The hierarchical network of arteries, veins and capillaries of our cardiovascular system is laid out during development, and further expanded and matured postnatally. Precise regulation of endothelial proliferation and behavior is needed during development and throughout life to maintain proper architecture of the vasculature. In this issue, Alsina-Sanchis and colleagues report that phosphatase and tensin homolog (PTEN) connects bone morphogenic protein-9 (BMP9) activation of activin-receptor-like kinase-1 (ALK1) to phosphatidylinositol 3-kinase (PI3K) signaling in endothelial cells and implicate PI3K-stimulated endothelial proliferation in arteriovenous malformation (AVM) in hereditary hemorrhagic telangiectasia-2 (HHT2)1.
HHT is a rare autosomal dominant disorder that affects approximately 1/5000 people. HHT1 is caused by loss of function of one allele of a TGFβ co-receptor called endoglin while HHT2 is caused by loss of function of ALK1, a TGFβ family type I receptor known phosphorylate SMADs 1/5/8. In both HHT1 and HHT2, vascular overgrowth and malformations called telangiectasias occur focally throughout the body. The telangiectasias appear to arise from abnormal connections between enlarged post-capillary venules and arterioles, which results in fragile vessels prone to bleeding2.
Previous studies had revealed ALK1 suppresses endothelial proliferation and that lack of Alk1 in mice leads to vascular overgrowth and malformation3, 4, but precisely how AlK1 controls vascular growth and morphogenesis was unknown.
Alsina-Sanchis, Vinals and colleagues set out to solve this mystery. They observed retinal vascular development in Alk1+/− mice, manipulated BMP-9/ALK1 signaling in cultured human umbilical vein endothelial cells (HUVECs), analyzed HHT-2 patient-derived tissue specimens and went back to mice to test what they had learned. Alk1+/− mice showed increased endothelial proliferation and widened venous and arterial retinal vessels by post-natal day 9. In vitro, BMP9 decreased VEGF-stimulated HUVEC proliferation and pAKT and pERK levels. Pre-treatment experiments showed that BMP9 must precede VEGF-A addition by at least 2 hours to see these reductions, a clue that perhaps biosynthesis or stabilization of a regulator(s) might be needed for the BMP9 anti-angiogenic effects.
PTEN dephosphorylates phosphatidylinositol (3,4,5) P3 (PIP3) to phosphatidylinositol (4,5) P2 (PIP2), reversing the enzymatic action of PI3K, a potent brake on PI3K-AKT-mTOR signaling. Since mice with endothelial deletion of PTEN phenocopy vascular defects in Alk1+/− mice5, the authors speculated that PTEN might be involved in ALK1 function. Indeed, they found PTEN mRNA, protein and activity all increased in BMP9 treated HUVECs. siRNA knockdown PTEN substantially increased pAKT and rendered BMP9 unable to block VEGF-A proliferation. Taken together, the in vitro experiments show PTEN is needed to suppress pAKT and for BMP9 to inhibit endothelial proliferation.
With this discovery in hand, the investigators scoured public data bases on HHT2 tissue specimens, which revealed overexpression of genes related to the PI3K/AKT pathway. Immunostaining confirmed the data: endothelial proliferation and markers of PI3K activation (pAKT, pNDGR1 and pS6) were increased in HHT2 tissue sections. Significant correlation was found between pNDGR1 positivity and the severity of nosebleeds experienced by HHT2 patients from which tissue was obtained, linking PI3K activation to a common feature of HHT2. The authors returned to the Alk1+/− mice to assess PI3K contribution to the vascular hyperplasia in the post-natal retina. Endogenous PI3K activity was reduced genetically by replacement of one allele with a kinase-dead form of the PI3K catalytic subunit and PI3K was inhibited pharmacologically with a pan-PI3K inhibitor. In both experiments, retinal vessel hyperplasia at post-natal day 7, measured by vessel width, was reduced to wild-type levels. This in vivo data indicates PI3K inhibition is sufficient to reverse the Alk1+/− phenotype, and thus may be a strategy to treat HHT2.
