Abstract
Objective:
To evaluate the dosimetric ramifications of simultaneously irradiating the prostate and pelvic lymph nodes (PLNs) with a stereotactic body radiotherapy approach based on rigid registration to intraprostatic markers (IPMs).
Methods and materials:
Nineteen patients received concurrent SBRT to the prostate and PLNs on a phase II clinical trial. The translational and rotation shifts required for rigid registration to bony anatomy and changes in bladder and rectal anatomy were compared between patients with > 90% and < 90% coverage of the nodal clinical target volume (CTVN ) as drawn on fractional kilovoltage cone-beam CTs. Stepwise multivariable regression models evaluated relationships between these anatomical parameters and the change in V100%CTVN.
Results:
The average V100%CTVN per patient was 92.4 % (IQR, 90.2 – 96.4 %). For five patients (26.3%), the average was 85.0 % (IQR, 82.4–88.3 %). The left-right and superior-inferior translational shifts, sagittal rotational shift, and change in bladder volume were significantly different ( p < 0.05 for all via Student’s t-test). Changes in bladder height, left/right shift, superior/inferior shift, 3-D shift, and axial rotation as significant predictors of change in dosing of V100%CTVN.
Conclusion:
While simultaneous SBRT to the prostate and PLNs based on rigid registration to IPMs provides adequate PLN coverage in most instances, overall coverage may be lower than anticipated if anatomy is unstable. Careful evaluation of bladder filling on kV-CBCT before treatment may be the most practical method for estimating accuracy prior to treatment.
Advances in knowledge:
Simultaneous SBRT to the prostate and PLNs based on rigid registration to IPMs provides adequate PLN coverage in most instances.
INTRODUCTION
Inter- and intrafractional prostatic motion is typically accounted for by rigid registration to implanted intraprostatic markers (IPMs).1,2 Due to the independent movement of IPMs and pelvic lymph nodes (PLNs), this may lead to nodal underdosing when the PLNs are being irradiated simultaneously.3–6 This risk may be amplified in stereotactic body radiotherapy (SBRT) regimens.7–9 Here, we present a dosimetric analysis of nodal coverage among 19 patients who received simultaneous SBRT to the prostate and PLNs on a phase II prospective trial (NCT02296229).
Methods and materials
Nineteen patients who received SBRT to the prostate and PLN on the aforementioned phase II trial were identified for analysis. For all patients, two IPMs were implanted in the base of the prostate and one in the apex. All planning CT simulation scans were obtained on a Siemens SOMATOM Definition AS scanner (Siemens Healthcare Diagnostics, Los Angeles, CA) utilizing 1.5 mm thick slices. The prostate was contoured and expanded by 5 mm isotropically to form a prostate planning target volume (PTVP). Nodal clinical target volumes (CTVNs) were contoured as per the RTOG consensus guidelines and expanded by 4–5 mm to create nodal planning target volumes (PTVNs), with the expansion derived per treating radiation oncologist. Plans were designed to deliver 40 Gy to the prostate and 25 Gy to the pelvic lymph nodes, such that 95% of each PTV received the prescription dose. Volumetric modulated arc therapy plans were generated utilizing four half-arcs. All patients underwent a kilovoltage cone beam CT (kV-CBCT) prior to treatment initiation to verify bladder and rectal filling. The IPMs on the kV-CBCTs were rigidly aligned with those on the planning CT prior to each fraction and after each half-arc. Patients received treatment on either a NovalisTx (Varian Medical Systems, Palo Alto, CA) or TrueBeam (Varian Medical Systems). All patients were instructed to void their bladders one hour before treatment and then drink 16 to 24 oz of water to maintain a reproducible and comfortably full bladder. They were also asked to do an enema the morning of treatment to ensure an empty rectum.
