Abstract
Objective:
In this study, we aimed to clarify the effects of long noncoding ribonucleic acid prostrate androgen-regulated transcript-1 on bladder cancer cell proliferation and apoptosis.
Methods:
Microarrays were implemented to investigate the long noncoding ribonucleic acid expression profiles in bladder cancer tissue (N = 9) and in noncancer bladder tissue (N = 5). Relative prostrate androgen-regulated transcript-1 expression levels in tissue samples or cell lines were detected by real-time quantitative reverse transcription-polymerase chain reaction. Prostrate androgen-regulated transcript-1 expression was enhanced by the transfection of pcDNA3.1-prostrate androgen-regulated transcript-1 and downregulated by the infection with pcMV-sh prostrate androgen-regulated transcript-1. Additionally, cell proliferation and apoptosis were measured by the cell counting kit-8 assay and flow cytometry, respectively. Cell invasion was determined by a Transwell assay.
Results:
Prostrate androgen-regulated transcript-1 expression was upregulated in bladder cancer tissues compared to adjacent nontumor tissues. Furthermore, prostrate androgen-regulated transcript-1 levels were successfully upregulated by pcDNA3.1-prostrate androgen-regulated transcript-1 and depleted by pCMV-sh prostrate androgen-regulated transcript-1 in bladder cancer cell lines (5637, T24). Enhanced prostrate androgen-regulated transcript-1 expression promoted cell proliferation and invasion and inhibited cell apoptosis. However, knockdown of prostrate androgen-regulated transcript-1 expression inhibited cell proliferation and invasion and induced cell apoptosis.
Conclusion:
In summary, these data suggest that the knockdown of prostrate androgen-regulated transcript-1 represents a tumor suppressor player in bladder cancer and contributes to the inhibition of tumor proliferation, the promotion of cell apoptosis, and the suppression of cell invasion. Prostrate androgen-regulated transcript-1 may function as a new prognostic biomarker and as a feasible therapeutic target for patients with bladder cancer.
Keywords: bladder cancer, LncRNA, knockdown, PART1, prognostic biomarker, therapeutic target
Introduction
Bladder cancer is a common malignancy of the genitourinary system.1 Bladder cancer is the fourth most commonly diagnosed cancer2 found among males and is nearly 5 times more common in male than female patients worldwide.3 Given its high morbidity and mortality, bladder cancer has received a great amount of attention recently. Despite a variety of effective treatments, such as operation, iatrochemistry, and radiation treatment, for patients with bladder cancer, the 5-year cancer-specific survival rate remains fairly low.4-7 Moreover, profound insight into the etiology of bladder cancer is lacking and its pathogenic mechanism has become one of the most vital reasons for its high-fatality rate.4 Thus, there is an urgent need for developing a more effective therapeutic target for bladder cancer.
Long noncoding RNAs (LncRNAs) are transcripts (>200 nucleotides) that have not been translated into proteins. Long noncoding RNAs function as carcinogens or tumor suppressors and play a central part in the progression of malignancies.8 Furthermore, with the rapid development of second-generation sequencing technology, dysregulation of a wide range of lncRNAs has been found to be implicated in proliferation, chemoresistance, metastasis, and other pathological stages of different human diseases,9 particularly in cancers.10 For instance, using small interfering RNAs, the lncRNA AATBC was revealed to induce intrinsic apoptosis in bladder cancer cells.11 Li et al found that knockdown of the lncRNA GHET1 suppressed the growth and invasion of bladder cancer cells in vitro.12 In addition, Xue et al reported that the lncRNA UCA1 played a crucial role in regulating the invasion of the 5637 bladder cancer cell line.13 Moreover, it was demonstrated that knockdown of lncRNA prostrate androgen-regulated transcript-1 (PART1) was an effective therapy in inducing apoptosis of prostate cancer cells.11
The lncRNA PART1, namely, PART1, is regarded as a novel androgen-responsive gene.14 Prostrate androgen-regulated transcript-1 is highly expressed in the prostate and is regulated by androgens in human prostate cancer cells.15 Additionally, it was reported that PART1 might be a new-found prostatic cancer biomarker that is dependent on androgenic hormones.16 However, the expression and physiologic function of PART1 in urinary bladder tumors has yet to be clarified.