In summary, data from mouse models, in vitro experiments and patient samples has revealed a critical link between ALK1 and PI3K that is needed to properly regulate endothelial proliferation and vessel morphogenesis in vivo; in vitro experiments show PTEN is the link. Extrapolating to HHT2, ALK1 haploinsufficiency should reduce the ability of the ALK1 ligand, BMP9, to increase PTEN sufficiently to dampen down PI3K-pAKT signaling in pro-angiogenic settings. Further experiments are needed to determine if PTEN is indeed deficient or reduced in HHT2.
The reliance on the retina as the vascular test-bed is an important limitation of this study as it relates to HHT2. The still-developing postnatal retinal vessels may not fully reflect the vascular microenvironments where telangiectasias and AVMs typically develop in HHT patients. Telangiectasias occur on the face and oral and nasal mucosal membranes; endangering AVMs can form in the lungs, liver, gastrointestinal tract, and brain. HHT2 patients are more likely to have AVMs in the liver while HHT1 patients are more likely to have AVMs in the lung and brain. Clearly the vascular bed plays a role in the pathogenesis of HHT. Another aspect is that HHT vascular lesions do not occur everywhere in the body despite haploinsufficiency in every cell. It would be of interest to learn if inherently low PTEN levels in subsets of endothelial cells might contribute to localized AVM formation.
Enormous strides have been made in recent years towards identifying molecular drivers of vascular malformations6. Increased endothelial PI3K/AKT/mTOR signaling is center-stage in venous malformations7–11, lymphatic malformations12, 13 and vascular tumors14 and now the pathway appears activated in HHT2 as well. Activating mutations in TIE2 or mutations downstream in the PI3K catalytic domain drive venous malformations, which can be reversed in animal models with PI3K or mTOR iinhibitors8, 10, 11. Yet major questions remain: how does increased PI3K/pAKT cause different types of vascular malformation? In venous malformations, activating mutations in TIE2 or PI3K over-activate pAKT whereas increased pAKT in HH2 appears to result from loss of an inhibitor, PTEN. Perhaps upstream regulators titrate the PI3K signaling to regulate endothelial behavior and morphogenesis in nuanced ways. It is clear that such mechanisms must be maintained throughout life as vascular overgrowths and malformations often worsen over a lifetime. As investigators elucidate molecular mechanisms and identify potential drugs to treat vascular malformations such as AVMs, it will be important to develop means for localized delivery to reduce potential harm to unaffected endothelium, as life-long therapy may be necessary for these genetic disorders.
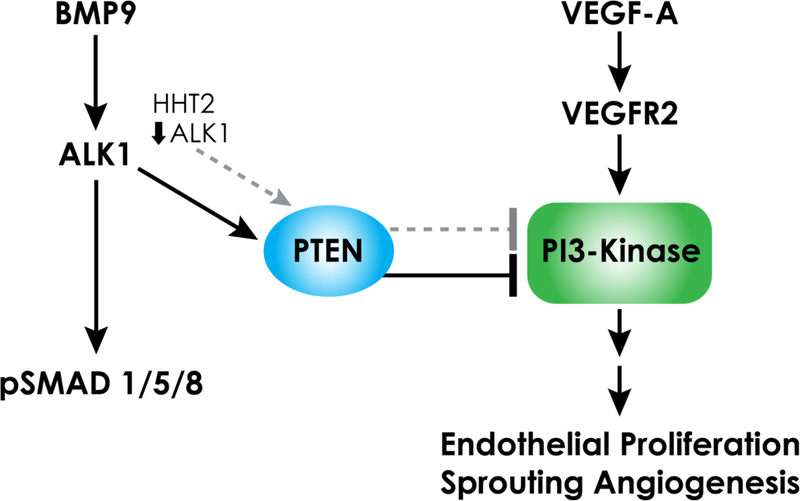
Figure 1.
