Abstract
Adequate salivary secretion is crucial to both oral and general health, since it provides a complex milieu for support of the microbial populations of the mouth, while at the same time containing antimicrobial products that help control these microbial populations. This paper summarizes several aspects of salivary component function, gland secretion mechanisms, and immuno-pathogenesis as related to oral health and disease. Salivary components mediate microbial attachment to oral surfaces, and also interact with planktonic microbial surfaces to facilitate agglutination and elimination of pathogens from the oral cavity. Adhesive interactions are often mediated by lectin-like bacterial proteins that bind to glycan motifs on salivary glycoproteins. An important salivary antimicrobial protein is histatin 5 (Hst 5), which shows potent and selective antifungal activity and also susceptibility to proteolytic degradation. Coupling of Hst 5 with the carrier molecule spermidine significantly enhanced killing of C. albicans and resistance to proteolytic degradation, compared with the parent peptide. Loss of salivary secretion may be caused by disorders such as Sjögren's syndrome (SS) or ectodermal dysplasia, or may be a side-effect of radiation therapy. Two new approaches to the treatment of salivary gland dysfunction include the use of resolvins and the creation of differentiated acinar structures to construct an artificial salivary gland. B-cells contribute to the pathogenesis of SS by releasing cytokines and autoantibodies and by influencing T-cell differentiation. CXCL13, a potent B-cell chemokine associated with autoimmune diseases, is elevated locally and systemically in SS and may represent a novel biomarker or therapeutic target in the management and treatment of SS.
Keywords: Sjögren's syndrome, bacterial adhesion, glycoproteins, CXCL13, Histatin 5, salivary proteome
Introduction
This article explores some important interactions between salivary components and the oral microbiota and assesses their potential therapeutic implications. Recent research on salivary gland dysfunction, including new information on immunopathogenesis and approaches to treatment and diagnosis, is also presented.
The present stage of knowledge about bacterial interactions with salivary proteins is reviewed in the following. The initial mechanisms of bacterial adhesion in the oral cavity are of interest because they provide a basis for the subsequent stepwise formation of multi-species oral biofilms, which have the potential to eventually lead to the onset of dental caries or periodontal disease. A further example of interaction between saliva and micro-organisms, also addressed in this paper, is the antifungal activity of the salivary protein Histatin 5. The natural fungicidal effects of this molecule have been harnessed and amplified by coupling with a carrier molecule to create a potential new agent for the treatment of oral candidiasis.
Salivary gland dysfunction may occur as a result of developmental or immune disorders, or as an unwelcome outcome of cancer therapy. Recent studies have identified new avenues of research that may facilitate the development of novel treatments to restore salivary function, including the use of resolvins and the development of differentiated acinar structures. In addition, fresh data are emerging about the pathogenic mechanisms underlying Sjögren's syndrome, an autoimmune disorder affecting the salivary and lacrimal glands. These insights may lead to the identification of disease biomarkers to aid in the diagnosis of Sjögren's syndrome.
The Salivary Proteome-Oral Microbiome Interface
The commensal microbiota of the oral cavity have evolved to adapt to this unique environment in part through their interaction with salivary molecules. Besides scavenging salivary components as metabolic substrates, some oral bacteria are able to attach to the exposed mineralized surfaces of teeth. Prerequisite in many cases is that oral surfaces are covered by a layer of adsorbed salivary proteins, the so-called salivary pellicle. This process allows certain commensal bacteria to colonize these surfaces and thus provides them with a selective advantage for survival in the fluid environment of the mouth (Gibbons, 1989).
The binding of salivary proteins by oral commensal bacteria has been most thoroughly studied with oral strains of actinomyces and viridans streptococci. These bacteria carry fimbrial adhesins on their surfaces that bind to peptide or glycan motifs on salivary proteins and glycoproteins (Takahashi et al., 2002; Mishra et al., 2011; Pyburn et al., 2011). Less is known about the corresponding receptors in saliva to which these adhesins preferentially bind, but several different receptors and the corresponding bacterial adhesin activities have been identified (Ruhl et al., 2004; Takamatsu et al., 2006). One investigative technique used was an adaptation of the original bacterial overlay method (Prakobphol et al., 1987), modified for the use of biotin- or fluorescence-labeled bacteria and with adhesin-deficient bacterial mutants as probes (Ruhl et al., 2004; Walz et al., 2005). Major salivary receptors were found to be salivary mucin-7 and secretory immunoglobulin A1, and their respective recognition by actinomyces and streptococci was shown to be dependent on the absence or presence of terminal sialic acid residues on their glycan side-chains (Ruhl et al., 2004; Walz et al., 2005).
These findings prompted the question of whether pathogenic bacteria that transiently or permanently colonize the oral cavity also bind to salivary proteins. The stomach pathogen Helicobacter pylori has been shown to colonize the oral cavity, and it was hypothesized that the mouth could serve as a reservoir for oral-oral transmission or gastric re-infection (Bürgers et al., 2008). To help identify possible counter-receptors in saliva to which H. pylori can bind, a further refinement of the bacterial overlay technique was used (Walz et al., 2009). The binding specificities of wild-type and adhesin-deficient mutants were initially tested by bacterial overlays on arrays containing a number of natural glycoproteins and synthetic neo-glycoproteins with well-defined glycans attached (Walz et al., 2005). Further, two-dimensional proteome maps of whole saliva as well as parotid and submandibular/sublingual glandular secretions, with well-defined strains as probes in bacterial overlays on 2-D transfers, enabled several glycoprotein receptors in saliva to be identified for H. pylori (Walz et al., 2006). By this technique, corresponding bacterial adhesins binding to their respective salivary receptor counterparts were also identified (Walz et al., 2009) (Table 1).
