Abstract
Objective:
To report treatment outcomes of stereotactic ablative radiation therapy (SABR) for non-spinal bone metastases in a single institution, and to compare assessments of Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 and the University of Texas MD Anderson Cancer Center (MDA) criteria.
Methods:
From July 2011 to January 2017, 33 patients with 38 non-spinal bone metastatic lesions were treated using SABR. Treatment intent was categorized as follows: single metastasis or oligo-metastases; oligo-progression; and dominant areas of progression. Tumor responses were evaluated according to the RECIST and MDA criteria. Local control (LC) was defined as lesions that were not classified as progressive disease on both criteria.
Results:
The median follow-up period was 10.4 months (range, 2.5–47.4). Both 1- and 2 year LC rates were 94.2 %. The median overall survival (OS) was 20.2 months, and the median progression-free survival (PFS) was 6.9 months. Treatment intent was a significant factor for OS in multivariate analysis. The 1 year OS rates for single metastasis or oligo-metastasis, for oligo-progression, and for dominant areas of progression were 84.2%, 66.7%, and 0.0%, respectively ( p < 0.001). Overall response rate was 86.8 % according to MDA criteria, and 75.7 % according to RECIST criteria. When using MDA criteria, there appeared to be significant associations both between response and PFS (median 7.6 months for responders vs 2.5 months for non-responders; p = 0.036) and between response and OS. In contrast, when using RECIST criteria, the associations were significant neither between response and PFS (median 5.8 months for responders vs 9.3 months for non-responders; p = 0.522) nor between response and OS (25.7 months for responders vs 18.5 months for non-responders; p = 0.811).
Conclusion:
SABR for non-spinal bone metastases demonstrated high LC rates with acceptable toxicity. The MDA criteria demonstrated advantages in predicting survival outcomes.
Advances in knowledge:
SABR for non-spinal bone metastases is a promising treatment option to achieve good local control. The MDA criteria, which is a newly proposed response evaluation criteria for bone metastases, has advantages in predicting survival outcomes compared to other established criteria.
Introduction
Bone metastasis occurs in up to 70% of cancer patients.1 Palliative radiotherapy has been the mainstay of treatment for painful bone metastases. In cases in which effective systemic therapy prolongs patient survival, high local control is imperative for maintaining quality of life. Recent advances in radiation technology, such as stereotactic ablative radiation therapy (SABR), enable the delivery of high conformal radiation dose to the target, and has been widely adopted for spinal metastases. Local control rates for SABR in spinal metastasis have been reported to be approximately 90%.2
However, the literature pertaining to SABR with regard to non-spinal bone metastatic disease is sparse. The goal of treatment for bone metastasis is diverse, and is dependent on disease state and tumor burden. To compare treatment outcomes, indications for SABR in non-spinal bone metastasis should be clearly defined. Single or oligo-metastasis refers to the state between locoregional and widespread metastatic disease, in which metastases are limited in number and location, whereas oligo-progression refers to a scenario in which cancer progression occurs in a limited number of tumors while the majority of other metastases are responding to or are stable while on a systemic therapy strategy.
Second, there is no consensus regarding the end point of SABR for bone metastasis.3 In conventional radiation settings, an international consensus on bone metastases has been developed for clinical end points based on pain scores and analgesic consumption.4 However, SABR is indicated in patients with single metastasis, or oligo-metastatic, oligo-progressive or traditionally radio-resistant disease, who often present with minimal or no associated symptoms. Assessing response to treatment is, therefore, difficult and must rely on evaluation of changes in the tumor itself and any related symptoms.
