Abstract
Background: Preoperative serum tumor markers have been widely used to predict prognosis in stage II and III colorectal cancer (CRC). However, few previous studies addressed the effect of increased preoperative numbers of tumor markers.
Methods: Patients with stage II and III CRC who underwent curative resection were included from January 2009 to October 2015. The relationship between serum tumor markers and clinicopathological parameters was analyzed. DFS and OS were compared in stage II and III CRC.
Results: The median follow-up was 45 months. In this study, 735 enrolled patients were assessed based on the numbers of increased tumor markers. We found that these increased tumor markers were closely associated with clinical stage, T stage, N stage, tumor location, pathology type, differentiation, lymphatic invasion and vascular invasion (all p values < 0.05). Furthermore, the number of increased tumor markers directly affected the survival of patients with CRC after curative surgery. The 3-year DFS and OS of patients with a score of 0 were 84.0% and 91.0%, respectively, which are much higher than those of patients with a score of 4 (42.9% and 37.8%, respectively) (p < 0.05). The 5-year DFS and OS of patients with a score of 0 were 75.9% and 77.9%, respectively, which are much higher than those of patients with a score of 4 (31.7% and 23.6%, respectively). Interestingly, our results suggested that stage III CRC patients with a score of 0 had longer DFS and OS times than stage II patients with scores of 3 and 4. Further analysis revealed statistically significant differences in OS (p < 0.05) but not in DFS.
Conclusions: The number of increased tumor markers could significantly predict prognosis in stage II and III CRC. In addition, these increased tumor markers had direct impacts on metastasis as well as the recurrence status and survival time of stage II and III CRC patients.
Keywords: CRC, CEA, CA19-9, CA242, CA125, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors with the third highest morbidity rate and the second highest mortality rate among males and females combined in the United States1. In China, CRC is the fifth most commonly diagnosed tumor, and the mortality rate of CRC has increased in recent years due to changes in lifestyle2, 3. Despite great advances in surgery and drug therapy, the five-year relative survival rate is only 65%, ranging from 90% to 14% in different stages4. The prognosis of CRC is affected by many factors, such as the quality of surgery, patients' compliance, and adjuvant therapy. After curative surgery, postoperative pathological reports are usually the main references to predict the prognosis. In addition, preoperative serum tumor markers are classic and important components with referenced value.
The detection of preoperative tumor markers is widely used in clinical practice because of its great convenience and acceptability. Serum carcinoembryonic antigen (CEA) has always been at a core position as a reliable tumor marker in CRC, which is recommended by the NCCN guidelines as a prognostic and monitoring indicator. CEA is an acid glycoprotein involved in cell recognition and cell adhesion and is secreted by solid tumors. The level of preoperative CEA influences the prognosis of multiple tumors, such as gastric cancer, lung cancer and CRC5, 6, 7. Furthermore, CEA levels are correlated with the metastasis and recurrence of CRC after curative surgery8, 9. CA19-9 can be elevated in digestive system tumors. For CRC, the preoperative increased level of CA19-9 predicts poor survival of CRC, and postoperative CA19-9 is a valuable prognostic index for monitoring lung and liver metastasis10, 11, 12. CA125 is shown to be expressed in ovarian cancer and gynecological diseases13. Furthermore, CA125 is also an independent factor for the prognosis of CRC14. CA242 has high specificity and sensitivity in CRC. CA242 is expressed independently of CEA, and the combination of CEA and CA242 has more sensitivity in CRC than either alone15.
Overall, many studies have confirmed the significance of serum tumor markers in the prognosis of CRC. However, few studies have focused on the elevated numbers of preoperative serum tumor markers in CRC. Therefore, in this study, the relation between preoperative tumor markers and clinicopathological characteristics was analyzed. We also investigated the effect of increased numbers of tumor markers on the prognosis of stage II and III CRC.
Materials and Methods
Patients
This study was a single-center retrospective clinical study and was registered in the Chinese Clinical Trial Registry (Approval No. ChiCTR1800016906). The studies on human subjects were approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (Approval No. 2018-KY-031K). Patients with pathologically diagnosed stage II and III CRC who underwent curative resection at the Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital were selected for participation from January 2009 to October 2015. Written informed consents were gained from all patients in this study. Patients under age 18 and those with emergency operations, incompatible pathological types, multiple primary tumors, unclear causes of death or death within 30 days, or incomplete data as well as those who were lost to follow-up were excluded.
Data collection and outcome definition
The clinical characteristics of the patients, including gender, age, tumor location and pathological reports, were obtained from electronic patient records and the departmental database. Pathological stage was assessed according to the 8th AJCC criterion for CRC. The results of four preoperative tumor markers (CEA, CA125, CA19-9 and CA242) were collected from biochemistry reports of the Laboratory Medicine Department, and no patients received any adjuvant radiotherapy or chemotherapy before these four tumor markers were determined. The upper normal limits of CEA, CA125, CA19-9 and CA242 were 5 ng/ml, 15 U/ml, 27 U/ml and 15 U/ml, respectively. If the values of these tumor markers were above the upper limit, they were considered positive. To analyze the effect of the numbers of increased preoperative tumor markers on the prognosis of stage II and III CRC, the weight of each marker was first evaluated. Subsequently, the patients were scored as 0, 1, 2, 3 and 4 according to the positive numbers of tumor markers; thus, there were five groups in this study.
Follow-up
All patients were followed up according to current guidelines, including blood laboratory testing, physical examination, colonoscopy, chest X-ray and CT (or MRI). The deadline for follow-up was October 31, 2017. Survival status and metastasis status were updated by telephone, email and medical history. Disease-free survival (DFS) was defined as the time from surgery to tumor metastasis or recurrence. Overall survival (OS) was defined as the time from surgery to death.
Statistical analysis
We first summarized the patients' basic information and then analyzed the factors affecting positive tumor markers. Then, the relationship between each group (different scores) and the clinical pathological indicators was tested, and we studied the survival differences among groups. Furthermore, we separately examined the survival differences among different groups with stage II and III CRC.