Institutional board review was in place for all analyses. Gastrointestinal Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria and patient-reported bowel quality of life domains cores (measured by the Expanded Prostate Index Composite [EPIC]) were extracted from the electronic medical record. After applying the clinically utilized shifts, the kV-CBCTs were fused to the planning CTs in MIMVista (MIM Software, Cleveland, OH), and the CTVNs were re-drawn on the kV-CBCTs. The dose distribution was transferred to the kV-CBCT and V100%CTVN (percentage of CTVN receiving the nodal CTV dose that was planned for that given patient) was computed (Figure 1). The translational and rotational shifts that would be required to rigidly register the pelvic bones after registering to IPMs, were extracted. Rectal diameter at mid-prostate, bladder height on the anterior-most coronal plane of the simulation scan of CBCT, and the total bladder volume were recorded. We considered V100%CTVN <90% to be suboptimal for the purposes of our analyses. The Student's t-test was used to make pairwise comparisons, and stepwise multiple linear regression was performed to evaluate the significance of associations between variables of interest and V100%CTVN.
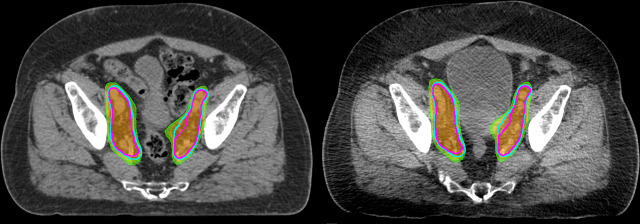
Figure 1.
Representative planning CT (left) and kilovoltage cone-beam CT (right) from a representative patient. The planned nodal clinical target volumes are show in cyan, with the re-drawn nodal target volume on the cone-beam CT shown in magenta. Orange, 100% isodose line; green, 95% isodose line.
Results
The median clinical follow-up was 21 months (IQR 16.5–28.7 months). No patient experienced acute or late grade ≥2 gastrointestinal toxicity and the average decline in EPIC bowel domain scores was 1.8 points (on a scale of 0–100).
When examining delivered doses and normalizing to the minimum dose received by the CTVN in any given patient’s plan (median of 24.5 Gy, interquartile range [IQR] 24.3–24.7 Gy], the average V100%CTVN was 92.4% (interquartile range [IQR] 90.2–96.4%). Five patients (26.3%) had mean V100%CTVN <90%; for this group, the average V100%CTVN was 85.0% (IQR, 82.4–88.3%), while for the other fourteen patients it was 95.1% (IQR 94.0–97.3%; p < 0.05 by Student’s t-test). The bony-to-fiducial translation in the left-right direction and superior-inferior direction, as well as the change in bladder volume and sagittal rotation were significantly different between groups (p < 0.05 for all via Student’s t-test, Table 1). When only assessing the magnitude of changes (i.e. the absolute value), no significant differences were found.
Table 1. .
Target Coverageand Associated Variables
| V100%CTVN > 90% | V100%CTVN ≤ 90% | p-value | |
| Raw Values | |||
| Change in Bladder Volume (%, average, IQR) | 9.8% (-74.4–24.8%) | −25.1% (-55.3– 1.40) | .01 |
| Change in Bladder Height (%, average, IQR) | −0.1% (-23.5– 18.9%) | −16.3% (-32.2– 3.1%) | .006 |
| Change in rectal diameter (%, average, IQR) | 5.9% (-4.2–20.3%) | 0.3% (-11.5–12.4%) | 0.17 |
| Left/Right Shift (cm, average, IQR) | 0.0 (-0.03– 0.11) | −0.1 (-0.14– 0.007) | < 0.001 |
| Anterior/Posterior Shift (cm, average, IQR) | −0.1 (-0.38–0.15) | 0.0 (-0.17–0.19) | 0.30 |
| Superior/Inferior Shift (cm, average, IQR) | 0.0 (-0.16– 0.26) | 0.3 (0.16– 0.53) | 0.003 |
| 3-D Shift (cm, average, IQR) | 0.4 (0.35–0.61) | 0.5 (0.34–0.60) | 0.66 |
| Axial Rotation (degrees, average, IQR) | 0.1 (-0.26–0.34) | 0.1 (-0.76–0.47) | 0.89 |
| Sagittal Rotation (degrees, average, IQR) | −1.0 (-1.86– 0.05) | −0.2 (-1.52– 0.03) | 0.04 |
| Coronal Rotation (degrees, average, IQR) | −0.1 (-0.4–0.23) | 0.0 (-0.34–0.23) | 0.70 |
| Absolute Values | |||
| Change in Bladder Volume (%, average, IQR) | 59.2% (18.3–65.6%) | 42.9% (25.3–61.3%) | .08 |
| Change in Bladder Height (%, average, IQR) | 25.8% (13.3–32.5%) | 21.2% (7.1–32.1%) | .21 |
| Change in rectal diameter (%, average, IQR) | 14.9% (4.4–24.0%) | 11.7% (3.7–15.4%) | 0.22 |
| Left/Right Shift (cm, average, IQR) | 0.1 (0.04–0.11) | 0.1 (0.02–0.14) | 0.89 |
| Anterior/Posterior Shift (cm, average, IQR) | 0.3 (0.10–0.43) | 0.0 (-0.17–0.19) | 0.40 |
| Superior/Inferior Shift (cm, average, IQR) | 0.3 (0.09–0.39) | −0.4 (0.29–0.53) | 0.05 |
| Axial Rotation (degrees, average, IQR) | 0.4 (0.15–0.54) | 0.8 (0.31–1.1) | 0.91 |
| Sagittal Rotation (degrees, average, IQR) | 1.2 (0.46–1.86) | 1.5 (0.61–1.95) | 0.31 |
| Coronal Rotation (degrees, average, IQR) | 0.4 (0.1–0.58) | 0.4 (0.08–0.5) | 0.84 |
Stepwise multiple linear regression modeling identified changes in bladder height, left/right shift, superior/inferior shift, 3-D shift, and axial rotation as significant predictors of change in dosing of V100%CTVN, with a coefficient of multiple determination (R2) of 0.45 (Table 2 and Figure 2A). A stepwise multiple regression model that considered all pairwise interactions and second order terms had similar results, with the identification of additional significant higher-order interaction terms and an R2 of 0.73 (Table 2 and Figure 2B).
Table 2. .
Stepwise Multivariable Regression Models
| Estimate | Standard Error | P-value | Variance Inflation | |
| Linear Regression | ||||
| Change in Bladder Height | 0.04 | 0.02 | 0.02 | 1.06 |
| Left/Right Shift | 19.33 | 4.34 | <0.0001 | 1.07 |
| Superior/Inferior Shift | −2.93 | 1.26 | 0.02 | 1.04 |
| 3-D Shift | −6.88 | 1.88 | 0.00 | 1.02 |
| Axial Rotation | −2.57 | 0.66 | 0.00 | 1.02 |
| Higher Order (Quadratic) Regression | ||||
| Change in Bladder Volume | 0.06 | 0.01 | <.0001 | 1.23 |
| Left/Right Shift | 11.87 | 3.25 | 0.00 | 1.18 |
| Superior/Inferior Shift | −3.58 | 0.91 | 0.00 | 1.08 |
| 3-D Shift | −5.31 | 1.42 | 0.00 | 1.14 |
| Axial Rotation | 2.23 | 0.47 | <.0001 | 1.03 |
| Change in Bladder Volume * Left/Right Shift | −0.60 | 0.10 | <.0001 | 1.32 |
| Left/Right Shift * Superior/Inferior Shift | 32.75 | 9.47 | 0.00 | 1.22 |
| Left/Right Shift * Axial Rotation | −20.65 | 6.58 | 0.00 | 1.29 |
| Change in Bladder Volume * Axial Rotation | −0.05 | 0.02 | 0.02 | 1.22 |
Figure 2.
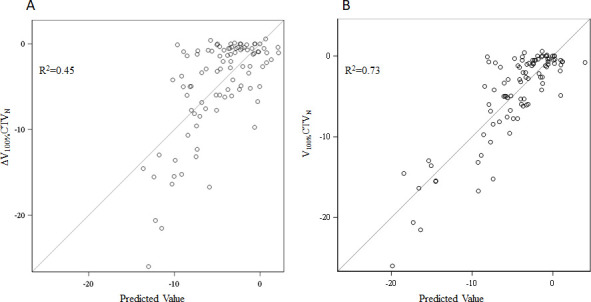
Stepwise regression models to estimate undercovering nodal clinical target volumes. The graphs indicate the model’s predicted value of the change in nodal coverage (ΔV100%CTVN) on the x-axis, versus the actual value of V100%CTVN on the y-axis. The model evaluated on the left (A) is a standard linear regression model, whereas the model evaluated on the right (B) accounts for higher-order interactions.