Table 1.
Salivary Counter-receptors Bound by H. pylori
| Bound Counter-receptorsa | Presence of Lewis B or H type 1 Glycansa | Presence of Sialic Acidsa | Corresponding H. pylori Adhesinsa |
|---|---|---|---|
| Mucin MUC5Bb | (+) | + | SabA / BabA |
| Mucin MUC7b | - | + | SabA |
| Salivary agglutinin DMBT1/gp340b | + | + | BabA |
| Proline-rich glycoprotein (PRG) | ? | (+) | BabA |
| Carbonic anhydrase VI | - | + | SabA |
| Parotid secretory protein (PSP) | - | + | SabA |
| Zinc-α2-glycoprotein | - | + | SabA |
| Secretory component | - | + | SabA |
| S-IgA heavy chain | - | + | SabA |
Data are summarized from Walz et al. (2005, 2009>).
Counter-receptors also identified by others (Veerman et al., 1997; Prakobphol et al., 2005).
Another medical pathogen that was recently investigated for its saliva-binding properties is Staphylococcus aureus (Heo et al., 2013). Identical strains of this bacterium were found in the infected lungs and oral cavities of hospitalized patients, suggesting that the oral environment may play a role in the infection or transmission process (Heo et al., 2008). To investigate the binding of salivary proteins to the surface of this bacterium, investigators used an in-suspension bacterial binding assay in which bacteria were exposed to fluorescence-labeled salivary proteins to evaluate binding (Heo et al., 2013). This allowed them to discriminate between truly saliva-derived components and bacterial contaminants in the eluates and also increased the sensitivity of detection in the assay. With this improved assay, a limited number of salivary proteins was identified that bound to the surface of S. aureus (Heo et al., 2013) (Fig. 1). It was also found that the binding of a subset of salivary immunoglobulin-related components to S. aureus was mediated by staphylococcal protein A (SpA), a member of the “surface components recognizing adhesive matrix molecules” (MSCRAMM) family of proteins.
Figure 1.
Salivary proteins in eluates released from the surface of S. aureus strain 8325. Positions and identities of bound proteins are derived from Heo et al. (2013).
What are the possible biological consequences of these different types of adhesive interactions of bacteria with salivary proteins? On one hand, binding to host-surface-associated salivary proteins allows bacteria to attach to surfaces in the oral cavity, thus favoring their colonization and survival. This mechanism is most likely used by commensal oral bacteria. Conversely, binding of salivary proteins to planktonic bacteria in saliva can lead to agglutination of the bacteria and their elimination from the oral cavity. This mechanism is most likely relevant for the clearance of undesirable pathogens. Thus, saliva may act as a gatekeeper at the entrance to both gastrointestinal and respiratory tracts by retaining a beneficial resident microflora in the oral cavity and at the same time eliminating potential pathogens that traverse the oral environment before they can reach their target tissue site and cause disease (Ruhl, 2012). Certain oral bacteria and pathogens bind salivary proteins to their surfaces in the suspended state, the biological consequences of which are less clear. It has been suggested that it could facilitate colonization by bacteria through the establishment of inter-bacterial adhesive interactions. It may also help bacteria to achieve a selective advantage by improving access to salivary compounds as metabolic substrates (Kolenbrander, 2011). Finally, it could also be speculated that bacteria, such as the pathogen S. aureus, decorate themselves with salivary proteins as a method of molecular mimicry to evade recognition by the host's immune system (Ruhl, 2012; Heo et al., 2013).
In the future, as the mechanisms of bacterial interactions with salivary proteins are unraveled, it may become possible to interfere with the bacterial adhesion process in the oral cavity in a selective manner by blocking the interaction of pathogenic bacteria with specifically designed inhibitors of salivary receptor-adhesin interactions.
Histatin 5 Uptake Into C. Albicans is Essential for Fungicidal Activity and Efficacy as a Topical Therapeutic for Oral Candidiasis
Fungal pathogens are a worldwide threat to human health. Candida albicans, which is normally a commensal resident of the oral cavity, can cause oropharyngeal candidiasis (OPC) in individuals whose immune system is compromised by AIDS, chemotherapy, or the use of immunosuppressant drugs. Although our understanding about factors that tip the balance from com-mensalism to virulence is incomplete, disruption of normal bacterial flora or host epithelial barriers (such as burns, catheters, or ventilation tubes) are predisposing circumstances for OPC and deep invasion to organs or blood by C. albicans.
The pipeline for new antifungal drugs for the treatment of OPC has been nearly empty for several years, as reflected by the dearth of new antifungal drugs or therapeutic innovations. It is generally agreed that new drugs should be rapidly fungicidal rather than fungistatic, and their use in new combination regimens is needed to improve clinical outcomes (Revanker and Sobel, 2002). Naturally occurring host peptides meet these criteria as a novel class of antimicrobial agents.
