Abstract
Introduction
Prior studies have found heightened negative affect following tobacco abstinence in women compared to men. However, experimental work addressing whether these findings generalize across racial groups is scarce. This study investigated whether race (non-Hispanic White vs. non-Hispanic African American) moderated gender differences in abstinence-induced negative affect and smoking behavior.
Methods
Data were collected from 2010 to 2017 from two separate laboratory studies investigating experimentally manipulated tobacco abstinence. Following a baseline session, adult daily smokers (≥10 cigarettes/day; women: n = 297, 83.8% non-Hispanic African American; men: n = 492, 86.2% non-Hispanic African American) attended two counterbalanced lab sessions (16 hours abstinent vs. non-abstinent) and completed self-report measures of negative affect followed by a laboratory analogue smoking reinstatement task.
Results
We found a gender × race interaction for several negative affect states and composite negative affect (βs = −.12 to −.16, Ps < .05). Analyses stratified by race showed that non-Hispanic White women compared to non-Hispanic White men exhibited greater abstinence-induced increases in anger, anxiety, and composite negative affect (βs = −.20 to −.29, Ps < .05). No significant gender differences in abstinence-induced negative affect were found for non-Hispanic African American smokers (βs = .00 to − .04, Ps > .05).
Conclusion
These findings suggest that negative affect during acute tobacco abstinence may be a clinically important and intervenable factor that can inform cessation interventions specifically for non-Hispanic White women smokers. Further empirical exploration of mechanisms underlying interactions of gender and race in tobacco addiction may benefit smoking cessation efforts in non-Hispanic African American women smokers.
Implications
This study contributes to a scant body of research examining the intersectional influence of race and gender on abstinence-induced negative affect—a central, motivationally prepotent feature of tobacco withdrawal. Using a laboratory-based design to experimentally manipulate abstinence, we provide evidence of a gender × race interaction on negative affect–related withdrawal. Our findings suggest that gender differences in abstinence-induced negative affect observed among non-Hispanic White smokers may not generalize to non-Hispanic African American smokers, highlighting the need for future work to address potential mechanisms underlying the racially discrepant impact of gender on affective tobacco withdrawal.
Introduction
A large body of evidence on tobacco-related health disparities suggests that women1–3 and non-Hispanic African American cigarette smokers4,5 are at heightened risk of developing smoking-related diseases. In addition, women (vs. men) and non-Hispanic African American (vs. other racial groups) smokers are also less likely to successfully quit smoking.6–10 Thus, it is important that health disparities research agendas advance theoretical and clinical knowledge regarding mechanisms that may underlie smoking behaviors and outcomes in women versus men and whether these gender differences differ across racial groups.
Tobacco withdrawal is an important factor that maintains tobacco addiction and impedes smoking cessation success.11,12 One motivationally prepotent feature of the tobacco withdrawal syndrome is negative affect, which is predictive of increased risk of smoking reinstatement in the lab and smoking maintenance and relapse during cessation attempts.12,13 Given its formative influence on the motivational salience of smoking reinstatement among chronic smokers, it is possible that withdrawal-related negative affect experienced during tobacco abstinence may underlie the relatively higher odds of cessation failure and tobacco-related health problems observed among both women (vs. men) and African Americans (vs. other racial backgrounds).
Several laboratory and clinical studies indicate that women compared to men report greater abstinence-induced severity of negative affect states, withdrawal-related distress, and expectations that smoking relieves negative affect.13–16 Moreover, recent epidemiological data suggest that women are more likely than men to endorse a greater variety of tobacco withdrawal symptoms (eg, anxiety, irritability) and may also exhibit greater withdrawal-provoked discomfort and relapse.10 Gender differences in withdrawal-related negative affect have been shown to mediate the effects of gender on smoking behavior.13,14 Hence, extant work indicates that changes in negative affect states during tobacco abstinence may be particularly important for understanding gender-related health disparities in smoking behaviors and cessation success. Yet, it remains unknown whether these gender differences in tobacco withdrawal generalize across different racial groups.
