Version Changes
Revised. Amendments from Version 1
This manuscript has been revised to address the comments of the reviewers. In the revised manuscript, we reworked the section on Africa and added a section that briefly describes how HTS leads to the rapid detection of the spread of the newly identified colistin resistance risk after reviewing the articles that had been suggested. We also reworked the sections that had been hinted upon as being too long and, lacking clarity, precision, and completeness, we made these sections shorter, more clear, precise and, complete. We also ultimately highlighted the potential of WGS in the identification of chromosomally mediated resistance mechanisms as well as acquired resistance mechanisms.
Abstract
Bacterial infections involving antibiotic-resistant gram-negative bacteria continue to increase and represent a major global public health concern. Resistance to antibiotics in these bacteria is mediated by chromosomal and/or acquired resistance mechanisms, these give rise to multi-drug resistant (MDR), extensive-drug resistant (XDR) or pan-drug resistant (PDR) bacterial strains. Most recently, plasmid-mediated resistance to colistin, an antibiotic that had been set apart as the last resort antibiotic in the treatment of infections involving MDR, XDR and PDR gram-negative bacteria has been reported. Plasmid-mediated colistin resistant gram-negative bacteria have been described to be PDR, implying a state devoid of alternative antibiotic therapeutic options. This review concisely describes the evolution of antibiotic resistance to plasmid-mediated colistin resistance and discusses the potential role of high-throughput sequencing technologies, genomics, and bioinformatics towards improving antibiotic resistance surveillance, the search for novel drug targets and precision antibiotic therapy focused at combating colistin resistance, and antibiotic resistance as a whole.
Keywords: Antibiotic resistance, Colistin resistance, Pan-drug resistance, Gram negative bacteria, Genomics, Bioinformatics
Introduction
In the recent past, old antibiotic classes previously deemed unfit for treatment of bacterial infections due to associated toxicity concerns have been recommended for the treatment of the same infections 1, 2. This has been attributed to the emergence of resistance to the most recently considered last line antibiotics, the carbapenems 1, 2. Carbapenem resistance has been documented in bacteria belonging to the Enterobacteriaceae family, Acinetobacter baumannii and Pseudomonas aeruginosa 1, 2. The adoption of the old antibiotic agent category in routine empirical treatment has witnessed the use of a number of antibiotics such as colistin 1, 2.
Despite this reversion, gram-negative bacteria continue to undergo chromosomal mutations, which render their respective treatments virtually impossible and hence a major threat to global public health. The effects of these antibiotic resistance mutations are further exacerbated by horizontal transfer of antibiotic resistance genes in the same bacteria. As such, this paper explores the current documented trends of colistin resistance in several African settings. Additionally, it also describes the evolution of antibiotic resistance to plasmid-mediated colistin resistance and the potential role of genomics and bioinformatics in precision antibiotic therapy targeted towards combating colistin resistance and antibiotic resistance.
Colistin resistance trends in Africa
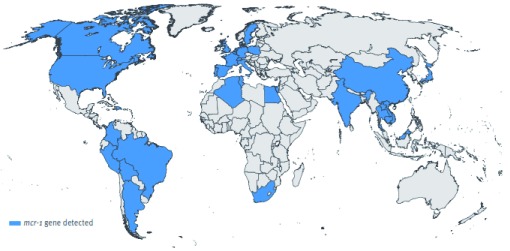
Data on the antimicrobial resistance burden, particularly colistin resistance, in Africa remains limited 3. In 2014, the World Health Organization (WHO) reported that antimicrobial resistance surveillance in Africa was a particularly difficult feat due to the scarcity of viable medical data, statistical information and unreliable laboratory capacity 3. Despite this, African countries remain un-exempted from this worldwide antibiotic resistance trend that has emerged not only within hospital settings but also disseminated within communities. The available literature from African settings (i.e. South Africa, Algeria, Tunisia, and Egypt) has reported colistin resistance ( Figure 1) 4– 7. Beyond these settings, colistin resistance has also been reported in Uganda and Rwanda 8– 10.
Figure 1. Data collected from 30 countries acknowledging the existence of the colistin resistant mcr-1 gene isolated from humans, the environment and animals.
Reproduced from Xavier et al. 4 under a CC-BY 4.0 license.
