Abstract
Objective
To evaluate a VEGF targeted gene therapy for the treatment of endometriosis.
Design
Analysis of the VEGF gene expression and promoter activity in ectopic and eutopic endometrium. Evaluation of the specific replication and cell killing effect of a VEGF targeted adenovirus (Ad5VEGFE1) in endometriotic cells.
Patients
4 patients who underwent hysterectomy for benign disease, 30 women with moderate superficial and 30 women with deep infiltrating endometriosis
Interventions
VEGF immunostaining and gene expression was examined in eutopic endometrium, endometriotic lesions and normal peritoneum. VEGF promoter activity was evaluated in eutopic endometrium and endometriotic lesions. A VEGF targeted conditionally replicative adenovirus (Ad5VEGFE1) was evaluated with regard to specific viral replication in endometriosis cells and induction of apoptosis. The biodistribution of the VEGF targeted CRAD was examined in a mouse model.
Results
The VEGF gene is highly expressed in ectopic endometrium compared to eutopic endometrium and normal peritoneum. The VEGF promoter is active in endometriotic cells. Ad5VEGFE1 shows efficient viral replication and induction of apoptosis in purified primary endometriotic cells and demonstrated a comparable lower targeting to the liver and the uterus in a mouse model.
Conclusions
Ad5VEGFE1 is a promising candidate for treating endometriosis and holds potential for clinical testing.
Keywords: endometriosis, gene therapy, replicating adenovirus, targeting
Introduction
Endometriosis is defined as the presence of endometrial tissue outside the uterine cavity (1). Endometriosis is a common chronic gynaecologic disease characterized by pelvic pain and infertility. The most widely accepted hypothesis for the development of endometriosis is retrograde menstruation. In addition, other factors render certain women susceptible to the implantation and re-growth of ectopic endometrium. It has been shown, that endometrium is rich in angiogenic growth factors which play an important role in the survival, adhesion and invasion of exfoliated endometrial cells (2).
The treatment of pelvic pain and infertility caused by endometriosis is surgical removal of endometriotic implants and adhesiolysis to restore the normal anatomy. Medical treatment of endometriosis aims at suppressing circulating estrogen to a postmenopausal level by analogues of gonadotropin-releasing hormone (GnRH), oral contraceptives or progestin (3). In humans estrogen stimulates the growth of endometriotic implants, whereas aromatase inhibitors that block estrogen formation are beneficial, as are antiprogestins. Unfortunately, this therapeutic approach has been shown to be of limited benefit and is associated with severe side effects such as loss of bone mineral density (4), suppression of ovulation and postmenopausal, vasomotor symptoms, weight gain and mood disorders. Therefore, it is recommended to limit the use of anti-estrogens to a maximum of 6 month although longer treatment periods are possible if add back strategies are used. In addition, a recent systematic Cochrane Database review did not convey any benefit for ovulation suppression in the treatment of endometriosis associated infertility (5).
Consequently, there is a need to develop new therapeutic approaches to endometriosis. In this regard, molecular therapies have been proposed as a treatment alternative for recurrent endometriosis. The use of conditionally replicative adenoviruses (CRADs) that are designed to exploit key differences between target cells and normal cells to allow specific viral replication, has been proposed an emerging strategy for cancer therapy. Until today, the use of CRADs has never been explored for the therapy of endometriosis. However, adenovirus based gene therapy approaches seem rational as a novel targeting approach for this disease and have shown promising results in vitro (6, 7). For the treatment of advanced or recurrent endometriosis intraperitoneal delivery schemes would be desirable but are potentially associated with ectopic side effects, especially to the liver (8). This ectopic liver delivery is the major predicate of Ad-vector-induced toxicity. To achieve a significant level of target cell selectivity, transcriptional targeted viruses have been developed. In transcriptional targeting, the expression of the gene of interest is placed under control of a tumor-specific promoter (TSP). It is rational to choose a TSP that is highly expressed in the target cell but has potentially low activity in normal cells including the liver. Vascular endothelial growth factor (VEGF) is a heparin binding glycoprotein with potent angiogenic and vascular permeability activities and is considered one key mediator of physiologic and pathologic angiogenesis. VEGF is found in women with endometriosis at elevated levels in peritoneal fluid and macrophages, where it appears to be regulated by ovarian steroids (9, 10). Immunohistochemical and biochemical studies have shown VEGF to be involved in both the pathogenesis and maintenance of endometriosis (9–11). Hull et al. (12) found a large number of pericyte-free vessels in endometriotic lesions compared with the superficial eutopic endometrium. Ectopic endometrial tissue triggers an angiogenic response by attracting endothelial cells to migrate into the graft and pericytes are recruited to stabilize vessels (14). Other authors have found that vascularisation and VEGF expression are significantly higher in patients with deep infiltrating endometriosis (13). A recent immunohistochemical evaluation of VEGF expression in women with endometriosis showed a significantly higher expression rate in ectopic compared to eutopic endometrium.