Salivary histatins (Hsts) are a family of related proteins whose primary function is inhibition of the growth and viability of C. albicans (Xu et al., 1991). Hsts are histidine-rich cationic peptides secreted by human parotid and submandibular-sublin-gual salivary glands (Oppenheim et al., 1998) with high antifungal activity (MIC50 ≈ 10 μM) and little microbicidal activity against oral bacteria (Groenink et al., 2003). Among at least 50 Hst peptides derived from post-translational proteolytic processing, Hst 5 (24 amino acids) and its 12-amino-acid subunit Hst 54-15 (AKRHHGYKRKFH) have the most potent fungicidal activity against C. albicans in vitro (Rothstein et al., 2001).
A major barrier to the use of Hst 5 as a topical drug for OPC is that Hsts exhibit only 10-15% of their in vitro fungicidal activity when tested in whole saliva. For example, while some individuals had salivary fungicidal activity that positively correlated with the quantity of Hsts, several others had very high concentrations of Hsts but with low salivary microbial killing activity (Conti et al., 2011). Two major processes are likely to account for these differences. First is the dynamic turnover of salivary proteins, which balances secretion with proteolytic degradation. Second, Hst binding to the microbial surface may be “masked” by interaction with salts and perhaps with various salivary metals, including zinc and copper (Melino et al., 1999; Grogan et al., 2001; Helmerhorst et al., 2004; Porciatti et al., 2010).
Unlike other antimicrobial peptides in the human innate defense arsenal, e.g., defensins, which insert into the membrane for killing activity (Fig. 2), native Hst 5 lacks amphiphilicity, precluding its entry into microbial membranes. Hst 5 has a unique mechanism of action that requires binding to C. albicans cell wall components, including Ssa1 proteins (Li et al., 2003, 2006) and β-glucans (Jang et al., 2010), for its stabilization prior to uptake. However, binding alone does not cause cell death, since fungicidal activity occurred only after the C. albicans cytosol was reached. Hst 5 translocation requires a membrane proton electrochemical gradient; disruption of the gradient by sodium azide (NaN3) prevented Hst 5 uptake, although it remained bound to the cell wall. Fungal uptake of Hst 5 was not due to endocytosis, since endocytotic mutants or pre-treatment with endocytosis inhibitors did not alter Hst 5 activity (Jang et al., 2010). Instead, Hst 5 is transported into the cell via C. albicans polyamine (spermidine) transporters Dur3 and Dur31 (Kumar et al., 2011). Lack of intracellular transport is the basis for Hst 5 resistance in Candida glabrata; expression of C. albicans Dur3 and Dur31 within C. glabrata resulted in both higher killing and uptake of Hst 5 (Tati et al., 2013), underscoring the key role of these transporters for Hst 5 translocation.
Figure 2.
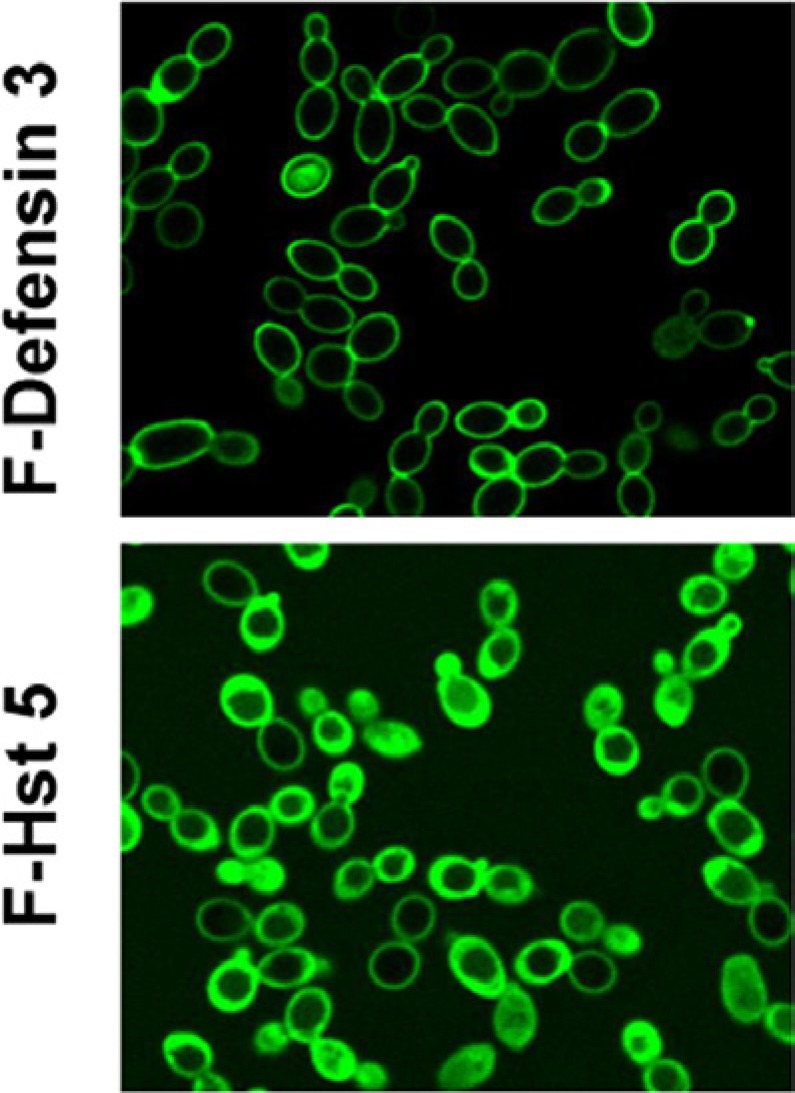
Localization of fluorescein isothiocyanate (FITC)–labeled peptides in C. albicans.
