Abstract
Notwithstanding recent advances, cognitive impairments are among the most difficult-to-treat symptoms in neuropsychiatric disorders. Deficits in information processing contributing to memory and sociability impairments are found across neuropsychiatric-related disorders. Previously, we have shown that mutations in the DTNBP1 gene (encoding dystrobrevin-binding protein 1 [dysbin-din-1]) lead to abnormalities in synaptic glutamate release in the prefrontal cortex (PFC) and hippocampus and to cognitive deficits; glutamatergic transmission is important for cortical recurrent excitation that allows information processing in the PFC. To investigate possible means of restoring glutamate release and improving cognitive impairments, we assess the effects of increasing endogenous levels of brain-derived neurotrophic factor (BDNF) in a dysbindin-1-deficient mouse model. Increasing endogenous levels of BDNF may aid in remediating cognitive deficits, given the roles of BDNF in synaptic transmission, plasticity, and neuroprotection. To increase BDNF, we use a novel strategy, repeated intraperitoneal injections of fingolimod (Gilenya). Sphingolipids have recently been shown to have therapeutic value in several neurology-related disorders. Both wild-type (WT) and mutant (MUT) genotypes were tested for sociability and recognition memory, followed by measuring endogenous BDNF levels and presynaptic [Ca2+]i within the PFC. Both genotypes were treated for 1 week with either saline or fingolimod. Relative to WT mice, MUT mice demonstrated impairments in sociability and recognition memory and lower presynaptic calcium. After fingolimod treatment, MUT mice exhibited significant improvements in sociability and recognition memory and increases in presynaptic calcium and endogenous concentrations of BDNF. These results show promise for counteracting the cognitive impairments seen in neuropsychiatric disorders and may shed light on the role of dysbindin-1.
Keywords: fingolimod, glutamate, dysbindin, prefrontal cortex
Despite their distinction in modern psychiatry, cognitive disabilities in neuropsychiatric and neurodevelopmental disorders share commonalities in phenomenology (Couture et al., 2011) and pathophysiology (Pinkham et al., 2008). Impairments in information processing, particularly social information processing, are some of the most commonly noted deficits shared by neurodevelopmental and neuropsychiatric disorders (Sugranyes et al., 2011). Despite the relevance of cognitive disabilities, there are few therapeutic options to treat them. With the increasing recognition that severity of cognitive disabilities may have the greatest impact on the long-term outcome of neuropsychiatric and neurodevelopmental patients, there is a requirement to develop treatments to improve cognitive function.
A potential therapeutic target, the prefrontal cortex (PFC), is a highly interconnected structure involved with top-down processing of sensorimotor information, and integration of this information affords complex cognitive functions (Miller, 2000; Fuster, 2001). Neuronal microcircuits within the PFC have essential roles in encoding, processing, and maintaining working memory (Goldman-Rakic, 1995; Miller, 2000). PFC neurons show increased activity during memory maintenance, and this memory-related activity has been attributed to glutamate-dependent recurrent excitation within local circuits (Compte et al., 2000). Compromised glutamate release may diminish the ability of cortical circuits to maintain recurrent excitation and may cause failure during information maintenance mechanisms.
Several susceptibility genes associated with deficits in cognition have been targeted as contributing factors in symptom pathogenesis (Modinos et al., 2013). DTNBP1 encodes dystrobrevin-binding protein 1 (dysbindin-1) and is located in the “vulnerability locus” on chromosome 6p24–22 (Straub et al., 1995). Spread across many diverse populations, genome-wide association studies and family-based pedigree studies have made genetic correlations between the DTNBP1 gene and the etiology of schizophrenia (Straub et al., 2002). Although variants of the DTNBP1 gene cannot be linked to every case of schizophrenia, there is evidence that negative and cognitive deficits may be attributed to diminished expression of the dysbindin-1 protein (DeRosse et al., 2006; Donohoe et al., 2007).
Dysbindin-1 is part of the biogenesis of lysosome-related organelle complex 1 (BLOC-1; Starcevic and Dell’Angelica, 2004). This complex is involved in multiple cellular functions, including synaptic vesicle dynamics (Larimore et al., 2011; Mullin et al., 2011). At the presynaptic level, its deficiency affects synaptic homeostasis, synaptic vesicle composition, and vesicle fusion (Ji et al., 2009; Newell-Litwa et al., 2009). Previously, using dysbindin-1 deficient mice (henceforth, dysbindin-1 mice), we found decreases in the replenishment of the readily releasable pool of synaptic glutamate vesicles in the PFC, decreases in quantal size and probability of glutamate release, deficits in the rate of endo- and exocytosis, and deficits in memory and working memory tasks (Jentsch et al., 2009; Karlsgodt et al., 2011; Glen et al., 2014; Saggu et al., 2013). Here, we hypothesize that restoring glutamate levels in the PFC may alleviate some of the cognitive deficits exhibited by dysbindin-1 mice. To increase endogenous levels of glutamate, we use a novel drug, fingolimod.