Third, there are several criteria which define tumor response by imaging modalities, but no consensus has been reached as to the optimal assessment system for bone metastasis.3 Currently, the Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 5 are the most commonly used method for objectively evaluating tumor response, however, they only includes measurable soft tissue components as target lesions, and the size change of the measurable lesions is the only consideration in evaluating the response. The University of Texas MD Anderson Cancer Center (MDA, Houston, TX, USA) developed bone-specific response criteria in 2004, which include qualitative changes as well as measurable changes in response evaluation.6
The objectives of the present study were to report treatment outcomes of SABR for non-spinal bone metastases in a single institution and to compare assessments using the RECIST v. 1.1 and the MDA criteria in evaluating tumor response for non-spinal bone metastases after SABR. We analyzed treatment response using the MDA and RECIST response criteria, and survival differences according to treatment indications for SABR in patients with non-spinal bone metastasis.
methods and materials
Population
From July 2011 to January 2017, 33 patents with non-spinal bone metastatic disease were treated using SABR. This study was approved by the Institutional Review Board of our institute (IRB no. D-1903-029-002). Medical information, including patient age, sex, tumor histology and treatment intent, was retrospectively collected. Treatment intent was categorized as follows: single metastasis or oligo-metastases, for which the goal was to irradiate all sites of disease; oligo-progression, for which the goal was to irradiate only tumors that were progressing while systemic therapy was controlling all other disease sites; and dominant areas of progression, for which the goal was to irradiate dominant tumors usually for palliation.7 Patients with ≤5 sites of metastases were categorized as oligo-metastatic, and oligo-progressive if ≤5 sites of metastases progressed while other sites including the primary disease remained stable.
Treatment
Simulation CT, with a slice thickness of 1.25 mm, was performed. Four-dimensional CT (4DCT) was used for lesions of the chest wall and sternum to calibrate respiratory motion. The gross target volume (GTV) was defined as radiologically evident disease based on CT and/or MRI results. The clinical target volume (CTV) was generated (usually GTV plus a 1 to –2 cm margin), and the internal target volume (ITV) was generated if 4DCT was used. The planning target volume (PTV) included an additional margin of 1– to 3 mm according to the target site. SABR plans were generated using Oncentra 4.3 (Eleckta) and Monaco 5.1 (Eleckta) for Linac SABR and Multiplan 3.5.1 (Accuray) for Cyberknife treatment. SABR was delivered via dynamic conformal arcs or multiple non-coplanar static beams using 6 to 15 MV of photons.
Dynamic conformal arcs therapy was delivered with 180–360° depending on the anatomic location of the tumor. 95% of the prescription dose covered 100% of the PTV. Maximum dose did not exceed 110% of the prescribed dose. In Cyberknife treatment, one or two collimator size of 7.5–15 mm was used depending of the size of the PTV. Maximum dose usually defined as 125% of the prescription dose and 80% of the maximum dose covered 100% of the PTV. Various prescription dose and fractionation was used. Because there is no established dose/fractionation in SABR of non-spine bone metastasis, various radiation doses used in previously published studies of spine, lung and liver SABR according to the size and location of the lesion were used. Fraction did not exceed five times. Daily image guidance by con-beam CT imaging or orthogonal X-rays verified the target position before each treatment delivery. SABR was administered either in a single fraction or 3- to 5-fraction course to be completed in no more than 2 weeks, but typically within 1 week.
Evaluation and follow up
Treatment responses were evaluated using ≥1 imaging modalities at 2–3 months after the beginning of SABR and other various time points. A response was assessed in each patient based on two sets of response criteria (i.e. RECIST and MDA criteria, Table 1)3,6 Responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Local control was assessed independently for each bone metastasis and was defined as long as the treated lesion did not exhibit progression according to both response criteria on serial imaging. Overall survival (OS) was defined as the time from the start day of SABR to death or last follow-up. Progression-free survival (PFS) was defined as the time from the start day of radiotherapy to disease progression or last follow-up. Toxicity was recorded according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v. 4.0).
Table 1.