Based upon these findings, a conjugate peptide was constructed with spermidine (Spd) linked to Hst 54-15. Hst 54-15-Spd was significantly more effective in killing both planktonic cells and biofilms of C. albicans and retained high fungicidal activity in both serum and saliva that was not dependent on protease inhibitors. Hst 54-15-Spd had very high efficacy in clearing OPC by topical application to tongues of immunosuppressed mice (Fig. 3), while sparing oral Streptococcal commensal bacteria and lacking hemolytic activity. Thus, we found a conjugate bio-peptide that appears to be protease-resistant and specific to fungi over oral Streptococcal sp. Hst 54-15-Spd conjugates are a new class of peptide-based drugs with high selectivity for C. albicans and potential as topical therapeutic agents for oral candi-diasis. Although further clinical studies with Hst 54-15-Spd conjugate peptides in humans are required, histatin-polyamine conjugates show promise as potent anti-fungal drugs.
Figure 3.

Topical treatment of candidiasis. (A) Clinical appearance of fungal lesions on animal tongues is shown on day 3 and day 5 postinfection with C. albicans. Tongues treated with SHAM control have abundant white patches that coalesce into a solid white layer by day 5. Tongues of animals treated with Hst 5 had fewer white lesions, while topical application of Hst 54-15-Spd resulted in nearly complete clearance of fungal lesions by day 5. (B) Microscopic imaging (10X) of tongue tissues stained with Periodic Acid Schiff shows extensive colonization by C. albicans on the dorsal epithelium of the tongue, inflammatory cell infiltration, as well as loss of epithelial architecture with SHAM treatment. Box shows typical fungal hyphal invasion of the superficial epithelium. Hst 5-treated mouse tongues had much lower fungal mass on the dorsum surface of the tongue (magnified box and arrowhead show reduced thickness of colonized candidal cells and lack of invasion). Hst 54-15-Spd-treated tongue tissues in mice did not have visible Candida cells and showed normal lingual papillae.
New Approaches to the Treatment of Salivary Gland Dysfunction
Differentiation is a highly regulated process by which a less specialized cell becomes a more specialized cell type. Differentiation dramatically changes a cell's size, shape, and function. In the case of salivary glands, they must be anatomically positioned and appropriately sized. In addition, salivary glands must be close to nerves, blood, and lymphatic vessels, as well as bone and muscle. For instance, acetylcholine (a major salivary secretion agonist) should be able to reach the basolateral side of the salivary glands to induce increases in intracel-lular calcium. This triggers the opening of chloride channels to create a transepithelial gradient that drives sodium and water to the lumen of the acinus, leading to the formation of primary saliva (Melvin and Turner, 1992; Melvin et al., 2005). Salivary gland tight junctions provide a barrier that allows for unidirectional fluid secretion (Nelson et al., 2013). These tight junctions are composed of three major transmembrane proteins – JAMS, claudins, and occludin – which are linked to the cytoskeleton by the adaptor protein ZO-1 (Nelson et al., 2013).
There are several causes of salivary hyposecretion in humans, such as Sjögren's syndrome (SS) (Mathews et al., 2008), ecto-dermal dysplasia (ED) (Singh and Warnakulasuriya, 2004), and the side effects of radiation therapy (Redman, 2008). What all these examples have in common is the loss of size, shape, and function of the salivary glands. For instance, patients with SS might display a persistent enlargement of the parotid gland, accompanied by lymphocytic infiltration, which destroys the branched salivary glands, and loss of saliva secretion (Mathews et al., 2008). Patients with ED, a developmental disorder, might have salivary gland hypoplasia and aplasia that is associated with secretory dysfunction (Singh and Warnakulasuriya, 2004). Finally, patients with head-and-neck cancer who have undergone irradiation display salivary acinar atrophy, which is associated with the presence of fibrotic tissue in the gland and loss of salivary gland function (Redman, 2008).
Despite recent improvements in the treatment of xerostomia, there has been little scientific advancement toward a clinically useful approach to the restoration of compromised salivary gland function. Attempts have been made to advance restorative treatments (via the development of an artificial salivary gland in a variety of extracellular matrices). Nonetheless, such restorative treatment models have proved to be inadequate due to poor differentiation and specification of the mechanisms underlying secretion. Here we present two new approaches for the treatment of salivary gland dysfunction: (a) the use of resolvins and (b) the creation of differentiated acinar structures.
(a) Use of Resolvins
Several studies have demonstrated that human and animal cells convert omega-3 polyunsaturated fatty acids into resolvins (Rvs), which are highly potent, short-lived, anti-inflammatory agents that control the duration and magnitude of inflammation in models of complex diseases (Serhan et al., 2002). Resolvin D1 (RvD1) is produced in resolving exudates in vivo and is a product of transcellular biosynthesis with human leukocytes and endothe-lial or epithelial cells. Aspirin, an anti-inflammatory drug that acetylates cyclooxygenase-2 (COX-2) and changes COX-2 function from a cyclooxygenase to a lipoxygenase, generates the aspirin-triggered (AT) form AT-RvD1, which appears to be more biologically stable than RvD1 (Sun et al., 2007). RvD1's potent anti-inflammatory actions in many tissues prompted the investigation of its impact on salivary epithelial integrity under physiological and inflammatory conditions in the polarized rat parotid cell line Par-C10 (Odusanwo et al., 2012).