In the present study, we observed that PART1 was upregulated in bladder cancer. Moreover, we found that overexpression of PART1 increased cell viability and invasion and diminished the rate of cell apoptosis; however, depleted PART1 suppressed cell growth and invasion and promoted cell apoptosis. Taken together, our findings demonstrated that PART1 might play a crucial role in promoting the tumorigenesis and progression of urinary bladder cancer through the regulation of proliferation, invasion, and apoptosis.
Materials and Methods
Tissue Samples
Thirty bladder cancer samples and matched adjacent normal bladder tissues were obtained from patients who experienced surgical resection of primary bladder cancer at The First Affiliated Hospital of Nanjing Medical University (Nanjing, China). The diagnosis of all specimens was based on histopathological examination by the experienced pathologists of The First Affiliated Hospital of Nanjing Medical University. The tissues were obtained before chemotherapy, radiation therapy, or any other treatments for tumors. Upon removal of the surgical specimen, each sample was frozen in liquid nitrogen directly and stored at −80°C before being used for RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis. This study was undertaken with approval from the Ethics Committee (approval no. 2018-SRFA-176) of The First Affiliated Hospital of Nanjing Medical University (Nanjing, China) and was performed in accordance with the Declaration of Helsinki (2008) of the World Medical Association. All individual participants of the study provided their written informed consent.
Cell Culture
Human bladder cancer cell lines (5637, T24) were purchased from the BeNa Culture Collection (Beijing, China). The RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) was used to culture the 5637 and T24 cells, and the cells were cultured in incubator under humidified conditions with 5% CO2 at 37°C.
RNA Extraction and Microarray Experiments
Total RNA from 9 bladder cancer tissues and 5 normal tissue specimens was extracted by the RNeasy Mini Kit (QIAGEN, Duesseldorf, Germany) with the RNase-Free DNase Set (QIAGEN), then were mixed and labeled with a spike in the control RNA, the Low Input Quick Amp Labeling Kit, and the One-color kit respectively according to the protocols from the kits. Each slide was hybridized with cyanine 3-CTP using the Gene Expression Hybridization Kit. After hybridization, slides were washed with the Gene Expression Wash Buffer Kit following the instructions from the manufacturer. Microarray slides were scanned by a High Resolution Microarray Scanner C. All the kits were purchased from Agilent Technologies (Santa Clara, California). The obtained array images were examined via the Agilent Feature Extraction software (version 12.0.3.2). Quantile normalization and successive data manipulation were conducted through the Gene Spring Software 11.5 (Agilent Technologies).
Microarray Analysis
GSE100926 was downloaded from the GEO website (https://www.ncbi.nlm.nih.gov/geo/). GSE100926 contained 6 bladder samples with 3 bladder cancer tissues and 3 normal tissues. Processing of gene expression data was carried out using the Limma package in the R software. Data in each array were normalized using the quantile normalization procedure. Our experiment was divided into 2 groups. We applied a t test in order to filter the differentially expressed lncRNAs in bladder cancer. Long noncoding RNAs in GSE100926 were considered as significantly different if they meet the conditions of having a P value <.05 (Benjamini-Hochberg method) and |log2FC| > 1.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA in cell lines and tissue samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, California). First-strand complementary DNA (cDNA) was transcribed using SYBR Green Master Mix Applied Biosystems (Thermo Fisher Scientific, Waltham, Massachusetts). After reverse transcription of the total RNA, PCR amplification was conducted for 40 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds to examine the expression of PART1 using SYBR Green PCR Master Mix (Roche, Basel, Switzerland) on a Light Cycler 480 Real-Time PCR instrument (Roche). glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. In addition, relative quantitative expression of PART1 was determined using the 2−ΔΔ C T method.
Cell Transfection
To construct the PART1 overexpressed plasmid, the full-length cDNA (GenBank accession: NR_024617.1, 2495 bp) was cloned into the pcDNA3.1 vector (GenePharma, Shanghai, China) and termed as pcDNA3.1-PART1. In addition, shRNA that specifically targeted PART1 was cloned into the pCMV vector (GenePharma) and termed as pCMV-shPART1. One day prior to transfection, 5637 or T24 cells were seeded in 6 well plates and transfected with pcDNA3.1-PART1 or pCMV-shPART1 plasmid using Lipofectamine 3000 Reagent (Life Technologies, Gaithersburg, Maryland) according to the manufacturer’s manuals. Cells were cultured in an incubator with 5% CO2 at 37°C. After incubating for 48 hours, cells were lysed with the TRIzol LS Reagent (Invitrogen) directly. The expression level of PART1 was determined by qRT-PCR.
