Abstract
Percutaneous microwave ablation of liver tumours is a well-established technique that has been proven to be effective in the curative and palliative treatment of small volume primary and secondary liver tumours. Microwave ablation is designed to achieve larger areas of necrosis compared to radiofrequency ablation and has a good safety profile among liver tumour treatments. Mortality is unreported and major complications are rare. Knowledge of potential complications is essential for interventional radiologists performing liver ablation in order to reduce patient morbidity. The aim of this review is to illustrate major complications post microwave ablation in a pictorial format as well as a discussion on how best to avoid these complications
INTRODUCTION
Microwave ablation (MWA) has been increasingly used to treat primary and secondary hepatic tumours, replacing radiofrequency ablation (RFA). Like RFA, MWA is a thermal ablation technique, which destroys tumour cells by coagulative necrosis while causing minimal damage to surrounding tissue.1 Advantages over RFA include shorter ablation time, more consistent intratumoural temperature and potential to treat larger lesions.2 A meta-analysis including 2062 patients comparing MWA and RFA for treatment of hepatic lesions showed that MWA has significantly better 6 year overall survival than RFA with a similar safety profile.3
The majority of MWA is performed via an imaging-guided percutaneous approach, although it can also be performed intraoperatively at the time of open or laparoscopic resection. When performed percutaneously, patients can either be under local or general anaesthetic using ultrasound or computed tomography (CT) as imaging guidance. Currently, the main indications of MWA to treat intrahepatic lesions are in the treatment of hepatocellular carcinoma (HCC) and metastatic liver disease. The European association for the study of liver disease (EASL) guidelines suggest ablative techniques in curative therapy for treating HCCs smaller than 3 cm. In patients with early HCC eligible for liver transplantation, MWA may be used as a bridging therapy while patients await transplant. MWA can be used in hepatic oligometastatic disease for curative intent, alternatively as an adjunctive therapy to liver resection for treatment of bilobar liver metastatic disease. It is also a treatment option in patients not fit enough to undergo surgical treatment. The efficacy of MWA has been reported with 5–10% local recurrence rates in completely ablated primary and secondary hepatic metastasis.4,5
MWA is a safe technique with “no major safety concerns” reported by National Institute for Health and Care Excellence (NICE).6 Studies have reported mortality rates between 0 and 0.36%.7 Major complications have been reported post MWA with figures between 2.6 and 4.6%.7–9 The major complications belong to four categories: vascular, biliary, mechanical and infectious. The aim of this pictorial review is to demonstrate these complications, discuss their mechanisms and how best to avoid them.
Vascular
Vascular complications include bleeding, pseudoaneurysm formation and thrombosis. Bleeding and pseudoaneurysm are results of vessel injury by mechanical force from the needle or indirect thermal injury. Vascular injury can present either as frank haemorrhage or pseudoaneurysm (Figure 1), the latter leading to increased risk of delayed haemorrhage due to rupture. The reported incidence of major haemorrhage is around 0.1–0.4%.7,9 Patients may present with acute drop in haemoglobin or severe abdominal pain following the procedure, which warrants an urgent triple phase CT scan. Sometimes, evidence of vascular injury such as a new pseudoaneurysm is found on the follow-up scan as an incidental finding. Small subcapsular or intraparenchymal haematomas (Figure 2) may be present on the follow-up imaging, which often requires no active treatment. Direct injury to the vessel can also present as arterial-portal shunting, which in chronic liver disease patients may lead to alteration of flow dynamics and hepatic decompensation. CT will demonstrate new development of ascites (a sign of decompensation) and early filling of the portal vein on the arterial phase imaging (Figure 3). In most cases, high resolution thin slice CT also allows direct visualisation of the arterioportal fistula, allowing for proper treatment planning. Macroscopic shunts may require angiography to embolise the shunt and prevent pre-hepatic portal hypertension and its complications.
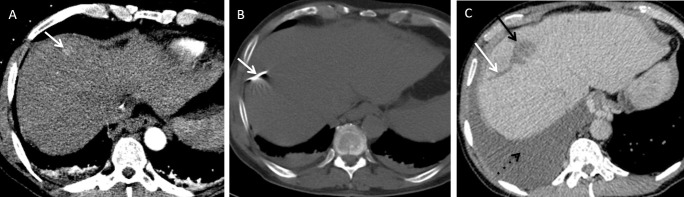
Figure 1.