Studies investigating racial differences in tobacco abstinence–related negative affect have been mixed. Studies in adults have found that non-Hispanic White (vs. non-Hispanic African American) cigarette smokers report experiencing more negative affect–related withdrawal symptoms17,18 and show a stronger relationship between number of withdrawal symptoms endorsed and reduced odds of quitting.17 In contrast, one laboratory study examining experimentally induced abstinence effects did not find any race differences in tobacco abstinence–related negative affect.19 Although inconsistencies among these findings remain, these results tend to suggest either no race effects or comparatively greater effects of withdrawal-related negative affect in non-Hispanic White versus non-Hispanic African American smokers.
Research examining whether gender differences in affect following acute tobacco abstinence distinctly manifest or generalize across racial groups remains scarce. However, a “sociopharmacological” model of tobacco-related health disparities20 proposes that psychobiological and sociocultural factors that differ by race or gender (eg, nicotine metabolism, expression of affect) may moderate both the overall averseness of the withdrawal experience and the extent to which tobacco’s affect-modulating effects translate into conscious motivation to relieve affective distress via reinstating smoking. Hence, it is possible that race and gender may have an interactive effect on affective withdrawal symptoms during abstinence. An initial test of identifying gender by race interactions on tobacco abstinence–related negative affect will have important clinical and research implications in beginning to understand the intersection of gender and race on underlying tobacco-related health disparities.
This study investigated whether race (non-Hispanic White vs. non-Hispanic African American) moderated associations between gender and abstinence-induced changes in negative affect and smoking behavior in a laboratory analog of smoking reinstatement in a community sample of non-treatment-seeking adult daily cigarette smokers. On the basis of prior work, we hypothesized that gender differences in abstinence-induced negative affect and smoking reinstatement behavior would be greater in non-Hispanic Whites than non-Hispanic African Americans.
Methods
Participants
The current report is a secondary analysis of data from two laboratory studies performed at the same study site between 2010 and 2017.21,22 Participants were non-treatment-seeking daily cigarette smokers recruited from the Los Angeles, California, area via paper (eg, newspaper, flyers, business cards, magazines, community periodicals) and online (eg, Craigslist, Facebook, Backpage, ClinicalConnection) advertisements for tobacco withdrawal studies. Across both studies, participants were required to (1) be 18 years of age or older; (2) be a regular cigarette smoker (≥10 cigarettes/day) for at least the past 2 years; (3) be fluent in English; and (4) report normal or corrected-to-normal vision. In both studies, participants were excluded for (1) current Diagnostic and Statistical Manual of Mental Disorders-IV non-nicotine substance dependence (including alcohol); (2) breath carbon monoxide (CO) levels less than 10 ppm at study intake; (3) current, regular use of non-cigarette forms of tobacco or nicotine replacement therapy; (4) current (Study 1) or recent (Study 2) use of psychiatric or psychoactive medications implicated in smoking cessation (eg, Chantix); (5) desire to quit or substantially reduce smoking in next 30 days; and (6) current or recent pregnancy/breastfeeding. Study 1 had an additional exclusion criterion restricting individuals with current Diagnostic and Statistical Manual of Mental Disorders-IV mood disorder or psychotic symptoms from participation. Study 2 additionally required that participants self-report having ancestry of either non-Hispanic African American or non-Hispanic European-American descent in both biological parents and also excluded those who participated in Study 1 and/or reported use of acute-acting anxiolytic medications more than once per week.
Following an initial phone screening to determine preliminary eligibility, participants attended an in-person baseline session at the laboratory involving additional eligibility assessments (eg, CO level analyses), structured clinical interviews, and questionnaires that assessed demographic and smoking characteristics. To facilitate the distinction between race and ethnicity, the current analyses were then limited to those self-reporting either non-Hispanic White or non-Hispanic African American that completed both experimental sessions. Prior work13,19 investigated main effect of gender differences in withdrawal across all races in the first 199 participants of Study 1 and main effect of race/ethnic differences (ie, non-Hispanic Whites, non-Hispanic African Americans, Hispanics) in withdrawal in 324 participants of Study 1. The University of Southern California Internal Review Board approved the studies and participants were compensated approximately $200 for the completing all three sessions.