Cell Proliferation and Apoptosis Assay
Cells were seeded in 96-well plates at 3000 viable cells/well. At 24 hours, 48 hours, 72 hours, and 96 hours, 10 µL of cell counting kit-8 (CCK-8) solution (Beyotime Biotechnology, Shanghai, China) was added to each well, and the plate was incubated for an additional 4 hours at 37°C. A microplate reader was used to evaluate the absorbance at 450 nm. The experiment was carried out in 6 replicates.
After 24-hour transfection, cells were detached using trypsin, collected by centrifugation at 15 00 rpm for 5 minutes and incubated with 250 µL Annexin V binding buffer and 10 µL freshly prepared Annexin V/propidium iodide (PI) reagent (Beyotime); the reaction was completed in the dark at room temperature for 15 minutes. Finally, samples were analyzed within 30 minutes on a FACScan flow cytometer (Becton Dickin-son, Mountain View, California). In addition, FlowJo version 6.0 was used to analyze the apoptosis rate of cells.
Cell Invasion Assay
Cell invasion assay was enacted within a 24-well chamber containing a membrane with an 8 µm pore size Matrigel coat (Corning Incorporated, Corning, New York). After transfection with either pcDNA3.1-PART1 or pcMV-PART1, 5637 or T24 cells were resuspended in serum-free medium and concentrated to 1 × 105 cells/mL, respectively. Then, 200 µL cell suspension was transferred to the upper chamber, and the bottom chamber was filled with 500 µL culture medium supplemented with 10% FBS. After 12 hours, the cells did not invade remained on the upper membrane surface and were separated with a cotton tip, and the cells that gone through the filter were fixed and stained with 0.1% crystal violet. Invaded cells were counted under a light microscope (Nikon, Tokyo, Japan).
Statistical Analysis
Statistical analysis was evaluated using GraphPad Prism version 6.0 software (La Jolla, California). The difference between bladder cancer tissues and adjacent cancer tissues was analyzed with Student t test. Cell counting kit-8 assay data were analyzed by analysis of variance, and independent samples t test was utilized to analyze other data. Values of P < .05 were considered statistically significant.
Results
Long Noncoding RNAs PART1 was Upregulated in Bladder Cancer
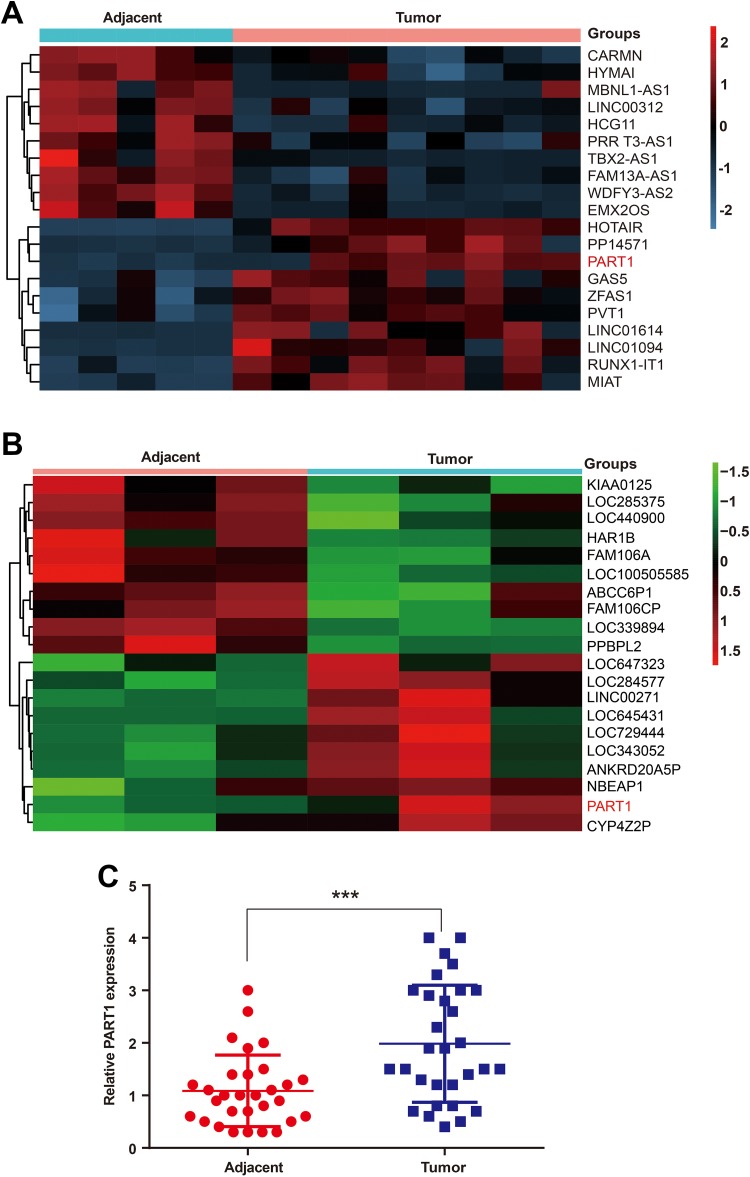