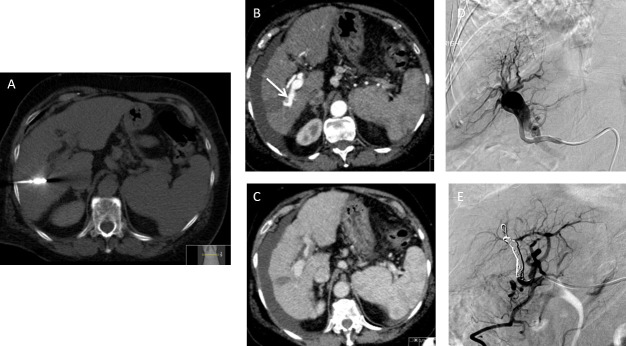
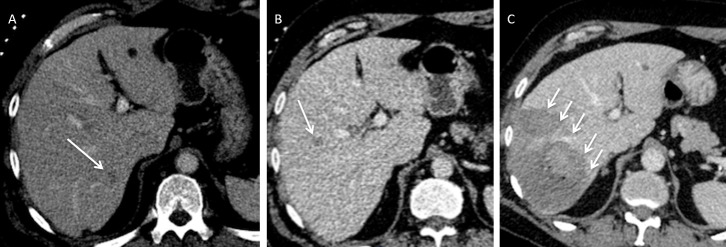
A 66-year-old male with chronic liver disease who developed a 26 mm current hepatocellular carcinoma in the left lateral liver was treated with microwave ablation. There was no immediate post-procedural complications. Patient presented 4 weeks later with acute drop in haemoglobin. (A) Axial contrast-enhanced CT demonstrates the position of the lesion (arrow). (B) Axial contrast-enhanced CT demonstrates the position of the microwave needle. (C) Arterial phase CT shows pseudoaneurysm which was confirmed on angiography (D) and the pseudoaneurysm was coil embolised successfully (E).
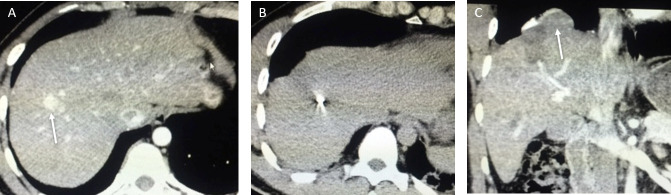
Figure 2.
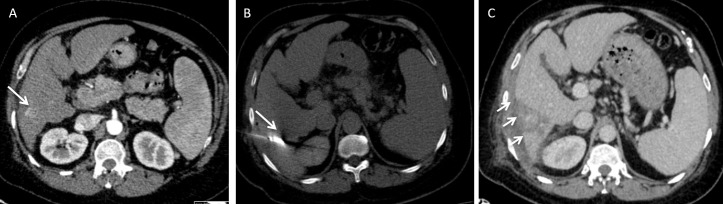
A 51-year-old male with chronic viral hepatitis B and C infection developed a small hepatocellular carcinoma recurrence in Segment VI after previous ablation. 3 days later, he developed right upper quadrant pain. (A) Axial arterial phase CT prior to MWA shows a small arterialized nodule (arrow) representing a recurrent hepatocellular carcinoma. (B) Axial non-contrast-enhanced CT shows the position of the needle (arrow). (C) The repeat CT shows the presence of a small subcapsular haematoma (arrows). This was treated conservatively and the patient recovered with no further complications.
Figure 3.