Procedure
Following the baseline session, participants attended two counterbalanced, in-person experimental sessions starting around noon: abstinent (following 16 hours of overnight smoking abstinence) and non-abstinent (following ad libitum smoking). Non-abstinent and abstinent session protocols were identical, except that participants were administered a cigarette of their preferred brand at the outset of the non-abstinent session to standardize smoking recency across the sample. Experimental sessions began with a breath alcohol analysis to confirm 24-hour abstinence from alcohol (BrAC = 0.000). Following the breath alcohol analysis at the abstinent session and the cigarette administration at the non-abstinent session, participants were administered a breath CO assessment. In adherence with published recommendations that expired CO concentrations of less than 10 ppm are indicative of recent smoking abstinence,23 participants with CO measurements at least 10 ppm at their abstinent session were considered non-abstinent and were rescheduled for a second attempt to complete their abstinent experimental visit. Participants who failed to meet CO criteria for abstinence at their second attempt were discontinued from the study (non-Hispanic White women n = 1; non-Hispanic White men n = 0; non-Hispanic African American women n = 7; non-Hispanic African American men n = 10). Following the CO assessment, participants completed self-report measures, which were administered at a single timepoint. Participants subsequently underwent a smoking reinstatement task described below, which assessed the motivational value of initiating smoking.
Baseline Session Measures
Author-constructed questionnaires assessed demographic characteristics including gender (forced choice: female, male), ethnicity (forced choice: Hispanic or Latino/Latina vs. non-Hispanic or Latino/Latina), and race (select all that apply: American Indian or Alaskan Native, Asian, African American, Middle Eastern, Pacific Islander, and White); smoking characteristics (eg, cigarettes per day). The 6-item Fagerström Test of Nicotine Dependence24 was administered to assess nicotine dependence severity (range 0–10 with higher scores indicating greater nicotine dependence severity).
Experimental Session Measures
The Profile of Mood States25 was used to measure the extent to which participants experienced Negative Affect “right now” on a five-point Likert scale from 0 (Not at All) to 4 (Extremely). Mean scores were computed for five separate negative affect subscales (ie, Anger, Anxiety, Confusion, Depression, and Fatigue). A composite negative affect score (ie, mean of the five negative affect subscale scores) was also computed.
The Analogue Smoking Reinstatement Task26,27 is a behavioral economic task that measures acute smoking reinstatement motivation under conditions when abstaining from smoking reinstatement is monetarily reinforced. Prior work has shown that responses on this task are sensitive to tobacco abstinence.13,26 Participants received eight preferred brand cigarettes and an ashtray. In the first part of the task—the 50-minute “delay period”—participants were told they could begin smoking any time during the next 50 minutes, but that for each 5 minutes that they delayed smoking reinstatement, they would earn $0.20 USD (for a maximum of $2.00 USD). The delay period ended when participants indicated that they would like to reinstate smoking or at the end of the 50 minutes for participants who delayed for the full delay period (total delay length range: 0–50 minutes). In the second part of the task—a 60-minute “self-administration period”—participants were given their lighter and told that they had a $1.60 credit from which they would pay $0.20 for each cigarette they wished to smoke (number of cigarettes smoked range: 0–8). Following the self-administration period, participants entered a rest period for the remainder of the session, during which they were not allowed to smoke. This served to standardize session length for participants who chose not to fully delay, as well as to minimize the influence of participants’ impending ability to smoke following session completion. During the delay and self-administration periods, eating, sleeping, drinking, and using personal belongings were not permitted. Participants could earn between $0.00 to a maximum of $3.60 USD (see Liautaud et al.28 for details about piloting of payment amounts), and payment was provided in cash (Study 1) and ClinCard Prepaid Mastercards (Study 2) at the end of the session to participants who completed the Analogue Smoking Reinstatement Task.