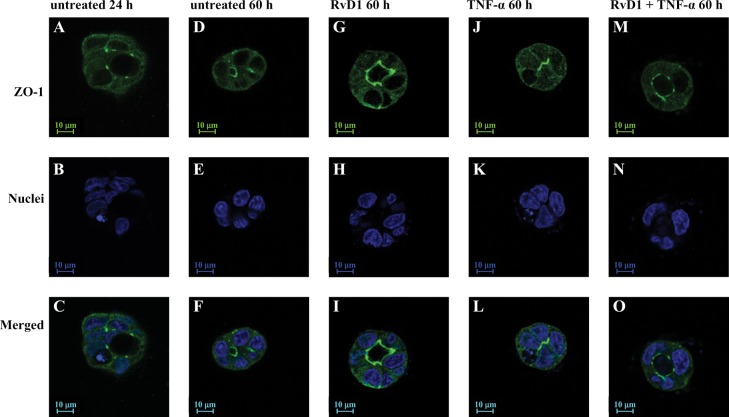
These studies demonstrated that RvD1 receptor (ALXR/ FPR2) activation enhances epithelial integrity in salivary glands (Odusanwo et al., 2012). RvD1 signaling is initiated by its binding to the high-affinity G protein-coupled receptor ALX/FPR2. The ALX/FPR2 receptor signals via modulation of the phospha-tidylinositol 3-kinase (PI3K)/Akt signaling pathways (Odusanwo et al., 2012). RvD1 not only blocks inflammatory responses caused by the pro-inflammatory cytokine TNFα but also is able to restore and enhance tissue architecture in salivary cells (Fig. 4). Activation of ALXR with RvD1 regulates signaling pathways that mediate cell proliferation and migration and the maintenance of tight junctions (TJ) required for normal saliva secretion, suggesting a new pro-resolving role for RvD1 in salivary epithelium (Odusanwo et al., 2012).
Figure 4.
RvD1 treatment enhances acinar formation in Par-C10 cells grown on GFR-Matrigel. Par-C10 cells grown on GFR-Matrigel in 8 well chambers as described in “Materials and Methods” and incubated in the absence (A-C; 24 h and D-F; 60 h) or presence of RvD1 (100 ng/ ml; G-I; 60 h), TNF- (10 ng/ml; J-l; 60 h), or RvD1 and TNF- (100 ng/ml; M-O; 60 h) added at plating. Acinar spheres were subjected to immunofluorescence using goat anti-rabbit anti-ZO-1 (A, D, G, J, and M; green) followed by Hoechst nuclear stain (B, E, h, K, and N; blue). XY images were obtained and analyzed using a Carl Zeiss 510 confocal microscope. Image taken from Odusanwo et al., 2012.
(b) Creation of Differentiated Acinar Structures
Salivary gland cell differentiation has presented a recurring challenge for researchers, since primary salivary cells show a loss of phenotype in culture. Particularly, parotid cells show a marked decrease in amylase expression, and loss of tight junction organization and proper cell function. More recently, a 3D cell culture model was developed to support parotid gland cell differentiation with a combination of fibrin hydrogel (FH) and growth-factor-reduced Matrigel (GFR-MG) (McCall et al., 2013). Furthermore, FH polymerized with a combination of EGF and IGF-1 induced formation of 3D spheroids capable of amylase expression and an agonist-induced increase in the intra-cellular Ca2+ concentration ([Ca2+]i) in salivary cells. These studies represent an initial step toward the construction of an artificial salivary gland to restore salivary gland dysfunction.
Immunopathogenesis of Sjögren's Syndrome
Sjögren's syndrome (SS) is an autoimmune disease that primarily affects salivary and lacrimal tissue. Like many autoimmune conditions, SS has a strong female predilection. SS occurs in two forms: primary (pSS) and secondary (sSS). In pSS, patients exhibit xerostomia and xerophthalmia in isolation, in the absence of other autoimmune dyscrasias, although additional serious systemic disease manifestations may be seen (Seror et al., 2010). The secondary form occurs with other autoimmune connective tissue disorders, most commonly rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Diagnosis of SS is challenging (Vitali et al., 2002), and there are no treatments for SS that target disease etiology. Therefore, it is critical to identify biomarkers that address mechanisms and may aid in the diagnosis and treatment of this debilitating disease.
B-cells Contribute to SS Pathogenesis
Historically, T-cells were thought to drive SS pathogenesis, but many studies in recent years have demonstrated an important role for B-cells as well (Cornec et al., 2012). Notably, B-cells secrete pro-inflammatory cytokines and produce antibodies that contribute to inflammation. Moreover, B-cells are able to influence the differentiation of T-cell subsets, thereby shaping and directing immune responses.