We first investigated the expression pattern (|fold changes| > 2 and P < .05) of lncRNAs in bladder cancer tissues and adjacent normal tissues obtained from 30 specimens by the microarray assay. Then, 10 highly expressed lncRNAs and 10 lowly expressed lncRNAs in urinary tumor tissues were screened. We determined that lncRNA PART1 was overexpressed in bladder tissues compared to the para-carcinoma normal tissues (Figure 1A). In addition, the public databases of GEO (https://www.ncbi.nlm.nih.gov/geo/; GSE100926) were used to confirm that the differential expression of PART1 is reproducible. Long noncoding RNAs PART1 was highly expressed in bladder cancer tissues in GSE100926 (Figure 1B). Quantitative reverse transcription-polymerase chain reaction was used to measure PART1 expression levels in a total of 30 bladder cancer specimens. Long noncoding RNAs PART1 was significantly overexpressed in clinical bladder cancer specimens compared to adjacent normal tissues (Figure 1C).
Figure 1.
The long noncoding RNA PART1 is overexpressed in bladder cancer. (A) The heatmap represents unsupervised hierarchical clustering of lncRNA expression in bladder cancer tissues compared with adjacent nontumor tissues. Each column represents the indicated tissue sample, and each row indicates one lncRNA. The red color scale represents higher expression levels, and the blue color scale represents lower expression levels. (B) The heatmap of the 20 differentially expressed lncRNAs from the GEO microarray GSE100926. PART1 is upregulated in bladder cancer (|fold changes|>2, p.value<0.05). The red color scale represents higher expression levels, and the green color scale represents lower expression levels. (C) The relative PART1 expression levels were determined using qRT-PCR in 30 samples of bladder cancer tissues. PART1 is upregulated in bladder cancer tissues. (***p<0.001). lncRNAs indicates long noncoding RNAs
Prostrate Androgen-Regulated Transcript-1 Enhanced Cell Proliferation in Bladder Cancer
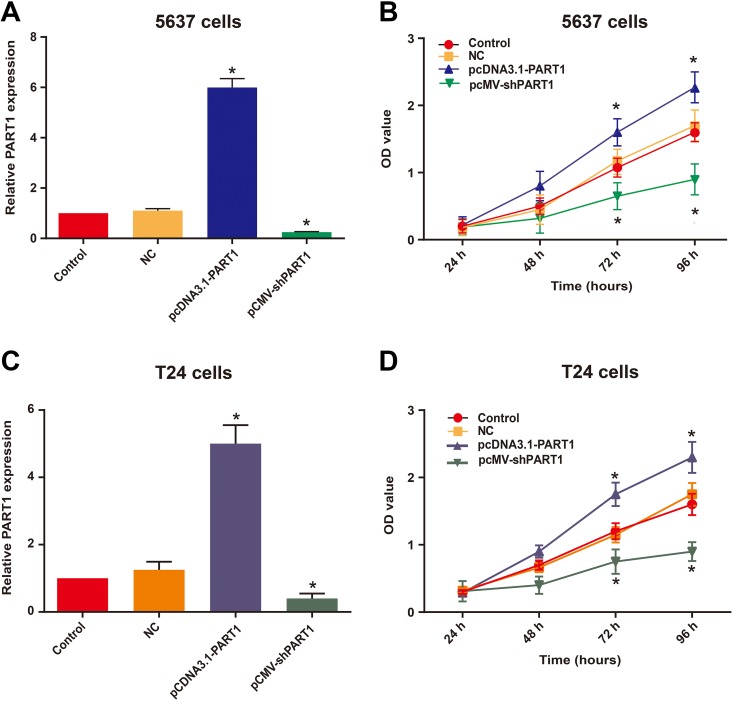
Specific plasmid vectors were used to interfere with the expression of PART1. Bladder cancer cell lines 5637 and T24 were cultured and transfected with pcDNA3.1-PART1, pCMV-shPART1 or negative control (NC). After 48 hours from the transfection, the relative PART1 expression levels were analyzed by qRT-PCR, and the results demonstrated that pcDNA-PART1 significantly enhanced the expression of PART1, while pCMV-shPART1 silenced the expression of PART1 (Figure 2A and C). To further examine the potential impact of PART1 on the development of bladder cancer cells, the cell proliferation of 5637 and T24 cells was assessed by the CCK-8 assay. The results suggested that pcDNA3.1-PART1 markedly enhanced cell growth, whereas pCMV-shPART1 significantly reduced cell viability in bladder cancer cells (Figure 2B and D). These data implied that the bladder cancer cell proliferation ability was greatly repressed by downregulated PART1.
