments. They were evaluated by SEM and EDX. The surface of the ceramic specimen from each surface treatment was analyzed using SEM at a magnification of ×500 and ×2,000, and their elemental compositions analyzed using EDX spectroscopy. The data were obtained by an SEM (JSM-5800LV, JEOL, Japan, and ISIS Series 300, Oxford, England) fitted with an EDX spectrometer. The primary electron energy used varied from 5 to 20 keV. Test parameters were set to the following: working distance (WD) = 15 mm, process time = 5 s, live time = 60 s, and dead time = 30%–40%. Three different areas were selected from each specimen and each area was scanned at five separate times.
Data Analysis
The shear bond strengths of all groups were analyzed statistically with SPSS 20.0 software for Windows (SPSS Inc, Chicago, Illinois, United States). A normal distribution of error was found with Kolmogorov–Smirnov test. The bond strength value was further analyzed by two-way ANOVA. Tukey's HSD test was applied to determine the significant differences between surface treatment groups at the confidence level of 95%. The surface morphology and elemental occurrence were analyzed using descriptive statistics.
Results
The means and standard deviations of shear bond strength are reported in Table 2 . Two-way ANOVA showed significant difference in shear bond strength values between different surface conditioning methods and simulated aging conditions. No significant differences were detected among all of the HF-etched porcelain groups ( p > 0.05). HF-treated groups revealed significantly higher shear bond strength than the control group in both thermocycled and nonthermocycled conditions ( p < 0.05).
Table 2. The means and standard deviations of shear bond strength.
| Surface treatments | Mean ± SD | |
|---|---|---|
| No-aging ( n = 10) | Aging ( n = 10) | |
| The same superscript indicates no significant difference. Abbreviations: SD, standard deviation; HF, hydrofluoric. | ||
| Group 1: Control | 7.49 ± 0.98 C | 2.93 ± 1.35 D |
| Group 2: HF | 19.44 ± 3.54 A | 12.84 ± 2.87 B |
| Group 3: HF + calcium hydroxide | 19.32 ± 2.82 A | 13.31 ± 2.97 B |
| Group 4: HF + calcium carbonate | 21.33 ± 2.93 A | 11.83 ± 2.34 B |
| Group 5: HF + calcium gluconate | 21.16 ± 3.54 A | 14.07 ± 3.31 B |
| Group 6: HF + ultrasonic | 20.69 ± 3.17 A | 13.64 ± 2.70 B |
After stimulated aging, the control group produced the lowest value (2.93 MPa) and was significantly different from other groups. All aged HF-etched porcelain groups (groups 2–6) had significantly higher shear bond strength values (11.83–14.07 MPa) than that of the control group without significant difference between groups.
The shear bond strength of the nonaged control group was significantly higher (7.49 MPa) than the aged control group. However, this control group still presented significantly lower bond strength than both aged and nonaged HF-etched porcelain groups (groups 2–6). The highest values (19.32–21.33 MPa) were found among nonaged HF-etched porcelain groups with no significant difference between groups. Significant differences were found between all thermocycled group and nonthermocycled group receiving the same treatment.
The control groups demonstrated adhesive failure at the resin/ceramic interface at both time periods, immediately EDX after surface treatment, and after simulated aging. On the other hand, failure analysis of all etched groups revealed predominantly cohesive failure in ceramic and very few mixed failures at both the composite resin and ceramic surface ( Table 3 ).
Table 3. Mode of failure for the different porcelain surface treatments.
| Surface treatments | Without thermocycling ( n = 10) | With thermocycling ( n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|
| Adhesive | Cohesive (composite) | Cohesive (ceramic) | Mixed | Adhesive | Cohesive (composite) | Cohesive (ceramic) | Mixed | |
| Abbreviation: HF, hydrofluoric. | ||||||||
| Group 1: Control | 100 | 100 | ||||||
| Group 2: HF | 80 | 20 | 100 | 0 | ||||
| Group 3: HF + calcium hydroxide | 90 | 10 | 80 | 20 | ||||
| Group 4: HF + calcium carbonate | 100 | 0 | 90 | 10 | ||||
| Group 5: HF + calcium gluconate | 100 | 0 | 100 | 0 | ||||
| Group 6: HF + ultrasonic | 90 | 10 | 100 | 0 | ||||
The SEM images at ×500 and ×2,000 magnification of the samples from each experimental group after surface treatment are shown in Figs. 1 2 . The nonetched porcelain surface of the control group exhibited a smooth-flat surface with slight porosity and polishing groove formations ( Figs. 1a 2a ).