Evolution to plasmid-mediated colistin resistance in gram-negative bacteria
Colistin (polymyxin E) is part of an old generation of antibiotics 11 that form a family of cationic polypeptides. These are characterised by having a lipophilic fatty acyl side chain 12– 14. No exact mechanism of bacterial killing has been documented for polymyxins, especially in Acinetobacter spp 14, 15. However, a two-step mechanism has been described to elucidate their possible mechanism of action 13, 14.
The two-step mechanism involves i) initial binding to and permeabilization of the outer membrane and ii) the destabilization of the cytoplasmic membrane of the bacteria 12– 14. As a consequence, colistin functions by intercalating into the inner membrane following diffusion from the outer membrane across the periplasm and consequently causing the formation of pores, a phenomenon that results in bacterial lysis, which follows initial binding to bacterial surfaces 12– 14. Initial binding of colistin to the bacterial surface chiefly depends on the electrostatic interaction between the positively-charged colistin and the negatively charged phosphate group of lipid A, an endotoxic component on the lipopolysaccharide localised on the outer leaflet of the bacterial outer membrane 12, 14.
The modifications of the lipid A which reduce and/or abolish the initial charge-based interaction of the polymyxins withbacteria and also the addition of either/or the 4-amino-4-deoxy-L-arabinose (L-Ara4N) and the phosphoethanolamine (PEtn) that ultimately form the basis of colistin resistance in bacteria are mediated by chromosomally encoded genes 13– 16. These genes are involved in the modulation of two-component regulatory systems; PmrA/PmrB and PhoP/PhoQ and mgrB, a negative regulator of the PhoP/PhoQ signaling system 13– 16 .
Although initially thought that this resistance could not be spread from cell to cell (plasmid-mediated) 16, currently studies have shown otherwise. These have alluded transfer of colistin resistance among bacteria via plasmids in horizontal gene transfer 4, 16, 17.
Pan-drug resistance and characteristics of colistin-resistant gram-negative bacteria
The treatment of infections involving antibiotic-resistant gram-negative bacteria has become increasingly difficult overtime, a factor that has greatly contributed to high morbidity, mortality and high costs of health care 18, 19.
Currently, antibiotic resistance in these bacteria spans across several classes but likely follows a precise hierarchy of acquisition; this is mostly characterized by acquisition of “enhanced resistance” against more potent antibiotics following primary acquisition of “weaker resistance” against the less potent antibiotics alongside intrinsic resistance mechanisms in these bacteria, a trend that follows a Darwin’s like fashion 20– 23. These changes are a function of horizontal gene transfer, via conjugation, transformation and transduction 24– 27.
Resistance in gram-negative bacteria has been seen to transit from being mediated by the extended spectrum β-lactamases, a group of enzymes that can be disseminated among bacteria 28, 29; these chiefly confer resistance against broad-spectrum cephalosporins. However, they also confer resistance to penicillins, monobactams and some carbapenems, particularly the Klebsiella pnemoniae carbapenemase, KPC 28, 30, 31. In the same hierarchy are AmpC β-lactamases that form another group of β-lactamases, derived from older broad spectrum β-lactamases. These provide an even more extended activity that includes resistance against the cephamycins alongside resistance to penicillins, monobactams and cephalosporins 32– 34. These enzymes have in recent times been shown to not only be limited to being encoded on the chromosomes of bacteria, but have also been documented to have the potential of being disseminated via plasmids in horizontal gene transfer 28, 34, 35 and also to co-exist with the extended spectrum β-lactamases 36, 37; factors that have made these bacteria “better resistant” to antibiotics. Next in the hierarchy are the carbapenemases, these enzymes are chiefly acquired in horizontal gene transfer and confer resistance to carbapenems alongside resistance to penicillins, broad-spectrum cephalosporins including cefepime, a fourth-generation cephalosporin, monobactams, aminoglycosides, quinolones and fluoroquinolones 28, 38. The development of resistance mediated by these enzymes to the different classes of antibiotics in these bacteria has been attributed to various factors among which is their use in therapy. This has not only abetted maintenance of resistance via selecting for resistance to these antibiotics in these bacteria but has also created a gap, a need for alternative antibiotics in therapy to replace the penicillins, β-lactams, carbapenems and the other classes of antibiotics used in the treatment of infections involving the drug-resistant gram-negative bacteria 4, 16.
Colistin, a polypeptide antibiotic, a relatively old antibiotic, has been currently relied upon to provide the ultimate line of refuge against infections caused by antibiotic-resistant gram-negative bacteria despite its previously documented impacts on health 4, 16. Colistin also appears to offer a choice in the face of almost no new antibiotics in production pipelines 4, 16, 17.