To verify these data and to evaluate the usefulness of the VEGF promoter for a targeting approach of endometriosis we have performed immunohistochemical examinations and RT-PCR to examine gene expression profiles in eutopic and ectopic endometrium and in peritoneal tissue. In addition we evaluated adenoviral constructs with the VEGF promoter controlling the expression of a marker gene. We have demonstrated replication of VEGF targeted adenoviruses and induction of apoptosis in endometriosis cells. Finally we have evaluated the toxicity of a systemic administration of a VEGF Crad with regard to liver and eutopic endometrium.
Material & Methods
Cell preparation and culture
All tissue samples were obtained following institutional review board approval. Normal endometrial stroma cells were prepared from hysterectomy specimen biopsies taken from three premenopausal women (age: 38 – 42 years) who underwent hysterectomy in the early proliferation phase for subserous myomas. Patients had regular 26- to 32-d menstrual cycles and had received no hormonal medication in the preceding 3 months. Endometrial tissue samples were taken from visually normal sites in the uterine fundus to avoid regional variabilities. Representative areas of eutopic and ectopic endometrium were prepared from 30 women with moderate superficial endometriosis revised American Fertility Society (rAFS) score I-II and 30 women with deep infiltrating endometriosis (rAFS score III–IV) who underwent hysteroscopy and laparoscopic surgery for primary treatment. Endometrium and endometriotic tissue was dissected away from the adjacent host tissue and diagnosis was confirmed by a clinician pathologist. The tissues were rinsed with phosphate-buffered saline (PBS) and endometrial or endometriotic tissue was minced and digested with collagenase (2mg/ml) at 37°C in 5% CO2/air for 60 min. Epithelial glands, blood and stromal cells were separated from stromal cells and debris by serial filtration using narrow gauge sieves with apertures of 38 – 105 μm as described previously (14). Endometrial and endometriosis stroma cell cultures were allowed to proliferate in α-Minimum Essential Medium supplemented with 10% fetal calf serum (FCS), nucleosides, and amino acids. Stromal cell viability was assessed by trypan blue exclusion and was similar in endometrial and endometriotic cells (83.2% and 85.6%, respectively). The purity of the isolated cells was assessed by staining with Thy-1 and vimentin. The percentages of Thy-1- and vimentin-positive cells were 89% and 93%, respectively.
Viruses
AdVEGFluc and AdCMVluc are replication-defective adenoviruses with a luciferase reporter gene, driven by the VEGF or CMV promoter, respectively, in the E1 region. Ad5VEGFE1 is a replication competent adenovirus and has been described previously (15). All replicative adenoviruses were amplified on HeLa cells to avoid wild-type contamination. Replication-deficient viruses were propagated on 293 cells and purified by double CsCl density centrifugation. Physical particle concentration (virus particles (vp/ml) was determined by OD260 reading and functional virus titers (plaque-forming units (pfu)/ml) were determined by plaque assay in 293 cells.
Immunohistochemistry
Immunohistochemistry was performed on 5 μm paraffin-embedded tissue sections of ectopic endometrium. After heat drying, sections were deparaffinized in xylene and sequentially rehydrated in gradients of ethanol. After 3 washes with H2O2, the sections were incubated overnight at 4º C with the monoclonal antibody SC-7269 (Santa Cruz Biotechnology, Santa Cruz, CA at 1:100 dilution. Incubations were carried out overnight and then revealed using SreptAB Complex/HPR Duet Kit (Dako). Binding of antibodies was visualized by a peroxidase catalysed colour reaction using 3′,3′-diaminobenzidine tetrahydrochloride. Both positive and negative controls were included with each group of slides to ensure consistency of the staining process and to monitor the presence of any non-specific staining. Negative staining was defined as no VEGF signal at either the stromal or glandular cells (Immunohistochemical (IHC) score 0) or with weak signal in < 20% of cells in either the stromal or glandular cells (IHC score 1). Positive VEGF staining was defined as moderate signal intensity in 20%–60% of the stromal or glandular cells (IHC score 2), strong VEGF signal intensity >60% of stromal and glandular cells (IHC score 3), or strong signal intensity in >90% of either the stromal or glandular cells.