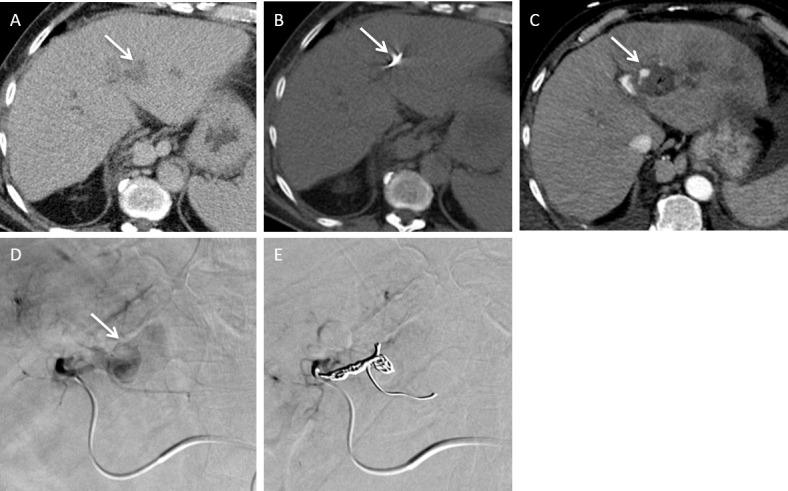
A surveillance CT of a 73-year-old female with non-alcoholic fatty liver disease related cirrhosis showed a 20 mm hepatocellular carcinoma in Segment V. This was treated with microwave ablation. (A) Axial non-contrast-enhanced CT demonstrates the position of the microwave needle. (B) Follow up axial arterial and (C) Axial portovenous post contrast-enhanced CT shows new onset of moderate ascites with blood results demonstrating worsening of hepatic function in keeping with decompensated portal hypertension. Note there is early opacification of the right portal vein on the arterial phase imaging (arrow). (D) Catheter hepatic angiography demonstrates an arterioportal shunt. (E) Angiography images demonstrate successful closure of arterioportal shunt with coil. The patient’s ascites and patient’s liver function improved.
Risk of haemorrhage is increased in patients with severe coagulopathy. Blood count and coagulation should be checked prior to the procedure and severe coagulopathy should be corrected. The cut off for acceptable haemoglobin (Hb) and coagulation may vary depending on institutional guidelines. As a guide, Hb <8 g dl−1, platelet <30 × 10^9, or INR >1.5 will require correction prior to percutaneous MWA. Lesions that are closer to the main vessels and are subcapsular in location also have increased risk of bleeding. Targeting the lesion obliquely through the capsule will increase the length of needle traversing healthy liver which reduces the risk of haemorrhage. Alternative non-thermal based techniques such as irreversible electroporation (IRE) may represent an alternative form of ablation.
The risk of complete or partial portal vein thrombosis (Figure 4) has been reported to increase when treating lesions close to the main portal vein or its major branches. Development of portal vein thrombosis will lead to worsening of hepatic function. In some cases, recanalisation of the portal vein may occur. However, long-term occlusion of portal vein branch will lead to atrophy of the hepatic tissue (Figure 4). Hepatic arterial and hepatic venous thrombosis have also been reported but their incidence was much lowered compared to portal vein thrombosis.10 This is likely due to the difference in their flow rate and volume. Patients who are treated for early HCC often have background chronic liver disease or cirrhosis. In these patients, portal vein velocities are often reduced making them prone to thrombosis. The reduced portal vein flow also reduces the protective “heat sink” effect from cooling of the flowing vessels.
Figure 4.
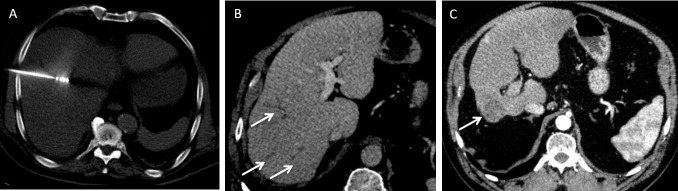
A 76-year-old male with crytogenic cirrhosis was treated for a 18 mm hepatocellular carcinoma found in Segment VIII on surveillance scan. (A) Axial non-contrast-enhanced CT demonstrates the position of the microwave needle. (B) Axial contrast-enhanced CT demonstrates initial segmental peripheral biliary duct dilation on an immediate follow-up study (arrows). (C) Axial portovenous phase contrast-enhanced CT demonstrates the treated tumour (arrow) and marked atrophy of the right lobe of the liver as a result of right portal vein thrombosis 18 months after the initial procedure.
