Abstract
Purpose of review:
Primary central nervous system lymphoma (PCNSL) is an aggressive malignancy confined to the brain, spinal cord, leptomeninges, and eyes. Due to its rarity, there is a paucity of randomized trials and a varied approach to its management in the oncologic community. This review summarizes recent literature guiding current clinical practice.
Recent findings:
The presentation, work up, and management of PCNSL are discussed. Induction therapy incorporates a methotrexate-based chemotherapy regimen and is generally followed by a consolidation regimen including high dose chemotherapy (with or without autologous stem cell rescue). Whole brain radiation therapy (WBRT) is a potential additional consolidation strategy. Management of relapsed and refractory disease poses a special challenge due to poor outcomes. Immunotherapy and targeted treatments are promising novel strategies for recurrent/refractory patients.
Summary:
Currently, there is little consensus in the management of PCNSL. Treatment recommendations should be tailored to the individual patient, with consideration for risk of neurotoxicity. New, exciting strategies are in development and when feasible, enrollment in a clinical trial should be considered.
Keywords: Primary central nervous system lymphoma, primary CNS lymphoma, PCNSL, treatment
Introduction
Primary central nervous system lymphoma (PCNSL) is an extra-nodal variant of non-Hodgkin lymphoma with disease limited to the brain, spinal cord, leptomeninges, or eyes, without evidence of systemic involvement. PCNSL is a highly aggressive but potentially curable malignancy, responsive to chemotherapy and radiation. While treatment response rates can be as high as 90%, relapses are frequent and prognosis after recurrence is poor. Approaches to this disease vary widely by institution and practitioner. Here, we will review the clinical presentation, work up, and general principles underlying the management of PCNSL.
Epidemiology and Outcomes
PCNSL is a rare malignancy with an incidence of 0.4–0.5 per 100,000 per year [1, 2]. Presently, it accounts for 4% of newly diagnosed brain tumors and 4% to 6% of all extranodal lymphomas [1]. While it can occur at any stage of adulthood, the median age of diagnosis is 65, and the incidence in the elderly population has been rising [3, 4].
Two models have been developed to predict outcomes. The Memorial Sloan Kettering Cancer Center (MSKCC) prognostic model factors age and Karnofsky Performance Status (KPS) at diagnosis to predict median progression-free and overall survivals[5]. The International Extranodal Lymphoma Study Group (IELSG) identified age, Eastern Cooperative Oncology Group (ECOG) performance status, serum lactate dehydrogenase (LDH), cerebrospinal fluid (CSF) protein concentration, and presence of deep brain structure involvement as independent predictors of survival and developed a similar prediction model [6]. Treatment delay more than 30 days after diagnosis is also associated with a poorer prognosis [7].
PCNSL can occur in the setting of immunosuppression (HIV/AIDS, congenital immunodeficiency, post-transplant immunosuppression) or in immunocompetent individuals. This review will focus on the presentation and management of immunocompetent patients.
Clinical Presentation
Diagnosis of PCNSL can require a high level of suspicion as clinical presentations are varying and dependent upon involved compartments. Focal neurologic deficits, resulting from involvement of the parenchyma or leptomeninges will prompt rapid imaging but are only seen in 70% of patients [8]. Up to 43% of individuals have behavioral or neuropsychiatric changes which are nonspecific and can lead to a delay in medical evaluation. Signs of elevated intracranial pressure (ICP) such as headache, nausea, and vomiting, are also common (33%). Fewer patients will present with seizures (14%), thought due to the relative sparing of the cortex. Visual symptoms at presentation are rare (4%) [8] despite the frequency of ocular involvement (20–25%)[9, 10]. Patients may complain of blurred vision, decreased acuity, or floaters; however, these symptoms are often subtle and asymptomatic ocular involvement is common. Ocular lymphoma resembles uveitis and may be misdiagnosed if visual complaints are the only clinical manifestation.
Diagnosis and Staging
Patients with symptoms concerning for PCNSL should undergo brain imaging. Magnetic resonance imaging (MRI) with and without contrast is the modality of choice. In patients who are unable to undergo MRI, a computed tomography (CT) scan with and without contrast, is indicated. PCNSL can appear as a solitary lesion or multifocal disease. Lesions are often periventricular, involving the deep white matter, basal ganglia, or corpus callosum. Classically, the lesions are isodense to hyperdense on CT and isointense to hyperintense on T2 MRI sequencing (Figure 1). Lesions are homogeneously enhancing with a mild amount of edema and are often associated with DWI restriction abnormality [11]. It is not uncommon for PCNSL to be confused with a demyelinating process or even stroke. Rarely, PCNSL can present with leptomeningeal or ocular involvement alone so special attention should be paid to the leptomeninges and ventricular size. If suspicion warrants, a normal appearing MRI should not preclude CSF and ocular examinations.
Figure 1. Characteristic PCNSL imaging pattern.