Worryingly, the use of colistin is under threat due to the emergence of plasmid-mediated colistin resistance involving mcr gene families 4, 16. This provides a new challenge as bacteria that express these resistance genes assume the lead in the antibiotic resistance hierarchy and are distinctively XDR or worse PDR 16, 39– 42.
Molecular studies previously done have reported colistin resistant gram-negative bacteria to also be resistant to an array of antibiotics 43, 44. These bacteria have also been reported to carry plasmids that have been found to carry alongside colistin resistance genes, β-lactamases 43, 44, carbapenemase encoding genes 45 and genes that code for resistances to other antibiotic classes that may include quinolones, fluoroquinolones and aminoglycosides 13. Additionally, the carriage of mcr-1 has been documented as a possible indicator of resistance to the third generation cephalosporins and carbapenems 38, 44. Furthermore, these genes have been found to be co–carried with other resistance determinants in plasmids 13, 44, 46, 47; these genes represent a novel mechanism of antibiotic resistance in bacteria and a threat to the existing antibiotic therapy. Worsening the situation is the ability of selection for colistin resistance via the use of the extended-spectrum cephalosporins. Additionally, the use of tetracycline and sulphonamides has also been reported to contribute to the dissemination of colistin mobile gene carrying plasmids 44, 46. Also, worth noting is plasmids that carry colistin resistance genes have also been found to mostly carry other antibiotic resistant genes 13, 44– 46.
The role of high-throughput sequencing technologies and bioinformatics
Advances in technology including the rapidly growing field of genomics, are transforming clinical medicine 48 and high-throughput sequencing technology (HTS) is increasingly being used in clinical microbiology 49. HTS, with relatively simple benchtop technology and efficient genomic library preparation protocols, has significantly improved the capacity to perform low-cost, efficient whole-genome sequencing (WGS), and has made it a feasible tool to enhance clinical diagnostic investigations in near real-time 48. The processes generally involve culture-free parallel sequencing, producing vast quantities of genomic data that require modern computation techniques to assemble the genomic sequence reads as well as performing ensuing analyses that range from identifying the bacterial species or strain, antibiotic resistance mutations in the bacterial genomes, while ensuring the highest possible discriminatory power ever achieved by any technology 49. Apart from this, WGS of bacteria can identify genes associated with virulence and pathogenicity as well as discover new genetic mechanisms for virulence, pathogenicity and antibiotic resistance 48, 50, 51.
The identification and prediction of antibiotic-resistant microorganisms in clinical specimens solely by molecular means in the diagnostic microbiology laboratory is not novel 52. HTS technologies and computational tools offer unprecedented ability to sequence multitudes of bacterial genomes and enable interpretation of the resultant sequence information in near “real-time” 52.
WGS represents the pinnacle for bacterial strain characterization and epidemiological analyses. It is rapidly replacing traditional typing methods, antibiotic resistance gene detection and other molecular-based investigations in the near future. HTS technologies are rapidly evolving and their implementation in clinical and public health microbiology laboratories is increasing at a similar pace. These require standardized sample quality control, data interpretation, bioinformatics expertise, and infrastructure. The term ‘bioinformatics’ encompasses the handling and analysis of genomic sequence data, usually with the assistance of computer-based algorithms. Both ‘open source’ and commercially available bioinformatics programs/tools have been specifically developed for use in a clinical setting. However, many of practicing healthcare workers in current practice have limited bioinformatics knowledge 48.
Furthermore, genome plasticity, as well as pan-genomes that have the ability to influence bacterial resistome can only effectively be investigated using HTS and bioinformatics analyses 48. Understanding the bacterial genome dynamics is an important step in identifying the forces behind phenotypicantibiotic resistance and therefore allows for effective management of the antibiotic-resistant infections 48.
HTS and bioinformatics analyses have made it possible to employ comprehensive WGS-based surveillance of colistin resistance and antimicrobial resistance as a whole by enabling the rapid detection of colistin resistance (i.e. both chromosomal and plasmid-mediated) as well as resistance to other antibiotics; these have also made it possible to map how resistance spreads in a One-Health perspective in a way that was not possible before 53, 54. Furthermore, these have also allowed for rapid re-analysis of large datasets in silico, this has enabled the early detection as well as risk assessment particularly when new genes emerge 53, 54.