Figure 2.
PART1 downregulation inhibits bladder cancer cell proliferation. (A and C) qRT-PCR was conducted to evaluate the relative expression of PART1 in 5637 (A) and T24 (C) cells after transfection. (B and D) The CCK-8 assay was used to investigate the effects of altered the expression of PART1 on 5637 (B) and T24 (D) cell proliferation. The results are expressed as the means ± SD for 4 replicate experiments. (*p<0.05 vs. NC group). CCK-8, cell counting kit-8
Overexpressed PART1 Suppressed Cell Apoptosis in Bladder Cancer Cells
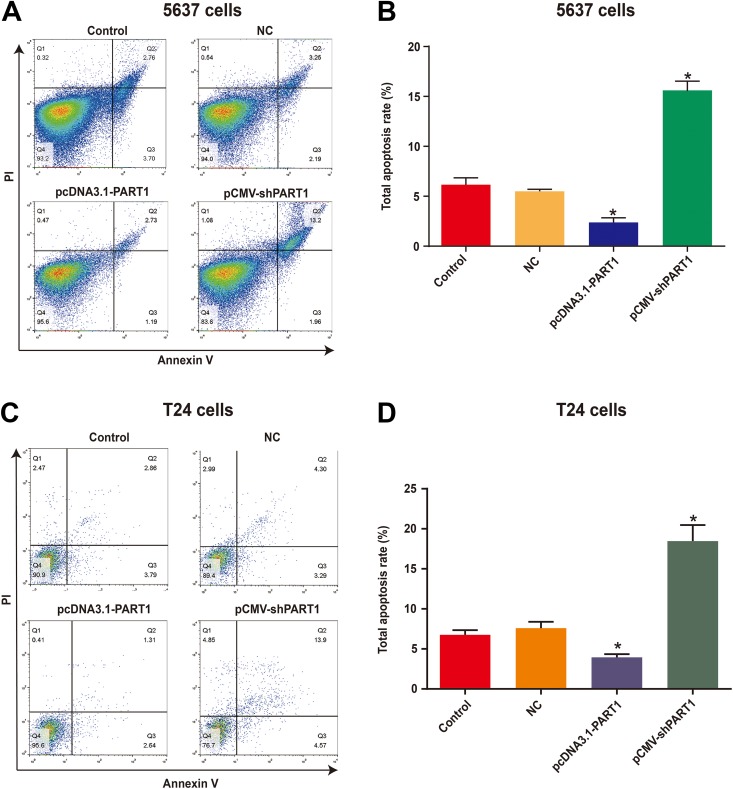
Furthermore, we investigated the effect of PART1 on cell apoptosis in bladder cancer. Annexin V-FITC/PI flow cytometry was performed to measure the cell apoptosis in 5637 and T24 cells. As shown in Figure 3A and B, the percentage of 5637 cells apoptosis in the pcDNA3.1-PART1 group was lower than that in the NC group. Additionally, the proportion of apoptotic 5637 cells in the pCMV-shPART1 group was markedly enhanced compared to that in the NC group. Furthermore, we had the same results in theT24 cells. The apoptosis rate of T24 cells in the pcDNA3.1-PART1 group was reduced compared to the NC group. Moreover, the apoptosis rate of T24 cells in the pCMV-shPART1 group was significantly increased compared to that in the NC group (Figure 3C and D). The results suggested that the downregulated PART1 causes cells apoptosis in bladder cancer cells. There were no significant differences between the control and NC groups.