Analytical Approach
Data were analyzed using IBM SPSS (v.25). Preliminary analyses involved reporting sample characteristics and determining whether characteristics differed by gender, race, or gender × race interaction. To determine the effect of abstinence, single-sample t-tests were run to determine whether abstinence-induced changes (ie, abstinent score–non-abstinent score) significantly differed from zero. Primary analyses utilized individual linear regression models to test associations between gender × race interactions and abstinence-induced changes on outcome variables. Significant interactions were followed up with individual regression models investigating gender differences stratified by race. All models controlled for smoking and demographic variables that differed by gender, race, or gender × race interaction (ie, age, Fagerström Test of Nicotine Dependence, cigarettes per day, menthol) and the corresponding non-abstinent score.
Results
Demographic and smoking characteristics are reported for the full sample and by gender and race in Table 1. Of the 1489 participants who underwent an initial phone screen, 376 were found ineligible due to low baseline CO (n = 209), current psychiatric disorder or use of psychiatric medications (n = 89), or other criteria (n = 78). After limiting analyses to participants who self-reported either Non-Hispanic White or Non-Hispanic African American and completed both experimental sessions, the final sample consisted of 789 participants (37.6% women; Non-Hispanic African American women N = 249; Non-Hispanic African American men N = 424; Non-Hispanic White women N = 48; Non-Hispanic White men N = 68). Non-Hispanic African Americans compared to non-Hispanic Whites were significantly older (P < .001), were more nicotine dependent (P = .002), and smoked fewer cigarettes per day (P = .01). Men compared to women smoked significantly more cigarettes per day (16.2 vs. 14.4; P = .03) and were more likely to smoke menthol cigarettes (55% vs. 44%: P = .004). There was a significant gender × race interaction for smoking menthol cigarettes (P = .02) with non-Hispanic African American men more likely to smoke menthol cigarettes than non-Hispanic African American women, but no gender effect for non-Hispanic White smokers (Table 1). There was no other gender × race differences in demographic and smoking characteristics (Ps > .05). Within the full sample, abstinence increased four of the negative affect subscales (ie, Anger, Anxiety, Confusion, Depression), composite negative affect, and number of cigarettes smoked, and decreased the amount of time willing to delay smoking (Ps > .001). Abstinence did not significantly increase fatigue in the full sample (P = .099).
Table 1.
Sample Characteristics
| Non-Hispanic White | Non-Hispanic African American | ||||||
|---|---|---|---|---|---|---|---|
| Overall samplea | Womenb | Menc | Overall sample | Womend | Mene | Overall sample | |
| Variable | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Demographics | |||||||
| Agef | 48.27 (11.18) | 39.79 (12.01) | 39.60 (10.90) | 39.68 (11.32) | 47.81 (10.72) | 50.89 (10.17) | 49.75 (10.47) |
| Smoking characteristics | |||||||
| Age of regular smoking (years) | 19.71 (5.96) | 19.29 (6.39) | 19.13 (5.09) | 19.20 (5.64) | 20.00 (6.08) | 19.67 (5.97) | 19.79 (6.01) |
| FTNDf | 5.83 (1.82) | 5.54 (1.64) | 5.15 (1.80) | 5.31 (1.74) | 5.95 (1.83) | 5.89 (1.82) | 5.92 (1.82) |
| Cigarettes per dayf,g | 15.49 (7.06) | 16.26 (5.35) | 17.48 (4.60) | 16.97 (4.94) | 14.02 (6.51) | 15.93 (7.70) | 15.24 (7.34) |
| Mentholg,e | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| Non-menthol | 365 (48.7) | 27 (60) | 34 (50.7) | 61 (54.5) | 124 (54.6) | 177 (43.5) | 304 (47.6) |
| Menthol | 385 (51.3) | 18 (40) | 33 (49.3) | 51 (45.5) | 103 (45.4) | 230 (56.5) | 334 (52.4) |
FTND = Fagerström Test of Nicotine Dependence (range = 0–10), SD = standard deviation.
a n’s ranged from 750 to 788 due to missing data.
b n’s ranged from 45–48 due to missing data.
c n’s ranged from 67–68 due to missing data.
d n’s ranged from 227 to 249 due to missing data.
e n’s ranged from 407–424 due to missing data.
fSignificant effect of race.
gSignificant effect of gender.
eSignificant effect of gender × race.