RNA preparation and quantitative RT-PCR
Total cellular RNA was extracted from 2×105 cells from purified deep infiltrating endometriosis cells, purified eutopic endometrium cells and peritoneal tissue samples using the RNeasy mini prep kit (Qiagen, Santa Clarita, CA) and treated with DNase I (Life Technologies Inc; Rockville, MD) for 30 min. PCR products from VEGF were used for creation of the standard curve. GeneAmp RNA PCR core kit (Applied Biosystems) was used for cDNA synthesis and PCR amplification of cDNA products. TaqMan primers and probes were designed by the Primer Express 1.0 software and synthesized by Applied Biosystems (Foster City, CA). Oligonucleotide sequences for amplification of the VEGF gene were forward primer, CAT GCA GAT TAT GCG GAT CAA reverse primer TTT GTT GTG CTG TAG GAA GCT CA and probe 6FAM- CCT CAC CAA GGC CAG CAC ATA GGA GA-TAMRA. Human house keeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as house keeping gene for internal control. The sequences to amplify GAPDH gene were forward primer GGT TTA CAT GTT CCA ATA TGA TTC CA, reverse primer ATG GGA TTT CCA TTG ATG ACA AG and probe 6FAM-CGT TCT CGC CTT GAC GGT GCC AT-TAMRA. With optimized concentration of primers and probe, the components of Real-Time PCR mixture were designed to result in a master mix with a final volume of 9 μl per reaction containing 1X TaqMan® EZ RT-PCT Kit (Applied Biosystems, Foster City, CA), 100 nM forward primer, 100 nM reverse primer, 100 nM probe and 0.025%BSA. For the assay, known amount of CMV template DNA (108, 106, 104 and 102 copies/μl) was amplified to generate a standard curve for quantification of the CMV copy numbers of unknown samples. The sequences to amplify CMV gene were forward primer ATA TGC CAA GTA CGC CCC CTA T, reverse primer CCA TAA GGT CAT GTA CTG GGC ATA A and probe 6FAM-TCA ATG ACG GTA AAT GGC CCG CCT-TAMRA Known amount of human total RNA (200, 20, 2 and 0.2 ng/μl) was amplified to generate a standard curve for determination of the concentration of unknown samples. 1μl of total RNA sample was added to 9 μl of PCR mixture in each reaction capillary. A no template control received 1 μl of water. All capillaries were then sealed and centrifuged using LC Carousel Centrifuge (Roche Molecular Biochemicals, Indianapolis, Indiana) to facilitate mixing. All PCR was carried out using a LightCycler™System (Roche Molecular Biochemicals, Indianapolis, Indiana). Thermal cycling conditions were subjected to 2 minutes at 50 °C, 30 minutes at 60 °C, 5 minutes at 95 °C and 40 cycles of 20 seconds at 94 °C and 1 minute at 60 °C. Data was analyzed with LightCycler software.
TUNEL assay
Primary endometriotic stroma cells were purified and cultured overnight. The next day, cells were infected for 1 hour at 37º C with Ad5VEGFE1 or Ad5CMV at an MOI of 50 PFU/cell. After 48 hours, cells were fixed and treated with protease for 2 min at room temperature. A fluorometric TUNEL assay (Promega, Madison, WI) was used to detect cells with apoptotic cell fragmentation. Negative control slides were prepared by omitting the TdT enzyme during labelling and positive control slides were pretreated with Dnase1. To determine the percentage of cells undergoing apoptosis, we stained cells with Sytox® Orange (Invitrogen, Carlsbad, CA).
In vitro gene transfer
Purified endometriotic stroma cells were plated on day 1 at 10000 cells/well on 96 well plates in 100 μl GM on a rocker. On day 2, cells were infected with 10 pfu/cell for 2 h in 20 μl of 2% GM on a rocker. Afterwards, cells were washed once with PBS, and 60 μl 10% GM was added per well. After 24 h, the GM was removed; cells were washed once with PBS and lysed with 20 μl of lysis buffer (Reporter Lysis Buffer; Promega Corp., Madison, WI) and freeze-thawed once. Twenty μl of these samples were mixed with 100 μl of luciferase assay reagent (Reporter Lysis Buffer; Promega Corp.) and measured with Berthold Lumat LB 9501. Standardization was accomplished by setting values obtained with AdCMVLuc as 100% for each cell line.