Figure 3.
Knockdown of lncRNA PART1-induced apoptosis in bladder cancer cells. (A and C) Apoptosis in 5637 (A) and T24 (C) cells was determined by Annexin V-FITC/PI flow cytometry. (B and D) The total proportion of apoptotic 5637 (B) and T24 (D) cells (Q2 + Q3) is shown. Data are shown as the mean ± SD. (*p<0.05 vs. NC group). PI, propidium iodide.
Downregulation of PART1 Attenuates Invasive Properties of Bladder Cancer Cells
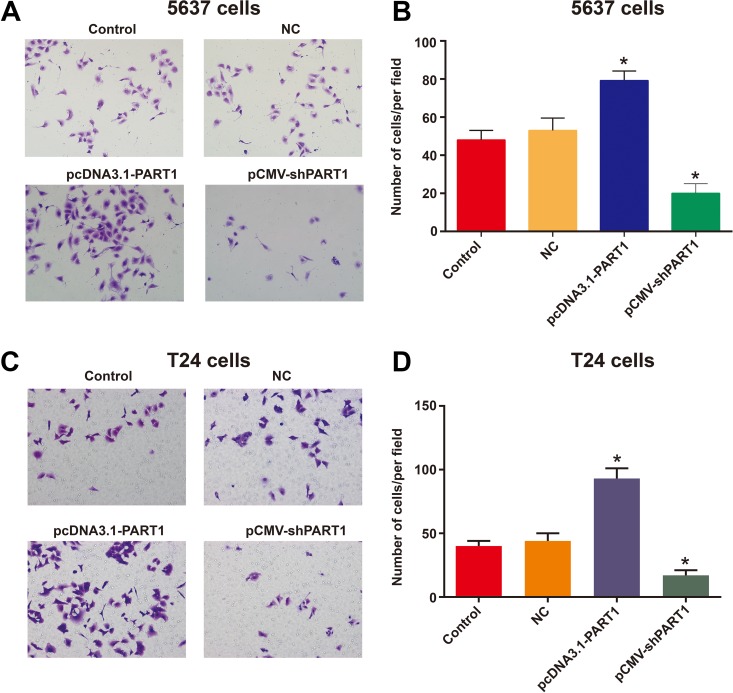
The effects of lncRNA PART1 on the invasive capacities of bladder cancer cells (5637, T24) were further analyzed. Transwell assay was applied to detect the invasive capacities of 5637 and T24 cells. The results showed that the invasive capacities of 5637 (Figure 4A and B) and T24 (Figure 4C and D) cells in the overexpressed lncRNA PART1 groups were markedly higher than those in the NC group at 12 hours. However, traversed cells that invaded through the Matrigel membrane in the PART1 reduced groups were reduced relative to those in the NC groups. The findings demonstrated that the knockdown of PART1 in bladder cancer cells significantly attenuated the cell invasive properties.
Figure 4.
Downregulated PART1 reduces invasion of bladder cancer cells. Invasion of bladder cancer cells in the Matrigel matrix was measured in a Transwell system. Both 5637 and T24 cells were transfected with pcDNA3.1 plus pCMV (NC), pcDNA3.1-PART1 and pCMV-shPART1. Then, 12 h after seeding on the matrix, invaded 5637 and T24 cells were counted. (A and C) 5637 (A) and T24 (C) cells that grew through the matrix were visualized under a light microscope (200 × magnification). (B and D) The numbers of 5637 (B) and T24 (D) cells that grew through the Matrigel were counted. The bar shows the mean ± SD. (*p<0.05 vs. NC group).