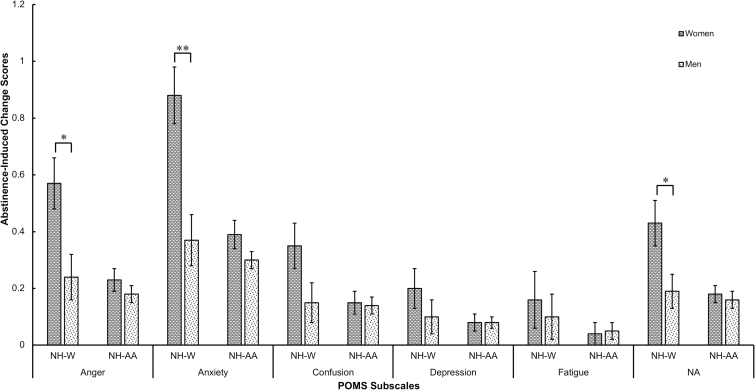
There was a significant interaction of gender × race on anger, anxiety, and composite negative affect (βs= −.12 to −.16, Ps < .05; Table 2). Analyses stratified by race showed that non-Hispanic White women compared to non-Hispanic White men had greater abstinence-induced negative affect, but there were no significant gender differences among non-Hispanic African Americans (Figure 1). There were no significant gender × race interactions on smoking outcomes during the Analogue Smoking Reinstatement Task (ie, latency to smoke, number of cigarettes smoked; Table 2).
Table 2.
Primary Analysis on Affect and Smoking Reinstatement
| Main effect of gender stratified by race | ||||||
|---|---|---|---|---|---|---|
| Gender × race interaction | Non-Hispanic White | Non-Hispanic African American | ||||
| β | P | β | P | β | P | |
| POMS | ||||||
| Negative affect | −0.12 | 0.03 | −0.20 | 0.03 | 0.00 | 0.94 |
| Anger | −0.12 | 0.04 | −0.21 | 0.03 | −0.03 | 0.43 |
| Anxiety | −0.16 | 0.003 | −0.29 | 0.002 | −0.04 | 0.30 |
| Confusion | −0.11 | 0.05 | — | — | — | — |
| Depression | −0.06 | 0.28 | — | — | — | — |
| Fatigue | −0.04 | 0.46 | — | — | — | — |
| Smoking reinstatement | ||||||
| Latency to smoke | 0.01 | 0.85 | — | — | — | — |
| Cigarettes smoked | 0.07 | 0.15 | — | — | — | — |
All models control for gender, race, age, Fagerström Test of Nicotine Dependence, cigarettes per day, menthol, and the corresponding non-abstinent rating. POMS = Profile of Mood States.
Figure 1.
Estimated marginal means by gender and race for Profile of Mood States (POMS). Estimated marginal means controlling for age, Fagerström Test of Nicotine Dependence, cigarettes per day, menthol, and the corresponding non-abstinent score. NH-W = non-Hispanic White; NH-AA = non-Hispanic African American; NA = composite negative affect.*P < .05; **P < .01.
Discussion
We previously reported that women compared to men showed greater abstinence-induced increases in several negative affect states and composite negative affect.13 In this study, we expand these findings to show that non-Hispanic White women compared to non-Hispanic White men reported greater abstinence-induced increases in several negative affect states (ie, anger, anxiety) and composite negative affect, but found no gender differences among non-Hispanic African American smokers. Prior research has found that women compared to men may experience increased negative affect during acute tobacco abstinence13,14 and have greater expectations that smoking relieves negative affect.16,29,30 This study extends extant research to suggest that gender differences in abstinence-induced negative affect may be primarily driven by increased abstinence-induced negative affect in non-Hispanic White women smokers.