Animal experiments
Mice were obtained at 4–6 weeks and quarantined at least 2 weeks before the study. Mice were kept under pathogen-free conditions according to the American Association for Accreditation of Laboratory Animal care guidelines. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of UAB. For determination of virus toxicity in the mouse uterus and liver female C57/BL6 mice (Charles River Laboratories, n=6/group) received 1×109 PFU of Ad5VEGFluc or Ad5CMVluc intraperitoneally. After 48h, mice were sacrificed and livers were harvested and representative sections were snap frozen. The uterine horns were opened longitudinally and biopsies of uterine tissue was isolated and snap frozen. The frozen organ samples were ground to a fine powder using a mortar and pestle cooled in a dry-ice ethanol bath. Organ powders were lysed using Cell Culture Lysis Buffer (Promega) at room temperature for 20 min. Lysates were frozen once, then centrifuged at 10,000 g for 15 min. Luciferase activity was measured as described before. Mean background luciferase activity was subtracted from the data. The luciferase activity was normalized by protein concentration in the tissue lysate.
Statistics
Data are presented as mean values ± standard deviation. Statistical differences among groups were assessed with a two-tailed student’s t-test. P (*) < 0.05 was considered significant.
Results
Immunohistochemical Analysis
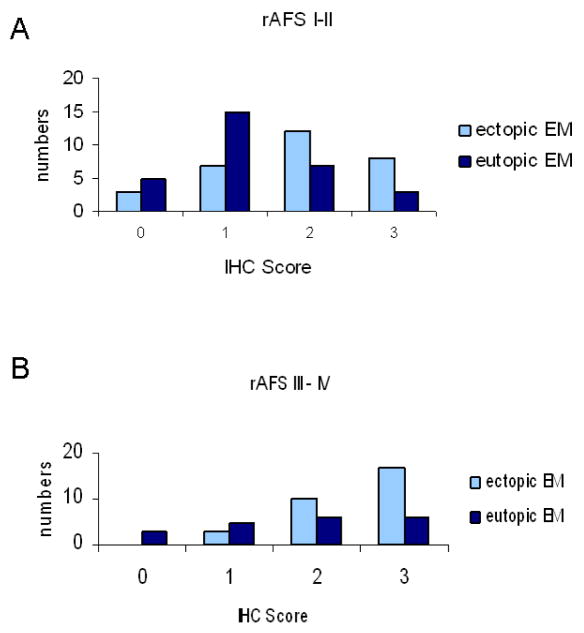
To evaluate VEGF expression in eutopic endometrium and endometriotic implants, we examined 30 women with moderate superficial endometriosis (rAFS score I–II) and 30 women with deep infiltrating endometriosis (rAFS score III–IV). All patients underwent hysteroscopy and endometrial biopsy and laparoscopy with resection of endometrial implants. All patients examined were in the early proliferative phase. All ectopic endometriotic implants and tissue samples of eutopic endometrium were examined for VEGF immunoreactivity. As shown in figure 1, VEGF expression in ectopic endometrium was significantly higher in both, women with moderate superficial endometriosis and advanced endometriosis with deep infiltrating implants. Interestingly, VEGF staining in patients with advanced endometriosis was not only increased in ectopic endometrial tissue (VEGF IHC score 3: 17 versus 8 patients) compared with patients with moderate superficial endometriosis but also in eutopic endometrium (VEGF IHC score 3: 6 versus 3 patients).
Figure 1.
Immunohistochemical analysis of VEGF expression in eutopic endometrium and ectopic endometriosis in 30 patients with moderate superficial endometriosis rAFS score I–II (A) and 30 patients with deep infiltrating endometriosis (B).