Fig. 1.
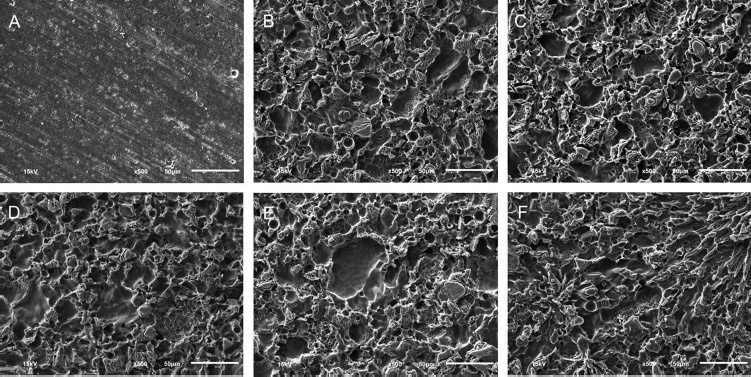
The scanning electron microscope images of the porcelain with different surface treatments at ×500: (a) Control, (b) hydrofluoric, (c) hydrofluoric and calcium hydroxide, (d) hydrofluoric and calcium carbonate, (e) hydrofluoric and calcium gluconate, and (f) hydrofluoric and ultrasonic cleaning.
Fig. 2.
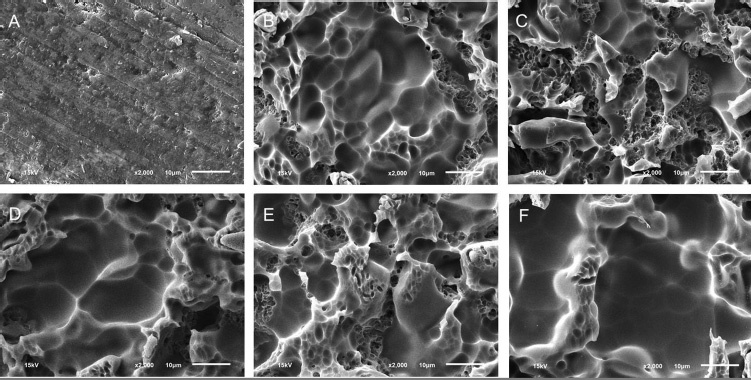
The scanning electron microscope images of the porcelain with different surface treatments at ×2000: (a) Control, (b) hydrofluoric, (c) hydrofluoric and calcium hydroxide, (d) hydrofluoric and calcium carbonate, (e) hydrofluoric and calcium gluconate, and (f) hydrofluoric and ultrasonic cleaning.
Etching with 9.5% HF acid for 90 s resulted in a morphological honeycomb-like surface with shallow irregularities, microporosities, and grooves as shown in Figs. 1b 2b . SEM photographs of experimental Groups 3–6 showed irregular morphology similar to that of Group 2 ( Fig. 1c-f ). In addition, high magnification of the surface irregularities of all experimental groups demonstrated no debris or acid-etched residue ( Fig. 2c-f ).
EDX analyses of the treated surfaces of each group are shown in Fig. 3 . The control group demonstrated peaks in C, O, Na, Mg, Al, Si, K, and Ca spectra at 0.2774 (Kα), 0.5249 (Kα), 1.0410 (Kα), 1.2536 (Kα), 1.4866 (Kα), 1.7398 (Kα), 3.3129 (Kα), and 3.6905 (Kα) keV, respectively. In addition, HF-etched porcelain showed identical spectra to the control group. However, F element, with a peak at 0.6768 (Kα) keV, was not found on any of the specimens.