There are a number of possible biological and sociocultural mechanisms that might account for elevated sensitivity to tobacco abstinence–related negative affect among non-Hispanic White women smokers. Prior research has found that hormonal factors in women (eg, menstrual cycle, ovarian hormones, hormonal medication) impact negative affect during acute tobacco abstinence31–34 and that hormone dynamics may differ between non-Hispanic African American and non-Hispanic White women.35 One limitation of this study is its lack of direct hormonal assessment, and thus, we are unable to determine the potential influence of hormones in this study. It will be important for future studies to address the potential role of racial differences in hormonal factors on tobacco abstinence–related negative affect withdrawal. Sociocultural factors may also potentially underlie racial differences in the impact of gender on tobacco abstinence–related negative affect. Factors related to cultural socialization may render African Americans (vs. White Americans) less likely to present emotional disturbance in terms of negative affect.36 Moreover, a prior study found gender differences in emotional expressions among Whites, but not non-Hispanic African Americans.37 Thus, it is possible that differences in cultural norms surrounding affective expression may account for the race–gender patterns of tobacco abstinence–related negative affect observed in our sample.
We did not find a significant gender by race interaction on smoking reinstatement behavior. Previous research has found that gender differences in abstinence-induced negative affect mediated latency to smoke following periods of abstinence,13,14 but has not shown racial differences in laboratory-based smoking reinstatement behavior.19 While we are unable to draw conclusions as to why increased gender differences in abstinence-induced negative affect among non-Hispanic White (vs. non-Hispanic African American) smokers does not generalize to smoking reinstatement behavior, it is possible that the study was limited by a non-treatment-seeking sample undergoing temporary abstinence as a part of a study protocol. As such, it is unclear the extent that anticipation of smoking at the end of the experimental study sessions may have mitigated smoking behavior during the Analogue Smoking Reinstatement Task. It will be important for future studies to investigate whether and how gender and race interact to impact smoking reinstatement risk during an actual cessation attempt.
The findings of this study should be interpreted in the context of its limitations. First, this study was restricted only to individuals who reported non-Hispanic White or non-Hispanic African American ethnic/racial identities and cis-male or cis-female gender identities. Thus, more research is needed to determine whether these findings generalize to other socioculturally vulnerable groups—particularly those with high prevalence of cigarette smoking (eg, American Indian, mixed race),38 and non-binary gender identities.39 In addition, we found race differences on a number of baseline characteristics (ie, age, nicotine dependence, cigarettes per day). Prior work suggests that non-Hispanic African American smokers compared to non-Hispanic White smokers tend to exhibit higher behavioral and biological indicators of nicotine dependence40,41 despite smoking fewer cigarettes per day.42,43 It will be important for future studies to address gender differences in populations with lighter/intermittent smoking. Finally, this study also included a number of tests, which increase the chance of Type I error. However, the consistent pattern of results specific to negative affect decreases the likelihood that the effects found were a result of this type of error.
In summary, non-Hispanic White women experience increased exacerbations of negative affect following acute tobacco abstinence in comparison to non-Hispanic White men, but these gender differences were not found in non-Hispanic African American smokers. Given recent findings suggesting that slower dissipation of negative affect (particularly anxiety and anger) may impact smoking cessation outcomes,44 these results are significant because they provide further evidence that non-Hispanic White women (vs. non-Hispanic White men) smokers may be susceptible to smoking cessation failure due to increased negative affect. Although we found smaller-sized abstinence-induced changes in negative affect in our study and are currently unaware of an exact cutoff for changes that would be of clinical significance, prior cessation studies have demonstrated that small changes in affect predict early versus late lapsers during cessation;45,46 thus, one clinical implication of this finding is that small to modest changes in negative affect could precipitate earlier relapse during cessation among non-Hispanic White women. These studies also suggest that prior findings demonstrating the central role of negative affective withdrawal as a driving factor of smoking maintenance in women compared to men may not generalize to non-Hispanic African American smokers. However, several studies in samples of non-Hispanic African American smokers have found that women report expectations of smoking-induced negative affect relief and stress reduction.29,47,48 Thus, even though non-Hispanic African American women did not report greater abstinence-induced negative affect in this study, it is possible that negative reinforcement-mediated smoking may still be an important mechanism underlying tobacco addiction in African American women, and it is essential that future work continue to explore etiological mechanisms underlying tobacco addiction among non-Hispanic African American women smokers. In addition, future research should address whether the racially discrepant patterns of gender differences in negative affect–related tobacco withdrawal reported in this study are found during self-motivated quit attempts. Such work may inform empirical efforts to develop tailored, gender- and race-specific cessation strategies to improve smoking outcomes in vulnerable groups subject to tobacco-related disparities.