The RECIST and MDA criteria for bone response assessment
| Response category | RECIST 1.1 (5) | MDA (6) |
| Complete response | Disappearance of all target lesions FDG PET can be used |
Complete sclerotic fill-in of lytic lesions Normalization of bone density on XR/CT, signal intensity on MRI, tracer uptake on SS |
| Partial response | At least a 30% decrease in the sum of diameters of target lesions A response of non-PD in non-target lesions |
Development of a sclerotic rim or partial sclerotic fill-in of lytic lesions Decrease in the size of measurable lesions or ill-defined lesions Decrease in tracer uptake on SS (excluding rapid regression a ) |
| Stable disease | No change or between 20% increase or 30% decrease in diameter sum | No change |
| Progressive disease | Increase in target lesion diameter sum by ≥20% ≥5 mm increase in target lesion diameter sum New malignant FDG uptake Unequivocal progression of non-target lesions |
Increase in size of any measurable lesions on XR/CT/MRI Increase in tracer uptake on SS New bone lesion (excluding flare phenomena b ) |
FDG, fludeoxyglucose; MDA, MD Anderson Cancer Center; PET, positron emission tomography;RECIST, the Response Evaluation Criteria in Solid Tumors; SS, skeletal scintigraphy; XR, plain radiography.
Rapid osteolytic progression may show decreased osteoblastic activity, resulting in regression of hot spots on SS. XR or CT can be helpful in detecting progressive osteolysis.
Osteoblastic flare is an interval visualization of lesions with a sclerotic rim or new sclerotic lesions in the setting of other signs of response to treatment.
Statistical analysis
The Kaplan–Meier method was used to analyze whether the use of a particular response classification would distinguish responders and non-responders in terms of PFS or OS and survival differences according to treatment indication using a two-sided log-rank test; p < 0.05 was considered to be statistically significant in all tests. The log-rank test was used for univariate analyses and the Cox proportional hazards regression model was used for multivariate analyses. Variables with associations with OS at p < 0.05 in univariate analysis were retained in the multivariable models. All analyses were performed using SPSS v. 18 (IBM Corporation., Chicago, IL, USA).
Results
Patient and treatment characteristics
The median age of the 33 patients was 59 years (range, 36–75 years) and 20 patients were male. Patient and treatment characteristics are summarized in Table 2. The most common primary cancer was non-small cell lung cancer (n = 12), followed by breast cancer (n = 8), hepatocellular carcinoma (n = 3), and renal cell carcinoma (n = 3). A total of 38 lesions were treated. 25 (66%) lesions were located in the pelvis, followed by the femur (n = 5), and ribs (n = 5). The majority of lesions were lytic bone metastases (n = 25 [66%]). Treatment intent of radiotherapy for 13 lesions was single metastasis, oligo-metastases for 16 lesions, oligo-progression for three lesions, and dominant area of progression for six lesions. Prescribed radiation doses and fractions were: 18 Gy/1 (n = 3); 24–60 Gy/3 (n = 16); 28–48 Gy/4 (n = 15); and 40–50 Gy/5 (n = 4) fractions. The mean and median biologic equivalent dose using an α/β of 10 Gy in the linear quadratic model were 71.8 Gy and 64.8 Gy, respectively (range, 43.2–180 Gy).
Table 2. .
Patient and treatment characteristics
| Characteristics |
Single or oligo-metastasis
(n = 29) |
Oligoprogression
(n = 3) |
Dominant areas of progression
(n = 6) |
| Age (range) (years) | 55 (36–75) | 59 (53–62) | 67 (59–75) |
| Sex | |||
| Male | 13 (54%) | 1 (33%) | 6 (100%) |
| Female | 11 (46%) | 2 (67%) | 0 (0%) |
| Primary cancer | |||
| Non-small cell lung cancer | 8 (33%) | 1 (33%) | 3 (50%) |
| Breast cancer | 6 (25%) | 2 (67%) | 0 (0%) |