Fig. 3.
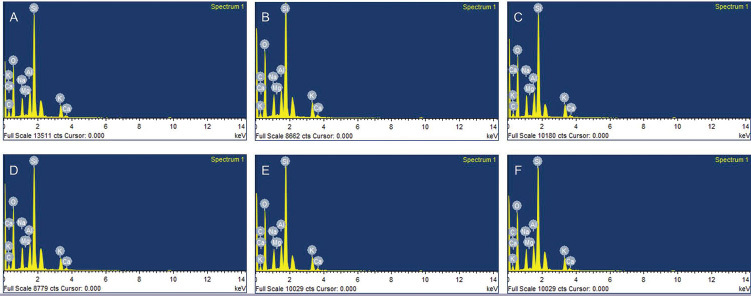
The energy-dispersive X-ray spectra of the porcelain with different surface treatments: (a) Control, (b) hydrofluoric, (c) hydrofluoric and calcium hydroxide, (d) hydrofluoric and calcium carbonate, (e) hydrofluoric and calcium gluconate, and (f) hydrofluoric and ultrasonic cleaning.
Discussion
This study investigated the effect of different surface treatments on the shear bond strength between HF-etched feldspathic porcelain and resin composite at two different time periods, immediately after surface treatment, and after simulated aging. Immediately after surface treatment, all five experimental groups showed higher bond strength values compared to the nonetched control group. Furthermore, there was no significant difference in shear bond strength between any experimental groups. After simulated aging, all specimens demonstrated a significant drop in shear bond strength compared with their nonaged counterparts. Similarly, all aged experimental groups demonstrated higher bond strength values compared with the control group but with no significant difference between the individual experimental groups. Therefore, the first hypothesis was rejected. All HF-etched groups demonstrated similar patterns of irregularities on the surface micromorphology and identical elements on the specimen surfaces. As a result, the second hypothesis was also rejected.
HF acid etching and silanization can improve the optimal bond strength of repaired feldspathic porcelain. 5 6 9 11 13 19 20 Etching porcelain with HF acid selectively dissolves the glassy phase, resulting in microporosities, thus creating mechanical interlock between resin composite and ceramic. 19 Silane coupling agent is then used to provide chemical bonding to the ceramic surface. 5 19 21 The focus of this study is to evaluate the effectiveness of HF acid when used to etch the porcelain surface to improve the mechanical bond. Thus, the use of chemical bonding agents such as silane was excluded to isolate the effect of HF acid in increasing mechanical interlock for investigation in this study.
HF acid is a weak acid when compared to other etching agents. However, it has high toxicity. When dissolved, the fluoride ion is released and can penetrate tissue. The deeply penetrated ion can bind to calcium and magnesium ions in bone and blood. 14 22 An effective treatment to alleviate HF acid toxicity is to use calcium gluconate. When dissolved, the calcium ions bind to fluorine to form an insoluble calcium fluoride salt. 12 14
Generally, neutralizing agents are used to neutralize the pH of a substance and make it less hazardous. A reaction will result from the acid–base reaction, forming water and salt. The objective of using neutralizing agent against HF acid is to reduce the toxicity of fluorine. The calcium-containing compounds used in this research were calcium hydroxide and calcium carbonate, two materials that can be easily found in every dental clinic. Calcium gluconate is mainly used to treat HF burns. 22
Özcan and Volpato 23 proposed that neutralizing agents could prevent the toxic effects of HF acid by eliminating its residuals within pores on the ceramic surface and by neutralizing its pH. On the other hand, Canay et al 24 stated that etching ceramic with HF acid would result in fluorosilicate crystalline precipitates that were insoluble in water. Saavedra et al 18 and Amaral et al 17 reported that using neutralizing agents after HF treatment decreased the bond strength between adhesive cement and ceramic due to the precipitation on the etched surface. They suggested that the precipitation was a result of the reaction between HF acid and neutralizing agents. Bottino et al 16 mentioned that the use of neutralizing agents decreased the surface energy of ceramic as it created a precipitate at the etched region, thus decreasing both the bonding capability between resin cement and ceramic and the microtensile bond strength.