Discussion
In this dosimetric analysis of 19 patients who prospectively received simultaneous SBRT to the prostate and PLNs, we demonstrate that the PLNs receive an adequate dose of radiation despite rigid registration to IPMs, with an overall mean V100%CTVN of 92.4%. However, a subset of five patients (26.3%) had suboptimal PLN coverage, with a mean V100%CTVN of 85%; this subset of patients had significantly greater changes in bladder volume and shifts of the bony pelvis with respect to the IPMs. Regression modeling confirmed the importance of these variables, particularly of translational shifts in the left/right direction and superior/inferior direction, and rotations in the axial plane. A multiple linear model incorporating higher-order interactions achieved an R2 of 0.73, suggesting that accounting for a complex relationship between these anatomical parameters would allow a high-fidelity prediction of nodal coverage. While such modeling would be impractical in the clinical setting, the totality of these results suggest that simultaneous SBRT to the prostate and PLNs can be performed with confidence, provided that changes in bladder filling (particularly, underfilling of the bladder) are taken into account with on-board imaging.
The results are highly concordant with our prior dosimetric analysis which found an average V100%CTVN of 92.6% among 10 patients who received conventional fractionation—using weekly CBCTs to model SBRT fractions—and two patients receiving SBRT. The shifts in the superior/inferior axis and the overall 3-D translational shift were the major predictors of nodal underdosing. The present study constitutes a more rigorous analysis of anatomic parameters in a larger cohort of patients who actually received SBRT. The regression modeling confirmed the importance of maintaining a stable anatomy in multiple planes. Notably, in the present analysis, shifts in the left-right axis are also strong predictors of undercoverage. This is likely explained by the sharp dose gradients in the axial plane that are designed to spare the bowel (Figure 3).
Figure 3.
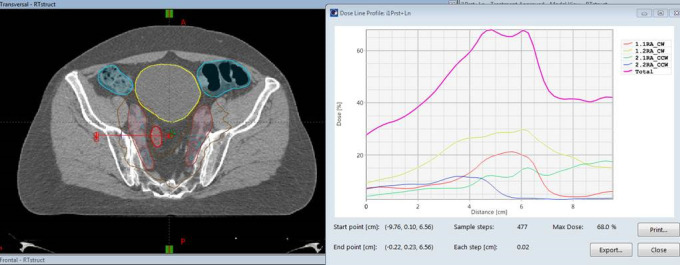
Representative planning CT with dose distribution (left) and the corresponding axial dose gradient (right). A shift of this dose distribution to the patient’s left (as indicated by the large red arrow) would lead to a sharp decrease in the dose over the span of a few millimeters, whereas a shift to the patient’s right would not cause a sharp decrease in the dose. In this case, the sharp dose gradient was designed to spare a loop of small bowel (outline in crimson) immediately adjacent to the nodal contour.
There are a few limitations associated with this study. Dosimetric changes to critical organs such as bowel were not evaluated in this study due to poor soft tissue contrast and image artifacts on the kV-CBCTs. With new development in fast image acquisition and advanced reconstruction algorithm, improvement in image quality may allow accurate delineation of daily anatomic change on CBCT images. Superior soft tissue contrast of MR image guidance makes it a potential tool to accurately assess these changes and potentially allow adaptive replanning. To account for daily position variation of the PLNs, one of practical solutions is to expand the nodal PTV margin. However, due to close proximity of bowel to nodal CTV, additional expansion of PTV margin very likely leads to exceeding of bowel constraints and is not feasible for all patients (e.g. of the five patients in the current study who had mean CTVN V100% < 90%, three already met bowel constraints, and an expansion of more than 5 mm would have led to violating constraints in the remaining two). Careful review of patient setup image and rigorous control of bladder filling are essential to reduce the daily positioning variations of PLNs. Finally, intrafractional motion may also contribute to underdosing, and was not examined in this study.
In summary, simultaneous SBRT to the prostate and PLNs based on rigid registration to IPMs provides adequate PLN coverage in most instances. Careful evaluation of bladder filling on kV-CBCT before treatment may be the most practical method for estimating accuracy prior to treatment. Alternative approaches, such as adaptive planning,5,10,11 may be useful as well.
Contributor Information
Amar U Kishan, Email: aukishan@gmail.com.
Marguerite Tyran, Email: marguerite.tyran@gmail.com.
Julius Weng, Email: JuliusWeng@mednet.ucla.edu.
Shrinivasa Upadhyaya, Email: skupadhyaya@ucdavis.edu.
James Lamb, Email: JLamb@mednet.ucla.edu.
Michael Steinberg, Email: MSteinberg@mednet.ucla.edu.
Christopher King, Email: crking@mednet.ucla.edu.
Minsong Cao, Email: minsongcao@mednet.ucla.edu.
REFERENCES
- 1. Schallenkamp JM , Herman MG , Kruse JJ , Pisansky TM . Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. International Journal of Radiation oncology, biology . Physics 2005. ; 63 : 800 – 11 . [DOI] [PubMed] [Google Scholar]
- 2. Dang A , Kupelian PA , Cao M , Agazaryan N , Kishan AU . Image-guided radiotherapy for prostate cancer . Transl Androl Urol 2018. ; 7 : 308 – 20 . doi: 10.21037/tau.2017.12.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsu A , Pawlicki T , Luxton G , Hara W , King CR . A study of image-guided intensity-modulated radiotherapy with fiducials for localized prostate cancer including pelvic lymph nodes. International Journal of Radiation oncology, biology . Physics 2007. ; 68 : 898 – 902 . [DOI] [PubMed] [Google Scholar]
- 4. Rossi PJ , Schreibmann E , Jani AB , Master VA , Johnstone PAS . Boost first, eliminate systematic error, and individualize CTV to PTV margin when treating lymph nodes in high-risk prostate cancer . Radiother Oncol 2009. ; 90 : 353 – 8 . doi: 10.1016/j.radonc.2008.09.021 [DOI] [PubMed] [Google Scholar]
- 5. Ferjani S , Huang G , Shang Q , Stephans KL , Zhong Y , Qi P , et al. . Alignment focus of daily image guidance for concurrent treatment of prostate and pelvic lymph nodes. International Journal of Radiation oncology, biology . Physics 2013. ; 87 : 383 – 9 . [DOI] [PubMed] [Google Scholar]
- 6. Lecavalier-Barsoum M , Souhami L , Cury F , Duclos M , Ruo R , Faria S . Pelvic lymph node displacement in high-risk prostate cancer patients treated with image guided intensity modulated radiation therapy with 2 independent target volumes . Pract Radiat Oncol 2015. ; 5 : 406 – 10 . doi: 10.1016/j.prro.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 7. Kaidar-Person O , Roach M . 3rd, Crehange G. Whole-pelvic nodal radiation therapy in the context of hypofractionation for high-risk prostate cancer patients: a step forward. International Journal of Radiation oncology, biology . Physics 2013. ; 86 : 600 – 5 . [DOI] [PubMed] [Google Scholar]
- 8. Lyons CA , King RB , Osman SOS , McMahon SJ , O'Sullivan JM , Hounsell AR , et al. . A novel CBCT-based method for derivation of CTV-PTV margins for prostate and pelvic lymph nodes treated with stereotactic ablative radiotherapy . Radiat Oncol 2017. ; 12 : 124 . doi: 10.1186/s13014-017-0859-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kishan AU , Lamb JM , Jani SS , Kang JJ , Steinberg ML , King CR . Pelvic nodal dosing with registration to the prostate: implications for high-risk prostate cancer patients receiving stereotactic body radiation therapy . Int J Radiat Oncol Biol Phys 2015. ; 91 : 832 – 9 . doi: 10.1016/j.ijrobp.2014.11.035 [DOI] [PubMed] [Google Scholar]
- 10. Li T , Thongphiew D , Zhu X , Lee WR , Vujaskovic Z , Yin F-F , et al. . Adaptive prostate IGRT combining online re-optimization and re-positioning: a feasibility study . Phys Med Biol 2011. ; 56 : 1243 – 58 . doi: 10.1088/0031-9155/56/5/002 [DOI] [PubMed] [Google Scholar]
- 11. Qi P , Pouliot J , Roach M , Xia P . Offline multiple adaptive planning strategy for concurrent irradiation of the prostate and pelvic lymph nodes . Med Phys 2014. ; 41 : 021704 . doi: 10.1118/1.4860663 [DOI] [PubMed] [Google Scholar]