ALK1 signaling decreases PI3-Kinase signaling and endothelial proliferation. In vitro studies show BMP9/ALK1 increases PTEN, a phosphatase that reverses the action of PI3-kinase. This implicates PTEN as an endogenous brake to PI3K/pAKT signaling, endothelial proliferation and angiogenesis. In HHT2, reduced ALK1 coincides with increased PI3K/AKT signaling (dashed gray lines), which may be caused, as in cultured endothelial cells, by reduced PTEN.
Abbreviations
- PTEN
phosphatase and tensin homolog
- BMP9
bone morphogenic protein-9
- ALK1
activin-receptor-like kinase-1
- PI3K
phosphatidylinositol 3-kinase
- AVM
arteriovenous malformation
- HHT-2
hereditary hemorrhagic telangiectasia-2
- HUVEC
human umbilical vein endothelial cells
References
- 1.Alsina-Sanchis E, Garcia-Ibanez Y, Figueiredo AM, Riera-Domingo C, Figueras A, Matias-Guiu X, Casanovas O, Botella LM, Pujana MA, Riera-Mestre A, Graupera M and Vinals F. ALK1 (Activin-Receptor Like Kinase 1) Loss Results in Vascular Hyperplasia in Mice and Humans Through PI3K (Phosphatidylinositol 3-Kinase) Activation. Arterioscler Thromb Vasc Biol 2018. [DOI] [PubMed]
- 2.Andrejecsk JW, Hosman AE, Botella LM, Shovlin CL, Arthur HM, Dupuis-Girod S, Buscarini E, Hughes CCW, Lebrin F, Mummery CL, Post MC and Mager JJ. Executive summary of the 12th HHT international scientific conference. Angiogenesis 2018;21:169–181. [DOI] [PubMed] [Google Scholar]
- 3.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW and ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 2007;120:964–72. [DOI] [PubMed] [Google Scholar]
- 4.Urness LD, Sorensen LK and Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 2000;26:328–31. [DOI] [PubMed] [Google Scholar]
- 5.Serra H, Chivite I, Angulo-Urarte A, et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat Commun 2015;6:7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen HL, Boon LM and Vikkula M. Vascular Anomalies Caused by Abnormal Signaling within Endothelial Cells: Targets for Novel Therapies. Semin Intervent Radiol 2017;34:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uebelhoer M, Natynki M, Kangas J, Mendola A, Nguyen HL, Soblet J, Godfraind C, Boon LM, Eklund L, Limaye N and Vikkula M. Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Hum Mol Genet 2013;22:3438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boscolo E, Limaye N, Huang L, et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. The Journal of Clinical Investigation 2015;125:3491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limaye N, Kangas J, Mendola A, Godfraind C, Schlogel MJ, Helaers R, Eklund L, Boon LM and Vikkula M. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am J Hum Genet 2015;97:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castel P, Carmona FJ, Grego-Bessa J, Berger MF, Viale A, Anderson KV, Bague S, Scaltriti M, Antonescu CR, Baselga E and Baselga J. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci Transl Med 2016;8:332ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo SD, Tzouanacou E, Zaw-Thin M, et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci Transl Med 2016;8:332ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscolo E, Coma S, Luks VL, Greene AK, Klagsbrun M, Warman ML and Bischoff J. AKT hyper-phosphorylation associated with PI3K mutations in lymphatic endothelial cells from a patient with lymphatic malformation. Angiogenesis 2015;18:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn AJ, Dickie P, Neilson DE, Glaser K, Lynch KA, Gupta A and Dickie BH. Activating PIK3CA alleles and lymphangiogenic phenotype of lymphatic endothelial cells isolated from lymphatic malformations. Hum Mol Genet 2015;24:926–38. [DOI] [PubMed] [Google Scholar]
- 14.Phung TL, Du W, Xue Q, et al. Akt1 and akt3 exert opposing roles in the regulation of vascular tumor growth. Cancer Res 2015;75:40–50. [DOI] [PubMed] [Google Scholar]