The future direction of HTS
Antibiotic resistance in bacteria is generally a natural phenomenon 55– 57 though augmented by human behavior. Therefore, it is imperative to harness the best HTS technologies that sequence DNA at unprecedented speed, to enable previously unimaginable scientific achievements and novel biological applications 53. Such applications of genomics tools have revolutionized microbial ecological studies and drastically expanded our view on the previously underappreciated microbial world 54 including acquisition and transmission dynamics of antibiotic resistance. Single-Molecule Real-Time (SMRT) sequencing (Pacific Biosciences Inc.) in clinical microbiology has finally been realized at many levels in health care systems in the developing world and relatively only used during isolated scenarios of disease outbreaks in the less developed countries. These developments in HTS must be matched with continued efforts to improve the current bioinformatics analytic pipelines. Applying SMRT while genome sequencing to investigate bacterial colistin resistance would be made possible to predict resistance mutations, resistance mechanisms, trends, and patterns enabling efficient management of the colistin resistance by healthcare providers and pharmaceutical companies.
Conclusions
It is known that host, bacterial and environmental factors interact collectively to bring about antibiotic resistance. Therefore, HTS should be applied to a wide range of global collections of bacterial whole genomes to identify and predict new antibiotic drug resistance mutations using appropriate computational and bioinformatics algorithms.
Computational algorithms and tools offer the ability to simulate bacterial genomic mutations while also offering possible clues on the mechanisms that may be shaping these mutations. These can as well be utilized to develop therapeutic interventions that may be used to target both the current and future acquired antibiotic drug resistance mutations.
Data availability
No data are associated with this article
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved
References
- 1. Giske CG: Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect. 2015;21(10):899–905. 10.1016/j.cmi.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 2. Sonnevend A, Ghazawi A, Alqahtani M, et al. : Plasmid-mediated colistin resistance in Escherichia coli from the Arabian Peninsula. Int J Infect Dis. 2016;50:85–90. 10.1016/j.ijid.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization: Antimicrobial resistance: global report on surveillance. World Health Organization.2014. Reference Source [Google Scholar]
- 4. Xavier BB, Lammens C, Ruhal R, et al. : Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27). 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- 5. Lowe M, Ehlers MM, Ismail F, et al. : Acinetobacter baumannii: Epidemiological and Beta-Lactamase Data From Two Tertiary Academic Hospitals in Tshwane, South Africa. Front Microbiol. 2018;9:1280. 10.3389/fmicb.2018.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alonso CA, Zarazaga M, Ben Sallem R, et al. : Antibiotic resistance in Escherichia coli in husbandry animals: the African perspective. Lett Appl Microbiol. 2017;64(5):318–334. 10.1111/lam.12724 [DOI] [PubMed] [Google Scholar]
- 7. Perreten V, Strauss C, Collaud A, et al. : Colistin Resistance Gene mcr-1 in Avian-Pathogenic Escherichia coli in South Africa. Antimicrob Agents Chemother. 2016;60(7):4414–15. 10.1128/AAC.00548-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sofiane B, Olaitan AO, Ammari H, et al. : Emergence of Colistin- and Carbapenem-Resistant Acinetobacter baumannii ST2 Clinical Isolate in Algeria: First Case Report. Microb Drug Resist. 2015;21(3):279–85. 10.1089/mdr.2014.0214 [DOI] [PubMed] [Google Scholar]
- 9. Carroll M, Rangaiahagari A, Musabeyezu E, et al. : Five-Year Antimicrobial Susceptibility Trends Among Bacterial Isolates from a Tertiary Health-Care Facility in Kigali, Rwanda. Am J Trop Med Hyg. 2016;95(6):1277–1283. 10.4269/ajtmh.16-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sserwadda I, Lukenge M, Mwambi B, et al. : Microbial contaminants isolated from items and work surfaces in the post- operative ward at Kawolo general hospital, Uganda. BMC Infect Dis. 2018;18(1):68. 10.1186/s12879-018-2980-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paterson DL, Harris PN: Colistin resistance: a major breach in our last line of defence. Lancet Infect Dis. 2016;16(2):132–133. 10.1016/S1473-3099(15)00463-6 [DOI] [PubMed] [Google Scholar]