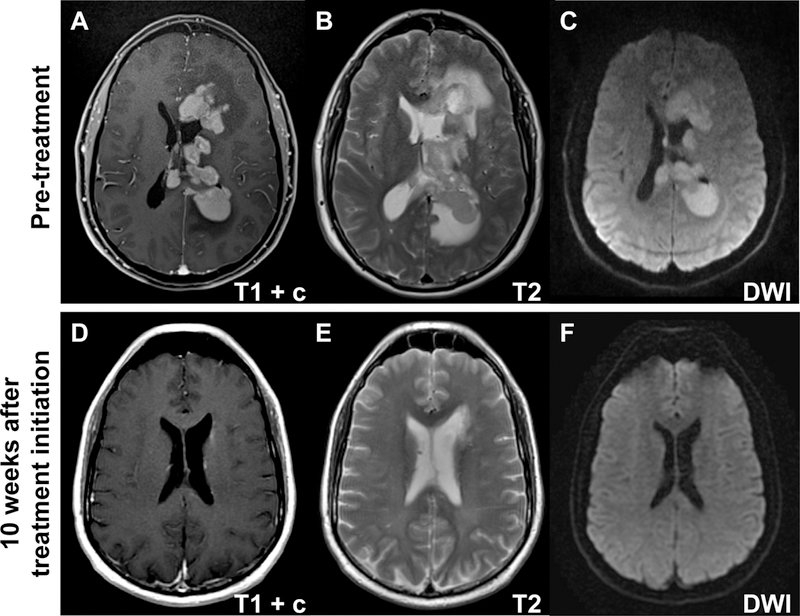
A) Magnetic resonance imaging (MRI) T1 sequence with gadolinium contrast (T1+c) reveals homogeneously enhancing deep lesions. B) Lesions are iso- to hyperintense on T2 imaging with a relatively small amount of edema. C) Diffusion-weighted imaging (DWI) demonstrates restricted diffusion in the tumor. D-F) PCNSL is highly chemosensitive with dramatic response on T1 with gadolinium (D), T2 (E), and DWI sequences (F) 10 weeks after initiation of methotrexate combination therapy.
Definitive diagnosis requires pathologic confirmation which often necessitates brain biopsy. Corticosteroids are lymphotoxic and can obscure pathology results [12, 13]. Their use can yield false negative results, delaying diagnosis and treatment. Corticosteroids should always be deferred until tissue is obtained except in cases of life threatening mass effect and edema.
Leptomeningeal involvement is present in up to 20% of patients at the time of diagnosis [14–17] and a lumbar puncture should be performed for staging when safe. Testing should include a cell count, measurements of protein and glucose concentrations, as well as cytology, flow cytometry and immunoglobulin heavy chain gene rearrangement. While lumbar puncture alone can be diagnostic, it is not generally advised to delay brain biopsy for results of CSF testing. A complete ophthalmologic evaluation, including slit lamp examination should always be performed even in the absence of visual symptoms. Diagnosis can be made via biopsy of the vitreous, retina, or choroid though again, we do not recommend delaying brain biopsy for these results.
While stereotactic biopsy is often required for diagnosis of PCNSL, further resection is rarely pursued. Lesions tend to involve deep brain structures and are often multifocal, making surgery impractical. Even when disease is isolated to a single focus on radiographic imaging, autopsy studies suggest tumor cells are infiltrative throughout the whole brain, indicating a surgical cure is not achievable[18]. Moreover, as PCNSL is highly sensitive to both, chemo and radiation therapies, the potential for surgical morbidity is thought an unnecessary risk. In concordance with this, retrospective data have largely failed to demonstrate a survival benefit with surgical resection [8, 19, 20].
This view has been recently called into question by a retrospective subset analysis of the German PCNSL Study Group-1 phase 3 trial which seemed to suggest patients undergoing subtotal or gross total resections had significantly prolonged progression-free survival (PFS) and overall survivals (OS) compared to those who were biopsied [21]. When adjusted for number of lesions, improved PFS was maintained but OS benefit was no longer statistically significant. It is not our practice to recommend debulking surgery for these patients.
Once CNS lymphoma is confirmed, whole body staging should be completed to rule out systemic disease with secondary CNS involvement. As outlined by the International PCNSL Collaborative Group [22], a whole body PET scan and bone marrow biopsy should be performed. If PET is unavailable, a CT chest, abdomen and pelvis with and without contrast can be obtained in conjunction with a testicular ultrasound in men. Routine laboratory evaluation should include a complete blood count, a metabolic panel with baseline liver function tests, serum LDH, and HIV testing.
Newly Diagnosed PCNSL
Treatment of PCNSL consists of induction therapy, with the goal of achieving a complete response (CR), followed by a consolidation regimen intended to eliminate remaining microscopic disease and maintain remission. Given the rarity of PCNSL and a paucity of phase 3 randomized clinical trials, there are different approaches to induction and consolidation regimens within the neuro-oncologic community. While it is generally agreed upon methotrexate (MTX) is the backbone of therapy, there is controversy surrounding the choice of additional chemotherapeutic agents, as well as the role for radiation therapies.
Until the early 1980s, whole brain radiation therapy (WBRT) was the mainstay of PCNSL treatment, resulting in dramatic radiographic and clinical improvement with 90% overall response rates (ORR) [23, 24]. Unfortunately, treatment with radiation alone is insufficient and long term control is poor, with OS of only 12–18 months [23, 24]. The addition of cyclophosphamide, doxorubicin, vincristine, and prednisone, agents used in the management of systemic lymphoma, did not confer a survival benefit, perhaps due to poor penetration of the blood brain barrier[25, 26].