Biliary
Biliary complications manifest as biliary stricture (Figures 4 and 5), bile leak, biloma formation or acute cholecystitis (Figure 6). Biliary strictures present with peripheral segmental bile duct dilatation may not require any active treatment depending on the volume of liver affected. However, some of these patients may present with recurrent ascending cholangitis, which requires endoscopic or percutaneous biliary drainage. Bile leak can occur as a result of direct injury to the bile duct which can be transient, however, formation of biloma increases the risk of secondary infection. Large volume biloma will require percutaneous drainage. MRI with both MR cholangiopancreatography (MRCP) and 20–30 min delayed excretory phase imaging following administration of Gadoxetic acid (Gd-EOB) is the imaging choice to localize bile leak. Treating lesions adjacent to the gallbladder has increased risk of gallbladder perforation or acute cholecystitis. Rarely, when treating lesions superiorly in the liver, damaging of the biliary duct and pleura may result in a biliary-pleural fistula between the biliary system and the pleura (Figure 7) which can be confirmed on pleural fluid analysis. Patients with existing cholangiopathy or biliary obstruction are at a increased risk of biliary complications. The risk for pleurobiliary fistula is higher when treating lesions within segments VII and VIII. Care should be taken to avoid transgressing the pleura with the microwave needle. Where available, non-thermal techniques such as IRE may be considered when treating central tumours that are close to bile duct or major vessels.
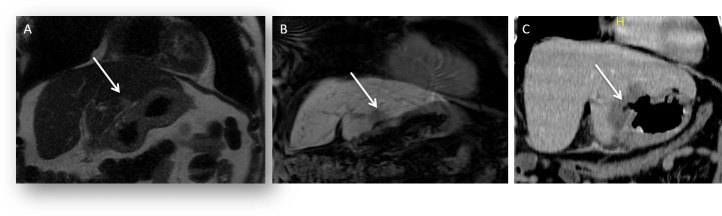
Figure 5.
A 55-year-old male developed a solitary colorectal metastatic deposit. (A) Axial post contrast-enhanced CT shows the position of the liver metastasis (arrow) in the left liver lobe (Segment II/III) in close relation to the left portal vein. This was ablated under ultrasound guidance. (B) Follow-up imaging from axial T2 weighted fat suppressed image shows lobar intrahepatic biliary dilatation (arrow) post-ablation secondary to a thermal biliary stricture. (C) Axial 20 min delayed hepatobiliary phase post-administration of gadoxetic acid image shows end-stage atrophy of segments II–III and no excretion of hepatobiliary contrast medium (arrow).
Figure 6.
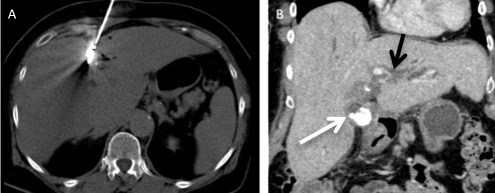
A 73-year-old female with chronic viral hepatitis B infection had viable hepatocellular tumour in segment IV following trans-arterial chemo-emobolisation was treated with microwave ablation. (A) Axial non-contrast-enhanced CT shows the centre of the microwave needle which is within close proximity to the gallbladder. (B) Coronal contrast enhanced CT shows thickened gallbladder wall with calculi (white arrow) and intrahepatic biliary duct dilatation (black arrow). Patient was clinically symptomatic and a diagnosis of acute cholecystitis was made.
Figure 7.
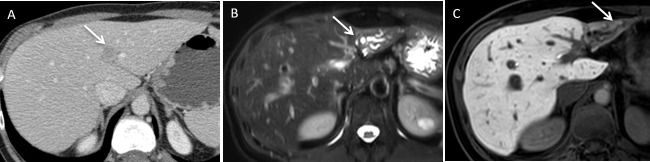
A 68-year-old male with viral hepatitis C related cirrhosis developed a 1.4 cm hepatocellular carcinoma within Segment VIII. (A) Axial contrast enhanced CT demonstrates a small hepatocellular carcinoma (arrow). (B) Axial CT (bone window) demonstrates the microwave needle position during ablation. (C) Axial post contrast enhanced CT demonstrates ablation zone (black arrow) with adjacent dilated biliary duct (white arrow) and associated large right pleural effusion (dotted black arrow). The appearance suggests an underlying biliary pleural fistula.
Mechanical
Mechanical complications include diaphragmatic injury, perforation of the gallbladder, colon, and stomach and breeching the pleural with the ablation needle causing pneumothorax or haemothorax (Figure 8). Injury to the diaphragm may be caused by thermal injury when treating an adjacent lesion. It may manifest as pleural effusion due to diaphragm irritation, diaphragmatic thickening and diaphragmatic hernia (Figure 9).
Figure 8.