- 12. Poirel L, Jayol A, Nordmann P: Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin Microbiol Rev. 2017;30(2):557–596. 10.1128/CMR.00064-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao R, Hu Y, Li Z, et al. : Dissemination and Mechanism for the MCR-1 Colistin Resistance. PLoS Pathog. 2016;12(11):e1005957. 10.1371/journal.ppat.1005957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moffatt JH, Harper M, Harrison P, et al. : Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54(12):4971–4977. 10.1128/AAC.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams MD, Nickel GC, Bajaksouzian S, et al. : Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009;53(9):3628–3634. 10.1128/AAC.00284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu YY, Wang Y, Walsh TR, et al. : Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 17. Schwarz S, Johnson AP: Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother. 2016;71(8):2066–2070. 10.1093/jac/dkw274 [DOI] [PubMed] [Google Scholar]
- 18. Nathan C, Cars O: Antibiotic resistance--problems, progress, and prospects. N Engl J Med. 2014;371(19):1761–1763. 10.1056/NEJMp1408040 [DOI] [PubMed] [Google Scholar]
- 19. Ferri M, Ranucci E, Romagnoli P, et al. : Antimicrobial resistance: A global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876. 10.1080/10408398.2015.1077192 [DOI] [PubMed] [Google Scholar]
- 20. Shallcross LJ, Howard SJ, Fowler T, et al. : Tackling the threat of antimicrobial resistance: from policy to sustainable action. Philos Trans R Soc Lond B Biol Sci. 2015;370(1670):20140082. 10.1098/rstb.2014.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmes AH, Moore LS, Sundsfjord A, et al. : Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 22. Redgrave LS, Sutton SB, Webber MA, et al. : Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22(8):438–45. 10.1016/j.tim.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 23. Blázquez J, Oliver A, Gómez-Gómez JM: Mutation and evolution of antibiotic resistance: antibiotics as promoters of antibiotic resistance? Curr Drug Targets. 2002;3(4):345–9. 10.2174/1389450023347579 [DOI] [PubMed] [Google Scholar]
- 24. Barlow M: What antimicrobial resistance has taught us about horizontal gene transfer. Methods Mol Biol. 2009;532:397–411. 10.1007/978-1-60327-853-9_23 [DOI] [PubMed] [Google Scholar]
- 25. Davies J, Davies D: Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynch JP, III, Clark NM, Zhanel GG: Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother. 2013;14(2):199–210. 10.1517/14656566.2013.763030 [DOI] [PubMed] [Google Scholar]
- 27. Martínez JL: Natural antibiotic resistance and contamination by antibiotic resistance determinants: the two ages in the evolution of resistance to antimicrobials. Front Microbiol. 2012;3:1. 10.3389/fmicb.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aruhomukama D: Phenotypic Assays for Detection of Extended Spectrum B-Lactamases and Carbapenemases: A Laboratory Guide for Microbiologists.2018. 10.20944/preprints201809.0448.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaikh S, Fatima J, Shakil S, et al. : Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. 10.1016/j.sjbs.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munoz-Price LS, Poirel L, Bonomo RA, et al. : Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pitout JD, Nordmann P, Poirel L: Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob Agents Chemother. 2015;59(10):5873–84. 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomson KS: Controversies about extended-spectrum and AmpC beta-lactamases. Emerg Infect Dis. 2001;7(2):333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lobkovsky E, Moews PC, Liu H, et al. : Evolution of an enzyme activity: crystallographic structure at 2-A resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc Natl Acad Sci U S A. 1993;90(23):11257–11261. 10.1073/pnas.90.23.11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Philippon A, Arlet G, Jacoby GA: Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother. 2002;46(1):1–11. 10.1128/AAC.46.1.1-11.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohamudha PR, Harish BN, Parija SC: Molecular description of plasmid-mediated AmpC β-lactamases among nosocomial isolates of Escherichia coli & Klebsiella pneumoniae from six different hospitals in India. Indian J Med Res. 2012;135(1):114–9. 10.4103/0971-5916.93433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghosh B, Mukherjee M: Emergence of co-production of plasmid-mediated AmpC beta-lactamase and ESBL in cefoxitin-resistant uropathogenic Escherichia coli. Eur J Clin Microbiol Infect Dis. 2016;35(9):1449–1454. 10.1007/s10096-016-2683-z [DOI] [PubMed] [Google Scholar]