Fingolimod (Gilenya; FTY720) is active as fingolimod phosphate (Groves et al., 2013; Martin and Sospreda, 2014). It has been shown that fingolimod selectively increases endogenous levels of brain-derived neurotrophic factor (BDNF; Deogracias et al., 2012). Evidence supporting a role for BDNF in synaptic transmission and neuronal excitability is strong (Pozzo-Miller et al., 1999; Tyler and Pozzo-Miller 2001, 2003; Chapleu et al., 2009). Activation of tropomyosin receptor kinase B (TrkB) receptors by BDNF induces new protein synthesis (Halegoua et al., 1991; Lewin and Barde, 1996), and Tyler and Pozzo-Miller (2001) have shown that BDNF increases release probability and the number of docked glutamate vesicles in the hippocampus. Furthermore, evidence indicates a link between dysbindin and BDNF. Among three proteins present in regulated secretory vesicles (VAMP7, P14IIα, and BDNF), only BDNF immunoreactivity is reduced in the absence of BLOC-1 (Larimore et al., 2013). Thus, fingolimod’s ability to increase BDNF expression may prove useful in the treatment of cognitive symptoms related to neuropsychiatric disorders. This study presents behavioral and biochemical indicators of the effects of fingolimod in social interaction in dysbindin-1 mice and tests the hypothesis that increasing endogenous levels of BDNF improves cognitive deficits in null mice.
MATERIALS AND METHODS
Subjects, Animal Handling, and Housing
All experiments used male wild-type (WT; dys+/+) C57Bl/6J mice and a genotype of C57Bl/6J mice carrying a genomic deletion exclusively within the DTNBP1 gene (Li et al., 2003), homozygous dysbindin-1 mutant (MUT; dys−/−). Mice were derived from back-crossing with C57Bl/6J (The Jackson Laboratory; Bar Harbor, ME) for at least 10 generations, and genotyping of mice was achieved with previously described protocols (Jentsch et al., 2009). All mice were caged in a temperature- and humidity-controlled 12-hr light-dark cycle housing facility and allowed ad libitum access to food and water. WT mice were littermates of the dysbindin-1 MUT mice. With the exception of three female mice that had to be added because of the small number of MUT males (three females used in our ELISA), all studies used male mice older than 40 days of age. Experimental protocols were approved by the institutional animal care and use committee at the Medical University of South Carolina. No data were excluded from the behavioral or qualitative assays.
General Experimental Setup
For all experiments, mice were divided into two groups; group 1 was injected intraperitoneally (IP) with saline for 7 days, and group 2 was treated with fingolimod (1 mg/kg IP; Sigma-Aldrich, St Louis, MO) dissolved in sterile saline for 7 days. The dose of fingolimod was determined from doses previously reported (Deogracias et al., 2012). To minimize stress, no testing, whether behavioral or molecular, was performed until 24 hr after the last injection.
Sample Preparation for Molecular Analysis
All mice used in behavioral tasks were deeply anesthetized with isoflurane (Abbot Laboratories, Lake Bluff, IL); the brain was rapidly extracted and bathed in a cold isolation buffer (differing per assay used), and the PFC (both infra- and prelimbic) was blocked off over an iced Petri dish. To make one testable sample, three animals’ PFCs per genotype were pooled in a preweighted Eppendorf tube; thus, every sample consisted of three cortices. Each pooled sample was homogenized by following the specific assay-dependent protocol.
ELISA: Measuring BDNF
After PFC dissection and sample preparation, concentration of endogenous BDNF in PFC tissue was measured across 30 mice (seven WT + saline, six WT + fingolimod, eight MUT + saline, nine MUT + fingolimod) with the BDNF Emax immunoassay system (Promega, Madison, WI). ELISA was performed with the kit protocol provided and referencing procedures, as described elsewhere (Ickes et al., 2000). Pooled tissue samples were homogenized with eight strokes in a Potter homogenizer with 0.5 ml cold isolation buffer containing distilled water (dH2O), 137 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, and 10% glycerol. To maximize free protein yield, homogenized samples were acidified with 1 N HCl and neutralized with 1 N NaOH. Before the protocol was started, Tris-buffered saline Tween 20 buffer (dH2O, 20 mM Tri-HCl, 150 mM NaCl, and Tween 20) was prepared for use as a washing agent before each major incubation step. A 96-well flat-bottom plate, carbonate coated (with added monoclonal anti- BDNF mAb) at room temperature (RT) overnight, was blocked for 1 hr at RT with the provided 1× block and sample buffer. To generate the standard curve, BDNF standard was serially diluted (500–0 pg) in duplicate. The standard and 150 μl of each sample (in duplicate) were incubated at RT for 2 hr on an orbital shaker. Next, the plates were incubated with antihuman BDNF pAb at RT for 2 hr and with anti-IgY HRP conjugate at RT for 1 hr. Finally, TMB One color developing solution was added to each well for 10 min, and the reaction was stopped with 1 N HCl. Plate readings were obtained within 30 min with a VersaMax ELISA microplate reader (absorbance recorded at 450 nm). BDNF levels in the PFC were compared among genotypes and treatment groups. ELISAs were performed by a person blind to the genotype and treatment of the animals.