| Others a | 10 (42%) | 0 (0%) | 3 (50%) |
| Symptom (pain) | |||
| Yes | 7 (24%) | 3 (100%) | 5 (83%) |
| No | 22 (76%) | 0 (0%) | 1 (17%) |
| Systemic treatment | |||
| Yes | 21 (88%) | 3 (100%) | 3 (50%) |
| No | 3 (13%) | 0 (0%) | 3 (50%) |
| Diagnostic image at diagnosis | |||
| CT | 100 (100%) | 100 (100%) | 100 (100%) |
| PET | 24 (83%) | 2 (67%) | 2 (33%) |
| MRI | 13 (45%) | 1 (33%) | 3 (50%) |
| Bone scintigraphy | 25 (86%) | 3 (100%) | 4 (67%) |
| Diagnostic image at follow-up | |||
| CT | 27 (93%) | 3 (100%) | 5 (83%) |
| PET | 6 (21%) | 0 (0%) | 1 (17%) |
| MRI | 7 (24%) | 0 (0%) | 0 |
| Bone scintigraphy | 23 (79%) | 3 (100%) | 3 (50%) |
| Treated site | |||
| Pelvis | 18 (62%) | 3 (100%) | 4 (67%) |
| Femur | 3 (10%) | 0 (0%) | 2 (67%) |
| Rib | 5 (17%) | 0 (0%) | 0 (0%) |
| Scapular | 1 (3%) | 0 (0%) | 0 (0%) |
| Skull | 1 (3%) | 0 (0%) | 0 (0%) |
| Sternum | 1 (3%) | 0 (0%) | 0 (0%) |
| Metastatic tumor type | |||
| Lytic | 20 (87%) | 0 (0%) | 5 (83%) |
| Sclerotic | 0 (0%) | 0 (0%) | 0 (0%) |
| Mixed | 3 (13%) | 3 (100%) | 1 (17%) |
| Prescription dose (Gy)/fraction | |||
| 18/1 | 26 (90%) | 0 (0%) | 0 (0%) |
| 24-60/3 | 10 (34%) | 1 (33%) | 4 (67%) |
| 28-48/4 | 11 (38%) | 2 (67%) | 2 (33%) |
| 40-50/5 | 5 (17%) | 0 (0%) | 0 (0%) |
| BED10Gy (Gy) | 79.6 (43.2–132) | 47.6 (43.2–47.6) | 43.2 (43.2–47.6) |
MUO, metastasis of unknown origin; PET, positron emission tomography; BED10Gy, biologically equivalent dose using an α/β of 10 Gy in the linear quadratic model.
Others include hepatocellular carcinoma, renal cell carcinoma, small cell lung cancer, rectal cancer, gastric cancer, cervical cancer, Ewing’s sarcoma, Melanoma, and metastasis of unknown origin.
Treatment outcome
23 (60.5%) lesions were asymptomatic before treatment. Among the 15 symptomatic lesions, the clinical response rate was 80.0% (12/15). The median follow-up period was 10.4 months (range, 2.5–47.4 months). The median OS was 20.2 months, and 1- and 2 year OS rates were 66.0 and 44.7%, respectively. Local recurrence was observed in 2 (5.3%) bone lesions. Both 1- and 2 year local control rates were 94.2%. The median PFS was 6.9 months, and 1- and 2 year PFS rates were 34.1 and 17.1%, respectively.
Survival difference according to treatment indication
Clinical factors significantly correlated with OS were symptom (p = 0.01) and treatment intent (p < 0.001) in univariate analyses (Table 3). Dominant areas of progression was confirmed to be inferior to single or oligo-metastasis or oligo-metastasis for OS in the multivariate analysis (Table 3). The 1 year OS rates for single metastasis or oligo-metastasis, for oligo-progression, and for dominant areas of progression were 84.2%, 66.7%, and 0.0%, respectively (p < 0.001) (Figure 1).
Table 3.
Univariate and multivariate analysis for survival
| Factors | MS (95% CI) (months) | P (UVA) | HR (95% CI) | P (MVA) |
| Age | 1.03 (0.97–1.10) | 0.361 | ||
| BED10Gy | 0.99 (0.96–1.02) | 0.521 | ||
| Primary cancer | 0.885 | |||
| Non-small cell lung cancer | 10.9 (0.0–27.2) | |||
| Breast | 41.5 | |||
| Others | 17.8 (9.2–26.5) | |||
| Symptom | 0.010 | 0.204 | ||
| Yes | 8.8 (0.0–20.3) | 1 | ||
| No | Not reached | 0.43 (0.12–1.58) | ||
| Tumor type | 0.269 | |||
| Lytic | 17.8 (11.1–24.6) | |||
| Sclerotic | Not reached | |||
| Mixed | 25.7 (0.0–58.8) | |||
| Treatment intent | <0.001 | 0.030 | ||
| Single or Oligo-metastasis | Not reached | 0.18 (0.04–0.85) | 0.031 | |
| Oligoprogression | 25.7 (0.0–54.2) | 0.10 (0.01–0.68) | 0.018 | |
| Dominant areas of progression | 2.0 (2.0–9.6) | 1 |
MS, median survival; 95% CI, 95% confidence interval; UVA, univariate analysis; HR, hazard ratio; MVA, multivariate analysis; BED10Gy, biologically equivalent dose using an α/β of 10 Gy in the linear quadratic model.