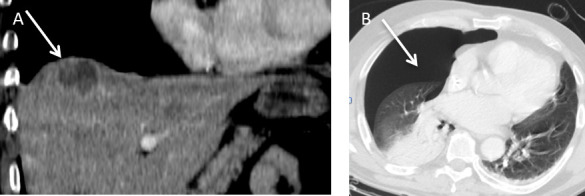
A 50-year-old male patient developed a colorectal liver metastasis in Segment VIII superiorly. (A) Coronal contrast enhanced CT demonstrates a hypoattenuate metastasis (arrow) which was subsequently treated with microwave ablation under ultrasound guidance. (B) Post procedure, the patient developed shortness of breath. Axial CT (lung window) revealed a moderate size pneumothorax (arrow).
Figure 9.
A 32-year-old female with a background of chronic viral hepatitis B infection developed marginal recurrence of hepatocellular carcinoma at the previous ablation site in Segment VIII. (A) Axial contrast-enhanced CT shows recurrent hepatocellular carcinoma (arrow). (B) Axial non-contrast-enhanced CT shows the tip of the microwave needle within the centre of the lesion. (C) Coronal image from contrast enhanced CT at 6 week follow-up scan shows a small diaphragmatic hernia (arrow).
Treatment of the left lobe of the lesion in close proximity to the stomach may cause gastric wall oedema or perforation (Figure 10). Colonic injury may also result from thermal injury when treating lesions in close proximity to the large bowel (Figure 11). Risk of bowel injury is increased if there were previous abdominal surgery with altered anatomy or adhesion between the liver capsule and the bowel. Fortunately, it occurs rarely with reported rates of 0.1–0.7%.7,9 Appropriate pre-procedure planning, patient selection and hydro-dissection11,12 might reduce incidence. Real time imaging using ultrasound may also help plan visceral avoidance.
Figure 10.
A 48-year-old male with a history of renal cell carcinoma presented with a solitary hepatic metastatic deposit located at the inferior free edge of the left liver lobe. This was ablated under ultrasound guidance. (A) Coronal T 2 and (B) T 1 coronal images shows location of the metastatic liver lesion (arrow). Patient developed epigastric pain 1 week after the procedure. (C) Coronal contrast enhanced CT shows a perforation at the lesser curvature of the stomach (arrow). Patient had a laparoscopic distal gastrectomy, with an uneventful post-operative recovery.
Figure 11.
A 63-year-old male developed a peripheral cholangiocarcinoma and was treated with ultrasound-guided microwave ablation. (A) Axial contrast-enhanced CT demonstrates the lesion is in a subcapsular location in segment IVa (arrow). (B) Axial CT image (bone window) demonstrates free intraperitoneal gas (arrows) likely a result from thermal injury to the adjacent hepatic flexure (arrow). (C) Coronal contrast enhanced CT demonstrates gas containing perihepatic fluid collection (arrow) as a result of colonic perforation. The patient was treated conservatively with percutaneous drainage and recovered fully.
It is also important to examine the ablation needle post procedure. Although it happens rarely the ablation needle tip can be broken and dislodged within the ablation track (Figure 12).
Figure 12.
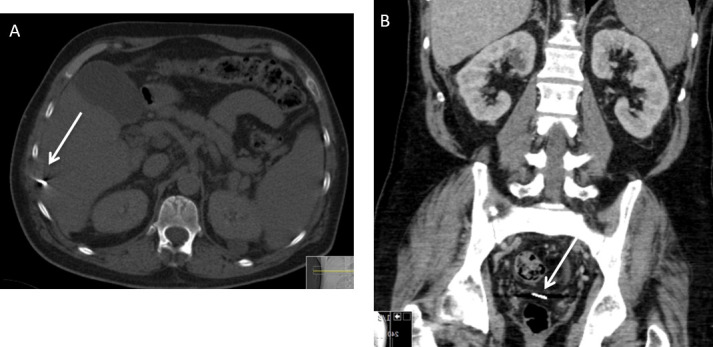
A 56-year-old male with cirrhosis due to alcohol liver disease developed an 18 mm hepatocellular carcinoma in Segment VI which was subsequently treated with ablation. (A) Axial CT on bone window demonstrates a dislodged ablation needle tip (arrow). The needle traverses the intercostal muscles and capsule of the liver in the region of Segment VI. After discussion with the surgical team, the needle was left in situ. (B) Subsequent follow-up post contrast enhanced coronal CT image shows that the dislodged needle has migrated into the pelvic peritoneal cavity (arrow).