In our study, the bond strengths of Groups 2–6 were not different. In contrast to Bottino et al, 16 their study demonstrated that neutralizing agents decreased the bond strength to porcelain. They hypothesized that the precipitate from the neutralizing agents interfered with the bond strength of porcelain.
However, according to the EDX readings of our study, only C, O, Na, Mg, Al, Si, K, and Ca ions were found in all groups. Fluorine ion was not found in any group. This might be because the fluorine and calcium ion present in the neutralizing agent were bound to the fluorine ion on the surface of the sample, which was rinsed away by the water spray or ultrasonic.
The SEM image showed that HF-etched porcelain, after neutralization with various substances and cleaned with water spray, demonstrated a clean surface with no acid–base reaction residue, similar to the group that was ultrasonically cleaned. This was significant as many studies have reported that ultrasonic cleaning after etching with HF resulted in maximum cleansing and optimal bond strength. 25 26 27
The reason for the disappearance of precipitate may have been from the water spray cleaning. Steinhauser et al 27 stated that 60 s of water spray cleaning provided comparable microshear bond strength to cleaning in an ultrasonic bath with distilled water for 5 min. They also reported that little to no residue was present on the ceramic surface after HF etching and ultrasonic cleansing. Still, this method may not be suitable for the direct repair technique. 22
The results from this study found that the unetched control group mainly demonstrated adhesive failure, whereas the remaining experimental groups mainly demonstrated either cohesive or mixed failure. The adhesive failure is mainly correlated with low shear bond strength as obtained from the unetched specimens. Cohesive failure in porcelain indicates that the cohesive strength of the porcelain is inferior to the bond of composite to porcelain. de Melo et al 28 have suggested that porcelain with lower crystalline phase content and higher glassy phase results in more cohesive failure. Cohesive or mixed failures are often correlated with high shear bond strength value as obtained from all HF-treated specimens.
This research used unfilled resin with low viscosity as the bonding agent. The unfilled resin provides better infiltration to the irregularities and improves adaptation between the resin and ceramic surface which may result in increased bond strength. 10 29 Previous studies used resin cement, which had high viscosity compared to unfilled resin. 16 This may result in less infiltration and poor adaptation and account for the resulting lower bond strengths of their studies.
Thermocycling is widely used to test the durability of adhesives and simulate intraoral aging. It significantly decreases the shear bond strength of all experimental groups compared to their nonaged counterparts. Due to the different coefficients of thermal expansion of porcelain and resin-based polymer, the series of alternations of compression and expansion may be responsible for the reduced bond strength at the interface of two materials. 30 31 This study showed all groups to have lower shear bond strength after aging.
Typically, it is recommended that the use of HF should be used in accordance with the manufacturer's instructions and that a rubber dam be used to protect the patient's adjacent hard and soft tissues. 22 This study found that the use of neutralizing agents did not negatively affect the bond strength of porcelain to resin composite. Therefore, it can be considered as an alternative to reducing the toxicity of HF. Further investigation is needed to test the effect of HF neutralizing agents on other physical properties (i.e., surface roughness and flexural strength) of feldspathic porcelain. Moreover, there should be studies on the effect of neutralizing agents of HF on other ceramic substrates as well.
Conclusion
Within the limitations of this in vitro study, the following can be concluded:
There is no significant difference in shear bond strength between plain etched porcelain, etched porcelain cleaned ultrasonically, or etched porcelain treated with neutralizing agents after cleaning with water spray for 60 s, given that unfilled resin is used.
The shear bond strength of every group decreases significantly when subjected to 5,000 cycles of thermocycling.
Neutralizing agents such as calcium carbonate, calcium hydroxide, and calcium gluconate can be considered as an alternative to reduce the toxicity of HF.