Figure 1.
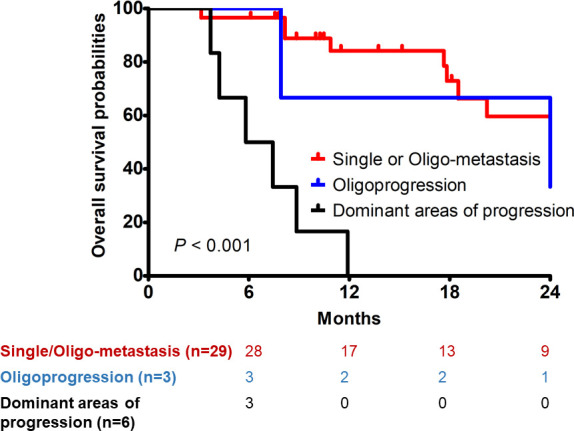
Overall survival of patients according to treatment indication.
MDA criteria vs RECIST v. 1.1 criteria
The overall response rate was 86.8% (33/38) at the last follow-up according to the MDA criteria, and 75.7% (28/37) according to the RECIST criteria. The responses over time are summarized in Table 4. There appeared to be a significant association between response and PFS when response status was determined by MDA criteria (median 7.6 months for responders vs 2.5 months for non-responders; p = 0.036) (Figure 2a). Also, response according to MDA criteria was associated with better OS (median 41.5 months for responders vs 8.2 months for non-responders; p = 0.004) (Figure 2b). In contrast, when response status was determined by RECIST criteria, the associations were significant neither between response and PFS (median 5.8 months for responders vs 9.3 months for non-responders; p = 0.522) (Figure 3a), nor between response and OS (25.7 months for responders vs 18.5 months for non-responders; p = 0.811) (Figure 3b).
Table 4.
Response according to RECIST 1.1 and MDA criteria.
| Months 3– 7 | Months 9– 13 | Months 17– 20 | Months 22– 24 | |||||
| MDA | RECIST | MDA | RECIST | MDA | RECIST | MDA | RECIST | |
| CR | 2 (5%) | 2 (5%) | 2 (8%) | 3 (12%) | 4 (25%) | 2 (13%) | 4 (31%) | 3 (23%) |
| PR | 24 (63%) | 20 (54%) | 22 (88%) | 15 (60%) | 10 (63%) | 9 (56%) | 9 (69%) | 7 (54%) |
| SD | 11 (29%) | 15 (41%) | 1 (4%) | 7 (28%) | 1 (6%) | 4 (25%) | 0 (0%) | 3 (23%) |
| PD | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6%) | 1 (6%) | 0 (0%) | 0 (0%) |
| Total a | 38 | 37 | 25 | 25 | 16 | 16 | 13 | 13 |
MDA, MD Anderson Cancer Center; RECIST, the Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The number of available patients
Figure 2.
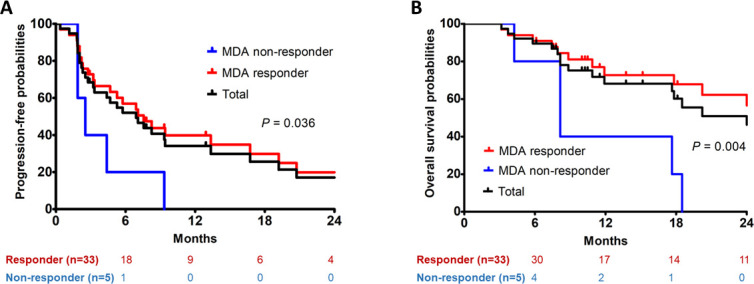
Progression-free survival (a) and overall survival (b) according to the University of Texas MDA Cancer Center (Houston, TX) criteria. MDA, MD Anderson Cancer Center.