Infectious
The ablation zone is susceptible to infection due to tissue necrosis (Figure 13). It has been reported infections occur 7–10 day after the MWA and delayed presentation can occur up to a month later. Depending on the size of the abscess, treatment may include percutaneous drain in addition to antibiotics. In our institution (King’s College Hospital, London UK), two doses of intravenous broad spectrum antibiotics are given immediately prior and 12 h post procedure. Patients with bilio-enteric anastomosis (e.g. post Whipple procedure) and those with a history of endoscopic sphincterotomy have a higher risk of infection of the ablation zone13 owing to reflux cholangitis and require a more rigorous antimicrobial prophylaxis.
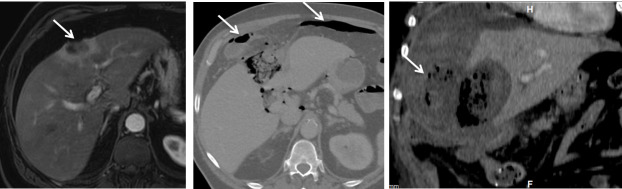
Figure 13.
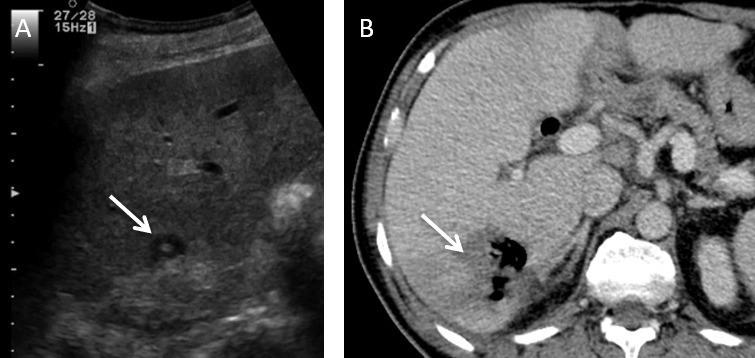
A 62-year-old male with an operable pancreatic head ductal adenocarcinoma was resected via a Whipple’s procedure. (A) Intercostal view of greyscale ultrasound shows a solitary metastatic deposit located in the medial subcapsular portion of Segment VI (arrow). An ultrasound guided microwave ablation was performed. Patient presented with chills and rigors post procedure. (B) Axial contrast enhanced CT shows a pyogenic liver abscess (arrow).
Functional
There are manufacturer’s charts for predicting ablation zone dimensions which allows the user to select the most appropriate ablation power and treatment time depending on the size of the lesion. However, the resultant ablation zone is occasionally larger than manufacturer’s chart would suggest (Figure 14). It is possible that the nature of the lesion and background liver tissue may contribute to the variation in the ablation zone size. Antibiotic treatment is often needed to prevent hepatic abscess formation within the large ablation zone. Early imaging in patients who developed post-operative fever and worsening of hepatic function is the key to identify these cases.
Figure 14.
A 64-year-old male with colorectal liver metastasis in segments VII and VIII were treated with microwave ablation. Post-procedure, he developed fever and the blood test showed an elevated level of aspartate aminotransferase. (A, B) demonstrates the low attenuation hepatic metastases (arrows) in segments VII and VIII respectively. (C) Subsequent CT demonstrates an unexpectedly large ablation zone (arrows).
There have been limited reports regarding tumour seeding along the ablation track using microwave ablation. Livraghi et al reported tumour seeding from the needle track occurred in 0.6% of patients.7 A study involving 711 patients with HCC showed incidence of 0.9% per tumour after being treated with RFA and the risk of needle track seeding is associated with large tumour size (>3 cm), subcapsular tumour and biopsy prior to treatment.14 Ablation of the needle track of 10–20 s while withdrawing the needle prior to exit from the liver capsule can be performed to reduce the risk of tumour seeding. However, caution should be taken to stop track ablation after exiting the capsule as this may cause skin burn.
CONCLUSION
Microwave ablation for hepatic lesions is a safe procedure with low incidence of major complications which can be further reduced by appropriate patient selection and pre-procedural planning. Care must be taken when treating central lesions close to major ducts or vessels. Contrast enhanced CT or ultrasound must be used after placing the needle and before ablation to ensure a safe distance is maintained to prevent biliary strictures, arterial complications and venous thrombosis.