Evaluation of VEGF mRNA Expression in eutopic endometrium, normal peritoneum and ectopic endometriosis
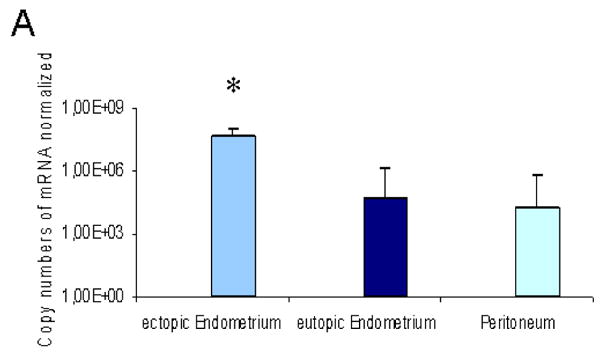
Quantitative RT-PCR was performed to examine the level of VEGF mRNA relative to the housekeeping gene, GAPDH in ectopic and eutopic endometrium and macroscopically unaffected peritoneum from women with deep infiltrating endometriosis (Fig. 2A). Whereas similar levels of expression were seen for the housekeeping gene GAPDH (data not shown), the average VEGF mRNA copy number was significantly (951-fold and 2390-fold, P<0.05) increased in endometiotic cells of patients with deep infiltrating endometriosis compared to normal endometrium and normal peritoneum, respectively.
Figure 2.
(A) The human VEGF gene is overexpressed in ectopic endometriosis. RNA was extracted from deep infiltrating endometriotic lesions, eutopic endometrium and normal peritoneum from four patients suffering from deep infiltrating endometriosis and reverse-transcribed into cDNA. Real-time PCR analysis was performed to quantify the expression of the VEGF gene. The gene copy numbers are normalized by the GAPDH copy number. Error bars indicate SD. *p<0.05 versus eutopic EM and Peritoneum
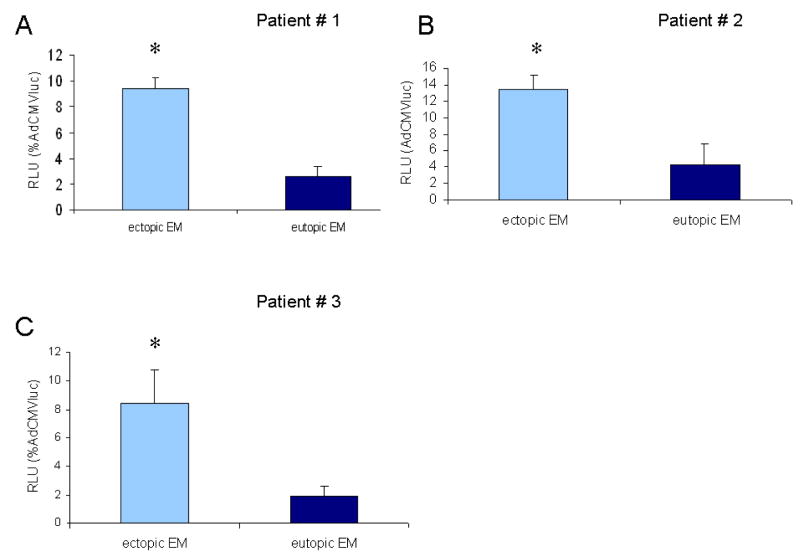
(B) The human VEGF promoter is active in endometriotic lesions. Ectopic endometrium cells from three women with endometriosis and eutopic endometrium from three healthy patients were infected with AdVEGFluc and AdCMVluc, respectively at an MOI of 10. Luciferase activity of the VEGF promoter is expressed as relative light units (RLU) as a percentage of the CMV promoter driven activity. Each point represents the mean of three experiments. Error bars indicate standard deviation. *p<0.05 versus eutopic endometrium
The VEGF promoter shows the highest activity in ectopic endometrium
To more closely model the clinical situation with the most stringent substrate, gene transfer experiments were performed using unpassaged human endometrium and endometriosis cells. In our experiments, primary human endometrium cells from three healthy patients and endometriotic cells from three women with deep infiltrating endometriosis were infected with AdVEGFluc and AdCMVluc, respectively. At a viral dose of 10 pfu/cell, gene expression controlled by the VEGF promoter resulted in 9.4, 13.4 and 8.4% of the expression achieved with the CMV promoter in the three endometriosis patient samples (Figure 2B). Promoter activity in eutopic endometrium cells from healthy patients were 2.6, 4.8, and 1.9 fold (P<0.05) lower. The differences of the promoter activity correlate with the results of the gene expression analysis.
