Abstract
Background
The use of T2* magnetic resonance imaging (MRI) has been promoted by recent studies as a noninvasive method for the detection of iron overload in thalassemia major patients. This study aims to estimate the iron load in the heart and liver of thalassemia major patients using T2* MRI and to determine its correlation with the left ventricle ejection fraction and serum ferritin level.
Methods
Forty β-Thalassemia major patients were included in the study. We evaluated the serum ferritin level, echocardiography, cardiac T2*, myocardial iron concentration (MIC), liver iron concentration (LIC) and hepatic T2* in all patients. CMR T2* findings were categorized as normal cardiac T2* (T2* >20 ms) or abnormal cardiac T2* (T2* <20 ms).
Results
The study found that 85% of patients had a normal cardiac T2* value. The median serum ferritin level was 2189. A significant inverse correlation was found between the serum ferritin level and the cardiac T2* (r=−0.381, =0.015); however, the correlations between serum ferritin and the hepatic T2* and liver iron concentration were statistically non-significant (P=0.539 and P=0.637, respectively). Additionally, the LVEF correlation was statistically non-significant with SF, hepatic T2* and cardiac T2*.
Conclusion
Regardless of the serum ferritin level or left ventricle function, a cardiac T2* MRI should be done for all patients with β-Thalassemia major in order to estimate the myocardial iron concentration.
Keywords: T2* MRI, thalassemia major, myocardial iron concentration, serum ferritin
Introduction
β-Thalassemia is a hereditary anemia caused by the absence (β0) or reduced synthesis (β+) of the β-globin chains of hemoglobin. Three hematological, clinical conditions of increasing severity are recognized: the β-thalassemia carrier state, thalassemia intermedia, and thalassemia major1. Patients with thalassemia major (TM) usually require frequent blood transfusions that lead to iron accumulation in various tissues and organs.2
The heart is one of the most sensitive and vulnerable organs to overload iron.3 Thus, myocardial sideriosis is still the major cause of death in transfusion dependent thalassemia major patients, so it is crucial to test for iron overload.4
Many options are available to determine cardiac iron levels, but not all are appropriate. The use of hepatic T2* and serum ferritin levels as predictors of myocardial iron concentration have been challenged by previous MRI studies.5–7
Serum ferritin is widely used as a surrogate marker for iron overload. However, the measurement of serum ferritin cannot be used to predict myocardial and liver iron content, as its level is also affected by inflammation, chelation therapy, vitamin C level, infection and liver damage.8,9
An echocardiograph is a valuable tool to assess cardiac function in clinical situations, but LVEF is not a reliable marker for the early detection of iron overload.10 This is due to the adaptation of cardiac function in the transfusion-dependent thalassemia patients in response to chronic anemia, which may overestimate LVEF and, therefore, neglect an underlying cardiac disorder.10 The risk of cardiac failure cannot be ruled out, even with normal LVEF.
The best method for the estimation of liver iron overload is a liver biopsy. However, this is an invasive method, cannot be used for long-term follow-up, and can also be erroneous due to the heterogeneous distribution of iron throughout the liver.11
Currently, the best noninvasive method to evaluate target organ hemosideriosis is T2* magnetic resonance imaging.12 However, several factors hinder the use of MRI; it is difficult to use with children, requires an expert radiologist to interpret its results, is not available in all locations, and is expensive.13
Several studies have demonstrated the association between serum ferritin level, MIC, LIC, cardiac T2* and hepatic T2* to determine whether ferritin levels could be used as a suitable index to assess iron overload status in such patients. However, conflicting results have been reported.2,14–16
Our study aimed to investigate the correlations between cardiac and hepatic iron concentration, myocardial and hepatic T2* values, along with serum ferritin level and left ventricle ejection fraction.
Patients and method
Study design and participants
We conducted a prospective cross-sectional study of 40 thalassemia major (TM) patients who were admitted to Beni-Suef University Hospital, Egypt. Participant criteria included patients who were older than 6 years of age, receiving regular blood transfusions at least once a month and undergoing chelation therapy. Patients were not included if they had suffered from heart failure (left ventricle ejection fraction less than 40%), valvar heart disease, congenital heart disease or infectious disease. Written informed consents were obtained from patients or their caregivers and approval was received from the ethical committee of Beni-Suef University of Medicine before the study began (Ethical committee code: FWA00015574). The study was conducted in accordance with the Declaration of Helsinki.