In SS, B-cells are clearly dysregulated. B-cells produce auto-antibodies, including anti-Ro (SSA) and anti-La (SSB), that are included as part of the diagnostic algorithm for SS (Vitali et al., 2002). In addition, many other autoantibodies are detected in SS patients, such as anti-nuclear antibodies and rheumatoid factor (Hansen et al., 2007). Interestingly, most autoantibodies in SS patients are directed against ubiquitous autoantigens, indicating that SS is a systemic disease with immune pathology that is not limited to salivary tissue. The systemic nature of B-cell dysfunction is also evidenced by the high incidence of B-cell malignancies in SS patients. While some of these lymphomas are reported in salivary tissue, many are located in extra-glandular sites, such as the stomach and the lung (Mariette, 1999). Analysis of these data, taken together, suggests that systemic disturbances in B-cells in SS contribute to disease pathogenesis.
CXCL13 Directs B-cell chemotaxis and Is Elevated in Autoimmunity
For B-cells to function most efficiently, they must be recruited to specific sites where they interact with T-cells and secrete cytokines and chemokines to orchestrate effective immune responses. CXCL13 is a potent B-cell chemokine that is secreted by follicular stromal cells, antigen-experienced T-cells, and follicular Th cells (Cyster et al., 2000; Finch et al., 2013). Importantly, overexpression of Cxcl13 (the mouse protein) causes formation of ectopic lymphoid tissue (Luther et al., 2000). Thus, under physiologic conditions, CXCL13 mediates migration of B-cells to peripheral lymphoid organs to ensure effective B-cell interactions.
Aside from its physiologic role as a homeostatic chemokine, CXCL13 is elevated in numerous autoimmune diseases, including RA and SLE (Rioja et al., 2008; Lee et al., 2010). Significantly, neutralization of Cxcl13 reduces disease severity in a mouse model of arthritis (Zheng et al., 2005). Thus, the presence of CXCL13 in autoimmune disease is well documented, and its neutralization ameliorates pathology.
CXCL13 Is Elevated Locally and Systemically in SS
CXCL13 recruits B-cells to ectopic locations. Notably, several studies have demonstrated elevated CXCL13 in the salivary glands of patients afflicted with SS (Amft et al., 2001; Salomonsson et al., 2002, 2003; Barone et al., 2005, 2008; Hjelmervik et al., 2005) (Table 2). Moreover, B-cell frequency in minor salivary glands of SS patients increases with disease severity (Christodoulou et al., 2010). Analysis of these data, together, implicates CXCL13 in the ectopic recruitment of B-cells to salivary tissue and the pathogenesis of SS.
Table 2.
CXCL13 is Elevated in Human Primary Sjögren's Syndrome Salivary Tissue
| Reference | Tissue | Methods | Source of CXCL13 |
|---|---|---|---|
| Barone et al., 2008 | MSG | IHC | CD20+ IgD+ B-cells and CD 21+ |
| qPCR | follicular dendritic cell-rich areas | ||
| Barone et al., 2005 | MSG | IHC | Periductal B-cell infiltrates, non-infiltrated ducts |
| Salomonsson et al., 2003 | MSG | IHC | Acini and ductal epithelium |
| Hjelmervik et al., 2005 | MSG | DNA | Whole MSG |
| microarray | |||
| Salomonsson et al., 2002 | MSG | IHC | Epithelial cells in acini and ducts |
| ISH | |||
| Amft et al., 2001 | MSG | IHC | Lymphoid aggregates, endothelium |
| IF |
MSG, minor salivary glands; IHC, immunohistochemistry; qPCR, quantitative PCR; ISH, in situ hybridization; IF, immunofluorescence.
While the presence of CXCL13 in SS disease is well documented, the role of this chemokine in disease progression remains unclear. Importantly, research demonstrates that Cxcl13 is elevated both locally and systemically in pSS and sSS models with advanced disease. Moreover, expression of Cxcl13 and its receptor Cxcr5 increases with disease progression within salivary gland tissue in a SS mouse model (NOD/ShiLtJ), suggesting that Cxcl13 may recruit Cxcr5-expressing lymphocytes to salivary tissue. Interestingly, the murine gene Cxcl13 is elevated in salivary tissue several weeks prior to disease onset in this model, indicating that its expression may be an early event in SS pathogenesis. Importantly, macrophages are the most significant source of Cxcl13 in SS salivary tissue. Studies in humans corroborate these findings, since CXCL13 is elevated in both sera and saliva of SS patients (Kramer et al., 2013). Analysis of these data, taken together, suggests that CXCL13 contributes to the pathogenesis of SS disease, and may be useful in the diagnosis of SS or as a potential therapeutic target.
Conclusion
Findings from studies of the salivary proteome-oral microbiome interface support the possible role of saliva as a gatekeeper for microbes at the entrance to the gastrointestinal and respiratory tracts (Ruhl, 2012). It is fortunate that, currently, both the salivary proteome and the oral microbiome are nearing completion. With such databases in hand, it will become possible to investigate interactions between salivary components and microbes in the human oral cavity under a new light. The ultimate hope is to find a mechanism for therapeutically blocking unwanted bacterial adhesion in the oral cavity.
Hst 54-15-Spd shows promise as a new topical therapy for oral candidiasis, with encouraging results in immunocompromised mouse models. Further research is required to ascertain its potential applicability in the clinical setting.
Two novel approaches to the treatment of salivary gland dysfunction have been presented. In vitro studies have shown that resolvin RvD1 may have a role in restoring the structural integrity of disordered salivary tissue, thus restoring normal function. At the same time, work toward the development of artificial salivary gland tissue is advancing.