Contributor Information
Cheng Fang, Email: chengfang@nhs.net.
Kelvin Cortis, Email: Kelvincortis@gmail.com.
Gibran T Yusuf, Email: gibran.yusuf@nhs.net.
Stephen Gregory, Email: stephengregory@nhs.net.
Dylan Lewis, Email: dylanlewis@nhs.net.
Pauline Kane, Email: p.kane2@nhs.net.
Praveen Peddu, Email: Praveen.peddu@nhs.net.
REFERENCES
- 1. Seki T , Wakabayashi M , Nakagawa T , Itho T , Shiro T , Kunieda K , et al. . Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma . Cancer 1994. ; 74 : 817 – 25 . doi: [DOI] [PubMed] [Google Scholar]
- 2. Simon CJ , Dupuy DE , Mayo-Smith WW . Microwave ablation: principles and applications . Radiographics 2005. ; 25 Suppl 1 S69 – S83 . doi: 10.1148/rg.25si055501 [DOI] [PubMed] [Google Scholar]
- 3. Huo YR , Eslick GD . Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis . J Vasc Interv Radiol 2015. ; 26 : 1139 – 46 . doi: 10.1016/j.jvir.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 4. Xu Y , Shen Q , Wang N , Wu P-P , Huang B , Kuang M , et al. . Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma . Chin J Cancer 2017. ; 36 : 14 . doi: 10.1186/s40880-017-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogata Y , Uchida S , Hisaka T , Horiuchi H , Mori S , Ishibashi N , et al. . Intraoperative thermal ablation therapy for small colorectal metastases to the liver . Hepatogastroenterology 2008. ; 55 ( 82–83 ): 550 – 6 . [PubMed] [Google Scholar]
- 6. Microwave ablation for treating liver metastases | Guidance and guidelines | NICE [Internet] .. Available from: https://www.nice.org.uk/guidance/ipg553 [ cited 2018 Oct 3 ].
- 7. Livraghi T , Meloni F , Solbiati L , Zanus G , Collaborative Italian Group using AMICA system . Complications of microwave ablation for liver tumors: results of a multicenter study . Cardiovasc Intervent Radiol 2012. ; 35 : 868 – 74 . doi: 10.1007/s00270-011-0241-8 [DOI] [PubMed] [Google Scholar]
- 8. Poggi G , Tosoratti N , Montagna B , Picchi C . Microwave ablation of hepatocellular carcinoma . World J Hepatol 2015. ; 7 : 2578 – 89 . doi: 10.4254/wjh.v7.i25.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang P , Wang Y , Yu X , Dong B . Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients . Radiology 2009. ; 251 : 933 – 40 . doi: 10.1148/radiol.2513081740 [DOI] [PubMed] [Google Scholar]
- 10. Chiang J , Cristescu M , Lee MH , Moreland A , Hinshaw JL , Lee FT , et al. . Effects of microwave ablation on arterial and venous vasculature after treatment of hepatocellular carcinoma . Radiology 2016. ; 281 : 617 – 24 . doi: 10.1148/radiol.2016152508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M , Liang P , Cheng Z-G , Yu X-L , Han Z-Y , Yu J . Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract . Int J Hyperthermia 2014. ; 30 : 134 – 41 . doi: 10.3109/02656736.2014.891765 [DOI] [PubMed] [Google Scholar]
- 12. Kitchin D , Lubner M , Ziemlewicz T , Hinshaw JL , Alexander M , Brace CL , et al. . Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection . Int J Hyperthermia 2014. ; 30 : 299 – 305 . doi: 10.3109/02656736.2014.936050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odisio BC , Richter M , Aloia TA , Conrad C , Ahrar K , Gupta S , et al. . Use of prophylactic antibiotics to prevent abscess formation following hepatic ablation in patients with prior Enterobiliary manipulation . J Gastrointest Surg 2016. ; 20 : 1428 – 34 . doi: 10.1007/s11605-016-3117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhong-yi Z , Wei Y , Kun Y , Ying D , Wei W , Jung-chieh L , et al. . Needle track seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma: 14-year experience at a single centre . Int J Hyperthermia 2017. ; 33 : 454 – 8 . doi: 10.1080/02656736.2017.1278630 [DOI] [PubMed] [Google Scholar]