Once patients met the inclusion criteria, they were subjected to a detailed history, clinical examination, echocardiograph, laboratory tests and MRI scans.
Serum ferritin
Estimation of the serum ferritin level was performed by enzyme immunoassay (ELISA), according to protocol provided by the manufacturer.
Cardiovascular magnetic resonance imaging (MRI)
In this research, MRI scans were performed according to the protocol used in previous studies.17 This method was used to measure heart and liver T2*, as well as myocardial and liver iron content. The MRIs for this study were performed in the Department of Radiology, Kasr el Aieny Hospital, using a four-element cardiac phased-array coil. Standard ECG gating was used to synchronize the scans to the cardiac cycle. The cardiac MRI was performed using a single 10-mm-thick short-axis mid-ventricular slice, which was positioned halfway between the base and the apex of the left ventricle (LV). It was acquired at eight echo times (TE =2.6–18.8 msec, with 2.02-msec increments) in a single breath-hold. For T2* and MIC analysis, a homogeneous full-thickness ROI was selected in the septum.18 The MRI T2* of the liver was measured using a single 10 mm slice positioned through the center of the liver, and was scanned at 8 different echo times (TE).
The TE used was 2.3–18 ms. The signal intensity of this area was determined for each of the images and the resulting data were plotted against the TE to draw an exponential decay curve. The cut-off points in this MRI instrument were as follows: Cardiac: normal >20 ms, mild: 14–20 ms, moderate: 10–14 ms, severe <10 ms; Liver: normal >6.3 ms, mild: 2.8–6.3 ms, moderate: 1.4–2.7 ms, severe <1.4ms.
The analysis was conducted using Thalassemia Tools Software, as explained by Carpenter et al (2012), for the measurements of the cardiac T2* and MIC while measurements of the liver T2* and LIC were carried out according to the protocol suggested by Garbowski and colleagues for the.19,20
Echocardiograph
Each patient underwent an echocardiographic examination using both the conventional and tissues Doppler echocardiography. All were performed by a single expert cardiologist. Normal systolic function was defined as ejection fraction >55% and fraction shortening >27%.
Statistical analysis
In this study, data were expressed as mean ± standard deviation for continuous variables; categorical variables were expressed as counts and percentages. Data were tested for normal distribution using the Kolmogorov-Smirnov test, while frequencies, means and standard deviations were calculated by descriptive statistics. Correlations of variables were evaluated using the Pearson’s or Spearman’s correlation analysis. A p-value <0.05 was considered statistically significant. Statistical analyses were conducted with a commercially available software package (SPSS version 23).
Results
The study was performed on 17 (42.5%) male and 23 (57.5%) female patients with a mean of age of 12.95±4.506 (ages 6–20). All patients were being treated with Deferasirox. The median serum ferritin level was 2189, while the mean left ventricle ejection fraction (LVEF) was 62.8±3.05%. All participants had normal systolic and diastolic function (Table 1). Twenty-one (52.5%) patients had serum ferritin levels >2000 ng/ml, of which 16 (47%) patients had normal cardiac T2* MRI; 19 (47.5%) participants had serum ferritin levels between 1,000 and 2,000 ng/ml, of which 18 (53%) had normal cardiac T2* MRI. Abnormal T2* was found in 83.3% of patients with a serum ferritin level >2,000 ng/ml and 16.7% of patients with a serum ferritin level between 1,000 and 2,000 (Table 2). An abnormal cardiac T2* was found in 2 (33.4%) females and 4 (66.6%) males (Table 3).
Table 1.