Ad5VEGFE1 induces apoptosis in endometriosis cells
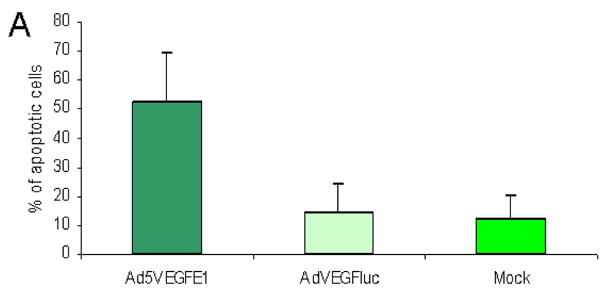
The induction of apoptosis in endometriosis cells was assessed with a TUNEL assay (Figure 3A). Endometriosis cells infected at an MOI of 50 pfu/cell showed a significantly higher apoptosis rate of 52.4% ± 17.1 compared to cells infected with the non-replicative control virus AdVEGFluc or no virus (14.2 ± 10.2 and 12.4 ± 8.2, respectively).
Figure 3.
(A) Induction of apoptosis in endometriosis cells after infection with the VEGF targeted CRAD. Endometriosis cells were infected at an MOI of 50 pfu/cells with Ad5VEGFE1, AdVEGFluc or no virus. Apoptosis was detected by TUNEL assay. Each bar represents the mean of three experiments. Error bars indicate standard deviation.
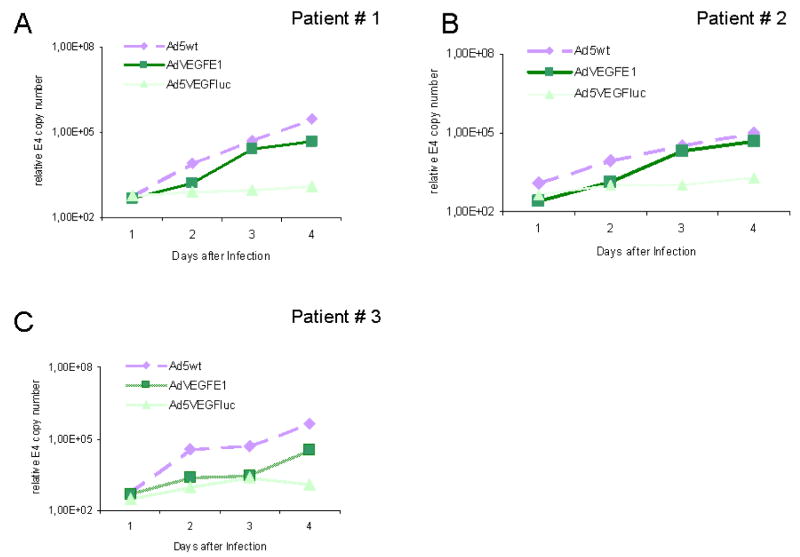
(B) Ad5VEGFE1 replicates in purified human endometriotic cells. Purified and unpassaged endometriotic cells were infected with 1000 vp/cell of Ad5VEGFE1, Ad5wt (wild type) and AdVEGFluc. Cells and growth medium were collected at indicated time points and virus copy number was measured with quantitative PCR.
Ad5VEGFE1 replicates in human endometriosis cells
We analysed three purified unpassaged human endometriosis samples for adenoviral replication. To evaluate viral copy number, we collected cells and growth medium at indicated time points and performed quantitative PCR for the adenoviral E4 gene (Figure 3B). To determine the relative increase in copies, we normalized E4 copy number at each time point to the copy number obtained with AdVEGluc at 24 hours. On day 4, Ad5VEGFE1 copy number had increased to 98-, 188- and 67-fold in patient samples 1 to 3, respectively. Thus, Ad5VEGFE1 showed continuous replication over time.