Figure 3.
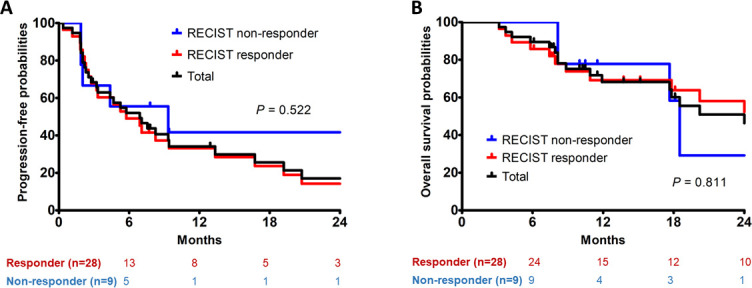
Progression-free survival (a) and overall survival (b) according to the RECIST v. 1.1. RECIST, Response Evaluation Criteria in Solid Tumors.
Toxicity
Three patients underwent treatment-related toxicity. One patient experienced Grade 2 diarrhea, one experienced edema-induced pain, and the other experienced a pain flare. No toxicity grade ≥3 was reported.
Discussion
Results of the present study suggest that SABR was an effective treatment for patients with non-spinal bone metastases. Although the patient population was heterogeneous, the use of SABR achieved a high local control rate (94.2%) at both 1 and 2 years, and approximately 50% of patients in the single- or oligo-metastatic group exhibited long-term survival. Other studies using 1 to 5 fractions of SABR for non-spinal bone metastases also reported 2 year local control rates of 83–100%.8–11
In the past, radiotherapy was not commonly recommended for asymptomatic bone metastases because the primary aim of the treatment was pain relief and functional preservation. Therefore, the response assessment was based on patient-reported pain scores and analgesic consumption. However, effective systemic agents prolonged the survival of patients, and recent technological advances in stereotactic radiotherapy have enabled improvement of local control; thus, SABR could shift the aim of the treatment to improve local tumor control and/or OS. In a prospective study investigating the efficacy of SABR for patients with oligo-metastases, the 2-, 4- and 6 year OS rates were 50%, 28% and 20%, respectively.10 These results suggest that patients could achieve long-term survival and disease control with aggressive radiotherapy for limited metastasis.
Similarly, the primary endpoint was local control in most recent studies investigating SABR for bone metastases, and SABR was also commonly performed in asymptomatic patients with bone metastasis.11,12 Our analysis demonstrated that 61% of patients who underwent bone SABR were asymptomatic before treatment. Therefore, the response assessment criteria should include the objective radiological image as well as the clinical response based on pain score. Currently, the RECIST criteria are the most commonly used method for objectively evaluating tumor response; however, they have limitations in evaluating the response of bone metastases to treatment.13 The RECIST criteria defines measurable lesions as those that can be accurately measured to within ≥10 mm of the greatest diameter using CT.5 Functional imaging, such as bone scan and positron emission tomography, is not considered to be useful for measuring bone lesions, but can be used only adjunctively to confirm the presence or disappearance of bone lesions. In contrast, the MDA criteria use bone scan as well as CT and MRI to assess response.6 In the MDA criteria, sclerotic rim or fill-in of lytic lesion and decrease or normalization of blastic lesions, which are not quantitative or measurable changes in bone lesions, were also considered as “responsive”. Regression on bone scan is also evaluated as “responsive”, except for rapid regression, which can be shown in rapid osteolytic progression. These qualitative elements of the evaluation criteria are not in the RECIST criteria, and have resulted in differences in response assessments (Table 4). For example, in our study, the change in the size of the mass portion of lytic bone lesions on CT was at a level conforming to “stable disease” according to the RECIST criteria, but the lesions were considered to be “responsive” according to the MDA criteria because there was a development of a sclerotic rim and significant decrease in tracer uptake on bone scan at 6 months after the treatment.