B-cells have been shown to contribute to Sjögren's syndrome (SS) disease through several well-defined mechanisms. Moreover, CXCL13 mediates B-cell movement and is dysregu-lated in SS. Laboratory studies demonstrate that Cxcl13 increases with disease progression both locally and systemically and thus may represent a novel biomarker or a therapeutic target in the management and treatment of SS.
Acknowledgements
The authors acknowledge the following financial support: National Institute of Dental and Craniofacial Research (NIDCR) grant 5R01 DE019807-02 (SR); NIDCR grants R21-DE19721-01A1, 1R01DE022971-01 and 1R01DE021697-01A1 (OB); a Mentored Clinical Scientist Research Career Development Award (K08 DE020882) from the NIDCR and a subproject award from grant M01 RR018535 from the U.S. National Institutes of Health (NIH), National Center for Research Resources (JMK); NIDCR NIH grant R01DE010641 (ME). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, et al. (2001). Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum 44:2633–2641. [DOI] [PubMed] [Google Scholar]
- Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, et al. (2005). Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren's syndrome. Arthritis Rheum 52: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. (2008). CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol 180: 5130–5140. [DOI] [PubMed] [Google Scholar]
- Bürgers R, Schneider-Brachert W, Reischl U, Behr A, Hiller KA, Lehn N, et al. (2008). Helicobacter pylori in human oral cavity and stomach. Eur J Oral Sci 116: 297–304. [DOI] [PubMed] [Google Scholar]
- Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. (2010). Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun 34: 400–407. [DOI] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, et al. (2011). New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol 4: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornec D, Devauchelle-Pensec V, Tobón GJ, Pers JO, Jousse-Joulin S, Saraux A. (2012). B cells in Sjögren's syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun 39: 161–167. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, et al. (2000). Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev 176: 181–193. [DOI] [PubMed] [Google Scholar]
- Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. (2013). Effects of CXCL13 on the inhibition of lymphoid follicles in models of autoimmune disease. Eur J Clin Invest 43: 501–509. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ. (1989). Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res 68: 750–760. [DOI] [PubMed] [Google Scholar]
- Groenink J, Ruissen AL, Lowies D, van ‘t Hof W, Veerman EC, Nieuw Amerongen AV. (2003). Degradation of antimicrobial histatin-variant peptides in Staphylococcus aureus and Streptococcus mutans. J Dent Res 82: 753–757. [DOI] [PubMed] [Google Scholar]
- Grogan J, McKnight CJ, Troxler RF, Oppenheim FG. (2001). Zinc and copper bind to unique sites of histatin 5. FEBS Lett 491: 76–80. [DOI] [PubMed] [Google Scholar]
- Hansen A, Lipsky PE, Dörner T. (2007). B cells in Sjögren's syndrome: indications for disturbed selection and differentiation in ectopic lym-phoid tissue. Arthritis Res Ther 9: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst EJ, Flora B, Troxler RF, Oppenheim FG. (2004). Dialysis unmasks the fungicidal properties of glandular salivary secretions. Infect Immun 72: 2703–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SM, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. (2008). Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis 47: 1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SM, Choi KS, Kazim LA, Reddy MS, Haase EM, Scannapieco FA, et al. (2013). Host defense proteins derived from human saliva bind to Staphylococcus aureus. Infect Immun 81: 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. (2005). Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum 52: 1534–1544. [DOI] [PubMed] [Google Scholar]
- Jang WS, Bajwa JS, Sun JN, Edgerton M. (2010) Salivary histatin 5 inter-nalization by translocation, but not endocytosis, is required for fungi-cidal activity in Candida albicans. Mol Microbiol 77:354–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE. (2011). Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. Int J Oral Sci 3: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Klimatcheva E, Rothstein TL. (2013). CXCL13 is elevated in Sjogren's syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol 94: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chadha S, Saraswat D, Bajwa JS, Li RA, Conti HR, et al. (2011). Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J Biol Chem 286: 43748–43758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Shiao YM, Wu TH, Chen WS, Hsu YH, Tsai SF, et al. (2010). Serum BLC/CXCL13 concentrations and renal expression of CXCL13/ CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol 37: 45–52. [DOI] [PubMed] [Google Scholar]
- Li XS, Reddy MS, Baev D, Edgerton M. (2003). Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem 278: 28553–28561. [DOI] [PubMed] [Google Scholar]
- Li XS, Sun JN, Okamoto-Shibayama K, Edgerton M. (2006). Candida albicans cell wall SSA proteins bind and facilitate import of salivary histatin 5 required for toxicity. J Biol Chem 281: 22453–22463. [DOI] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. (2000). BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-depen-dent lymphoid neogenesis. Immunity 12: 471–481. [DOI] [PubMed] [Google Scholar]
- Mariette X. (1999). Lymphomas in patients with Sjögren's syndrome: review of the literature and physiopathologic hypothesis. Leuk Lymphoma 33: 93–99. [DOI] [PubMed] [Google Scholar]
- Mathews SA, Kurien BT, Scofield RH. (2008). Oral manifestations of Sjogren's syndrome. J Dent Res 87: 308–318. [DOI] [PubMed] [Google Scholar]