Fluorescent Calcium Assay
Thirty mice (nine WT + saline, nine WT + fingolimod, six MUT + saline, six MUT + fingolimod) were used for quantification of presynaptic intracellular PFC [Ca2+]i with a fluorescent calcium assay (both fluo-3 and fluo-4). To measure only intracellular calcium, crude synaptosomes were prepared as previously described (Saggu et al., 2013) with the pooled PFC sample tissue. This was achieved by homogenizing tissue with a Potter homogenizer with 2 ml cold isolation buffer (dH2O, 320 nM sucrose, 1 mM EDTA, 10 mM Tri-HEPES, and protease cocktail inhibitor [catalog No. P8340; Sigma]) and then centrifuging at 4°C with a specific centrifugation regimen (600g for 10 min, then centrifuging supernatant at 9,200g for 15 min). A Bradford assay (Bio-Rad; Hercules, CA) was used to determine the specific protein concentration for each sample.
The fluorescent calcium assay was performed with 3–5 mg/ml crude PFC synaptosome preparations with either a fluo-3 AM calcium indicator (Molecular Probes/Life Technologies, Grand Island, NY) or a fluo-4 NW assay kit (Molecular Probes/Life Technologies), according to the company’s protocols provided with reference to the methods previously described (Saggu et al., 2013). The synaptosomal preparations were incubated with 5 μM fluo dye for 30 min at 37°C and centrifuged at 10,000g for 10 min. Supernatant was discarded, and the pellet was resuspended in prewarmed Krebs buffer. Newly fluo-loaded synaptosome preparations were placed in a black polystyrene, flat, clear-bottom 96-well plate (CellStar; Greiner Bio-One, Frickenhausen, Germany) along with set-aside WT synaptosome preparations treated with either 0.4% Triton X-100 or 7.5 mM EGTA (pH 8.0) to generate maximum and minimum values, respectively. Readings were obtained with a BioTek (Winooski, VT) FLx800 fluorescence and luminescence microplate reader, with excitation wavelength of 325 nm and emission at 526 nm (Kd = 325 nm) for fluo-3 or excitation wavelength of 494 nm and emission at 516 nm (Kd = 345 nm) for fluo-4. For both dyes, presynaptic intracellular synaptosomal [Ca2+] was calculated with the formula [Ca2+]i = Kd × B (R − Rmin)/(Rmax − R), with 0.4% Triton X-100 and 7.5 mM EGTA(pH 8.0) to calculate Rmax and Rmin values. Calcium levels were compared among the genotypes and the saline and fingolimod treatment groups.
Sociability Test Apparatus
Animals were subjected to the three-chamber sociability test. The three-compartment testing apparatus consisted of a Plexiglas box (41.9 cm long × 41.91 cm wide × 30.5 cm high) without a top. The sides of the box were covered to minimize environmental visual stimuli. Inverted custom wire-mesh cups (diameter 10.16 cm) were placed in each side of the compartments during testing sessions and housed the novel stimulus mouse. The apparatus and wire cups were thoroughly cleaned with distilled water between sessions and after each test mouse.
Sociability Test
Social interaction was measured across 49 mice (12 WT + saline, 12 WT + fingolimod, 12 MUT + saline, 13 MUT + fingolimod) with a social choice/approach task similar to that described elsewhere (Moy et al., 2004; O’Tuathaig and Waddington, 2010; Wilson and Koenig, 2014). In the first session, a test mouse was placed in the middle compartment and allowed to habituate to the apparatus for 5 min. The test mouse was then returned to its home cage for 10 min. A novel stimulus mouse (NM1) was place in an inverted wire cup in the side designated as the social compartment, and an empty inverted wire cup (novel object, NO) was placed in the side designated as the nonsocial compartment. The side opposite of inherent preference, which had been measured during a 5-min habituation period, determined the side designated for the location ofNM1.
The test mouse was then returned to the apparatus and left to explore both chambers for 10 min. Time spent in each chamber was measured. An entry into any of the three chambers was defined as all four paws past the doorway. Social interaction was measured as time spent on the side with NM1. Degree of social interaction was determined by comparing time spent in the presence of NM1 relative to time spent in the middle or the side with the NO. Normal social behavior is assumed to be a range between equal mouse-to-item interactions and a greater novel mouse interaction time. After measurement of social interaction, the mouse subject was returned to the home cage for 10 min, and the test chamber was cleaned with distilled water before the next behavioral assay.