Funding
This work was supported by funds from the National Institute on Drug Abuse at the National Institutes of Health (grant numbers K01-DA040043 and R01-DA026831), the American Cancer Society (grant number RSG-13-163-01), and the National Science Foundation Graduate Research Fellowship (grant number DGE-1418060). Funding sources had no role in the study design, collection, analysis, or interpretation of the data; writing the manuscript; or the decision to submit the article for publication.
Declaration of Interests
None declared.
References
- 1. Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. 2010;7(5):381–401. [DOI] [PubMed] [Google Scholar]
- 2. Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papadopoulos A, Guida F, Leffondré K, et al. . Heavy smoking and lung cancer: are women at higher risk? Result of the ICARE study. Br J Cancer. 2014;110(5):1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haiman CA, Stram DO, Wilkens LR, et al. . Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. [DOI] [PubMed] [Google Scholar]
- 5. Kytola V, Topaloglu U, Miller LD, et al. . Mutational landscapes of smoking-related cancers in Caucasians and African Americans: precision oncology perspectives at Wake Forest Baptist Comprehensive Cancer Center. Theranostics. 2017;7(11):2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker NJ, van Woerden HC, Kiparoglou V, Yang Y, Robinson H, Croghan E. Gender difference and effect of pharmacotherapy: findings from a smoking cessation service. BMC Public Health. 2016;16(1):1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith PH, Kasza KA, Hyland A, et al. . Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu PC, Hsueh KC, Mar GY, et al. . Gender differences in outcome of an attempt to stop smoking among smokers attending a smoking cessation clinic in Taiwan: 3-year follow-up Study. Eval Health Prof. 2016;39(3):317–325. [DOI] [PubMed] [Google Scholar]
- 9. Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: a review. Prev Med. 2016;92:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberger AH, Platt JM, Shuter J, Goodwin RD. Gender differences in self-reported withdrawal symptoms and reducing or quitting smoking three years later: a prospective, longitudinal examination of U.S. adults. Drug Alcohol Depend. 2016;165:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. [DOI] [PubMed] [Google Scholar]
- 12. Piper ME, Schlam TR, Cook JW, et al. . Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl). 2011;216(4):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol. 2013;21(4):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp Clin Psychopharmacol. 2007;15(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu J, Azizian A, Monterosso J, et al. . Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pang RD, Zvolensky MJ, Schmidt NB, Leventhal AM. Gender differences in negative reinforcement smoking expectancies. Nicotine Tob Res. 2015;17(6):750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinberger AH, Platt JM, Smith PH, Goodwin RD. Racial/ethnic differences in self-reported withdrawal symptoms and quitting smoking three years later: a prospective, longitudinal examination of US adults. Nicotine Tob Res. 2017;19(3):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson CD, Pickworth WB, Heishman SJ, Waters AJ. The acute tobacco withdrawal syndrome among black smokers. Psychol Addict Behav. 2014;28(1):173–181. [DOI] [PubMed] [Google Scholar]
- 19. Bello MS, Pang RD, Cropsey KL, et al. . Tobacco withdrawal amongst African American, Hispanic, and White smokers. Nicotine Tob Res. 2016;18(6):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leventhal AM. The sociopharmacology of tobacco addiction: implications for understanding health disparities. Nicotine Tob Res. 2016;18(2):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014;123(2):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bello MS, Pang RD, Chasson GS, Ray LA, Leventhal AM. Obsessive-compulsive symptoms and negative affect during tobacco withdrawal in a non-clinical sample of African American smokers. J Anxiety Disord. 2017;48:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benowitz NL, Jacob P III, Ahijevych K, et al. . Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. McNair DM, Lorr M, Droppelman LF.. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 26. McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl). 2006;189(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liautaud MM, Leventhal AM, Pang RD. Happiness as a buffer of the association between dependence and acute tobacco abstinence effects in African American smokers. Nicotine Tob Res. 2017. doi:10.1093/ntr/ntx216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulvers KM, Catley D, Okuyemi K, et al. . Gender, smoking expectancies, and readiness to quit among urban African American smokers. Addict Behav. 2004;29(6):1259–1263. [DOI] [PubMed] [Google Scholar]