- McCall AD, Nelson JW, Leigh NJ, Duffey ME, Lei P, Andreadis ST, et al. (2013). Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng Part A 19: 2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino S, Rufini S, Sette M, Morero R, Grottesi A, Paci M, et al. (1999). Zn(2+) ions selectively induce antimicrobial salivary peptide histatin-5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry 38: 9626–9633. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Turner RJ. (1992). Cl- fluxes related to fluid secretion by the rat parotid: involvement of Cl(-)-HCO3- exchange. Am J Physiol 262(2 Pt 1): G393–G398. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. (2005). Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469. [DOI] [PubMed] [Google Scholar]
- Mishra A, Devarajan B, Reardon ME, Dwivedi P, Krishnan V, Cisar JO, et al. (2011). Two autonomous structural modules in the fimbrial shaft adhesin FimA mediate Actinomyces interactions with streptococci and host cells during oral biofilm development. Mol Microbiol 81: 1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, Manzella K, Baker O. (2013). Current cell models for bioengineering a salivary gland: a mini-review of emerging technologies. Oral Dis 19: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. (2012). Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol 302: C1331–C1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, et al. (1988) Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungi-static effects on Candida albicans. J Biol Chem 263:7472–7477. [PubMed] [Google Scholar]
- Porciatti E, Milenkovic M, Gaggelli E, Valensin G, Kozlowski H, Kamysz W, et al. , (2010). Structural characterization and antimicrobial activity of the Zn(II) complex with P113 (demegen), a derivative of histatin 5. Inorg Chem 49: 8690–8698. [DOI] [PubMed] [Google Scholar]
- Prakobphol A, Murray PA, Fisher SJ. (1987). Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem 164: 5–11. [DOI] [PubMed] [Google Scholar]
- Prakobphol A, Borén T, Ma W, Zhixiang P, Fisher SJ. (2005). Highly glycosyl-ated human salivary molecules present oligosaccharides that mediate adhesion of leukocytes and Helicobacter pylori. Biochemistry 44: 2216–2224. [DOI] [PubMed] [Google Scholar]
- Pyburn TM, Bensing BA, Xiong YQ, Melancon BJ, Tomasiak TM, Ward NJ, et al. (2011). A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog 7: e1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS. (2008). On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem 83: 103–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revanker SG, Sobel JD. (2002). Mucosal candidiasis. In: Candida and can-didiasis. 2nd ed. Calderone RA, editor. Washington, DC: ASM Press, pp. 419–427. [Google Scholar]
- Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, et al. (2008). Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum 58: 2257–2267. [DOI] [PubMed] [Google Scholar]
- Rothstein DM, Spacciapoli P, Tran LT, Xu T, Roberts FD, Dalla Serra M, et al. (2001). Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother 45: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S. (2012). The scientific exploration of saliva in the post-proteomic era: from database back to basic function. Expert Rev Proteomics 9: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Sandberg AL, Cisar JO. (2004). Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J Dent Res 83: 505–510. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelmström P, Wahren-Herlenius M. (2002). Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren's syndrome. Scand J Immunol 55: 336–342. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, et al. (2003). Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum 48: 3187–3201. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. (2002). Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. (2010). EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 69: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Warnakulasuriya S. (2004). Aplasia of submandibular salivary glands associated with ectodermal dysplasia. J Oral Pathol Med 33: 634–636. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. (2007). Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem 282: 9323–9334. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Konishi K, Cisar JO, Yoshikawa M. (2002). Identification and characterization of Hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect Immun 70: 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. (2006). Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect Immun 74: 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tati S, Jang WS, Li R, Kumar R, Puri S, Edgerton M. (2013). Histatin 5 resistance of Candida glabrata can be reversed by insertion of Candida albicans polyamine transporter-encoding genes DUR3 and DUR31. PLoS One 8: e61480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman EC, Bank CM, Namavar F, Appelmelk BJ, Bolscher JG, Nieuw Amerongen AV. (1997). Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology 7: 737–743. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjögren's syndrome (2002). Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Odenbreit S, Mahdavi J, Borén T, Ruhl S. (2005). Identification and characterization of binding properties of Helicobacter pylori by glyco-conjugate arrays. Glycobiology 15: 700–708. [DOI] [PubMed] [Google Scholar]
- Walz A, Stühler K, Wattenberg A, Hawranke E, Meyer HE, Schmalz G, et al. (2006). Proteome analysis of glandular parotid and submandibu-lar-sublingual saliva in comparison to whole human saliva by two-dimensional gel electrophoresis. Proteomics 6: 1631–1639. [DOI] [PubMed] [Google Scholar]
- Walz A, Odenbreit S, Stühler K, Wattenberg A, Meyer HE, Mahdavi J, et al. (2009). Identification of glycoprotein receptors within the human salivary proteome for the lectin-like BabA and SabA adhesins of Helicobacter pylori by fluorescence-based 2-D bacterial overlay. Proteomics 9: 1582–1592. [DOI] [PubMed] [Google Scholar]
- Xu T, Levitz SM, Diamond RD, Oppenheim FG. (1991). Anticandidal activity of major human salivary histatins. Infect Immun 59: 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ozen Z, Zhang X, De Silva S, Marinova E, Guo L, et al. (2005). CXCL13 neutralization reduces the severity of collagen-induced arthritis. Arthritis Rheum 52: 620–626. [DOI] [PubMed] [Google Scholar]