- 37. Anusuya Devi D, Ramachander R: Detection of Extended-spectrum Beta-lactamases in AMPC Co-producing Bacteria by Disc Diffusion Method in Clinical Isolates of Enterobacteriacae. Indian J Public Health Res Dev. 2016;7(2):236–242. 10.5958/0976-5506.2016.00100.5 [DOI] [Google Scholar]
- 38. Capone A, Giannella M, Fortini D, et al. : High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19(1):E23–E30. 10.1111/1469-0691.12070 [DOI] [PubMed] [Google Scholar]
- 39. Ah YM, Kim AJ, Lee JY: Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2014;44(1):8–15. 10.1016/j.ijantimicag.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 40. Olaitan AO, Diene SM, Kempf M, et al. : Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44(6):500–507. 10.1016/j.ijantimicag.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 41. Qureshi ZA, Hittle LE, O'Hara JA, et al. : Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. 2015;60(9):1295–1303. 10.1093/cid/civ048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGann P, Snesrud E, Maybank R, et al. : Escherichia coli harboring mcr-1 and bla CTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother. 2016;60(7):4420–4421. 10.1128/AAC.01103-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Falgenhauer L, Waezsada SE, Yao Y, et al. : Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis. 2016;16(3):282–283. 10.1016/S1473-3099(16)00009-8 [DOI] [PubMed] [Google Scholar]
- 44. Malhotra-Kumar S, Xavier BB, Das AJ, et al. : Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect Dis. 2016;16(3):283–284. 10.1016/S1473-3099(16)00012-8 [DOI] [PubMed] [Google Scholar]
- 45. Poirel L, Kieffer N, Liassine N, et al. : Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis. 2016;16(3):281. 10.1016/S1473-3099(16)00006-2 [DOI] [PubMed] [Google Scholar]
- 46. Du H, Chen L, Tang YW, et al. : Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis. 2016;16(3):287–288. 10.1016/S1473-3099(16)00056-6 [DOI] [PubMed] [Google Scholar]
- 47. Monaco M, Giani T, Raffone M, et al. : Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19(42): pii: 20939. 10.2807/1560-7917.ES2014.19.42.20939 [DOI] [PubMed] [Google Scholar]
- 48. Kwong JC, McCallum N, Sintchenko V, et al. : Whole genome sequencing in clinical and public health microbiology. Pathology. 2015;47(3):199–210. 10.1097/PAT.0000000000000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rossen JWA, Friedrich AW, Moran-Gilad J: Practical issues in implementing whole-genome-sequencing in routine diagnostic microbiology. Clin Microbiol Infect. 2018;24(4):355–360. 10.1016/j.cmi.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 50. Nijhuis RH, Oueslati S, Zhou K, et al. : OXY-2-15, a novel variant showing increased ceftazidime hydrolytic activity. J Antimicrob Chemother. 2015;70(5):1429–1433. 10.1093/jac/dkv002 [DOI] [PubMed] [Google Scholar]
- 51. Ferdous M, Kooistra-Smid AM, Zhou K, et al. : Virulence, Antimicrobial Resistance Properties and Phylogenetic Background of Non-H7 Enteropathogenic Escherichia coli O157. Front Microbiol. 2016;7:1540. 10.3389/fmicb.2016.01540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dunne WM, Jr, Westblade LF, Ford B: Next-generation and whole-genome sequencing in the diagnostic clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis. 2012;31(8):1719–1726. 10.1007/s10096-012-1641-7 [DOI] [PubMed] [Google Scholar]
- 53. Hasman H, Hammerum AM, Hansen F, et al. : Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill.(Online Ed)2015;20(49):1–5. 10.2807/1560-7917.ES.2015.20.49.30085 [DOI] [PubMed] [Google Scholar]
- 54. Donà V, Bernasconi OJ, Pires J, et al. : Heterogeneous Genetic Location of mcr-1 in Colistin-Resistant Escherichia coli Isolates from Humans and Retail Chicken Meat in Switzerland: Emergence of mcr-1-Carrying IncK2 Plasmids. Antimicrob Agents Chemother. 2017;61(11): pii: e01245-17. 10.1128/AAC.01245-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chandra H, Bishnoi P, Yadav A, et al. : Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials-A Review. Plants (Basel). 2017;6(2): pii: E16. 10.3390/plants6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wright GD: Q&A: Antibiotic resistance: where does it come from and what can we do about it? BMC Biol. 2010;8(1):123. 10.1186/1741-7007-8-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prestinaci F, Pezzotti P, Pantosti A: Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]