Financial Support and Sponsorship
The study was supported by the Research Institute of Rangsit University, Thailand (Project No. 44/2559).
Footnotes
Conflicts of Interest None.
References
- 1.Ho G W, Matinlinna J P. Insights on ceramics as dental materials. Part I: ceramic material types in dentistry. Silicon. 2011;3(03):109–115. [Google Scholar]
- 2.Rashid H. The effect of surface roughness on ceramics used in dentistry: a review of literature. Eur J Dent. 2014;8(04):571–579. doi: 10.4103/1305-7456.143646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozcan M. Fracture reasons in ceramic-fused-to-metal restorations. J Oral Rehabil. 2003;30(03):265–269. doi: 10.1046/j.1365-2842.2003.01038.x. [DOI] [PubMed] [Google Scholar]
- 4.Pameijer C H, Louw N P, Fischer D. Repairing fractured porcelain: how surface preparation affects shear force resistance. J Am Dent Assoc. 1996;127(02):203–209. doi: 10.14219/jada.archive.1996.0170. [DOI] [PubMed] [Google Scholar]
- 5.Matinlinna J P, Lung C YK, Tsoi J KH. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent Mater. 2018;34(01):13–28. doi: 10.1016/j.dental.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Reston E G, Filho S C, Arossi G, Cogo R B, Rocha Cdos S, Closs L Q. Repairing ceramic restorations: final solution or alternative procedure? Oper Dent. 2008;33(04):461–466. doi: 10.2341/07-151. [DOI] [PubMed] [Google Scholar]
- 7.da Cunha L F, Reis R, Santana L, Romanini J C, Carvalho R M, Furuse A Y. Ceramic veneers with minimum preparation. Eur J Dent. 2013;7(04):492–496. doi: 10.4103/1305-7456.120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaghloul H, Elkassas D W, Haridy M F. Effect of incorporation of silane in the bonding agent on the repair potential of machinable esthetic blocks. Eur J Dent. 2014;8(01):44–52. doi: 10.4103/1305-7456.126240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian T, Tsoi J K, Matinlinna J P, Burrow M F. Aspects of bonding between resin luting cements and glass ceramic materials. Dent Mater. 2014;30(07):e147–e162. doi: 10.1016/j.dental.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Naves L Z, Soares C J, Moraes R R, Gonçalves L S, Sinhoreti M A, Correr-Sobrinho L. Surface/interface morphology and bond strength to glass ceramic etched for different periods. Oper Dent. 2010;35(04):420–427. doi: 10.2341/09-152-L. [DOI] [PubMed] [Google Scholar]
- 11.Barghi N, Fischer D E, Vatani L.Effects of porcelain leucite content, types of etchants, and etching time on porcelain-composite bond J Esthet Restor Dent 2006180147–52.discussion 53 [DOI] [PubMed] [Google Scholar]
- 12.Bertolini J C. Hydrofluoric acid: a review of toxicity. J Emerg Med. 1992;10(02):163–168. doi: 10.1016/0736-4679(92)90211-b. [DOI] [PubMed] [Google Scholar]
- 13.Ho G W, Matinlinna J P. Insights on ceramics as dental materials. Part II: Chemical surface treatments. Silicon. 2011;3(03):117–123. [Google Scholar]
- 14.Wang X, Zhang Y, Ni L. A review of treatment strategies for hydrofluoric acid burns: current status and future prospects. Burns. 2014;40(08):1447–1457. doi: 10.1016/j.burns.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Panah F G, Rezai S M, Ahmadian L. The influence of ceramic surface treatments on the micro-shear bond strength of composite resin to IPS Empress 2. J Prosthodont. 2008;17(05):409–414. doi: 10.1111/j.1532-849X.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 16.Bottino M A, Snellaert A, Bergoli C D, Özcan M, Bottino M C, Valandro L F. Effect of ceramic etching protocols on resin bond strength to a feldspar ceramic. Oper Dent. 2015;40(02):E40–E46. doi: 10.2341/13-344-L. [DOI] [PubMed] [Google Scholar]