Discussion
Bladder cancer is a common death-related malignancy worldwide.2,17 Currently, chemoradiotherapy as well as surgical procedures are the main approaches for treating bladder cancer. Even though the current treatment methods can prolong the lifespan of patients, the prognosis for bladder cancer remains poor.5-7 Therefore, the development of specific therapeutic targets and identification of a diagnostic and/or prognostic biomarkers for bladder cancer are needed. The initiation and progression of bladder cancer is a complicated process. Recently, lncRNAs were widely identified to play crucial roles in various diseases. For instance, MALAT1 promotes gastric cancer progression by regulating the expression of Gli2 through sponging miR-202.18 Furthermore, lncRNA FTH1P3 regulates the expression of fizzled 5 by miR-224-5p to strengthen oral cancer cell progression.19 More importantly, several lncRNAs, such as CALML3-AS1, LINC00641, and SNHG16, are involved in bladder cancer progression.20-22
In this study, we focused on the lncRNA PART1, a novel oncogene lncRNA in several cancers. Sun et al. reported that lncRNA PART1 was upregulated and enhanced TLR pathways, including TLR3, TNFSF10 and CXCL13, to promote cell proliferation in prostate cancer cells.23 Moreover, a recent study showed that patients with esophageal squamous cell carcinoma have an enhanced expression of the lncRNA PART1, which induces gefitinib resistance.24 These findings prompted us to investigate the expression and function of lncRNA PART1 in bladder cancer. In agreement with our expectation, we observed that lncRNA PART1 was overexpressed in bladder cancer tissues and cells, and these findings suggests that lncRNA PART1 may be an oncogenic player for bladder cancer.
After validating the expression of PART1 in bladder cancer, we performed a series of experiments to explore the biological activities that PART1 plays in bladder cancer cells. Gain- and loss-of-function assays were performed on 5637 and T24 cells. One of the most common characteristics of cancer is uncontrolled cell growth. In the current study, we measured the change in cell proliferation after silencing PART1 expression using CCK-8 assays. The results showed that silencing PART1 dramatically attenuated cell proliferation in the 5637 and T24 cells. We performed flow cytometric analyses to evaluate whether the promotion of cell apoptosis contributed to the antiproliferative effect of pcMV-shPART1. Data from apoptotic analysis demonstrated that cell apoptosis increased after PART1 downregulation. Additionally, we evaluated the alteration of invasive capacities of bladder cancer cells induced by overexpression or knockdown of PART1. As a result, we found that PART1 had an enhanced metastatic property. Knockdown of PART1 inhibited cells invasion significantly. This is the first study to determine the function of PART1 in bladder carcinoma. However, the possible targets and mechanisms underlying the regulatory actions of PART1 in bladder cancer need to be elucidated in future studies.
Conclusions
In conclusion, we identified that the knockdown of PART1 was implicated in the inhibition of cell proliferation, the induction of apoptosis, and the suppression of cell invasion in bladder cancer. In addition, this study extends our knowledge on the oncogenesis of bladder cancer and reveals the biological function of PART1 in urinary bladder cancer and its potential as a new molecular target for bladder cancer therapy.
Abbreviations
- CCK-8
cell counting kit-8
- lncRNA
long noncoding ribonucleic acid
- PART1
prostate androgen-regulated transcript 1
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
Footnotes
Authors’ Note: This study was undertaken with approval from the Ethics Committee (approval no. 2018-SRFA-176) of The First Affiliated Hospital of Nanjing Medical University (Nanjing, China) and was performed in accordance with the Declaration of Helsinki (2008) of the World Medical Association. All individual participants of the study provided their written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); Program for Development of Innovative Research Team in the First Affiliated Hospital of Nanjing Medical University; Provincial Initiative Program for Excellency Disciplines of Jiangsu Province; National Natural Science Foundation of China (81672531); Jiangsu Provincial Six talents peak Program (2016-WSW-011).