The comparisons between imaging assessment criteria in our study indicate that the MDA criteria are superior to the RECIST version 1.1 criteria in differentiating responders and non-responders among patients with non-spinal bone metastases in terms of PFS and OS rates. The inconsistency of response evaluation between different response criteria has also been reported in other studies.3,14,15 Hamaoka, et al compared two response criteria from the World Health Organization (WHO) and the MDA Cancer Center in stratifying breast cancer patients with bone-only metastases, and demonstrated the advantages of the MDA classification in differentiating between responders and non-responders among patients with bone metastases after systemic therapy.12 There were significant differences in PFS between responders and non-responders classified according to the MDA criteria (p = 0.025), as in our study; however, there were none using the World Health Organization criteria.
Similar to other studies investigating oligo-metastases, our study also demonstrated that at least half of patients with single- or oligo-metastasis or oligo-progression survived for up to 2 years.15,16 However, patients with widespread metastasis exhibited a desperate median survival of 5.8 months and no patients survived more than a year. Other studies have suggested the need for re-radiotherapy, oligo-metastatic disease, and oligo-metastatic progression as potential indications for SABR in bone metastases.17,18 As firm recommendations of indications are not yet to be established for SABR in bone metastases, all the above information should be comprehensively considered.
The current study had several limitations, the first of which was that it did not provide the highest level of evidence because of its retrospective design and relatively small sample size. The treatment details such as dose prescription and origin or location of tumors varied among the included patients, and these differences could affect treatment outcome. Additionally, a selection bias may have been introduced because SABR is performed in patients with fewer comorbidities and better performance status in general. The major end points were response assessments of the tumor, which can vary among different radiologists.19
Although the patient population was heterogeneous, SABR for non-spinal bone metastases exhibited a high local control rate with acceptable toxicity. The MDA classification had an advantage in differentiating between responders and non-responders compared with the RECIST criteria. Appropriate treatment indication and unified response evaluation are important for future clinical trials and wide implementation of SABR for non-spinal bone metastases in the clinic.
Footnotes
Acknowledgment: Kyung Su Kim currently works at Department of Radiation Oncology, Ewha Womans University College of Medicine, Seoul, Republic of Korea.
Contributor Information
Tosol Yu, Email: tosolyu@naver.com.
Chul-Won Choi, Email: drasdf19@gmail.com.
Kyung Su Kim, Email: happyend67@gmail.com.
REFERENCES
- 1. Coleman RE . Clinical features of metastatic bone disease and risk of skeletal morbidity . Clinical Cancer Research 2006. ; 12 ( 20 Pt 2 ): 6243s – 9 . doi: 10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- 2. Husain ZA , Sahgal A , De Salles A , Funaro M , Glover J , Hayashi M , et al. . Stereotactic body radiotherapy for de novo spinal metastases: systematic review . Journal of neurosurgery Spine 2017. ; 27 : 295 – 302 . doi: 10.3171/2017.1.SPINE16684 [DOI] [PubMed] [Google Scholar]
- 3. McDonald R , Probyn L , Poon I , Erler D , Brotherston D , Soliman H , et al. . Tumor response after stereotactic body radiation therapy to Nonspine bone metastases: an evaluation of response criteria . Int J Radiat Oncol Biol Phys 2015. ; 93 : 879 – 81 . doi: 10.1016/j.ijrobp.2015.07.2288 [DOI] [PubMed] [Google Scholar]