Baseline characteristics
| Characteristics | Patients (n=40) |
|---|---|
| Female, n (%) | 23 (57.5) |
| Male, n (%) | 17 (42.5) |
| Age | 12.95±4.5 |
| Splenectomy, yes, n (%) | 5 (12.5) |
| Pretransfusional hemoglobin | 7.35±1.44 |
| Age at onset of disease (months) | 10.55±8.918 |
| Duration of the disease (years) | 12.1450±4.54 |
| Number of blood transfusion/life time | 170.875±73.05396 |
| Deferasorix dose mg/day | 1175.6250±501.55 |
| Serum ferritin ng/ml | 2851±1696.7 |
| Cardiac T2 * MRI (ms) | 46.9275±27.48,791 |
| Cardiac T2* category, n (%) | |
| Abnormal Cardiac T2* (<20 ms) | 6 (15) |
| Normal Cardiac T2* (>20 ms) | 34 (85) |
| MIC mg/g/dry weight myocardial iron concentration | 0.7490±0.7490 |
| Hepatic T2 *MRI, ms | 27.663±20.225 |
| LIC mg/g/dry weight | 2.19±2.092 |
| LVEF | 62.8375±3.05167 |
Note: Data expressed as mean ± SD or n (%).
Abbreviations: MIC, myocardial iron concentration; LIC, liver iron concentration; MRI, magnetic resonance imaging; ms, milliseconds; SF, serum ferritin; LVEF, left ventricle ejection fraction.
Table 2.
Serum ferritin levels according to cardiac T2 * MRI findings
| Serum ferritin level (ng/ml) | Total | Chi square value | P-value | ||
|---|---|---|---|---|---|
| 1000–2000 | >2000 | ||||
| Normal cardiac T2* MRI | 18 (53%) | 16 (47%) | 34 (100%) | 2.691 | 0.1 |
| Abnormal cardiac T2* MRI | 1 (16.7%) | 5 (83.3%) | 6 (100%) | ||
| Total | 19 | 21 | 40 | ||
Table 3.
Cardiac T2 * MRI findings according to gender
| Gender | Total | Chi square value | P-value | ||
|---|---|---|---|---|---|
| male | Female | ||||
| Normal cardiac T2* MRI | 13 (38.2%) | 21 (61.8%) | 34 (100%) | 1.687 | 0.194 |
| Abnormal cardiac T2* MRI | 4 (66.6%) | 2 (33.4%) | 6 (100%) | ||
| Total | 17 | 23 | 40 | ||
The correlation of age with serum ferritin level in patients with TM showed a significant and moderate correlation (r=0.343, P=0.03). However, the correlations between age and myocardial iron concentration, liver iron concentration and ejection fraction were poor and statistically insignificant (Table 4).
Table 5.
Correlation between ferritin level, LVEF and T2* MRI values of the liver and heart in thalassemia major patients
| First parameter | Second parameter | R | P-value |
|---|---|---|---|
| Ferritin | LIC mg/g/dry weight | 0.077 | 0.637 |
| Ferritin | Cardiac T2*MRI, ms | −0.381-* | 0.015 |
| Ferritin | Hepatic T2 *MRI, ms | 0.1 | 0.539 |
| Cardiac T2* | LIC mg/g/dry weight | 0.051 | 0.754 |
| Hepatic T2* | Cardiac T2*MRI, ms | 0.342 | 0.056 |
| MIC | Cardiac T2*MRI, ms * | −0.802** | 0.001 |
| MIC | LIC mg/g/dry weight | −0.096 | 0.557 |
| LVEF | SF | 0.132 | 0.416 |
| LVEF | Cardiac T2*MRI, ms | −0.103 | 0.525 |
| LVEF | Hepatic T2 *MRI, ms | −0.114- | 0.843 |
Notes: *Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
Abbreviations: MIC, myocardial iron concentration; LIC, liver iron concentration; MRI, magnetic resonance imaging; ms, miliseconds; SF, serum ferritin.
Table 4.
Correlation of age with serum ferritin, LVEF, and T2* values of the heart and liver in patients with thalassemia major
| First parameter | Second parameter | R | P-value |
|---|---|---|---|
| Age | Serum ferritin | 0.343* | 0.030 |
| Age | Cardiac T2*MRI, ms | −0.217 | 0.179 |
| Age | MIC mg/g/dry weight | 0.292 | 0.068 |
| Age | Hepatic T2 *MRI, ms | 0.098 | 0.547 |
| Age | LIC mg/g/dry weight | −0.120 | 0.463 |
| Age | LVEF | 0.027 | 0.867 |
Notes: *Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
Abbreviations: MIC, myocardial iron concentration; LIC, liver iron concentration; MRI, magnetic resonance imaging; ms, milliseconds; LVEF, left ventricle ejection fraction.
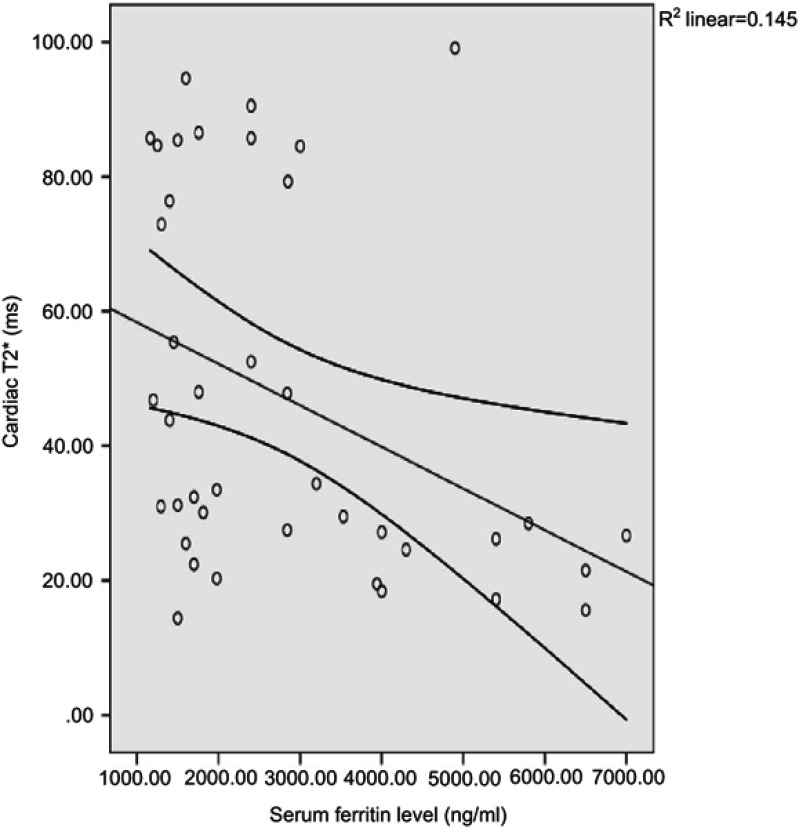
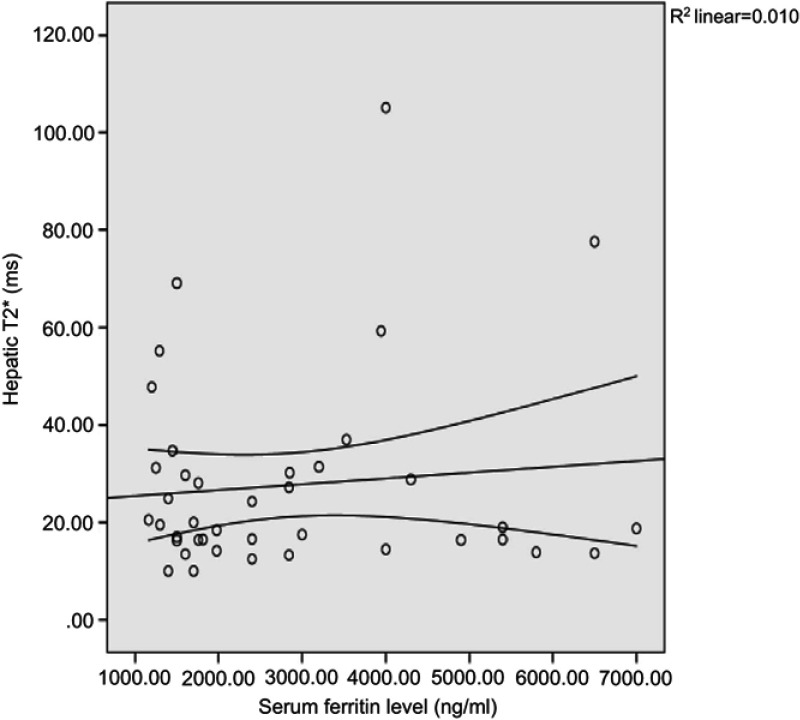
A significant inverse correlation was noted between serum ferritin level and cardiac T2* (r=−0.381, P=0.015) (Figure 1). In addition, the correlations between serum ferritin and the hepatic T2* and liver iron concentration were statistically non-significant (P=0.539 and 0.637, respectively) (Figure 2).
Figure 1.
Correlation between cardiac T2* and serum ferritin level.
Figure 2.
Correlation between hepatic T2* and serum ferritin level.
The left ventricle ejection fraction showed a weak insignificant correlation with serum ferritin level (r=0.132, P=0.416) and a weak inverse non-significant correlation with cardiac T2* and hepatic T2* (P=0.525 and 0.843, respectively).
A strong inverse correlation was found between cardiac T2* and myocardial iron concentration (r=−0.802, P=0.001). As the cardiac T2* increases, the myocardial iron concentration decreases in tandem. However, the association between myocardial and liver iron concentrations was weak and non-significant (P=0.557).
The association between hepatic T2* and liver iron concentration showed a negatively moderate and significant correlation (r=−0.440, P=0.005) (Table 5).
Discussion
A significant cause of death in patients with TM is a cardiac complication, such as heart failure or arrhythmia. The dysfunction of the heart in TM patients is multifactorial; however, iron toxicity is the major factor responsible for this failure.21 Patients with TM require frequent blood transfusions; therefore, the excess iron results from both increased iron absorption from the G.I.T. and repeated blood transfusions. Transfusional iron is deposited in the reticuloendothelial system (RES); when the stores of the RES become saturated, the excess iron is deposited in different parenchymal tissues, such as hepatocytes, myocardium and endocrine glands.22,23
Cardiomyopathy due to iron overload can be reversed if aggressive chelation therapy is initiated early.22,23 The non-chelated TM patients usually develop cardiomegaly by the age of 10 years, and by the age of 16, they experience heart failure (Engle, Erlandson, and Smith, 1964). Therefore, the early detection of iron deposition in the myocardium is imperative to prevent heart failure.
This study used cardiac T2* and functional CMR imaging, which are considered to be the best tools for the assessment of cardiac iron loading and cardiac function. The cardiac T2* value is inversely proportional to the myocardial iron concentration. An increase in the T2* value indicates good cardiac function.25
The serum ferritin level is sometimes used as a predictor for myocardial iron overload. A serum ferritin level of more than 1,800 mg/L is associated with an elevated cardiac iron concentration, while a level of more than 2,500 mg/L is associated with an increase in the prevalence of cardiac events.26 However, the serum ferritin level also increases due to inflammatory responses and liver disease, so its measurement is not a reliable marker for cardiac issues.15 In addition, the risk of cardiomyopathy due to iron overload cannot be ruled out by a low level of the serum ferritin.27
Eighty five percent of the patients included in this study had acceptable levels of cardiac and hepatic iron. We found a significant but inverse correlation between serum ferritin level and cardiac T2*. These findings are in accordance with earlier studies.11,17,26 this data indicates a large variability, confirming that the relationship is too weak to be useful in the clinical setting. Several previous studies reported that serum ferritin is not a valuable predictor of myocardial iron overload.25,28 Some studies found no association between serum ferritin and cardiac T2*.21,27 However, other large-scale studies reported a weak correlation between serum ferritin level and cardiac T2*.5,6,11,29 Our result conflicts with the study presented by Yang et al (2014), which reported a strong significant association between serum ferritin level and cardiac T2*; this may be due to the insufficient chelation therapy received by the majority of the patients in their study .30
The association between serum ferritin and hepatic T2* was also found to be insignificant; therefore, hepatic haemosideriosis cannot be ruled out by the measurement of serum ferritin level. Our results are in contrast to Eghbali’s study, which indicated a moderate correlation between serum ferritin levels and hepatic T2* levels (Eghbali, Ahmadi et al 2014). However, the correlation between hepatic T2* and LIC was a significant moderate inverse correlation, similar to Eghbali et al.15
A myocardial biopsy is an invasive method that can be used to determine a patient’s iron level, but it is not associated with cardiac iron levels or cardiac functions.19 which may be related to the non-homogenous distribution of myocardial iron deposition.31 As such, a myocardial biopsy is not recommended for evaluating cardiac iron overload.
Additionally, a liver biopsy is touted as the optimal method to measure iron level, but it is invasive and cannot predict the iron level of the heart.15 The electrocardiograph and echocardiography are only reliable in patients with advanced iron overloading.15
Our study detected no significant correlations between the LVEF and serum ferritin level, cardiac T2* or hepatic T2*. This may be because the patients in our study exhibited mild to moderate cardiac iron overload with normal left ventricle (LV) functions. Our results are thus in accord with previous studies that concluded that, even when LVEF is normal, the risk of cardiac iron overload cannot be ruled out.32 We do not mean to infer that we are ignoring the role of the echocardiograph in the detection of cardiac iron overload, but it is more reliable in advanced stages of the disease.15
The Thalassemia International Federation (TIF) recommends that the first assessment of myocardial iron concentration should be done at puberty for patients who received chelation therapy early and regularly.33
Conclusion
Serum ferritin level, hepatic T2* and LVEF are not reliable measures for the detection of myocardial iron concentration, as the association between the SF and hepatic T2* with cardiac T2* is poor, and no association has been found between cardiac T2* and LVEF. Therefore, it is very important to detect myocardial iron concentration in thalassemia major patients by using the noninvasive cardiac T2* procedure. Regular clinical monitoring of hepatic and cardiac iron is essential for primary prevention of cardiac events.
Acknowledgments
This research received no specific grant from a funding agency in the public, commercial, or not-for-profit sectors.
Study limitation
The main limitation of this study is its small scope, taking place at a single center with a relatively small sample size of 40 participants. These findings should be assessed by other studies with larger sample sizes.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Origa R. β-Thalassemia. Genet Med. 2017;19(6):609–619. doi: 10.1038/gim.2016.173 [DOI] [PubMed] [Google Scholar]
- 2.Taghizadeh Sarvestani R, Moradveisi B, Kompany F, Ghaderi E. Correlation between heart and liver iron levels measured by MRI T2* and serum ferritin in patients with β-thalassemia major. Int J Pediatr. 2016;4(3):1559–1567. doi: 10.22038/ijp.2016.6587 [DOI] [Google Scholar]
- 3.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56(13):1001–1012. doi: 10.1016/j.jacc.2010.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennell DJ, Porter JB, Piga A, et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am J Hematol. 2015;90(2):91–96. doi: 10.1002/ajh.23876 [DOI] [PubMed] [Google Scholar]
- 5.Tanner MA, Galanello R, Dessi C, et al. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8(3):543–547. doi: 10.1080/10976640600698155 [DOI] [PubMed] [Google Scholar]
- 6.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103(5):1934–1936. doi: 10.1182/blood-2003-06-1919 [DOI] [PubMed] [Google Scholar]
- 7.Aessopos A, Fragodimitri C, Karabatsos F, et al. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92(1):131–132. doi: 10.3324/haematol.10455 [DOI] [PubMed] [Google Scholar]
- 8.Voskaridou E, Douskou M, Terpos E, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126(5):736–742. doi: 10.1111/j.1365-2141.2004.05104.x [DOI] [PubMed] [Google Scholar]
- 9.Mavrogeni SI, Markussis V, Kaklamanis L, et al. A comparison of magnetic resonance imaging and cardiac biopsy in the evaluation of heart iron overload in patients with beta-thalassemia major. Eur J Haematol. 2005;75(3):241–247. doi: 10.1111/j.1600-0609.2005.00474.x [DOI] [PubMed] [Google Scholar]
- 10.Pennell DJ, Udelson JE, Arai AE, et al. Cardiovascular function and treatment in beta-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128(3):281–308. doi: 10.1161/CIR.0b013e31829b2be6 [DOI] [PubMed] [Google Scholar]
- 11.Wahidiyat PA, Liauw F, Sekarsari D, Putriasih SA, Berdoukas V, Pennell DJ. Evaluation of cardiac and hepatic iron overload in thalassemia major patients with T2* magnetic resonance imaging. Hematology. 2017;22(8):501–507. doi: 10.1080/10245332.2017.1292614 [DOI] [PubMed] [Google Scholar]
- 12.Youssef DM, Fawzy Mohammad F, Ahmed Fathy A, Aly Abdelbasset M. Assessment of hepatic and pancreatic iron overload in pediatric Beta-thalassemic major patients by t2* weighted gradient echo magnetic resonance imaging. ISRN Hematol. 2013;2013:496985. doi: 10.1155/2013/496985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltanpour MS, Davari K. The correlation of cardiac and hepatic hemosiderosis as measured by T2*MRI technique with ferritin levels and hemochromatosis gene mutations in Iranian patients with beta thalassemia major. Oman Med J. 2018;33(1):48–54. doi: 10.5001/omj.2018.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majd Z, Haghpanah S, Ajami GH, et al. Serum ferritin levels correlation with heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J. 2015;17(4):0–3. doi: 10.5812/ircmj.17(4)2015.24959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eghbali A, TaheraAhmadi H, Shahbazi M, Bagheri B, Ebrahimi L. Association between serum ferritin level, cardiac and hepatic T2-star {MRI} in patients with major β-thalassemia. Iran J Ped Hematol Oncol. 2014;4(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Azarkeivan A, Hashemieh M, Shirkavand A, Sheibani K. Correlation between heart, liver and pancreas hemosiderosis measured by MRI T2* Among thalassemia major patients from Iran. Arch Iran Med. 2016;19(2):96–100. doi:0161902/AIM.006 [PubMed] [Google Scholar]
- 17.He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T2* measurements in iron overloaded thalassemia. Magn Reson Med. 2008;60(5):1082–1089. doi: 10.1002/mrm.21744.Myocardial [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghugre NR, Enriquez CM, Coates TD, Marvin DN Jr, Wood JC. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging 2006;23:9–16.. doi: 10.1002/jmri.20467.Improved [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter J, He T, Kirk P, et al. On T2* magnetic resonance and cardiac iron. Circulation 2012;123(14):1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garbowski MW, Carpenter JP, Smith G, et al. Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J Cardiovasc Magn Reson. 2014;16(1):1–11. doi: 10.1186/1532-429X-16-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayraktaroǧlu S, Aydinok Y, Yildiz D, Uluer H, Savaş R, Alper H. T86. Diagnostic Interv Radiol. 2011;17(4):346–351. doi: 10.4261/1305-3825.DIR.3933-10.2 [DOI] [PubMed] [Google Scholar]
- 22.Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann N Y Acad Sci. 1998;850:191–201. [DOI] [PubMed] [Google Scholar]
- 23.Wood JC, Enriquez C, Ghugre N, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci. 2005;1054:386–395. doi: 10.1196/annals.1345.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engle MA, Erlandson M, Smith CH. Late cardiac complications of chronic, severe, refractory anemia with hemochromatosis. Circulation. 1964;30:698–705. doi: 10.1161/01.CIR.30.5.698 [DOI] [PubMed] [Google Scholar]
- 25.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–355. doi: 10.1111/j.1365-2141.2004.05202.x [DOI] [PubMed] [Google Scholar]
- 26.Yuksel IO, Koklu E, Kurtoglu E, et al. The association between serum ferritin level, tissue doppler echocardiography, cardiac T2* MRI, and heart rate recovery in patients with beta thalassemia major. Acta Cardiol Sin. 2016;32(2):231–238. doi: 10.6515/ACS20150824A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822 [DOI] [PubMed] [Google Scholar]
- 28.Noetzli LJ, Carson SM, Nord AS, et al. Longitudinal analysis of heart and liver iron in thalassemia major longitudinal analysis of heart and liver iron in thalassemia major. October. 2008;112(7):2973–2978. doi: 10.1182/blood-2008-04-148767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsella M, Borgna-Pignatti C, Meloni A, et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: a T2* magnetic resonance imaging study. Haematologica. 2011;96(4):515–520. doi: 10.3324/haematol.2010.025510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G, Liu R, Peng P, et al. How early can myocardial iron overload occur in beta thalassemia major? PLoS One. 2014;9(1). doi: 10.1371/journal.pone.0085379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51(2):209–221. [DOI] [PubMed] [Google Scholar]
- 32.Walker JM, Nair S. Detection of the cardiovascular complications of thalassemia by echocardiography. Ann N Y Acad Sci. 2010;1202:165–172. doi: 10.1111/j.1749-6632.2010.05643.x [DOI] [PubMed] [Google Scholar]
- 33.Cappellini MD, Cohen A, Eleftheriou A, et al. Thalassaemia International Federation; 2008. Guidelines for Clinical Management of Thalassaemia. 2nd Revised edition 2008. [PubMed] [Google Scholar]