ORCID iD: Chao Qin, PhD  https://orcid.org/0000-0001-8340-653X
https://orcid.org/0000-0001-8340-653X
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 2010;60(4):244–272. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
- 3. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4. He A, Liu Y, Chen Z, et al. Over-expression of long noncoding RNA BANCR inhibits malignant phenotypes of human bladder cancer. J Exp Clin Cancer Res. 2016;35:125 doi: 10.1186/s13046-016-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose TL, Milowsky MI. Improving systemic chemotherapy for bladder cancer. Curr Oncol Rep. 2016;18(5):27 doi: 10.1007/s11912-016-0512-2. [DOI] [PubMed] [Google Scholar]
- 6. Sofra M, Fei PC, Fabrizi L, et al. Immunomodulatory effects of total intravenous and balanced inhalation anesthesia in patients with bladder cancer undergoing elective radical cystectomy: preliminary results. J Exp Clin Cancer Res. 2013;32:6 doi: 10.1186/1756-9966-32-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Racioppi M, D’Agostino D, Totaro A, et al. Value of current chemotherapy and surgery in advanced and metastatic bladder cancer. Urol Int. 2012;88(3):249–258. doi: 10.1159/000335556. [DOI] [PubMed] [Google Scholar]
- 8. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 9. Hu Y, Ma Z, He Y, Liu W, Su Y, Tang Z. PART-1 functions as a competitive endogenous RNA for promoting tumor progression by sponging miR-143 in colorectal cancer. Biochem Biophys Res Commun. 2017;490(2):317–323. doi: 10.1016/j.bbrc.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 10. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2103;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao F, Lin T, He W, et al. Knockdown of a novel lincRNA AATBC suppresses proliferation and induces apoptosis in bladder cancer. Oncotarget. 2015;6(2):1064–1078. doi: 10.18632/oncotarget.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li LJ, Zhu JL, Bao WS, Chen DK, Huang WW, Weng ZL. Long noncoding RNA GHET1 promotes the development of bladder cancer. Int J Clin Exp Pathol. 2014;7(10):7196–7205. [PMC free article] [PubMed] [Google Scholar]
- 13. Xue M, Pang H, Li X, Li H, Pan J, Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107(1):18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu L, Blackburn GL, Zhou JR. Genistein and daidzein downregulate prostate androgen-regulated transcript-1 (PART-1) gene expression induced by dihydrotestosterone in human prostate LNCaP cancer cells. J Nut. 2003;133(2):389–392. doi: 10.1093/jn/133.2.389. [DOI] [PubMed] [Google Scholar]
- 15. Lin B, White JT, Ferguson C, et al. PART-1: a novel human prostate-specific, androgen-regulated gene that maps to chromosome 5q12. Cancer Res. 2000;60(4):858–863. [PubMed] [Google Scholar]
- 16. Sidiropoulos M, Chang A, Jung K, Diamandis EP. Expression and regulation of prostate androgen regulated transcript-1 (PART-1) and identification of differential expression in prostatic cancer. Br J Cancer. 2001;85(3):393–397. doi: 10.1054/bjoc.2001.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng L, Pan P, Chen J, Yu X, Wu J, Chen Y. A tetracycline-inducible CRISPR/Cas9 system, targeting two long non-coding RNAs, suppresses the malignant behavior of bladder cancer cells. Oncol Lett. 2018;16(4):4309–4316. doi: 10.3892/ol.2018.9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Chen Z, Li MJ, Guo HY, Jing NC. Long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 regulates the expression of Gli2 by miR-202 to strengthen gastric cancer progression. Biomed Pharmacother. 2017;85:264–271. doi: 10.1016/j.biopha.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 19. Zhang CZ. Long non-coding RNA FTH1P3 facilitates oral squamous cell carcinoma progression by acting as a molecular sponge of miR-224-5p to modulate fizzled 5 expression. Gene. 2017;607:47–55. doi: 10.1016/j.gene.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 20. Wang F, Zu Y, Huang W, Chen H, Xie H, Yang Y. LncRNA CALML3-AS1 promotes tumorigenesis of bladder cancer via regulating ZBTB2 by suppression of microRNA-4316. Biochem Biophys Res Commun. 2018;504(1):171–176. doi: 10.1016/j.bbrc.2018.08.150. [DOI] [PubMed] [Google Scholar]
- 21. Feng F, Chen A, Huang J, Xia Q, Chen Y, Jin X. Long noncoding RNA SNHG16 contributes to the development of bladder cancer via regulating miR-98/STAT3/Wnt/beta-catenin pathway axis. J Cell Biochem. 2018;119(11):9408–9418. doi: 10.1002/jcb.27257. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Hong S, Liu Z. LncRNA LINC00641 predicts prognosis and inhibits bladder cancer progression through miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem Biophys Res Commun. 2018;503(3):1825–1829. doi: 10.1016/j.bbrc.2018.07.120. [DOI] [PubMed] [Google Scholar]
- 23. Sun M, Geng D, Li S, Chen Z, Zhao W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol Chem. 2018;399(4):387–395. doi: 10.1515/hsz-2017-0255. [DOI] [PubMed] [Google Scholar]
- 24. Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 2018;37(1):171 doi: 10.1186/s13046-018-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]