Biodistribution of VEGF targeted adenoviruses after intraperitoneal administration in mice
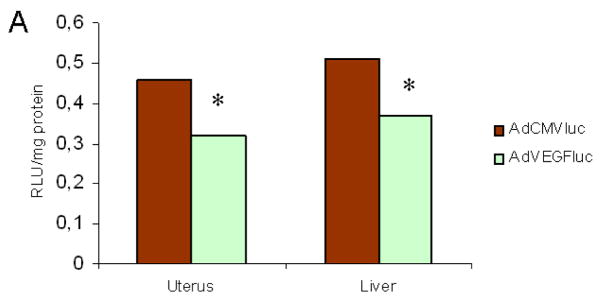
A key limitation to the use of a systemic gene therapy approach is the potential toxicity to non-target cells. Ad-based gene therapy vectors show a high tropism to the liver. In the context of endometriosis gene therapy tropism to the endometrium is another concern in woman at reproductive age. Therefore we were interested to determine the VEGF promoter activity in the liver and the eutopic endometrium in vivo. To this end, AdVEGFluc and AdCMVluc (as a positive control) were injected intraperitoneally (i.p.) into mice (Fig. 4). In this assay, transgene expression induced by the VEGF promoter in the liver was a mean 1.43-fold (P<0.05) lower compared to that that of AdCMVluc. Transgene expression in the eutopic endometrium was 1.37-fold (P<0.05) lower compared to AdCMVluc.
Figure 4.
The human VEGF promoter is repressed in the murine liver and endometrium. Fourthy-eight hours after intraperitoneal administration of VEGF or CMV promoter-driven expression vectors (AdVEGFluc, AdCMVluc; 109 pfu), Organs were harvested and luciferase activities were analysed in the liver and the uterus of the mouse. The luciferase activities are shown as RLU/mg protein. Error bars indicate SD. *p<0.05 versus AdCMVluc.
Discussion
The development of novel therapeutic strategies for the treatment of recurrent deep infiltrating endometriosis would be a welcome expansion of established treatment modalities. A radical surgical approach with resection of deep infiltrating endometriosis is considered the therapy of choice in the treatment of this disease. However, targeted therapy is a potentially effective therapeutic option for patients with recurrent endometriosis or patients with an increased risk for radical surgery. Adenoviral vectors have been used in a large number of gene therapy approaches predominantly for cancer treatment due to their capacity to accomplish effective in vivo gene delivery. Findings ways to target specific cells has become an important goal for gene therapy research. Former examinations have demonstrated a high VEGF expression in patients suffering from deeply infiltrating endometriosis affecting the rectum (13). We compared the VEGF expression in patients with deep infiltrating endometriosis and moderate superficial endometriosis. The first group of patients showed a significantly higher VEGF immunoreactivity. In a second step we evaluated the expression of VEGF mRNA in early proliferative phase endometrial cells, ectopic endometriotic tissue and unaffected peritoneal tissue samples from women with deep infiltrating endometriosis and normal peritoneal tissue samples. In our study VEGF showed significantly higher expression rates in ectopic endometriotic tissue compared to both, normal eutopic endometrium and peritoneum. With regard to these expression patterns a targeted therapy of endometriosis using the VEGF promoter seemed rational.
Targeting of endometriotic lesions with angiostatic agents has been demonstrated previously. Hull et al. (12) showed that antiangiogenic agents inhibit the growth of explants in an in vivo model of endometriosis by disrupting the vascular supply. Others (16) demonstrated that inhibition of angiogenesis interferes with the maintenance of endometriosis in a nude mouse model. Park et al (17) demonstrated an inhibition of endometriosis development in Rhesus monkeys by blocking the VEGF receptor (18).
To evaluate the usefulness of the VEGF promoter for a targeted therapy approach of endometriosis, we compared the activity of the VEGF promoter in an Ad construct with that of the ubiquitously expressed CMV promoter. Our results demonstrate a significantly higher activity in ectopic endometriotic tissue compared to eutopic endometrium and normal peritoneum respectively. In addition, our results show a strong correlation between VEGF expression level and the corresponding promoter activity. Thus, VEGF appears to be a warranted promoter with sufficient specificity for targeting of endometriotic cells. Alternative promoters for transcriptional targeting of adenoviral vectors to endometriosis cells are promoters for the secretory leucoprotease gene (SLPI) the heparanase gene (19). Both promoters demonstrated a high activity in endometriosis cells but activity in eutopic endometrium and normal peritoneum has not been examined to date.
After having identified the VEGF promoter as potentially useful for targeting endometriosis we did a preclinical evaluation of the VEGF targeted CRAD Ad5VEGFE1 in the context of endometriosis gene therapy. We demonstrated that AdVEGFE1 replicates in a short term culture of purified ectopic endometriosis cells. In addition, the virus induces apoptosis in endometriotic cells in vitro.