- 17.Amaral R, Ozcan M, Bottino M A, Valandro L F. Resin bonding to a feldspar ceramic after different ceramic surface conditioning methods: evaluation of contact angle, surface pH, and microtensile bond strength durability. J Adhes Dent. 2011;13(06):551–560. doi: 10.3290/j.jad.a19815. [DOI] [PubMed] [Google Scholar]
- 18.Saavedra G, Ariki E K, Federico C D. Effect of acid neutralization and mechanical cycling on the microtensile bond strength of glass-ceramic inlays. Oper Dent. 2009;34(02):211–216. doi: 10.2341/08-68. [DOI] [PubMed] [Google Scholar]
- 19.Matinlinna J P, Vallittu P K. Bonding of resin composites to etchable ceramic surfaces - an insight review of the chemical aspects on surface conditioning. J Oral Rehabil. 2007;34(08):622–630. doi: 10.1111/j.1365-2842.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen J H, Matsumura H, Atsuta M. Effect of etchant, etching period, and silane priming on bond strength to porcelain of composite resin. Oper Dent. 1998;23(05):250–257. [PubMed] [Google Scholar]
- 21.Lung C Y, Matinlinna J P. Aspects of silane coupling agents and surface conditioning in dentistry: an overview. Dent Mater. 2012;28(05):467–477. doi: 10.1016/j.dental.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan M, Allahbeickaraghi A, Dündar M. Possible hazardous effects of hydrofluoric acid and recommendations for treatment approach: a review. Clin Oral Investig. 2012;16(01):15–23. doi: 10.1007/s00784-011-0636-6. [DOI] [PubMed] [Google Scholar]
- 23.Özcan M, Volpato C A. Surface conditioning protocol for the adhesion of resin-based materials to glassy matrix ceramics: how to condition and why? J Adhes Dent. 2015;17(03):292–293. doi: 10.3290/j.jad.a34590. [DOI] [PubMed] [Google Scholar]
- 24.Canay S, Hersek N, Ertan A. Effect of different acid treatments on a porcelain surface. J Oral Rehabil. 2001;28(01):95–101. doi: 10.1046/j.1365-2842.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- 25.Martins M E, Leite F P, Queiroz J R, Vanderlei A D, Reskalla H N, Ozcan M. Does the ultrasonic cleaning medium affect the adhesion of resin cement to feldspathic ceramic? J Adhes Dent. 2012;14(06):507–509. doi: 10.3290/j.jad.a28625. [DOI] [PubMed] [Google Scholar]
- 26.Alex G.Preparing porcelain surfaces for optimal bonding Compend Contin Educ Dent 20082906324–335.quiz 336 [PubMed] [Google Scholar]
- 27.Steinhauser H C, Turssi C P, Franca F M, Amaral F L, Basting R T. Micro-shear bond strength and surface micromorphology of a feldspathic ceramic treated with different cleaning methods after hydrofluoric acid etching. J Appl Oral Sci. 2014;22(02):85–90. doi: 10.1590/1678-775720130339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Melo R M, Valandro L F, Bottino M A. Microtensile bond strength of a repair composite to leucite-reinforced feldspathic ceramic. Braz Dent J. 2007;18(04):314–319. doi: 10.1590/s0103-64402007000400008. [DOI] [PubMed] [Google Scholar]
- 29.Sundfeld Neto D, Naves L Z, Costa A R. The effect of hydrofluoric acid concentration on the bond strength and morphology of the surface and interface of glass ceramics to a resin cement. Oper Dent. 2015;40(05):470–479. doi: 10.2341/14-133-L. [DOI] [PubMed] [Google Scholar]
- 30.Gale M S, Darvell B W. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27(02):89–99. doi: 10.1016/s0300-5712(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 31.Yoo J Y, Yoon H I, Park J M, Park E J. Porcelain repair—Influence of different systems and surface treatments on resin bond strength. J Adv Prosthodont. 2015;7(05):343–348. doi: 10.4047/jap.2015.7.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]