- 4. Chow E , Hoskin P , Mitera G , Zeng L , Lutz S , Roos D , et al. . Update of the International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases . Int J Radiat Oncol Biol Phys 2012. ; 82 : 1730 – 7 . doi: 10.1016/j.ijrobp.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 5. Eisenhauer EA , Therasse P , Bogaerts J , Schwartz LH , Sargent D , Ford R , et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1 . European Journal of Cancer 2009. ; 45 : 228 – 47 . doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 6. Hamaoka T , Madewell JE , Podoloff DA , Hortobagyi GN , Ueno NT . Bone imaging in metastatic breast cancer . Journal of Clinical Oncology 2004. ; 22 : 2942 – 53 . doi: 10.1200/JCO.2004.08.181 [DOI] [PubMed] [Google Scholar]
- 7. Helou J , Thibault I , Poon I , Chiang A , Jain S , Soliman H , et al. . Stereotactic ablative radiation therapy for pulmonary metastases: histology, dose, and indication matter . International Journal of Radiation Oncology*Biology*Physics 2017. ; 98 : 419 – 27 . doi: 10.1016/j.ijrobp.2017.02.093 [DOI] [PubMed] [Google Scholar]
- 8. Erler D , Brotherston D , Sahgal A , Cheung P , Loblaw A , Chu W , et al. . Local control and fracture risk following stereotactic body radiation therapy for non-spine bone metastases . Radiotherapy and Oncology 2018. ; 127 : 304 – 9 . doi: 10.1016/j.radonc.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 9. Owen D , Laack NN , Mayo CS , Garces YI , Park SS , Bauer HJ , et al. . Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases . Practical Radiation Oncology 2014. ; 4 : e143 – 9 . doi: 10.1016/j.prro.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milano MT , Katz AW , Zhang H , Okunieff P . Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study . Int J Radiat Oncol Biol Phys 2012. ; 83 : 878 – 86 . doi: 10.1016/j.ijrobp.2011.08.036 [DOI] [PubMed] [Google Scholar]
- 11. Zelefsky MJ , Greco C , Motzer R , Magsanoc JM , Pei X , Lovelock M , et al. . Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma . Int J Radiat Oncol Biol Phys 2012. ; 82 : 1744 – 8 . doi: 10.1016/j.ijrobp.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muacevic A , Kufeld M , Rist C , Wowra B , Stief C , Staehler M . Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer . Urologic Oncology: Seminars and Original Investigations 2013. ; 31 : 455 – 60 . doi: 10.1016/j.urolonc.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 13. Costelloe CM , Chuang HH , Madewell JE , Ueno NT . Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST . J Cancer 2010. ; 1 : 80 – 92 . doi: 10.7150/jca.1.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamaoka T , Costelloe CM , Madewell JE , Liu P , Berry DA , Islam R , et al. . Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer . British Journal of Cancer 2010. ; 102 : 651 – 7 . doi: 10.1038/sj.bjc.6605546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milano MT , Philip A , Okunieff P . Analysis of patients with oligometastases undergoing two or more curative-intent stereotactic radiotherapy courses . Int J Radiat Oncol Biol Phys 2009. ; 73 : 832 – 7 . doi: 10.1016/j.ijrobp.2008.04.073 [DOI] [PubMed] [Google Scholar]
- 16. Ahmed KA , Barney BM , Davis BJ , Park SS , Kwon ED , Olivier KR . Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer . Front Oncol 2012. ; 2 : 215 . doi: 10.3389/fonc.2012.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahgal A , Roberge D , Schellenberg D , Purdie TG , Swaminath A , Pantarotto J , et al. . The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy . Clinical Oncology 2012. ; 24 : 629 – 39 . doi: 10.1016/j.clon.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 18. Bedard G , McDonald R , Poon I , Erler D , Soliman H , Cheung P , et al. . Stereotactic body radiation therapy for non-spine bone metastases--a review of the literature . Ann Palliat Med 2016. ; 5 : 58 – 66 . doi: 10.3978/j.issn.2224-5820.2015.07.01 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki C , Torkzad MR , Jacobsson H , Åström G , Sundin A , Hatschek T , et al. . Interobserver and intraobserver variability in the response evaluation of cancer therapy according to RECIST and WHO-criteria . Acta Oncologica 2010. ; 49 : 509 – 14 . doi: 10.3109/02841861003705794 [DOI] [PubMed] [Google Scholar]