Preference for Social Novelty Task
After 10 min of being in the home cage, test mice used in the social choice/approach task were tested for memory (as measured by recognition ratio) and preference for social novelty with the preference for social novelty task (PSNT) similar to that described elsewhere (Moy et al., 2004; O’Tuathaigh and Waddington, 2010; Wilson and Koening, 2014). Thirty-seven mice were used in this task (nine WT + saline, nine WT + fingolimod, nine MUT + saline, 10 MUT + fingolimod). First, the NM1 that had been used in the social choice/approach task was sequestered on the original side, and a second enclosed novel mouse (NM2) was placed on the side that had originally contained the NO. The mouse subject was place in the middle and given the option of interacting with either NM1 or NM2. Time spent in each chamber was measured for 10 min, and both preference for social novelty and recognition memory were assessed. Preference for social novelty was measured as time spent with NM2 relative to other activity. Recognition memory was measured as time spent with NM2 relative to NM1, i.e., the “recognition ratio.” Normally functioning memory (or WT memory) was assumed to be a recognition ratio greater than or equal to one. The basic layout for testing of individual subject mice was start → 5 min acclimation period → 10 min in home cage → 10 min social choice/approach task → 10 min in home cage → 10 min preference for social novelty task → returned to home cage.
Statistical Analysis
For all assays, comparisons are between genotypes and across treatment groups (vehicle and fingolimod). All data presented are mean ± SEM. Repeated measures (RM) ANOVA was used to assess differences following treatment. Prism 6 (GraphPad Software, La Jolla, CA) was used for all statistical comparisons. Statistical significance was defined as P < 0.05 (risk of type 1 error, α = 0.05).
RESULTS
Sociability Test
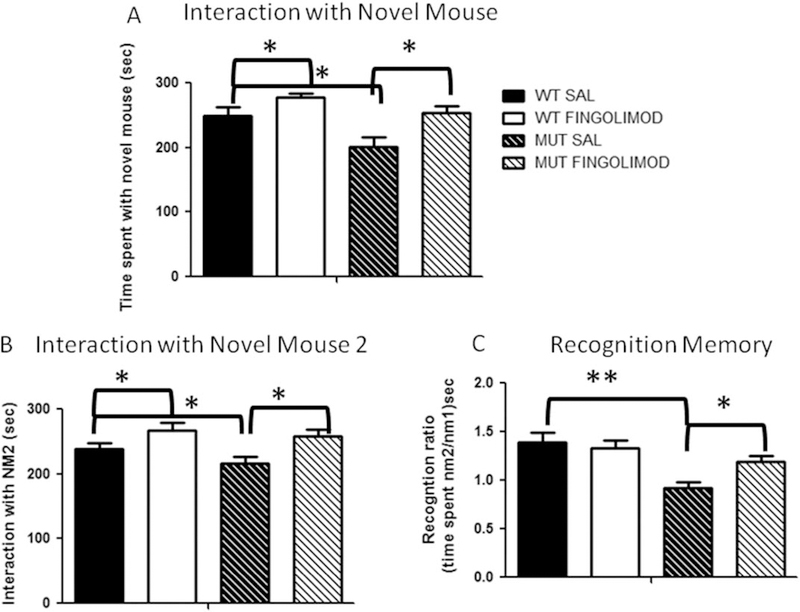
Forty-nine mice (24 WT and 25 dysbindin-1) were tested for sociability. Saline WT mice spent 205.9 ± 19.2 sec with NO vs. 248.9 ± 13.5 sec with NM1. There was a trend for significance in the time spent with NM1 vs. NO (P < 0.08, t-test; Fig. 1). After fingolimod treatment (7 days of daily IP fingolimod injections, 1 mg/kg), WT mice exhibited a significant increase in time spent with NM1 compared with saline mice, 278.0 ± 5.7 sec interacting with NM1 vs. 211.9 ± 9.6 sec interacting with NO (F3,11 = 5.81, P < 0.05, RM ANOVA; Fig. 1A).
Fig. 1.
A: Sociability of dysbindin-1 MUT mice as measured by the social choice/approach task. Relative to WT mice (n = 12), dysbindin-1 MUT mice (n = 12) exhibited lower interaction times with NM1 (★P < 0.0109). After fingolimod treatment, dysbindin-1 MUT mice (n = 13) showed normalized levels of interaction with NM1; the interaction is significantly greater compared with saline-treated counterparts (n = 12; ★P < 0.018). B: Assessing preference for social novelty in dysbindin-1 MUT mice. Given the choice between a past and a novel social interaction, dysbindin-1 MUT mice (n = 9) exhibited significantly lower interaction times with NM2 (★P = 0.0104) compared with WT mice (n = 9). After fingolimod treatment, dysbindin-1 MUT mice (n = 10) showed normalized levels of interaction with NM2, significantly greater compared with saline treatment (n = 9; ★P = 0.0173). C: Recognition ratio as a measure of memory function in dysbindin-1 MUT mice. WT mice showed no deficits in recognition memory when given the choice between a past and a novel social interaction, as measured by the recognition ratio. However, relative to WT mice, dysbindin-1 MUT mice exhibited significant reductions (★★P < 0.001) in recognition. After treatment with fingolimod, dysbindin-1 MUT mice showed significant improvement in recognition ratio (★P < 0.05) compared with the saline-treatment group. SAL, saline.