A major challenge for systemic administration of adenovirus-based gene therapy vectors is toxicity resulting from infection of normal cells. Endometriotic cells infected with replication-competent Ads may release new virions in vivo. If this were to occur there would be a potential for in vivo toxicity especially in the liver which is the predominant site of Ad vector localisation after systemic administration. In vivo, the liver is the major organ for Ad clearance in mice (19, 20). The comparison of a candidate promoter with the ubiquitously expressed CMV promoter in C57/BL6 mice is therefore regarded as standard experiment to evaluate tropism of candidate vectors to non target organs. Another important aspect in women at reproductive age is potential toxicity to the eutopic endometrium. In the present study VEGF promoter activity was low in both, the liver and endometrium following intraperitoneal Ad administration in the mouse. In addition, a previous study (15) demonstrated a good correlation in terms of replication rate and cytotoxicity for high versus low VEGF expressing cells for AdVEGFE1. Therefore we can expect a sufficient target/liver toxicity profile for this virus. A recently published study has shown a high activity of the expanded tropism adenovirus Ad5-RGD-luc in endometriosis cells. This adenovirus contains a targeting peptide (Arg-Gly-Asp) that is attached to the HI loop of the adenovirus fiber to redirect its entry through integrin receptors on the surface of the target cell. The liver activity of this fiber modified adenovirus was comparable to unmodified viruses resulting in an improved target/liver ratio. A combination of both targeting approaches is an interesting option to further improve the vector specificity for endometriosis gene therapy in the future. This is the first study which has evaluated a replicative Adenovirus for gene therapy of endometriosis. A previously published study has evaluated the therapeutic effect of angiostatin gene transfer in a mouse model of endometriosis. Intraperitoneal delivery of a nonreplicative adenovirus encoding for the angiogenesis inhibitor angiostatin induced transient production of bioactive angiostatin in vivo up to 10 days (21, 22) and resulted in a decrease of the number, size and density of blood vessels. More importantly, established endometriosis was eradicated in all treated mice within 18 days (18).
Fortin et al. evaluated the efficacy of HSV-TK/GCV for treatment of endometriosis. Human endometrial fragments were infected ex vivo with an adenovirus containing HSV-TK and injected subcutaneously into nude mice. GCV treatment induced a significant regression in endometrial implants (23).
Another approach which has recently been published (24) is the transfection of endometriotic cells by dominant negative estrogen receptor genes via adenovirus vector. Dominant negative mutants of the estrogen receptors are altered ER forms that are unable to activate transcription of estrogen responsive genes when estradiol binds to them. In this study endometriotic cells were effectively transfected in vitro resulting in decreased cell proliferation and increased apoptosis.
Gene therapy applications in which viral vectors are used for gene transfer have shown promise in several preclinical studies. Unfortunately, inefficient tumor transduction has often precluded significant benefit in clinical trials. Utilization of the oncolytic potential of viruses for killing of target cells predates the concept of gene therapy by more than half a decade (25). Nevertheless, due to safety concerns, most gene therapy approaches have been based on viruses that are unable to replicate in infected cells. Nevertheless, the main result from a generation of clinical trials with these agents is that the utility of replication-deficient viruses may be limited in the face of advanced and bulky disease such as advanced tumors or deep infiltrating endometriosis. The intratumoral diffusion of nanosize carriers such as viruses may be a limiting step. To help improve transduction, oncolytic viruses such as conditionally replicating adenoviruses (CRAds), have been developed. Ad5VEGFE1 allows specific replication and efficient killing of endometriotic cells. In addition the virus shows a comparably low tropism to the liver and eutopic endometrium in vitro and in a mouse model. In a clinical setting, VEGF targeted CRADs could be administered into the abdominal cavity following laparoscopic resection of deep infiltrating endometriosis.
Acknowledgments
This work was supported by the NIH (Grant R01 CA083821).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Ramon L, Gilabert-Estelles J, Castello R, Gilabert J, Espana F, Romeu A, et al. mRNA analysis of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis using a real-time quantitative RT-PCR assay. Hum Reprod. 2005;20:272–8. doi: 10.1093/humrep/deh571. [DOI] [PubMed] [Google Scholar]
- 3.Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Lupron Study Group Fertil Steril. 1990;54:419–27. doi: 10.1016/s0015-0282(16)53755-8. [DOI] [PubMed] [Google Scholar]
- 4.Whitehouse RW, Adams JE, Bancroft K, Vaughan-Williams CA, Elstein M. The effects of nafarelin and danazol on vertebral trabecular bone mass in patients with endometriosis. