Abstract
Introduction
Action observation neurorehabilitation systems are usually based on the observation of a virtual limb performing different kinds of actions. In this way, the activity in the frontoparietal Mirror Neuron System is enhanced, which can be helpful to rehabilitate stroke patients. However, the presence of limbs in such systems might not be necessary to produce mirror activity, for example, frontoparietal mirror activity can be produced just by the observation of virtual tool movements. The objective of this work was to explore to what point the presence of a virtual limb impacts the Mirror Neuron System activity in neurorehabilitation systems.
Methods
The study was conducted by using an action observation neurorehabilitation task during a functional magnetic resonance imaging (fMRI) experiment with healthy volunteers and comparing two action observation conditions that: 1 – included or 2 – did not include a virtual limb.
Results
It was found that activity in the Mirror Neuron System was similar during both conditions (i.e. virtual limb present or absent).
Conclusions
These results open up the possibility of using new tasks that do not include virtual limbs in action observation neurorehabilitation environments, which can give more freedom to develop such systems.
Keywords: fMRI, neurorehabilitation, action observation, virtual reality, virtual limbs, mirror neurons
Introduction
Mirror neurons, originally discovered by using intracranial electrodes in the premotor and the parietal cortex of monkeys, discharge not only when individuals perform a particular action (e.g. reaching for a piece of food) but also when they observe others performing the same or a similar action.1–3 This discovery was an important milestone in neuroscience because it showed that action perception and action execution were intrinsically linked from the neuronal level. Later research in humans with non-invasive neuroimaging and neurophysiological techniques showed evidence of the existence of a frontoparietal cortical network with the same property, which has been called the Mirror Neuron System (MNS).4
The essential property of mirror neurons (i.e. their activation by both executed and perceived actions) has a clinical application in the field of neurorehabilitation. This approach is based on the visual presentation of actions (e.g. using a mirror or virtual reality) to increase the activity in the MNS, as this activity (which has been found to be similar for virtual and for real stimuli5) can facilitate the reorganization of the brain motor regions affected by stroke.6–9 This kind of approach can be helpful to rehabilitate patients who cannot perform some active movements as a result of a cerebrovascular accident.
Action observation rehabilitation systems are usually based on the observation of a virtual limb performing different kinds of actions, for example patients can observe approaching virtual objects that are intercepted by virtual arms,10,11 thereby activating their MNS.12 However, representing the limbs in such systems may not be necessary to produce mirror activity: in a previous experiment, we have shown that extensive MNS activity can be produced just by the observation of a virtual paddle movement.13
The objective of the present research is to explore to what point the presence of a virtual limb is necessary to produce mirror activity in a neurorehabilitation system based on action observation. This was done by using an action observation neurorehabilitation task and comparing conditions that present or do not present a virtual limb to healthy volunteers in an a functional magnetic resonance imaging (fMRI) experiment. Based on previous research,12,13 we expect to find mirror activity in both conditions (note that these conditions were not directly compared in those experiments). Interestingly, if the activations are similar in both conditions, this would have important implications for the development of virtual environments: this would open up the possibility of using new tasks that do not include virtual limbs, giving more freedom to develop virtual environments.
Methods
Participants
Fourteen (4 female, 10 male) right-handed neurologically healthy subjects (mean age = 22.8, SD = 1.9) participated in this study. They had normal or corrected-to-normal vision. They gave their written informed consent. The study was approved by the local Ethics Committee (University of La Laguna; approval number: CEIBA2015-0178) and was conducted in accordance with the Declaration of Helsinki.
Neurorehabilitation task
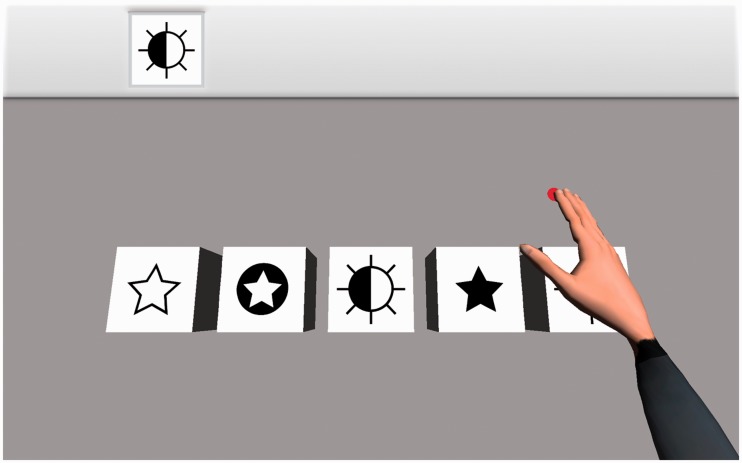
Participants were involved in an action observation neurorehabilitation task as shown in Figure 1. The task is a simplified version of the Reh@Task (Faria et al., 2016),14 a virtual reality system that presents a cancellation task with one image as target among four distractors. The task is solved by moving a virtual cursor and placing it over the target element for 5 s. The system is programmed to solve the tasks automatically (by using inverse kinematics, skeletal constraints are considered and the movement is physically correct and plausible), presenting to the user either (1) a virtual hand with a red dot under the tip of the middle finger or (2) just a red dot performing the selection process. After completion, the task restarts the process with a new randomly selected target and distractor elements.
Figure 1.
The action observation task. Participants observed movements of a virtual limb with a red dot under the tip of the middle finger (hand condition, shown here), or equivalent movements of the red dot alone (dot condition). In both cases, the red dot moves to reach the figure indicated in the left top corner of the screen.
Data acquisition
The fMRI run consisted of three conditions: dot, hand and fixation. The dot condition consisted of 6 blocks (58 s) of 11 trials each, where the participant observed the movements of the dot. The hand condition was similar, but in this case a virtual limb appeared above the dot (Figure 1). The dot and hand blocks were presented in random order and were preceded by a fixation task where the player stared at a gray cross in the middle of a black screen (baseline). The same random sequence of blocks was kept for all participants. The participants were instructed to focus on the movements of the dot and the hand during the corresponding conditions. Before the observation task, participants played a hand-controlled version of the same task for 6 min to link those actions to their motor repertory (by using a joystick). Visual stimuli were given via MRI compatible eyeglasses (Visuastim, Resonance Technology, Northridge, CA).
Axially oriented functional images were obtained by a 3T Signa HD MR scanner (GE Healthcare, Milwaukee, WI) using an echo-planar-imaging gradient-echo sequence and an eight-channel head coil (repetition time [TR] = 2000 ms, echo time [TE] = 21.6 ms, flip angle [FA] = 75°, matrix size = 64 × 64 pixels, 36 slices, 4 × 4 mm in plane resolution, spacing = 4 mm, slice thickness [ST] = 3.3 mm, interleaved acquisition). The slices were aligned to the anterior commissure – posterior commissure line and covered the whole brain. High resolution sagittally oriented anatomical images were also collected for anatomical reference. A three-dimensional fast spoiled-gradient-recalled pulse sequence was obtained (TR = 8.84 ms, TE = 1.75 ms, FA = 10°, matrix size = 256 × 256 pixels, 1 × 1 mm in plane resolution, spacing = 1 mm, ST = 1 mm).
Data analysis
Data were preprocessed and analyzed using SPM12 software (www.fil.ion.ucl.ac.uk/spm/). The images were spatially realigned, unwarped, normalized and smoothed using standard SPM12 procedures. The three conditions were modelled in the design matrix for each participant. Activation maps for the contrast dot > fix, hand > fix, hand > dot and dot > hand were generated for each subject by applying t statistics. These first-level contrast images were used in a random effects group analysis. Statistical maps were set at a voxel-level threshold of p < 0.05, false discovery rate corrected for multiple comparisons, and a minimum cluster size of 25 voxels.
Results
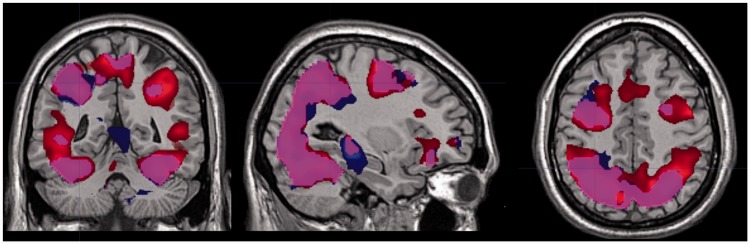
Figure 2 shows the brain regions that were activated by the action observation neurorehabilitation task. The task was associated with an increase of activity in bilateral frontoparietal regions of the MNS (parietal lobe, premotor cortex, caudal part of the inferior frontal gyrus)4 not only when the virtual hand was moving but also when the virtual hand was absent and only the dot was moving. Other regions outside the MNS, such as the occipital lobe and the cerebellum were also bilaterally activated in both conditions. Interestingly, no significant differences in brain activity were found between the hand and the dot conditions (hand > dot and dot > hand contrasts).
Figure 2.
The action observation neurorehabilitation task was associated with an increase of activity in the Mirror Neuron System when the virtual hand was moving but also when the virtual hand was absent and just the dot was moving. Blue voxels were activated only in hand condition (hand > fix contrast); red voxels were activated only in dot condition (dot > fix contrast); violet voxels depict regions activated in both conditions (both contrasts). The contrasts hand > dot and dot > hand did not show significant results. Group analysis, N = 14, threshold: p < 0.05 at the voxel level, false discovery rate corrected for multiple comparisons; minimum cluster size = 25 voxels.
Conclusions
As expected, the two main conditions (hand/dot) of the action observation task activated the MNS of the participants. Regarding the hand condition, the participants were observing the movements of a virtual arm, thus finding activity in the MNS is consistent with previous research on action observation rehabilitation systems that have presented virtual limbs in their tasks.12 Concerning the dot condition, we also found activity in the MNS although the participants were just observing the movements of a dot (it should be mentioned here that the presence of the red dot in the hand blocks of the practice period can help to associate the movements of the dot and the virtual arm, and this association may be a factor involved in this activity). This is also consistent with previous research showing that MNS activity can be produced just by the observation of virtual tool movements.13
More interestingly, we have directly compared two conditions that only differed in the presence or absence of a virtual limb, and we did not find significant differences in associated MNS activity. In this way, the activity we found during the hand condition does not appear to be so directly related with the observation of the limb but more with the observation of actions that had been previously linked to the observer’s motor repertory during the practice period.13 Therefore, the question arises regarding what would happen in the absence of such a practice period. Because the movements of the virtual limb were physically correct and plausible, they can be considered as belonging to the participant’s motor repertory; thus, mirror activity would also be expected in this case (as happens in some action observation experiments that do not use practice periods15). With respect to dot condition, mirror activity could also be expected due to previous experience with other related games or activities, such as moving a cursor on a screen. To what extent the mirror activity in dot and hand conditions would differ without a practice period is a question to be addressed in further research. In any case, if manual practice were not possible, a visual training period could be useful to enhance the link between the dot and the hand conditions and obtain more mirror activity if necessary.16
The results presented here may be of interest for researchers and developers of neurorehabilitation systems based on action observation, and could be used to make the systems more attractive for the patients (for example, combining different kinds of tasks including or not including virtual limbs), which therefore may help the patient to adhere to the therapy.
Acknowledgements
We acknowledge the support of Servicio de Resonancia Magnética para Investigaciones Biomédicas de la Universidad de La Laguna.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by: Spanish National Program (PTA2015-10703-I, Ministerio de Economía y Competitividad); European Union (INTERREG program, MAC/1.1.b/098; MACbioIDi project); Fundação para a Ciência e a Tecnologia (FCT, Portugal) (project UID/EEA/50009/2013); Cabildo de Tenerife (Programa Agustín de Betancourt 2016-2020; Código: 02-12).
Guarantor
CM.
Contributorship
CM, SB, MC, FP, FM, EHM, JPB, JLGM conceived and designed the experiments. CM, NP, DNP, JMPG performed the experiments. CM analyzed the data and wrote the paper. SB, TP, MC, FP, FM, EHM, CM, JLGM contributed materials/analysis tools.
References
- 1.Dipellegrino G, Fadiga L, Fogassi L, et al. Understanding motor events: a neurophysiological study. Exp Brain Res 1992; 91: 176–180. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, Fadiga L, Fogassi L, et al. Action recognition in the premotor cortex. Brain 1996; 119: 593–609. [DOI] [PubMed] [Google Scholar]
- 3.Gallese V, Fadiga L, Fogassi L, et al. Action representation and the inferior parietal lobule. Common Mech Percept Action 2002; 19: 334–355. [Google Scholar]
- 4.Cattaneo L, Rizzolatti G. The Mirror Neuron System. Arch Neurol 2009; 66: 557–560. [DOI] [PubMed] [Google Scholar]
- 5.Brihmat N, Tarri M, Quide Y, et al. Action, observation or imitation of virtual hand movement affect differently regions of the mirror neuron system and the default mode network. Brain Imaging Behav 2018; 12: 1363–1378. [DOI] [PubMed] [Google Scholar]
- 6.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol 2006; 19: 55–63. [DOI] [PubMed] [Google Scholar]
- 7.Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair 2010; 24: 404–412. [DOI] [PubMed] [Google Scholar]
- 8.Sale P, Franceschini M. Action observation and mirror neuron network: a tool for motor stroke rehabilitation. Eur J Phys Rehabil Med 2012; 48: 313–318. [PubMed] [Google Scholar]
- 9.Bhasin A, Padma Srivastava MV, Kumaran SS, et al. Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol India 2012; 60: 570–576. [DOI] [PubMed] [Google Scholar]
- 10.Cameirao MS, Bermudez i Badia S, Duarte Oller E, et al. Neurorehabilitation using the virtual reality based Rehabilitation Gaming System: methodology, design, psychometrics, usability and validation. J Neuroeng Rehabil 2010; 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameirao MS, Bermudez i Badia S, Zimmerli L, et al. A Virtual Reality System for Motor and Cognitive Neurorehabilitation. In: Challenges for Assistive Technology, 2007, pp.393–397. Amsterdam: IOS Press.
- 12.Prochnow D, Bermudez I Badia S, Schmidt J, et al. A functional magnetic resonance imaging study of visuomotor processing in a virtual reality-based paradigm: Rehabilitation Gaming System. Eur J Neurosci 2013; 37: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 13.Modroño C, Navarrete G, Rodriguez-Hernandez AF, et al. Activation of the human mirror neuron system during the observation of the manipulation of virtual tools in the absence of a visible effector limb. Neurosci Lett 2013; 555: 220–224. [DOI] [PubMed] [Google Scholar]
- 14.Faria AL, Cameirao MS, Couras JF, et al. Combined Cognitive-Motor Rehabilitation in Virtual Reality Improves Motor Outcomes in Chronic Stroke – A Pilot Study. Front Psychol 2018; 9: 854. [DOI] [PMC free article] [PubMed]
- 15.Plata-Bello J, Modroño C, Marcano F, et al. Modulation in the mirror neuron system when action prediction is not satisfied. Eur J Neurosci 2015; 41: 940–948. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci 2005; 17: 212–226. [DOI] [PubMed] [Google Scholar]