Contrary to the findings in WT, dysbindin-1 mice spent significantly more time interacting with NO than with NM1 (201.9 ± 14.6 sec with NM1 vs. 247.2 ± 10.7 sec with NO; F1,45 = 9.884, P = 0.0109, ANOVA; Fig. 1A). After fingolimod treatment, dysbindin-1 mice significantly increased the time spent with NM1 vs. NO (NM1 254 ± 10 sec vs. NO 219 ± 11.4 sec; P < 0.018, Kruskall-Wallis test, Dunn’s multiple-comparisons test; Fig. 1A). These results show that dysbindin-1 mice exhibited deficits in social interaction compared with WT mice. More importantly, fingolimod treatment reversed the deficits in social interaction exhibited by the dysbindin-1 mice.
PSNT
Thirty-seven mice were used in the PSNT (18 WT and 19 dysbindin-1). Preference for social novelty was measured as time spent with NM2 relative to other activity. Assessments of social novelty showed that dysbindin-1 mice exhibited significant deficits in the task (time of interaction with NM2; WT saline, 268.6 ± 9.2 sec; MUT saline, 215.5 ± 11.9 sec; n = 9; F2,49 = 6.09, P = 0.0104, RM ANOVA; Fig. 1B). After fingolimod treatment, dysbindin-1 mice showed significant improvements, reaching levels of social novelty similar to those of WT mice (WT fingolimod, 267.6 ± 12.1 sec; MUT fingolimod, 257.4 ± 10.4 sec; n = 9; Fig. 1B).
Recognition memory was measured as time spent with NM2 relative to NM1, i.e., the recognition ratio. Normally functioning memory (or WT memory) is assumed to be a recognition ratio greater than or equal to one. Analysis of the recognition ratio shows that dysbdindin-1 mice exhibited significant deficits compared with WT mice (WT saline, 1.3 ± 0.9; dysbindin-1 saline, 0.9 ± 0.16; WT vs. dysbindin-1, F2,49 = 8.954, P < 0.001; Fig. 1C). After fingolimod treatment, both genotypes improved significantly relative to saline treatment (WT, 1.3 ± 0.7; MUT, 1.1 ± 0.06; WT vs. MUT, F1,49 = 13.56, P < 0.05, RM ANOVA; Fig. 1C). These results show that dysbindin-1 mice exhibited significant deficits in social novelty and recognition memory and that fingolimod treatment improved performance in social and memory tests.
BDNF ELISA
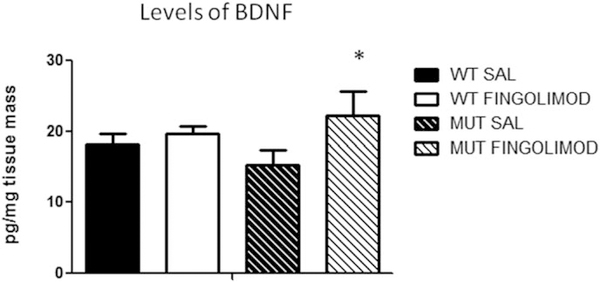
We used ELISA kits to measure the levels of BDNF in PFC of WT and dysbindin-1 mice. BDNF levels in WT mice were 18.1 ± 1.5 pg/mg tissue (n = 7; Fig. 2), and BDNF levels in dysbindin-1 mice were 15.2 ± 2.2 pg/mg tissue (n = 6; Fig. 2). After fingolimod treatment, the levels of BDNF did not change in WT mice (19.6 ± 1.1 pg/mg tissue, n = 6) and were significantly increased in WT mice (22.2 ± 3.5 pg/mg tissue, P < 0.02, paired t-test; n = 9; Fig. 2). We did not find any differences in the levels of BDNF measured in female vs. male mice. These results show that basal levels of BDNF in MUT mice were slightly decreased compared with those found in WT mice and that fingolimod treatment increased levels of BDNF.
Fig. 2.
ELISA measuring levels of endogenous BDNF in the PFC across both genotype and treatment groups. Dysbindin-1 MUT mice (n = 8) showed slightly lower levels of BDNF in the PFC compared with their WT counterparts (n = 7) and a significant increase (n = 9; paired t-test, ★P < 0.022) after fingolimod treatment. SAL, saline.
[Ca2+]i Levels in Cortical Synaptosomes
Previously, we reported that dysbindin-1 mice exhibited significant decreases in the levels of [Ca2+]i measured in synaptosomes of the PFC (Saggu et al., 2013). We proposed that the decrease in [Ca2+]i levels could underlie the decrease in glutamate release reported for the PFC of these mice (Chen et al., 2008; Jentsch et al., 2009). To investigate whether [Ca2+]i is affected by fingolimod treatment, we used fluo-3 and fluo-4 in combination with a colorimetric assay.