All data in this study were analyzed by IBM SPSS STATISTICS 22.0 software. Statistical methods mainly used the Pearson chi-square test (minimum theoretical frequency ≥ 5), continuous correction chi-square test (5>minimum theoretical frequency ≥ 1), Fisher's exact probability method (minimum theoretical frequency < 1) and rank sum test. Survival times, including 3- and 5-year survival rates, were assessed by the Kaplan-Meier method, and the log-rank test was used to compare the differences in survival rates among groups. Cox regression was used to detect the effect of various indicators on the prognosis of stage II and III colorectal cancer. P values were two-sided, with statistically significant differences at p < 0.05.
Results
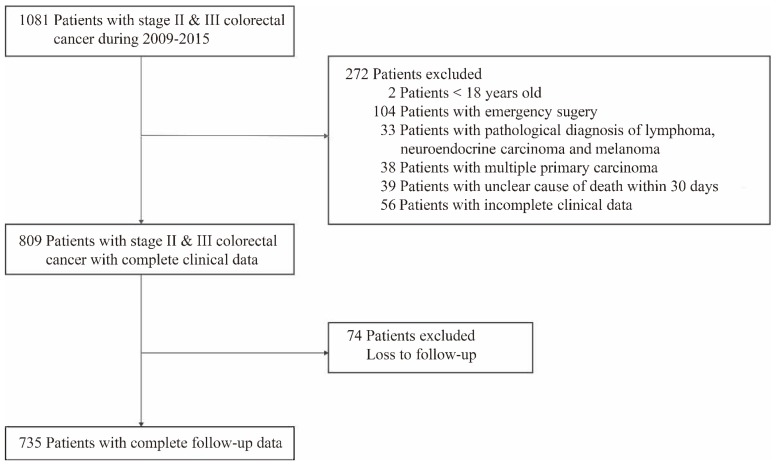
A total of 735 patients who underwent curative resection for stage II and III CRC with complete clinical and follow-up data were enrolled in our study. 346 Patients who met our exclusion criteria were excluded (Figure 1).
Figure 1.
Study design.
The patients in this study included 446 males (60.7%) and 289 females (39.3%). The median age was 66 years, ranging from 27 to 90. There were 403 patients (54.8%) older than 65, which was slightly higher than those younger than 65 years. A total of 428 patients (58.2%) were in clinicopathological stage II, while stage III accounted for 41.8%. A total of 716 patients had T3 (22.2%) and T4 (75.2%) primary tumors, while T1 and T2 (2.6% plus) tumors were less frequent. N0 was found in 428 patients (58.2%), and 307 patients (41.8%) had lymph node metastasis, with 176 N1 patients (23.9%) and 131 N2 patients (17.8%). In these cases, 461 patients (62.7%) had colon cancer, including 235 with right colon cancer (32.0%) and 226 with left colon cancer (30.7%), and 274 patients (37.3%) had rectal cancer. According to the pathological report, 689 patients (93.7%) had adenocarcinoma and signet ring cell carcinoma, and mucous adenocarcinoma occurred only in 46 cases (6.3%). A total of 435 carcinoma tissues (59.2%) were well or moderately differentiated, while the other 300 tissues (40.8%) were poorly differentiated. According to our data, perineural infiltration in stage II and III CRC tissues was more likely to occur, and 700 cases (95.2%) were observed. Furthermore, lymphatic invasion was found in 439 patients (59.7%), and vascular invasion was caught in 102 patients (13.9%). By the end of our follow-up time, 222 patients (30.2%) had recurrence or metastasis, and 235 patients (32%) had died (Table 1).
Table 1.
Patient demographics and clinicopathologic features
| Variables | Patients (N=735) |
|---|---|
| Gender | |
| Male | 446 (60.7%) |
| Female | 289 (39.3%) |
| Age, median (Range) | 66 (27 to 90) |
| <65 | 332 (45.2%) |
| ≥65 | 403 (54.8%) |
| Clinical stage | |
| II | 428 (58.2%) |
| III | 307 (41.8%) |
| T stage | |
| T1+T2 | 19 (2.6%) |
| T3 | 163 (22.2%) |
| T4 | 553 (75.2%) |
| N stage | |
| N0 | 428 (58.2%) |
| N1 | 176 (23.9%) |
| N2 | 131 (17.9%) |
| Tumor location | |
| Right colon | 235 (32.0%) |
| Left colon | 226 (30.7%) |
| Rectum | 274 (37.3%) |
| Pathological type | |
| Adenocarcinoma | 689 (93.7%) |
| Mucinous Adenocarcinoma | 42 (5.7%) |
| Signet ring cell carcinoma | 4 (0.6%) |
| Degree of differentiation | |
| Moderate and well | 435 (59.2%) |
| Poor | 300 (40.8%) |
| Lymphatic invasion | |
| Yes | 439 (59.7%) |
| No | 296 (40.3%) |
| Vascular invasion | |
| Yes | 102 (13.9%) |
| No | 633 (86.1%) |
| Perineural invasion | |
| Yes | 700 (95.2%) |
| No | 35 (4.8%) |
| Metastasis and recurrence | |
| Yes | 222 (30.2%) |
| No | 513 (69.8%) |
| Survival status | |
| Alive | 500 (68.0%) |
| Dead | 235 (32.0%) |
Then, the correlation between preoperative serum tumor markers and clinical and pathological parameters was analyzed. The Chi-square test results showed that the elevation of CEA was associated with clinical stage, T stage (tumor, the depth of primary tumor infiltration), N stage (lymph node, the number and extent of lymph node metastasis), tumor location and lymphatic invasion (all p values < 0.05; Table 2). In contrast, there was no significant difference in gender, age, pathology type, differentiation, vascular invasion or perineural invasion (p>0.05; Table 2). Our results also found that preoperative serum CA125 expression was significantly different in terms of T stage (T3 and T4), tumor location, pathology type and lymphatic invasion (p < 0.05; Table 2). However, there was no significant difference in terms of gender, age, clinical stage, N stage, differentiation, vascular invasion or perineural invasion (p>0.05; Table 2). Similar to CEA, the level of CA19-9 had statistical significance with clinical stage, N stage, pathology type, differentiation, lymphatic invasion and vascular invasion (p < 0.05; Table 2). However, no significant difference was obtained in terms of gender, age, T stage, tumor location and perineural invasion (p>0.05; Table 2). Furthermore, the increase in CA242 was related to clinical stage, N stage, tumor location, pathology type, differentiation and vascular invasion (p < 0.05; Table 2), while there was no significant difference in terms of gender, age, T stage, lymphatic invasion or perineural invasion (p>0.05; Table 2). In addition, the preoperative elevation of CEA, CA199 and CA242 was associated with metastasis and recurrence as well as poor survival status in stage II and III CRC (p < 0.05; Table 2). Interestingly, the increase in CA125 predicted poor survival status (p < 0.05; Table 2) rather than metastasis and recurrence (p>0.05; Table 2).
Table 2.
The association of demographics and clinicopathologic characteristics with different serum tumor markers (# means the p values compared T3 with T4)
| Variables | CEA | CA125 | CA19-9 | CA242 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | p-value | Positive | Negative | p-value | Positive | Negative | p-value | Positive | Negative | p-value | ||||
| (N=316) | (N=419) | (N=236) | (N=499) | (N=166) | (N=569) | (N=151) | (N=584) | ||||||||
| Gender | 0.380 | 0.973 | 0.301 | 0.234 | |||||||||||
| Male | 186 | 260 | 143 | 303 | 95 | 351 | 98 | 348 | |||||||
| Female | 130 | 159 | 93 | 196 | 71 | 218 | 53 | 236 | |||||||
| Age | 0.478 | 0.127 | 0.377 | 0.132 | |||||||||||
| <65 | 138 | 194 | 97 | 235 | 70 | 262 | 60 | 272 | |||||||
| ≥65 | 178 | 225 | 139 | 264 | 96 | 307 | 91 | 312 | |||||||
| Clinical stage | 0.000 | 0.177 | 0.000 | 0.003 | |||||||||||
| II | 159 | 269 | 129 | 299 | 76 | 352 | 72 | 356 | |||||||
| III | 157 | 150 | 107 | 200 | 90 | 217 | 79 | 228 | |||||||
| T stage | 0.012 | 0.095 | 0.250 | 0.305 | |||||||||||
| T1+T2 | 6 | 13 | 7 | 12 | 2 | 17 | 2 | 17 | |||||||
| T3 | 55 | 108 | 0.005# | 41 | 122 | 0.033# | 33 | 130 | 0.358# | 29 | 134 | 0.280# | |||
| T4 | 255 | 298 | 188 | 365 | 131 | 422 | 120 | 433 | |||||||
| N stage | 0.001 | 0.151 | 0.000 | 0.003 | |||||||||||
| N0 | 159 | 269 | 129 | 299 | 76 | 352 | 72 | 356 | |||||||
| N1 | 92 | 84 | 67 | 109 | 45 | 131 | 39 | 137 | |||||||
| N2 | 65 | 66 | 40 | 91 | 45 | 86 | 40 | 91 | |||||||
| Location | 0.006 | 0.011 | 0.319 | 0.008 | |||||||||||
| Right colon | 84 | 151 | 90 | 145 | 55 | 180 | 63 | 172 | |||||||
| Left colon | 114 | 112 | 75 | 151 | 57 | 169 | 45 | 181 | |||||||
| Rectum | 156 | 118 | 71 | 203 | 54 | 220 | 43 | 231 | |||||||
| Pathology type | 0.056 | 0.018 | 0.041 | 0.000 | |||||||||||
| Adenocarcinoma | 290 | 399 | 214 | 475 | 150 | 539 | 129 | 560 | |||||||
| Other | 26 | 20 | 22 | 24 | 16 | 30 | 22 | 24 | |||||||
| Differentiation | 0.447 | 0.453 | 0.044 | 0.004 | |||||||||||
| Moderate and well | 182 | 253 | 135 | 300 | 87 | 348 | 74 | 361 | |||||||
| Poor | 134 | 166 | 101 | 199 | 79 | 221 | 77 | 223 | |||||||
| Lymphatic invasion | 0.014 | 0.006 | 0.004 | 0.068 | |||||||||||
| Yes | 205 | 234 | 158 | 281 | 115 | 324 | 100 | 339 | |||||||
| No | 111 | 185 | 78 | 218 | 51 | 245 | 51 | 245 | |||||||
| Vascular invasion | 0.123 | 0.864 | 0.000 | 0.001 | |||||||||||
| Yes | 51 | 51 | 32 | 70 | 37 | 65 | 33 | 69 | |||||||
| No | 265 | 368 | 204 | 429 | 129 | 504 | 118 | 515 | |||||||
| Perineural invasion | 0.157 | 0.930 | 0.430 | 0.438 | |||||||||||
| Yes | 11 | 24 | 11 | 24 | 6 | 29 | 9 | 26 | |||||||
| No | 305 | 395 | 225 | 475 | 160 | 540 | 142 | 558 | |||||||
| Metastasis & recurrence | 0.001 | 0.094 | 0.000 | 0.000 | |||||||||||
| Yes | 116 | 106 | 81 | 141 | 71 | 151 | 71 | 151 | |||||||
| No | 200 | 313 | 155 | 358 | 95 | 418 | 80 | 433 | |||||||
| Survival status | 0.000 | 0.000 | 0.000 | 0.000 | |||||||||||
| Alive | 193 | 307 | 131 | 369 | 90 | 410 | 67 | 433 | |||||||
| Dead | 123 | 112 | 105 | 130 | 76 | 159 | 84 | 151 |
Our univariate analysis results indicated that clinical stage, N stage, pathological type, tumor differentiation, lymphatic invasion, vascular invasion and serum tumor markers (CEA, CA125, CA19-9, CA242) were prognostic factors of disease-free survival (DFS) and overall survival (OS). However, gender, age, T stage, tumor location and perineural invasion had no significance for DFS and OS. Multivariate analysis found that CA242 was independent prognostic factor for both DFS (HR=1.641, 95%CI, 1.178- 2.286, p=0.003; Table 3) and OS (HR=2.003, 95%CI, 1.471- 2.728, p=0.000; Table 3), and CA125 was independent prognostic factor for OS (HR=1.846, 95%CI, 1.416- 2.406, p=0.000; Table 3) but not DFS (HR=1.303, 95%CI, 0.983- 1.726, p=0.066; Table 3).
Table 3.
Univariate and multivariate Cox regression analysis for DFS and OS
| Variables | DFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Univariate analysis | ||||
| Gender (male vs. female) | 1.060 (0.809- 1.390) | 0.672 | 1.082 (0.831- 1.407) | 0.559 |
| Age (<65 vs. ≥65 ) | 0.842 (0.647- 1.095) | 0.199 | 1.104 (0.852- 1.429) | 0.454 |
| Clinical stage (II vs. III) | 2.242 (1.718- 2.926) | 0.000 | 2.412 (1.859- 3.131) | 0.000 |
| T stage | ||||
| T1+T2 | 1 (Referent) | 1 (Referent) | ||
| T3 | 0.599 (0.267- 1.347) | 0.215 | 0.836 (0.354- 1.976) | 0.684 |
| T4 | 1.013 (0.476- 2.156) | 0.973 | 1.404 (0.623- 3.167) | 0.413 |
| N stage | ||||
| N0 | 1 (Referent) | 1 (Referent) | ||
| N1 | 1.611 (1.160- 2.237) | 0.004 | 1.754 (1.276- 2.411) | 0.001 |
| N2 | 3.308 (2.426- 4.510) | 0.000 | 3.513 (2.597- 4.752) | 0.000 |
| Location | ||||
| Right colon | 1 (Referent) | 1 (Referent) | ||
| Left colon | 1.233 (0.880- 1.730) | 0.224 | 1.072 (0.775- 1.483) | 0.675 |
| Rectum | 1.218 (0.881- 1.684) | 0.234 | 1.086 (0.796- 1.482) | 0.603 |
| Pathology type (Adenocarcinoma vs. other) | 0.443 (0.292- 0.672) | 0.000 | 0.484 (0.322- 0.727) | 0.000 |
| Differentiation (Moderate and well vs. poor) | 1.475 (1.133- 1.919) | 0.004 | 1.547 (1.196- 1.999) | 0.001 |
| Lymphatic invasion (yes vs. no) | 1.362 (1.032- 1.797) | 0.029 | 1.383 (1.051- 1.819) | 0.020 |
| Vascular invasion (yes vs. no) | 1.998 (1.444- 2.765) | 0.000 | 1.878 (1.371- 2.573) | 0.000 |
| Perineural invasion (yes vs. no) | 1.557 (0.769- 3.155) | 0.219 | 2.162 (0.961- 4.864) | 0.062 |
| CEA (positive vs. negative) | 1.665 (1.279- 2.167) | 0.000 | 1.661 (1.286- 2.146) | 0.000 |
| CA125 (positive vs. negative) | 1.373 (1.045- 1.805) | 0.023 | 1.904 (1.472- 2.463) | 0.000 |
| CA19-9 (positive vs. negative) | 1.851 (1.396- 2.455) | 0.000 | 1.863 (1.417- 2.450) | 0.000 |
| CA242 (positive vs. negative) | 2.343 (1.765- 3.109) | 0.000 | 2.787 (2.132- 3.643) | 0.000 |
| Multivariate analysis | ||||
| N stage | ||||
| N0 | 1 (Referent) | 1 (Referent) | ||
| N1 | 1.854 (1.264- 2.719) | 0.002 | 1.997 (1.386- 2.876) | 0.000 |
| N2 | 3.653 (2.442- 5.465) | 0.000 | 4.298 (2.919- 6.328) | 0.000 |
| Pathology type (Adenocarcinoma vs. other) | 0.569 (0.362- 0.894) | 0.014 | 0.625 (0.403- 0.970) | 0.036 |
| Differentiation (M and W vs. poor) | 1.276 (0.958- 1.699) | 0.096 | 1.309 (0.989- 1.733) | 0.060 |
| Lymphatic invasion (yes vs. no) | 1.656 (1.139- 2.407) | 0.008 | 1.802 (1.254- 2.588) | 0.001 |
| Vascular invasion (yes vs. no) | 1.522 (1.086- 2.134) | 0.015 | 1.440 (1.038- 1.999) | 0.029 |
| CEA (positive vs. negative) | 1.282 (0.967- 1.700) | 0.084 | 1.238 (0.942- 1.627) | 0.125 |
| CA125 (positive vs. negative) | 1.303 (0.983- 1.726) | 0.066 | 1.846 (1.416- 2.406) | 0.000 |
| CA19-9 (positive vs. negative) | 1.156 (0.830- 1.609) | 0.391 | 1.113 (0.810- 1.529) | 0.510 |
| CA242 (positive vs. negative) | 1.641 (1.178- 2.286) | 0.003 | 2.003 (1.471- 2.728) | 0.000 |
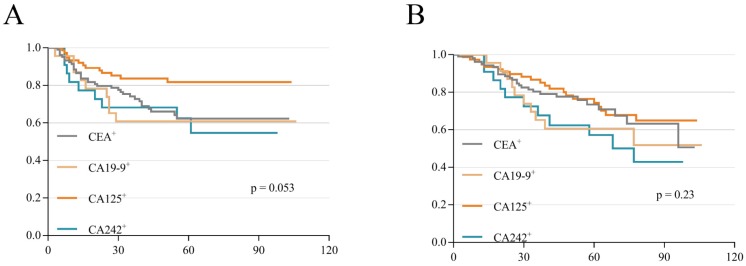
To verify the weight of each serum tumor marker on the prognosis of stage II and III CRC, we analyzed the differences among these increased tumor markers according to DFS and OS. The results showed no significant difference among these tumor markers between DFS (p=0.053; Figure 2A) and OS (p=0.23; Figure 2B), which meant that the weight difference of these tumor markers was narrow. Therefore, these 735 patients were scored on a scale of 0 to 4 (five groups) based on the number of preoperative increased tumor markers (if none/anyone/ any two/ any three/all among the 4 markers increased, the score is 0/1/2/3/4 respectively). Then, the association between different scores and clinicopathologic variables was revealed. We found that different scores were closely related to clinical stage, T stage, N stage, tumor location, pathology type, differentiation, lymphatic invasion and vascular invasion (all p values < 0.05; Table 4). There was no significant difference in gender, age, T3/T4, or perineural invasion (p>0.05; Table 4). In addition, these different scores significantly affected metastasis as well as the recurrence status and survival status (p < 0.05; Table 4). To further clarify which groups had differences, we selected the clinicopathologic variables with p values less than 0.05 for a subgroup analysis. As shown in Table 5, there was a pairwise comparison between different scores and variables (Table 5).
Figure 2.
K-M survival curves according to different single positive tumor markers. (A) DFS curves according to single positive tumor markers. (B) OS curves according to single positive tumor markers.
Table 4.
The association of demographics and clinicopathologic characteristics with different scores (# means the p value compared T3 with T4)
| Variables | 0 | 1 | 2 | 3 | 4 | p-value |
|---|---|---|---|---|---|---|
| (N=248) | (N=227) | (N=167) | (N=63) | (N=30) | ||
| Gender | 0.370 | |||||
| Male | 155 | 136 | 100 | 33 | 22 | |
| Female | 93 | 91 | 67 | 30 | 8 | |
| Age | 0.184 | |||||
| <65 | 119 | 110 | 67 | 22 | 14 | |
| ≥65 | 129 | 117 | 100 | 41 | 16 | |
| Stage | 0.000 | |||||
| II | 163 | 148 | 75 | 29 | 13 | |
| III | 85 | 79 | 92 | 34 | 17 | |
| T stage | 0.042 | |||||
| T1+T2 | 8 | 5 | 6 | 0 | 0 | |
| T3 | 66 | 54 | 28 | 12 | 3 | 0.054# |
| T4 | 174 | 168 | 133 | 51 | 27 | |
| N stage | 0.000 | |||||
| N0 | 163 | 148 | 75 | 29 | 13 | |
| N1 | 45 | 51 | 52 | 24 | 4 | |
| N2 | 40 | 28 | 40 | 10 | 13 | |
| Location | 0.002 | |||||
| Right colon | 82 | 68 | 43 | 30 | 12 | |
| Left colon | 66 | 64 | 68 | 21 | 7 | |
| Rectum | 100 | 95 | 56 | 12 | 11 | |
| Pathology type | 0.001 | |||||
| Adenocarcinoma | 239 | 218 | 153 | 56 | 23 | |
| Other | 9 | 9 | 14 | 7 | 7 | |
| Differentiation | 0.020 | |||||
| Moderate and well | 152 | 149 | 89 | 29 | 16 | |
| Poor | 96 | 78 | 78 | 34 | 14 | |
| Lymphatic invasion | 0.001 | |||||
| Yes | 131 | 126 | 116 | 44 | 22 | |
| No | 177 | 101 | 51 | 19 | 8 | |
| Vascular invasion | 0.020 | |||||
| Yes | 27 | 28 | 24 | 15 | 8 | |
| No | 221 | 199 | 143 | 48 | 22 | |
| Perineural invasion | 0.718 | |||||
| Yes | 236 | 216 | 157 | 62 | 29 | |
| No | 12 | 11 | 10 | 1 | 1 | |
| Metastasis and recurrence | 0.000 | |||||
| Yes | 52 | 65 | 59 | 28 | 18 | |
| No | 196 | 162 | 108 | 35 | 12 | |
| Survival status | 0.000 | |||||
| Alive | 204 | 157 | 101 | 29 | 9 | |
| Dead | 44 | 70 | 66 | 34 | 21 |
Table 5.
Further pairwise comparison of parameters with p values less than 0.05 in the Table 4
| Variables | 0 vs 1 | 0 vs 2 | 0 vs 3 | 0 vs 4 | 1 vs 2 | 1 vs 3 | 1 vs 4 | 2 vs 3 | 2 vs 4 | 3 vs 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | |||||||||||||
| Stage | 0.904 | 0.000 | 0.004 | 0.016 | 0.000 | 0.006 | 0.020 | 0.879 | 0.873 | 0.807 | |||
| T stage | 0.585 | 0.063 | 0.136 | 0.021 | 0.188 | 0.231 | 0.052 | 0.737 | 0.169 | 0.419 | |||
| N stage | 0.318 | 0.000 | 0.002 | 0.002 | 0.000 | 0.017 | 0.001 | 0.358 | 0.041 | 0.006 | |||
| Location | 0.764 | 0.010 | 0.006 | 0.748 | 0.033 | 0.003 | 0.533 | 0.005 | 0.139 | 0.175 | |||
| Pathology type | 0.848 | 0.038 | 0.037 | 0.000 | 0.065 | 0.059 | 0.000 | 0.522 | 0.034 | 0.218 | |||
| Differentiation | 0.326 | 0.105 | 0.028 | 0.400 | 0.013 | 0.005 | 0.186 | 0.326 | 0.997 | 0.510 | |||
| Lymphatic invasion | 0.003 | 0.000 | 0.000 | 0.001 | 0.005 | 0.041 | 0.063 | 0.955 | 0.670 | 0.729 | |||
| Vascular invasion | 0.622 | 0.289 | 0.007 | 0.030 | 0.555 | 0.023 | 0.065 | 0.089 | 0.158 | 0.765 | |||
| Metastasis and recurrence | 0.053 | 0.001 | 0.000 | 0.000 | 0.157 | 0.017 | 0.001 | 0.204 | 0.011 | 0.161 | |||
| Survival status | 0.001 | 0.000 | 0.000 | 0.000 | 0.073 | 0.001 | 0.000 | 0.049 | 0.002 | 0.142 | |||
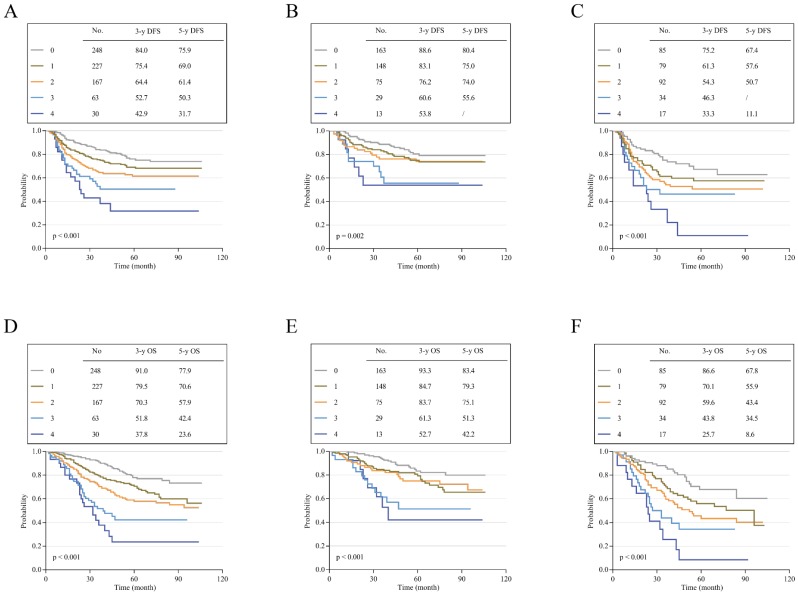
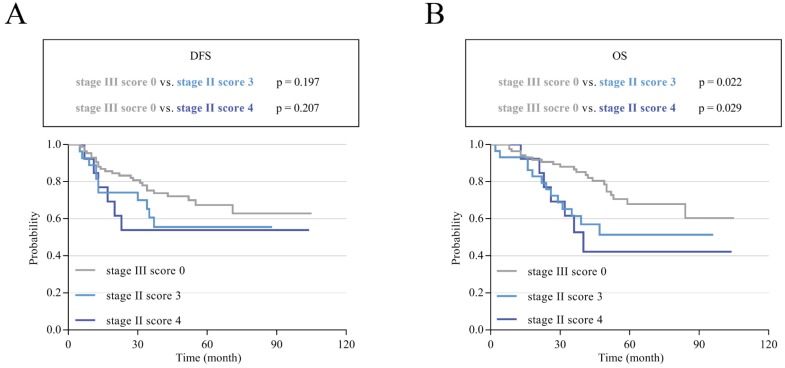
To assess the effects of different scores on the prognosis of stage II and III CRC, Kaplan-Meier survival curves were performed according to our follow-up data. The results showed that patients with high scores had poor DFS and OS; additionally, the higher the score was, the poorer the survival. The 3-year and 5-year DFS for scores 0-4 ranged from 84.0% and 75.9% to 42.9% and 31.7% (p<0.001; Figure 3A). For OS, the 3-year and 5-year rates for scores 0-4 decreased from 91.0% and 77.9% to 37.8% and 23.6% (p<0.001; Figure 3D). Subgroup analysis showed that the survival results of patients with stage II or III CRC also matched this finding. In stage II, the 3-year and 5-year DFS decreased from 88.6% and 80.4% to 53.8% (score=4) and 55.6% (score=3) (p=0.002; Figure 3B). The 3-year and 5-year OS for patients with a score of 0 were 93.3% and 83.4%, respectively, which were much higher than the values for those with a score of 4 (52.7% and 42.2%, respectively, p<0.001; Figure 3E). In stage III, this trend was more pronounced. The 3-year and 5-year DFS ranged from 75.2% and 67.4% to 33.3% and 11.1% (p<0.001; Figure 3C). For OS, the rates obviously decreased from 86.6% and 67.8% to 25.7% and 8.6% (p<0.001; Figure 3F). Mean survival time was also demonstrated for patients with different scores of stage II and III CRC. The overall mean DFS was 85.402 months, and the mean OS was 86.208 months for stage II patients. For stage III patients, the mean DFS was 65.997 months, and the mean OS was 65.769 months. In general, the overall DFS was 77.608 months, and the overall OS was 77.8 months for these 735 patients. Interestingly, we found that stage III patients with low scores had a better prognosis than stage II patients with high scores (Table 6). To verify whether there was a statistically significant difference between them, we performed a statistical analysis of DFS and OS. The results showed that stage III CRC patients with a score of 0 had a longer DFS and OS than stage II patients with scores of 3 and 4, and there was a statistically significant difference in OS (p < 0.05; Figure 4B) but not in DFS (p>0.05; Figure 4A).
Figure 3.
Disease-free survival and overall survival according to different scores. (A) K-M curves of DFS in all patients. (B) K-M curves of DFS in patients with stage II CRC. (C) K-M curves of DFS in patients with stage III CRC. (D) K-M curves of OS in all patients. (E) K-M curves of OS in patients with stage II CRC. (F) K-M curves of OS in patients with stage III CRC.
Table 6.
Means and 95% CI for DFS and OS in patients with stage II and III CRC
| Stage | Score | Mean disease-free survival time(months) | Mean overall survival time(months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | ||||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| II | 0 | 90.943 | 2.626 | 85.795 | 96.09 | 93.883 | 2.362 | 89.254 | 98.513 |
| 1 | 85.274 | 3.157 | 79.087 | 91.461 | 84.755 | 3.063 | 78.752 | 90.758 | |
| 2 | 81.966 | 4.531 | 73.085 | 90.846 | 83.354 | 4.143 | 75.234 | 91.475 | |
| 3 | 57.824 | 6.967 | 44.169 | 71.478 | 61.525 | 7.105 | 47.6 | 75.45 | |
| 4 | 63 | 12.325 | 38.843 | 87.157 | 60.116 | 11.137 | 38.288 | 81.945 | |
| Overall | 85.403 | 1.853 | 81.771 | 89.036 | 86.208 | 1.754 | 82.77 | 89.646 | |
| III | 0 | 77.497 | 4.579 | 68.522 | 86.472 | 81.298 | 4.236 | 72.995 | 89.601 |
| 1 | 67.027 | 4.919 | 57.385 | 76.668 | 68.136 | 4.337 | 59.635 | 76.636 | |
| 2 | 60.782 | 4.692 | 51.586 | 69.978 | 59.778 | 4.232 | 51.484 | 68.073 | |
| 3 | 46.062 | 6.206 | 33.898 | 58.227 | 43.123 | 5.413 | 32.514 | 53.732 | |
| 4 | 28.489 | 7.276 | 14.228 | 42.75 | 29.319 | 6.052 | 17.457 | 41.181 | |
| Overall | 65.997 | 2.616 | 60.869 | 71.124 | 65.769 | 2.407 | 61.051 | 70.488 | |
| Overall | Overall | 77.608 | 1.58 | 74.51 | 80.706 | 77.8 | 1.492 | 74.875 | 80.725 |
Figure 4.
K-M survival curves in stage III CRC patients with a score of 0 and stage II patients with scores of 3 and 4. (A) K-M curves of DFS comparing patients with stage III tumors and a score of 0 with patients with stage II tumors and scores of 3 and 4. (B) K-M curves of OS comparing patients with stage III tumors and a score of 0 with patients with stage II tumors and scores of 3 and 4.
Discussion
Preoperative serum tumor markers have long been used as prognostic indicators of CRC. Recently, the role of serum tumor markers has been underestimated due to more predictive indicators, especially the application of genetic testing. Serum tumor markers have often been used as references for the efficacy of postoperative chemotherapy. CEA is the most important serum tumor marker in the prognosis and therapeutic effect of CRC according to current guidelines16, 17. Nevertheless, other serum tumor markers also have significant implications for the prognosis of CRC. Several studies have found that combined detection of multiple tumor markers can improve the early detection of pancreatic cancer18 and colorectal cancer10.
In our study, 735 patients with stage II and III CRC were enrolled and analyzed based on their clinicopathological and follow-up data. We demonstrated the association between tumor markers and clinicopathological parameters, which was consistent with the findings of a previous study19. Further, patients were scored according to the number of preoperatively increased tumor markers. We also clarified the relationship between groups with different scores and clinicopathologic variables and confirmed that the higher the score was, the worse the survival. More importantly, we observed that patients with stage II with scores of 3 and 4 had shorter overall survival times than those with stage III with a score of 0, and this difference was statistically significant.
Many studies have focused on serum tumor markers in stages I to III CRC. Jung et al. analyzed 472 CRC patients and found that preoperative CEA was an independent prognostic factor with regard to CSS and DFS, and CA 19-9 also had prognostic value for CSS and DFS20. Another study that enrolled 237 patients found that CEA predicted OS (HR 2.50, 95% CI 1.17-5.36, P = 0.02) and DFS (HR 1.78, 95% CI 1.02-3.13, P = 0.04)7. Similar to our study, Gao et al. analyzed the relationship between serum tumor markers (including CEA, CA19-9, CA72-4 and CA125) and clinicopathologic factors and suggested that the combination of multiple preoperative tumor markers could improve the early diagnosis and treatment of CRC21. Additionally, Ning et al. found that the combined detection of serum tumor markers was useful not only in the diagnosis of CRC but also in gastric cancer22. A study from Japan and the United States focused on preoperative and postoperative CEA levels, and the results suggested that elevated postoperative CEA (hazard ratio, 2.0; 95% CI, 1.1-3.5) had a shorter RFS than normalized postoperative CEA (HR, 0.77; 95% CI, 0.45-1.30)23. Another innovative study demonstrated that the preoperative CEA cut-off point should be 2.35 ng/mL in stage I and II colon cancer24. In recent years, many studies have begun to explore the significance of postoperative tumor markers. A Japanese study suggested that the combination of post-CEA and post-CA 19-9 after R0 resection in stage IV CRC could predict the risk of recurrence25. Moreover, Araujo RL et al. showed that postoperative CEA ≥ 15 ng/ml strongly indicated recurrence after resection for colorectal liver metastases26. It can be seen that serum tumor markers are of great value in predicting the prognosis of CRC, and we still need to mine more data and conduct more research to show their value.
Nevertheless, few studies had been involved in the survival of patients with stage II and III CRC who were scored based on an increased number of serum tumor markers. As is known, according to the results of current large clinical trials27, 28, there are still many controversial points in the subsequent treatment of stage II and III CRC after curative resection. Our study found that stage III patients with low scores had longer DFS and OS times than stage II patients with high scores, which surprised us. Based on pathological reports, postoperative radiotherapy and chemotherapy may have defective aspects. Should we refer to the preoperative serum tumor markers when we give postoperative chemotherapy to stage II patients? In other words, should all stage III patients require whole-course chemotherapy? At the same time, our research has some shortcomings. This is a single-center retrospective case study with a limited number of cases included. In addition, the few numbers of patients with scores of 4 in the study restricted our study of these patients.
Despite some new findings from our study, it is important to note that tumor markers cannot replace the pathological criteria or the role of imaging examinations in the follow-up of CRC. However, as a supplement, serum tumor markers should be given more attention. We will further explore the significance of preoperative and postoperative tumor markers and use serum tumor markers to detect the metastasis or recurrence of CRC as soon as possible.
In conclusion, preoperative serum tumor markers are related to the prognosis of stage II and III CRC, and the number of increased tumor markers is closely related to the DFS and OS of CRC patients.
Acknowledgments
This work was supported by Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (no.20172023), Shanghai Science and Technology Commission Medical Project (no.16411953200), Shanghai Pujiang Program (no.16PJ1408200), Natural Science Foundation of Shanghai (no.16ZR1449600), National Natural Science Foundation of China (no.81602689).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Fang JY, Dong HL, Sang XJ. et al. CRC Mortality Characteristics and Predictions in China, 1991-2011. Asian Pacific Journal of Cancer Prevention. 2015;16(17):7991–7995. doi: 10.7314/apjcp.2015.16.17.7991. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fedewa SA. et al. CRC statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Feng XY, Li YF. et al. The prognosis of gastric cancer patients with marginally elevated carcinoembryonic antigen (CEA) values after D2 radical gastrectomy. J Surg Oncol. 2013;107(6):641–645. doi: 10.1002/jso.23300. [DOI] [PubMed] [Google Scholar]
- 6.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76(2):138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Gunawardene A, Larsen P, Shekouh A. et al. Pre-operative carcinoembryonic antigen predicts survival following CRC surgery with curative intent. ANZ J Surg. 2018;88(12):1311–1315. doi: 10.1111/ans.14723. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal C, Meropol NJ, Punt CJ. et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic CRC. Ann Oncol. 2013;24(2):420–428. doi: 10.1093/annonc/mds336. [DOI] [PubMed] [Google Scholar]
- 9.Primrose JN, Perera R, Gray A. et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of CRC: the FACS randomized clinical trial. JAMA. 2014;311(3):263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 10.Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor CRC. Clin CRC. 2014;13(4):239–244. doi: 10.1016/j.clcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Lin PC, Lin JK, Lin CC. et al. Carbohydrate antigen 19-9 is a valuable prognostic factor in CRC patients with normal levels of carcinoembryonic antigen and may help predict lung metastasis. Int J Colorectal Dis. 2012;27(10):1333–1338. doi: 10.1007/s00384-012-1447-1. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Tang J, Li L-H. et al. Serum Carbohydrate Antigen 19-9 as an Indicator of Liver Metastasis in Colorectal Carcinoma Cases. Asian Pacific Journal of Cancer Prevention. 2013;14(2):909–913. doi: 10.7314/apjcp.2013.14.2.909. [DOI] [PubMed] [Google Scholar]
- 13.Romagnolo C, Leon AE, Fabricio ASC. et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: An Italian multicenter study. Gynecol Oncol. 2016;141(2):303–311. doi: 10.1016/j.ygyno.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Yang XQ, Li Y, Chen C. et al. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in CRC. Med Oncol. 2011;28(3):789–795. doi: 10.1007/s12032-010-9518-z. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson O, Johansson C, Glimelius B. et al. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992;65(2):215–221. doi: 10.1038/bjc.1992.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Cervantes A, Adam R. et al. ESMO consensus guidelines for the management of patients with metastatic CRC. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 17.Provenzale D, Gupta S, Ahnen DJ. et al. NCCN Guidelines Insights: CRC Screening, Version 1.2018. J Natl Compr Canc Netw. 2018;16(8):939–949. doi: 10.6004/jnccn.2018.0067. [DOI] [PubMed] [Google Scholar]
- 18.Reitz D, Gerger A, Seidel J. et al. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J Clin Pathol. 2015;68(6):427–433. doi: 10.1136/jclinpath-2014-202451. [DOI] [PubMed] [Google Scholar]
- 19.Zhong W, Yu Z, Zhan J. et al. Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in CRC. Pathol Oncol Res. 2015;21(1):83–95. doi: 10.1007/s12253-014-9791-9. [DOI] [PubMed] [Google Scholar]
- 20.Giessen-Jung C, Nagel D, Glas M. et al. Preoperative serum markers for individual patient prognosis in stage I-III colon cancer. Tumour Biol. 2015;36(10):7897–7906. doi: 10.1007/s13277-015-3522-z. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Wang J, Zhou Y. et al. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for CRC. Sci Rep. 2018;8(1):2732. doi: 10.1038/s41598-018-21048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning S, Wei W, Li J. et al. Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels in gastric and CRC patients. J Cancer. 2018;9(3):494–501. doi: 10.7150/jca.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi T, Shimada Y, Hsu M. et al. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4(3):309–315. doi: 10.1001/jamaoncol.2017.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margalit O, Mamtani R, Yang YX. et al. Assessing the prognostic value of carcinoembryonic antigen levels in stage I and II colon cancer. Eur J Cancer. 2018;94:1–5. doi: 10.1016/j.ejca.2018.01.112. [DOI] [PubMed] [Google Scholar]
- 25.Abe S, Kawai K, Ishihara S. et al. Prognostic impact of carcinoembryonic antigen and carbohydrate antigen 19-9 in stage IV CRC patients after R0 resection. J Surg Res. 2016;205(2):384–392. doi: 10.1016/j.jss.2016.06.078. [DOI] [PubMed] [Google Scholar]
- 26.Araujo RL, Gonen M, Allen P. et al. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(9):3087–3093. doi: 10.1245/s10434-014-4358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonardi S, Sobrero A, Rosati G. et al. Phase III trial comparing 3-6 months of adjuvant FOLFOX4/XELOX in stage II-III colon cancer: safety and compliance in the TOSCA trial. Ann Oncol. 2016;27(11):2074–2081. doi: 10.1093/annonc/mdw404. [DOI] [PubMed] [Google Scholar]
- 28.Thierry A, Dewi V, Laurent M. et al. Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer: Disease-Free Survival Results From a Randomized, OpenLabel, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol. 2018;36(15):1469–1477. doi: 10.1200/JCO.2017.76.0355. [DOI] [PubMed] [Google Scholar]