High dose MTX (HD-MTX) is now considered the backbone of multimodality therapy for PCNSL. MTX penetrates the blood brain barrier when administered at doses >1.5mg/m2 as a rapid infusion [27, 28]. Single agent MTX (8g/m2) has proven efficacious with a 74% ORR [15]. In a randomized controlled trial, Ferreri et al demonstrated improved response rates (69% vs 40%) and a longer PFS (18 vs 3 months) with the addition of cytarabine to MTX, arguing for efficacy of polychemotherapy, despite a higher rate of hematologic toxicity (92% vs 15%) [29]. A number of trials have investigated various combinations with MTX (Table 1). The optimal dose of MTX is not agreed upon, with many regimens calling for 3.5mg/m2 to 8mg/m2. A retrospective analysis of 357 patients suggested a statistically significant survival benefit with regimens that include doses 3mg/m2 [30].
Table 1.
Prospective Upfront Treatment Trials in PCNSL
| Author | Year | Agents | Patients | Median Age | ORR (PR+CR) | Median PFS [m] | Median OS [m] |
|---|---|---|---|---|---|---|---|
| DeAngelis et al | 1992 | M(1)+RT(40+14 boost)+AraC(3) | 31 | 58 | 27/31 (87%) | 41 | 42.5 |
| Glass et al | 1994 | M(3.5)+RT(30–40) | 25 | 56 | 23/25 (90%) | 32 | 33 |
| O’Brien et al | 2000 | M(1)+RT (45+5.4 boost) | 46 | 58 | 44/46 (96%) | NR | 33 |
| Abrey et al | 2000 | M(3.5)+P(100)+V(1.4)+Ara-C(3)+IT M+IT A+RT(45) | 52 | 65 | 49/52 (94%) | NR | 60 |
| Ferreri et al | 2001 | M(3)+P(100)+V(1.4)+Ara-C(3)+RT(45) | 13 | 54 | 12/13 (92%) | 18 | 25+ |
| DeAngelis et al | 2002 | M(2.5)+V(1.4)+P(100)+AraC(3)+IT M+RT(45 or 36) | 102 (98 treated) | 56.5 | 47/50 (94%) | 24 | 37 |
| Herlinger et al | 2002 | M(8) | 37 | 60 | 13/37 (35%) | 10 | 25 |
| Abrey et al | 2003 | M(3.5)+AraC(3); BEAM | 28 (14 transplanted) | 53 | induction: 16/24 (57%), SCT 11/14 (77%) | 5.6 | not reached |
| Batchelor et al | 2003 | M(8) | 25 | 60 | 17/23 (74%) | 12.8 | 22.8+ |
| Pels et al | 2003 | M(5)+AraC(3)+V(2)+ifos(800)+dex(10)+cyclo(200)+IT M+IT A+IT P | 65 | 62 | 43/61 (71%) | 21 | 50 |
| Poortmans et al | 2003 | M(3)+Ten(100)+B(100)+MP(60)+IT M+IT A+RT(40) | 52 | 51 | 42/52(81%) | NR | 46 |
| Colombat et al | 2006 | M(3)+B(100)+eto(100)+pred (60); BEAM+RT(30) | 25 (17 transplanted) | 52 | induction: 2½5 (84%), SCT 16/16 (100%) | 40 | not reached |
| Illerhaus et al | 2006 | M(8)+AraC(3)+thio (40mg/m2); B(400)+thio(5mg/kg)+RT(45) | 30 (23 transplanted) | 54 | induction: 21/30 (70%), SCT 2½1 (100%) | NR | not reached |
| Ferreri et al | 2009 | M(3.5)+/−AraC(2)+RT(45) | 79 | 59/58 | 27/39 (69%) vs 16/40 (40%) | 3 vs. 18 | NR |
| Thiel et al | 2010 | M(3;+ifos) +/− RT(45) | 526 (all)/318 (TPP) | 61 | 283/526 (53%) | 18.3 vs. 11.9 | 32.4 vs. 37.1 |
| Morris et al | 2013 | R(500)+M(3.5)+V(1.4)+P(100)+RT(23.4) | 52 | 60 | 41/52 (78%) | 92.4 | not reached |
| Rubinstein et al | 2013 | R(375)+M(8)+T(150)+AraC(2) vs eto(40) | 44 | 61 | 34/47(72%) | 48 | not reached |
| Omuro et al | 2015 | R(500)+M(3.5)+V(1.4)+P(100); thio(250)+cyclo(60)+bus(3.2) | 32 (26 transplanted) | 57 | induction: 31/32 (97%), SCT 24/26 (92%) | not reached | not reached |
| Omuro et al | 2015 | M(3.5)+V(1.4)+P(100)+AraC(3) vs. M(3.5)+T(150) | 95 | 72/73 | 37/45(82%) vs. 34/42(74%) | 9.5 vs. 6.1 | 31 vs. 14 |
| Glass et al | 2016 | R(375)+M(3.5)+T(100)+RT(36) | 66 | 57 | 30/35 (86%) | 63 | 90 |
| Ferreri et al | 2016 | M(3.5)+AraC(2)+/−R(375)+/−thio(30) | 227 | 58/57/57 | 40/75(53%)/51/69(73%)/65/75 (86%) | 6/20/not reached | 12/30/not reached |
| Illerhaus et al | 2016 | R(375)+M(8)+AraC(3)+thio(40); R(375)+B(400)+thio(5mg/kg) | 79 (73 transplanted) | 56 | induction: 73/79 (92%), SCT: 72/79 (91%) | 74 | not reached |
| Fritsch et al | 2017 | R(375)+M(3)+P(60)+L(110) | 107 (all)/(69 R-MPL) | 73 | 53/107 (50%); 32/69 (46% R-MPL) | 10.3/(9.6 R-MPL) | 20.7/(15.4 R-MPL) |
AraC: cytarabine (g/m2); B: BCNU (mg/m2); BEAM: carmustine, etoposide, cytarabine, melphalan; bus: busulfan (mg/kg); CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; cyclo: cyclophosphamide (mg/m2); eto: etoposide (mg/m2); ifos: ifosfamide (mg/m2); IT A: intrathecal cytarabine; IT M: intrathecal methotrexate; IT P: intrathecal prednisone; M: methotrexate (g/m2); L: lomustine (mg/m2) NR: not reported; P: procarbazine (mg/m2/day), SCT: stem cell transplant; pred: methylprednisone (mg/m2); R: rituximab (mg/m2); RT: Whole brain radiation (dose used in Gy); T: temozolomide (mg/m2); Ten: teniposide (mg/m2); thio: thiotepa (mg/m2); V: vincristine (mg/m2)
Current common practice is to incorporate rituximab, an anti-CD20 monoclonal antibody, into regimens containing MTX. Multiple retrospective trials have suggested improved outcome with its addition [31–34]. Recently, IELSG32, a randomized study of 227 patients, investigated the addition of rituximab and/or thiotepa to a regimen of MTX and cytarabine [35]. Highest response rates were seen in patients who received HD-MTX/cytarabine/rituximab (MATRix, 86%) as compared to HD-MTX/cytarabine/rituximab without thiotepa (73%) and HD-MTX/cytarabine alone (53%). The role of rituximab in particular, is being further investigated by an ongoing European trial randomizing patients to receive HD-MTX, teniposide, carmustine (BCNU), and prednisone (MVBP) with or without rituximab (HOVON/ALLG; EduraCT, No.2009–014722-42). In general, induction regimens differ based on geography and physician preference but common induction combinations include rituximab, HD-MTX, procarbazine, and vincristine (R-MPV); rituximab, HD-MTX, and temozolomide, (R-MT); MATRix; and R-MVBP.
Toxicity related to induction chemotherapy varies based on regimen. In general, HD-MTX is relatively well tolerated and safe to administer, even in older patients with medical comorbidities though impaired renal function (creatinine clearance <30 mL/minute) is a contra-indication. Common systemic toxicities include renal failure, hepatitis, and myelosuppression. Patients may complain of nausea, vomiting, diarrhea or headache. Treatment is administered concurrently with leucovorin “rescue” to prevent systemic organ damage. As leucovorin has poor blood brain barrier penetration, use does not impede efficacy of MTX in the CNS. Neurologic toxicity is less common but can consist of stroke-like symptoms, an acute or sub-acute encephalopathy, and long term, a delayed multifocal leukoencephalopathy. Rituximab is also well-tolerated though can be associated with severe infusion reactions and bears the risk of reactivation of viral hepatitis. We therefore recommend hepatitis serologies on all patients prior to treatment. If past exposure is documented, patients can be prophylaxed with anti-viral agents.
There is a lack of consensus regarding the current role for WBRT. Long term survivors may develop radiation-related neurotoxicity which can manifest as cognitive decline, gait imbalance, and incontinence. Toxicity is thought to be higher in patients who also receive treatment with chemotherapy, particularly HD-MTX, and in those who received standard doses of WBRT (>42Gy)[36]. While the exact mechanism of decline is unclear, autopsy studies revealed white matter damage with gliosis, vessel thickening, and demyelination [37]. In an effort to reduce neurotoxicity, studies began to reduce the dose of WBRT or defer in favor of consolidation with high-dose chemotherapy.
Our institution conducted a single center phase 2 study of R-MVP followed by cytarabine and reduced-dose WBRT (23.4 Gy) for patients in whom a CR was achieved. Patients who completed the regimen had a median PFS of 7.7 years and a 3-year OS of 87%. Neuropsychological testing scores were stable at 48 months of follow up [38]. Based on these results RTOG is conducting a randomized phase 2 trial of this approach (NCT01399372). A similar phase 2 trial investigated treatment with R-MT followed by hyperfractionated WBRT (36 Gy) and ten months of maintenance temozolomide. This study similarly found no change in cognitive testing though longer follow up is needed [39].
The only phase 3 randomized study in PCNSL attempted to address the ongoing need for WBRT. All patients received HD-MTX with or without ifosfamide. Patients who achieved a CR were then randomized to receive 45 Gy WBRT or observation alone while those who did not achieve a CR were randomized to receive 45 Gy WBRT or cytarabine. The study did not meet its endpoint though revealed some notable findings. There was 34% noncompliance in the WBRT arm, as compared to complete compliance in the chemotherapy arm. While PFS was longer in patients who received WBRT (18 mo vs 12 mo) there was no statistically significant difference in OS (32.4 mo vs 37.1 mo). As expected neuro-toxicity rates were significantly higher in groups receiving WBRT (49% vs 26%)[17].
Increasingly, WBRT is omitted from treatment regimens in favor of chemotherapy consolidation. The multicenter Cancer and Leukemia Group B (CALGB) study 50202 treated patients with R-MT followed by consolidation with high-dose etoposide and cytarabine. Results were comparable to those achieved with chemoradiation with an ORR of 72% and a median PFS of 48 months [7]. More aggressive myeloablative chemotherapy regimens followed by autologous stem cell transplant rescue (HCD-ASCT) are available to younger patients with few medical comorbidities. Theoretically, increased chemotherapy doses offer better penetration of the blood brain barrier and increased drug concentrations in the CNS. There are different approaches to conditioning regimens though thiotepa-based treatments appear to have better clinical results than the commonly used regimens of BCNU, etoposide, cytarabine, melphalan (BEAM) or cyclophosphamide, etoposide, and BCNU (CBV)[40–42]. Two recent studies –one utilizing R-MPV induction therapy [43], and the other HD-MTX, thiotepa, and cytarabine[44], both demonstrated high ORR (>90%) and prolonged PFS (>74 mo) with HDC-ASCT.
Site-Specific Therapy
The role of intrathecal (IT) chemotherapy for PCNSL is unclear. Several retrospective studies did not demonstrate improved survival with its use [45–47]. However, a small single arm phase 2 study suggested higher rates of relapse when IT therapy was omitted [48]. Choice of IT agent varies between practitioners with some giving rituximab and others methotrexate. Less often, IT cytarabine may be used. IT delivery can occur via lumbar puncture or intraventricularly via an Ommaya catheter. To avoid toxicity, there should be unobstructed CSF flow through the ventricular system. Thus, IT chemotherapy should be deferred in cases of bulky leptomeningeal disease or in patients with elevated intracranial pressure. In general, systemic MTX doses>3g/m2 are thought to penetrate the CSF raising the question of need for IT chemotherapy.
The eyes are often considered a reservoir in PCNSL though experience with ocular-directed therapy is limited to retrospective data. While some studies suggest higher rates of intraocular failure when site-directed therapy is deferred [49], there is evidence that micromolar concentrations of MTX are detected in the eye after infusion of high doses (8g/m2)[50] and other studies did not demonstrate a difference in overall survival with ocular therapy [51]. Site directed therapy can consist of ocular radiation or intravitreal methotrexate or rituximab. While practices vary, it is our practice to defer local therapy to the time of relapse.
Treatment of Refractory and Relapsed Disease
Though initial response rates to treatment are high, 10–15% of cases remain refractory to upfront therapy. In those who do respond, relapse is common, seen in up to 50% of patients [52]. The median time to relapse is 10 to 18 months, with most cases occurring in the first 2 years, though relapses up to 15 years after diagnosis have been described [52, 53]. Prognosis at relapse is poor with an estimated overall survival of 3.7 months for relapsed patients and 2 months for those with primary refractory disease [54]. Restaging, including complete neuroaxis imaging, lumbar puncture, and ophthalmologic exam should be performed at the time of relapse. We also recommend repeating a body PET as up to 10% of PCNSL can relapse systemically [52].
There is no standard approach to patients with relapsed or refractory disease and there are currently no randomized trials in this patient population. Choice of therapeutic agent depends on age, performance status, prior treatments and response, and medical comorbidities. Rechallenge with MTX-based therapy is reasonable, particularly for those with initial durable benefit to MTX and has led to response rates of 85 to 91% with a median OS of 41 to 62 months [55, 56]. Prospective trials using other chemotherapeutic agents including pemetrexed, topotecan, temozolomide, and rituximab have all been performed and demonstrated an ORR of 31–55% with PFS of 1.6 to 5.7 months [57–61].
In patients for whom it is in an option, HDC-ASCT is promising at time of relapse. A French prospective multi-center study treated patients with refractory and recurrent PCNSL with high-dose cytarabine and etoposide (CYVE) followed by HDC-ASCT. Only 47% of patients responded to CYVE (20) of which 75% of patients proceeded to HDC-ASCT. An additional 12 patients who did not have radiographic response to induction CYVE also underwent HDC-ASCT. All but one of 27 total patients had a CR after transplant with median PFS and OS 41.4 and 58.6 months, respectively[62, 63]. In patients naïve to radiation in whom HDC-ASCT is not an option, WBRT remains an effective treatment strategy. Retrospective studies in recurrent disease suggest a ORR of 74 to 79% and median OS of 10–16 months, suggesting efficacy similar to use in upfront treatment [64, 65]. Unfortunately, neurocognitive impairment remains a concern.
A number of novel strategies are being investigated in the treatment of relapsed PCNSL including immunotherapy and targeted therapies. PCNSL has a high rate of PDL-1 expression [66] leading to the investigation of PD-1 antibodies such as pembrolizumab and nivolumab. In a small study, four patients with relapsed or refractory disease received nivolumab. All four patients responded with PFS ranging 14 to 17 months and two patients remained without disease at 13 and 17 months [67]. Currently, phase 2 trials of PD-1 blockade are ongoing (NCT02857426 and NCT02779101). Another promising therapeutic is lenalidomide, an agent with activity in systemic lymphoma that recently demonstrated an ORR of 67% in combination with rituximab [68].
Increased understanding of the pathophysiology of this disease has led to the investigation of targeted therapies, a relatively new strategy. The first targeted agent was the mammalian target of rapamycin inhibitor temsirolimus, studied in a German multicenter phase 2 trial. Response rates were relatively high at 54% but unfortunately were not durable (PFS 2.1 months) suggesting development of drug resistance [69].
Bruton tyrosine kinase (BTK) is a central signaling node in the B-cell receptor (BCR) pathway. Frequent mutations affecting the BCR have been found in PCNSL [70]. Ibrutinib is a BTK inhibitor and has been shown to induce significant responses (77%) in recurrent PCNSL [70]. Ibrutinib was detectable in CSF at a concentration sufficient to induce cell death in lymphoma cell lines [70, 71]. Moreover, a recent phase 1 trial incorporated ibrutinib with temozolomide, etoposide, liposomal doxorubicin, dexamethasone, and rituximab (DA-TEDDI-R) for treatment of both relapsed/refractory and newly diagnosed PCNSL. Patients received two weeks of single agent ibrutinib prior to starting the additional chemotherapeutic agents. During the ibrutinib monotherapy window, 94% of patients had a radiographic response, with 86% achieving CR before completion of study [71]. Unfortunately, a serious and sometimes fatal complication of pulmonary or cerebral aspergillosis was seen in this trial and caution should be used when ibrutinib is given in PCNSL patient with chronic corticosteroid use or immunosuppressed status as seen after multiple chemotherapy treatments.
Elderly Patients
While there is no standard definition of the term “elderly,” more than half of patients with PCNSL are aged 60 and greater. The optimal management in these patients is not well defined and this population poses a particular set of challenges given their medical comorbidities and increased risk of neurologic toxicity. Moreover, these patients do not seem to have as strong or durable of a response to therapy. A post-hoc analysis of patients aged 70 and older was performed in the G-PCNSL-SG-1 trial, evaluating HD-MTX with our without WBRT [17]. Older patients had a slightly lower ORR (44% vs 57%) with shorter OS (12.5 vs 26.2 mo). Interestingly in all patients who achieved CR, PFS was much shorter in those aged 70 and older (16.1 vs 35.0 mo) [72], suggesting poorer outcomes in this population regardless of initial response to treatment.
While age is an independent predictor of survival [5, 6], a systematic review of 783 patients aged 60 and older demonstrated KPS to be a stronger predictor [73]. A majority of patients in this series (73%) were treated with HD-MTX, which was associated with improved OS. There was no survival difference between groups who received HD-MTX plus oral alkylating chemotherapy and those receiving more aggressive HD-MTX based regimens. This is in contrast to a prospective multicenter study in elderly patients comparing HD-MTX and temozolomide to a regimen of HD-MTX, procarbazine, vincristine, and cytarabine. The study suggested a trend towards improvement in ORR (82% vs 71%) and overall survival (31 mo vs 14 mo) with the more aggressive regimen, though results were not statistically significant [74].
Once response is achieved, there is no consensus regarding consolidation therapy in this population. WBRT is associated with a high risk of neurologic toxicity in the elderly [73, 75–77] and should be avoided. HDC-ASCT has traditionally been reserved for younger patients though one prospective study included patients up to age 72 [43] and retrospective data supports its use to age 77 [78], suggesting KPS is a more important consideration when determining eligibility. At the other end of the spectrum, many argue against any consolidation regimen in elderly patients who achieve CR.
Post-Treatment Monitoring
PCNSL has a high rate of recurrence. While a majority of relapses occur in the first two years after diagnosis, late relapses have been described. National Comprehensive Cancer Network (NCCN) guidelines recommend surveillance brain imaging every three months for the first two years after treatment, then every six months [79]. After five years, MRIs can be annual but should continue indefinitely. Surveillance spine MRI and CSF sampling should be considered for patients with spinal and leptomeningeal disease. Patients with documented ocular involvement should undergo routine ophthalmologic exams.
Conclusions
While PCNSL is an aggressive malignancy, treatment has evolved in the last 40 years and survival outcomes have improved in most patient groups [2]. There is also a focus on minimizing toxicity and morbidity associated with therapy. Though exact treatment differs by institution and practitioner, a rituximab/MTX-based induction therapy should be used as first-line treatment. Recommendations regarding consolidation may be more variable and should take into consideration the individual patient. Promising new developments are emerging with the optimization of HDC-ASCT and the introduction of immunotherapy as well as agents targeted in BCR pathway. Enrollment in clinical trials should be encouraged when available.
Acknowledgments
Support: This research was supported by a NIH/NCI Cancer Center Support Grant (P30-CA008748) and supported by grants from the Lymphoma Research Foundation Career Development Award (C.G.), Susan and Peter Solomon Divisional Fund (C.G.), and Cycle for Survival Equinox Innovation Award (C.G.).
References
- 1.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–8. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendez JS, Quinn OT, Kruchko C, Barnholtz-Sloan J, Grommes C. Changes in survival of primary central nervous system lymphoma based on a review of national databases over 40 years. Journal of Clinical Oncology. 2017;35(15_suppl):2040-. doi: 10.1200/JCO.2017.35.15_suppl.2040. [DOI] [Google Scholar]
- 3.O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol. 2013;88(12):997–1000. doi: 10.1002/ajh.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eloranta S, Branvall E, Celsing F, Papworth K, Ljungkvist M, Enblad G et al. Increasing incidence of Primary CNS lymphoma but no improvement in survival in Sweden 2000–2013. Eur J Haematol. 2017. doi: 10.1111/ejh.12980. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 6.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–72. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–8. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–6. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 9.Hong JT, Chae JB, Lee JY, Kim JG, Yoon YH. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol. 2011;102(1):139–45. doi: 10.1007/s11060-010-0303-9. [DOI] [PubMed] [Google Scholar]
- 10.DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G. Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology. 1990;40(1):80–6. [DOI] [PubMed] [Google Scholar]
- 11.Coulon A, Lafitte F, Hoang-Xuan K, Martin-Duverneuil N, Mokhtari K, Blustajn J et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12(2):329–40. doi: 10.1007/s003300101037. [DOI] [PubMed] [Google Scholar]
- 12.Gametchu B Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987;236(4800):456–61. [DOI] [PubMed] [Google Scholar]
- 13.Weller M Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 1999;43(3):237–9. [DOI] [PubMed] [Google Scholar]
- 14.Korfel A, Weller M, Martus P, Roth P, Klasen HA, Roeth A et al. Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol. 2012;23(9):2374–80. doi: 10.1093/annonc/mdr627. [DOI] [PubMed] [Google Scholar]
- 15.Batchelor T, Carson K, O’Neill A, Grossman SA, Alavi J, New P et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96–07. J Clin Oncol. 2003;21(6):1044–9. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010;12(4):409–17. doi: 10.1093/neuonc/nop053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–47. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 18.Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59(10):1557–62. [DOI] [PubMed] [Google Scholar]
- 19.Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31(1):100–5. doi: 10.1016/j.ejso.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Reni M, Ferreri AJ, Garancini MP, Villa E. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8(3):227–34. [DOI] [PubMed] [Google Scholar]
- 21.Weller M, Martus P, Roth P, Thiel E, Korfel A, German PSG. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14(12):1481–4. doi: 10.1093/neuonc/nos159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–43. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 23.Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9–17. [DOI] [PubMed] [Google Scholar]
- 24.Shibamoto Y, Ogino H, Hasegawa M, Suzuki K, Nishio M, Fujii T et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62(3):809–13. doi: 10.1016/j.ijrobp.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Schultz C, Scott C, Sherman W, Donahue B, Fields J, Murray K et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88–06. J Clin Oncol. 1996;14(2):556–64. doi: 10.1200/JCO.1996.14.2.556. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill BP, Wang CH, O’Fallon JR, Colgan JD, Earle JD, Krigel RL et al. Primary central nervous system non-Hodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86–72-52. Int J Radiat Oncol Biol Phys. 1999;43(3):559–63. [DOI] [PubMed] [Google Scholar]
- 27.Borsi JD, Moe PJ. A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer. 1987;60(1):5–13. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293(4):161–6. doi: 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 29.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–20. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 30.Reni M, Ferreri AJ, Guha-Thakurta N, Blay JY, Dell’Oro S, Biron P et al. Clinical relevance of consolidation radiotherapy and other main therapeutic issues in primary central nervous system lymphomas treated with upfront high-dose methotrexate. Int J Radiat Oncol Biol Phys. 2001;51(2):419–25. [DOI] [PubMed] [Google Scholar]
- 31.Mocikova H, Pytlik R, Sykorova A, Janikova A, Prochazka V, Vokurka S et al. Role of rituximab in treatment of patients with primary central nervous system lymphoma: a retrospective analysis of the Czech lymphoma study group registry. Leuk Lymphoma. 2016;57(12):2777–83. doi: 10.3109/10428194.2016.1167203. [DOI] [PubMed] [Google Scholar]
- 32.Kansara R, Shenkier TN, Connors JM, Sehn LH, Savage KJ, Gerrie AS et al. Rituximab with high-dose methotrexate in primary central nervous system lymphoma. Am J Hematol. 2015;90(12):1149–54. doi: 10.1002/ajh.24204. [DOI] [PubMed] [Google Scholar]
- 33.Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83(3):235–9. doi: 10.1212/WNL.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol. 2013;15(8):1068–73. doi: 10.1093/neuonc/not032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–27. doi: 10.1016/S2352-3026(16)00036-3. ••This is a prospective randomized study that demonstrated improved response rates with the MATRix regimen. Offered support for the use of rituximab in combination with methotrexate.
- 36.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570–4. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 37.Omuro AM, Ben-Porat LS, Panageas KS, Kim AK, Correa DD, Yahalom J et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62(10):1595–600. doi: 10.1001/archneur.62.10.1595. [DOI] [PubMed] [Google Scholar]
- 38.Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–9. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass J, Won M, Schultz CJ, Brat D, Bartlett NL, Suh JH et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227. J Clin Oncol. 2016;34(14):1620–5. doi: 10.1200/JCO.2015.64.8634. •This is a prospective study of methotrexate, rituximab, and temozolomide followed by low dose whole brain radiation and adjuvant temozolomide. Early data suggests there is limited neurotoxicity.
- 40.Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151–6. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38(6):417–20. doi: 10.1038/sj.bmt.1705452. [DOI] [PubMed] [Google Scholar]
- 42.Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18(4):665–71. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 43.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–10. doi: 10.1182/blood-2014-10-604561. ••This is a prospective study of rituximab, methotrexate, procarbazine, and vincristine followed by HDC-ASCT in patients with newly diagnosed PCNSL.
- 44.Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3(8):e388–97. doi: 10.1016/S2352-3026(16)30050-3. ••This is a prospective study of methotrexate-based chemotherapy followed by HDC-ASCT in newly diagnosed PCNSL.
- 45.Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–20. [DOI] [PubMed] [Google Scholar]
- 46.Sierra Del Rio M, Ricard D, Houillier C, Navarro S, Gonzalez-Aguilar A, Idbaih A et al. Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol. 2012;106(1):143–6. doi: 10.1007/s11060-011-0649-7. [DOI] [PubMed] [Google Scholar]
- 47.Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58(2):175–8. [DOI] [PubMed] [Google Scholar]
- 48.Pels H, Juergens A, Glasmacher A, Schulz H, Engert A, Linnebank M et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: results of a phase II study. J Neurooncol. 2009;91(3):299–305. doi: 10.1007/s11060-008-9712-4. [DOI] [PubMed] [Google Scholar]
- 49.Ferreri AJ, Blay JY, Reni M, Pasini F, Gubkin A, Tirelli U et al. Relevance of intraocular involvement in the management of primary central nervous system lymphomas. Ann Oncol. 2002;13(4):531–8. [DOI] [PubMed] [Google Scholar]
- 50.Batchelor TT, Kolak G, Ciordia R, Foster CS, Henson JW. High-dose methotrexate for intraocular lymphoma. Clin Cancer Res. 2003;9(2):711–5. [PubMed] [Google Scholar]
- 51.Grimm SA, McCannel CA, Omuro AM, Ferreri AJ, Blay JY, Neuwelt EA et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology. 2008;71(17):1355–60. doi: 10.1212/01.wnl.0000327672.04729.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahnke K, Thiel E, Martus P, Herrlinger U, Weller M, Fischer L et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159–65. doi: 10.1007/s11060-006-9165-6. [DOI] [PubMed] [Google Scholar]
- 53.Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525–9. doi: 10.1093/neuonc/nor014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langner-Lemercier S, Houillier C, Soussain C, Ghesquieres H, Chinot O, Taillandier L et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–303. doi: 10.1093/neuonc/now033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plotkin SR, Betensky RA, Hochberg FH, Grossman SA, Lesser GJ, Nabors LB et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–6. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 56.Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117(1):161–5. doi: 10.1007/s11060-014-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nayak L, Abrey LE, Drappatz J, Gilbert MR, Reardon DA, Wen PY et al. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54(1):58–61. doi: 10.3109/10428194.2012.698736. ••This study suggested efficacy of nivolumab in relapsed/refractory PCNSL.
- 58.Fischer L, Thiel E, Klasen HA, Birkmann J, Jahnke K, Martus P et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–5. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 59.Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–7. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–30. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raizer JJ, Rademaker A, Evens AM, Rice L, Schwartz M, Chandler JP et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118(15):3743–8. doi: 10.1002/cncr.26709. [DOI] [PubMed] [Google Scholar]
- 62.Soussain C, Hoang-Xuan K, Levy V. [Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma]. Bull Cancer. 2004;91(2):189–92. [PubMed] [Google Scholar]
- 63.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26(15):2512–8. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 64.Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–82. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507–13. doi: 10.1200/JCO.2005.01.161. [DOI] [PubMed] [Google Scholar]
- 66.Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–81. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubenstein J, Fraser E, Formaker P, al. e. Phase I investigation of lenalidomide plus rituximab and outcomes of lenalidomide maintenance in recurrent CNS lymphoma [abstract 7502]. J Clin Oncol. 2016;34(suppl):7502. [Google Scholar]
- 69.Korfel A, Schlegel U, Herrlinger U, Dreyling M, Schmidt C, von Baumgarten L et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol. 2016;34(15):1757–63. doi: 10.1200/JCO.2015.64.9897. •Patients with relapsed/refractory PCNSL were treated with temsirolimus. The study yielded high ORR but results were not durable.
- 70.Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017;7(9):1018–29. doi: 10.1158/2159-8290.CD-17-0613. ••Study demonstarted the central role of the B-cell receptor signaling axis in PCNSL and clinical efficacy of ibrutinib targeting this pathway in patients with relapsed/refractory PCNSL.
- 71.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31(6):833–43 e5. doi: 10.1016/j.ccell.2017.04.012. ••Phase I study of ibrutinib in combination with chemotherapy (DA-TEDDI-R) suggested efficacy for relapsed/refractory PCNSL though with a risk for aspergillosis.
- 72.Roth P, Martus P, Kiewe P, Mohle R, Klasen H, Rauch M et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology. 2012;79(9):890–6. doi: 10.1212/WNL.0b013e318266fcb2. [DOI] [PubMed] [Google Scholar]
- 73.Kasenda B, Ferreri AJ, Marturano E, Forst D, Bromberg J, Ghesquieres H et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)--a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26(7):1305–13. doi: 10.1093/annonc/mdv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omuro A, Chinot O, Taillandier L, Ghesquieres H, Soussain C, Delwail V et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–9. doi: 10.1016/S2352-3026(15)00074-5. •This study compared two different methotrexate-based regimens in an elderly population. Results favored the use of methotrexate, procarbazine, and vincristine over methotrexate and temozolomide alone.
- 75.Ney DE, Reiner AS, Panageas KS, Brown HS, DeAngelis LM, Abrey LE. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2010;116(19):4605–12. doi: 10.1002/cncr.25363. [DOI] [PubMed] [Google Scholar]
- 76.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18(17):3144–50. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 77.Doolittle ND, Korfel A, Lubow MA, Schorb E, Schlegel U, Rogowski S et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84–92. doi: 10.1212/WNL.0b013e318297eeba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schorb E, Fox CP, Fritsch K, Isbell L, Neubauer A, Tzalavras A et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant. 2017;52(8):1113–9. doi: 10.1038/bmt.2017.23. [DOI] [PubMed] [Google Scholar]
- 79.Network NCC. Primary CNS Lymphma (Version 1.2017). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.