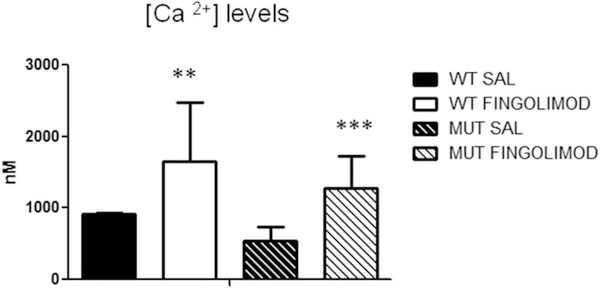
With fluo-4 in cortical synaptosomes of WT mice, the average concentration of [Ca2+]i was 912.3 ± 163.4 nM (n = 9), and in dysbindin-1 mice it was 544.3 ± 190.6 nM (n = 6). After fingolimod treatment, the levels of [Ca2+]i were increased in WT and in dysbindin-1 mice, with dysbindin-1 mice showing the greatest increase (WT, 1,649.9 ± 83.2 nM, an increase of 80%, n = 9; dysbindin-1 mice, 1,276.9 ± 452.3 nM, an increase of 134.6%, n = 6; Fig. 3).
Fig. 3.
Effect of fingolimod treatment on PFC presynaptic calcium concentration as measured by fluorescent calcium indicators. Baseline levels of intracellular calcium did not significantly differ between genotype, as measured by fluo-4 and fluo-3 (WT SAL, n = 9; MUT SAL, n = 6). After treatment, fluo-4 measures showed significant increases in intracellular calcium in both WT (n = 9; ★★P = 0.001) and dysbindin-1 MUT (n = 6; ★★★P < 0.0001) mice. Both dyes indicate a near doubling of intracellular calcium for both genotypes after treatment with fingolimod. SAL, saline.
Using fluo-3, we repeated the measures in a small group of mice; basal levels of [Ca2+]i in WT mice were 604.8 ± 108.8 nM (n = 2), and basal levels of [Ca2+]i in dysbindin-1 mice were 447.4 nM (n = 1). Fingolimod treatment increased the levels by 102.9% in WT mice (1,227.7 ± 519.2 nM, n = 2) and by 86.9% in dysbindin-1 mice (836.6 nM, n = 1).
By combining percentage measures of fluo-3 and fluo-4, the average increase in [Ca2+]i after fingolimod treatment was 91.9% in WT mice and 86.9% in dysbindin-1 mice. These results replicate our previous findings showing that dysbindin-1 deletions decrease [Ca2+]i. Furthermore, these results show that fingolimod treatment increased the basal level of [Ca2+]i.
DISCUSSION
We previously reported that dysbindin-1 mice exhibited decreases in glutamate release, decreases in basal levels of [Ca2+]i, and decreases in N-type Ca2+ channels (Saggu et al., 2013). Those data, together with evidence that dysbindin-1 mice exhibit deficits in working memory (Karlsgodt et al., 2011), allowed us to generate the hypothesis that the decrease in the basal levels of [Ca2+]i was underlying the decrease in glutamate release and the cognitive deficits exhibited by the dysbindin-1 mice.
As reviewed by Amaral and colleagues (2007), at presynaptic level, BDNF enhances excitatory synaptic transmission by direct presynaptic mechanisms (Lohof et al., 1993), an effect mediated by increased presynaptic Ca2+ levels and dependent on extracellular Ca2+ levels and presynaptic depolarization (Stoop and Poo, 1996). Enhancement of GABA-mediated inhibitory synaptic transmission by BDNF in cultured hippocampal neurons also seems to be mediated by presynaptic Ca2+ signaling because chronic BDNF treatment increases the expression and channel density of presynaptic P/Q- and N-type channels without affecting postsynaptic L-type channels (Baldelli et al., 2000, 2002).
To alleviate the decrease in [Ca2+]i levels present in dysbindin-1 mice and perhaps alleviate some of the cognitive deficits exhibited by these mice, we decided to increase the endogenous concentration of BDNF. However, BDNF cannot be administered systemically because it does not cross the blood-brain barrier. Deogracias and colleagues (2012) demonstrated that the sphingosine 1-phosphate (S1P) analog fingolimod (FTY720) crosses the blood-brain barrier, selectively increases endogenous BDNF levels, and activates the ERK-MAPK pathway (Deogracias et al., 2012; Doi et al., 2013) in the brain. We tested the effects of fingolimod treatment in WT and dysbindin-1 mice to rescue presynaptic Ca2+ levels and restore glutamatergic transmission in the PFC.
ELISA measures show that fingolimod treatment slightly increases levels of BDNF in dysbindin-1 mice but not in WT mice. Moreover, our data show that dysbindin-1 mice exhibit lower basal levels of BDNF compared with WT mice. We did not construct a dose/response curve. However, Deogracias and colleagues (2012) demonstrated a dose/response effect of fingolimod in the levels of BDNF in neuronal cultures; fingolimod at concentrations of 10 or 100 nM but not at doses of 1 μM increased BDNF levels. Perhaps increasing the IP dose of fingolimod could result in greater increases in endogenous levels of BDNF in dysbdindin-1 mice.
Our experiments did not measure the levels of other neurotrophic factors; however, Doi and colleagues (2013) have shown that fingolimod selectively increases BDNF mRNA (but not nerve growth factor or neurotrophin-3 mRNA) and increases neuronal BDNF production. When basal levels of [Ca2+]i were measured, we were able to replicate the results reported previously (Saggu et al., 2013), and we now show that fingolimod treatment improves the basal concentrations of [Ca2+]i measured in cortical synaptosomes of dysbindin-1 mice. These results suggest that fingolimod treatment, by increasing BDNF levels in dysbindin-1 mice, improves [Ca2+]i.
BDNF plays multiple roles in synaptic transmission and neuronal plasticity. As reported by Amaral and colleagues (2007), one of the prominent signaling cascades activated by TrkB receptors, the hydrolysis of phosphatidylinositol-4,5-biphosphate by activated phospholipase (PLC)-γ, is expected to cause Ca2+ elevations in neurons (Segal and Greenberg, 1996; Huang and Reichardt, 2003). The amplification of Ca2+ signals by mobilization from intracellular stores may allow the enhancement of processes as transmitter release and gene expression. It is possible that the increases in [Ca2+]i resulting from the fingolimod treatment are mediated directly by the mobilization of Ca2+ from the endoplasmic reticulum.
It is not possible to rule out completely a direct effect of fingolimod on [Ca2+]i. Fingolimod activates S1P1 receptors that are involved in many pathophysiological processes. S1P receptors couple to several G proteins, such as Ras, PLC, and phosphoinositide 3-kinase. Moreover, S1P activation increases glutamate release from presynaptic terminals in the hippocampus (Kanno et al., 2010). Hagihara et al. (2013) have shown that fingolimod stimulates Ca2+/calcineurin signaling in yeast.
More relevant was the finding that fingolimod treatment improves social cognition. Our results showed that dysbindin-1 mice exhibited significantly lowered scores in social tests compared with WT mice. Seven days of daily IP injections of fingolimod at 1.0 mg/kg significantly improved sociability, preference for social novelty, and recognition memory.
CONCLUSIONS
Fingolimod treatment improves social and memory deficits in dysbindin-1 mice via increases in BDNF that, in turn, improve [Ca2+]i and may normalize synaptic glutamate levels in the PFC.
SIGNIFICANCE.
Neurodevelopmental disorders are devastating health problems that produce enormous costs in terms of medical expenses, social and family stress, and loss of productivity; therefore, there is an urgent requirement to understand the mechanisms underlying these disorders and to explore new therapeutic interventions. To explore the cellular underpinnings of cognitive deficits associated with neurodevelopmental disorders, we use a mouse model, dystrobrevin-binding protein 1 (dysbindin-1) mutant mice, and evaluate the effects of fingolimod (FTY720), a sphingolipid that selectively increases endogenous levels of brain-derived neurotrophic factor. Systemic treatment with fingolimod improved social interaction and working memory in dysbindin-1 mice.
ACKNOWLEDGMENTS
The authors thank the Brain and Behavior Research Foundation for their support.
Contract grant sponsor: Brain and Behavior Research Foundation (to A.L.).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflicts of interest.
REFERENCES
- Amaral MD, Chapleau CA, Pozzo-Miller L. 2007. Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome. Pharmacol Ther 113:394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, Forni PE, Carbone E. 2000. BDNF, NT-3, and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci 12:4017–4032. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Novara M, Carabelli V, Hernández-Guijo JM, Carbone E. 2002. BDNF upregulates evoked GABAergic transmission in developing hippocampus by potentiating presynaptic N- and P/Q-type Ca2+ channels signalling. Eur J Neurosci 16:2297–2310. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1998. Neuronal calcium signaling. Neuron 21:13–26. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. 2000. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10:1078–1092. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L. 2009. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J Neurodev 1:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. 2008. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol 181:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. 2000. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10:910–923. [DOI] [PubMed] [Google Scholar]
- Couttas TA, Kain N, Daniels B, Lim XY, Shepherd C, Kril J, Pickford R, Li H, Garner B, Don AS. 2014. Loss of the neuroprotective factor Sphingosine 1-phosphate early in Alzheimer’s disease pathogenesis. Acta Neuropathol Commun 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Granholm EL, Fish SC. 2011. A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real-world functioning in schizophrenia. Schizophr Res 125: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, Barde YA. 2012. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci U S A 109:14230–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosse P, Funke B, Burdick KE, Lencz T, Ekholm JM, Kane JM, Kucherlapati R, Malhotra AK. 2006. Dysbindin genotype and negative symptoms in schizophrenia. Am J Psychiatry 163:532–534. [DOI] [PubMed] [Google Scholar]
- Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J, Jin S, Parajuli B, Sonobe Y, Mizuno T, Suzumura A. 2013. Fingolimod phosphate attenuates oligomeric amyloid β-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS One 8:e61988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, Garavan H, Robertson IH, Gill M, Corvin A. 2007. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia 45:454–458. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Mizoguchi H, Takeuchi H, Horiuchi H, Kawanokuchi J, Jin S, Mizuno T, Suzumura A. 2014. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid β-induced memory impairment. Behav Brain Res 268:88–93. [DOI] [PubMed] [Google Scholar]
- Fuster JM. 2001. The prefrontal cortex—an update: time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Glen WB Jr, Horowitz B, Carlson GC, Cannon TD, Talbot K, Jentsch JD, Lavin A. 2014. Dysbindin-1 loss compromises NMDAR-dependent synaptic plasticity and contextual fear conditioning. Hippocampus 24: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A, Kihara Y, Chun J. 2013. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 328:9–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Kita A, Mizukura A, Yao M, Kitai Y, Kunoh T, Masuko T, Matzno S, Chiba K, Sugiura R. 2013. Fingolimod (FTY720) stimulates Ca2+/calcineurin signaling in fission yeast. PLoS One 8:e81907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S, Armstrong RC, Kremer NE. 1991. Dissecting the mode of action of a neuronal growth factor. Curr Top Microbiol Immunol. 165:119–170. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. 2003. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. 2000. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol 164:45–52. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. 2009. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology 34:2601–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B. 2009. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A 106:19593–19598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T, Nakamura S. 2010. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience 171:973–980. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. 2009. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res 108: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. 2011. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry 69:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare JF, Smith Y, Faundez V. 2011. The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell 22:4854–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. 1996. Physiology of the neurotrophins. Annu Rev Neurosci. 19:289–317. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell’Angelica EC, Elliott RW, Mishra V, Kingsmore SF, Paylor RE, Swank RT. 2003. Hermansky-Pudlak syndrome type 7 (HPS-7) results from MUT dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 35:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. 1993. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363: 350–353. [DOI] [PubMed] [Google Scholar]
- Martin R, Sospedra M. 2014. Sphingosine-1 phosphate and central nervous system. Curr Top Microbiol Immunol 378:149–170. [DOI] [PubMed] [Google Scholar]
- Miller EK. 2000. The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. 2003. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem 10:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Iyegbe C, Prata D, Rivera M, Kempton MJ, Valmaggia LR, Sham PC, van Os J, McGuire P. 2013. Molecular genetic geneenvironment studies using candidate genes in schizophrenia: a systematic review. Schizophr Res 150:356–365. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3:287–302. [DOI] [PubMed] [Google Scholar]
- Mullin AP, Gokhale A, Larimore J, Faundez V. 2011. Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol 44:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Salazar G, Smith Y, Faundez V. 2009. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell 20:1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tuathaigh CM, Waddington JL. 2010. Mutant mouse models: phenotypic relationships to domains of psychopathology and pathobiology in schizophrenia. Schizophr Bull 36:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. 2008. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res 99:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. 1999. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci 19:4972–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA. 2011. Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: basic characterization and drug pharmacology. Brain Res 1401:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggu S, Cannon TD, Jentsch JD, Lavin A. 2013. Potential molecular mechanisms for decreased synaptic glutamate release in dysbindin-1 mutant mice. Schizophr Res 146:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. 1996. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19:463–489. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Sanger RH, Messerli MA. 2007. Principles, development, and applications of self-referencing electrochemical microelectrodes to the determination of fluxes at cell membranes In: Michel AC, Borlands LM, editors. Electrochemical methods for neuroscience. Boca Raton, FL: CRC Press. [PubMed] [Google Scholar]
- Starcevic M, Dell’Angelica EC. 2004. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem 279:28393–28401. [DOI] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Webb BT, Zhang J, Walsh D, et al. 1995. A potential vulnerability locus for schizophrenia on chromosome 6p24–22: evidence for genetic heterogeneity. Nat Genet 11:287–293. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo MM. 1996. Synaptic modulation by neurotrophic factors. Prog Brain Res 109:359–364. [DOI] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, O’Neill FA, Walsh D, Kendler KS. 2002. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and followup of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry 7:542–559. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. 2011. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One 6:e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, Kamins J, Hahn CG, Blake DJ, Arnold SE. 2006. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet 15: 3041–3054. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. 2001. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 21:4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. 2003. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 1:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Koenig JI. 2014. Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol 24:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. 2010. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70:304–322. [DOI] [PMC free article] [PubMed] [Google Scholar]