- 30. Wetter DW, Smith SS, Kenford SL, et al. . Smoking outcome expectancies: factor structure, predictive validity, and discriminant validity. J Abnorm Psychol. 1994;103(4):801–811. [DOI] [PubMed] [Google Scholar]
- 31. Pang RD, Liautaud MM, Kirkpatrick MG, Huh J, Monterosso J, Leventhal AM. Ovarian hormones and transdermal nicotine administration independently and synergistically suppress tobacco withdrawal symptoms and smoking reinstatement in the human laboratory. Neuropsychopharmacology. 2018;43(4):828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allen SS, Hatsukami DK, Bade T, Center B. Transdermal nicotine use in postmenopausal women: does the treatment efficacy differ in women using and not using hormone replacement therapy?Nicotine Tob Res. 2004;6(5):777–788. [DOI] [PubMed] [Google Scholar]
- 33. Wetherill RR, Franklin TR, Allen SS. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Rep. 2016;3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinberger AH, Smith PH, Allen SS, et al. . Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17(4):407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw ND, Srouji SS, Welt CK, et al. . Evidence that increased ovarian aromatase activity and expression account for higher estradiol levels in African American compared with Caucasian women. J Clin Endocrinol Metab. 2014;99(4):1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Consedine NS, Magai C. The uncharted waters of emotion: ethnicity, trait emotion and emotion expression in older adults. J Cross Cult Gerontol. 2002;17(1):71–100. [DOI] [PubMed] [Google Scholar]
- 37. Vrana SR, Rollock D. The role of ethnicity, gender, emotional content, and contextual differences in physiological, expressive, and self-reported emotional responses to imagery. Cognition and Emotion. 2002;16(1):165–192. [Google Scholar]
- 38. Jamal A, Philips E, Gentzke AS, et al. . Current cigarette smoking among adults —United States, 2016. MMWR Morbidity and Mortality Weekly Report. 2018;67(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edmiston EK, Donald CA, Sattler AR, Peebles JK, Ehrenfeld JM, Eckstrand KL. Opportunities and gaps in primary care preventative health services for transgender patients: a systemic review. Transgend Health. 2016;1(1):216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Branstetter SA, Mercincavage M, Muscat JE. Predictors of the nicotine dependence behavior time to the first cigarette in a multiracial cohort. Nicotine Tob Res. 2015;17(7):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pérez-Stable EJ, Benowitz NL. Do biological differences help explain tobacco-related disparities?Am J Health Promot. 2011;25(suppl 5):S8– S–10.. [DOI] [PubMed] [Google Scholar]
- 42. Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo Z, Alvarado GF, Hatsukami DK, Johnson EO, Bierut LJ, Breslau N. Race differences in nicotine dependence in the Collaborative Genetic study of Nicotine Dependence (COGEND). Nicotine Tob Res. 2008;10(7):1223–1230. [DOI] [PubMed] [Google Scholar]
- 44. Zuo Y, Rabinovich NE, Gilbert DG. Negative affect subtypes and craving differentially predict long-term cessation success among smokers achieving initial abstinence. Psychopharmacology (Berl). 2017;234(5):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, Wetter DW. Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week. J Abnorm Psychol. 2011;120(3):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Motschman CA, Germeroth LJ, Tiffany ST. Momentary changes in craving predict smoking lapse behavior: a laboratory study. Psychopharmacology. 2018:1–12. doi:10.1007/s00213-018-4898-4. [DOI] [PubMed] [Google Scholar]
- 47. Shervington DO. Attitudes and practices of African-American women regarding cigarette smoking: implications for interventions. J Natl Med Assoc. 1994;86(5):337–343. [PMC free article] [PubMed] [Google Scholar]
- 48. Ahijevych K, Wewers ME. Factors associated with nicotine dependence among African American women cigarette smokers. Res Nurs Health. 1993;16(4):283–292. [DOI] [PubMed] [Google Scholar]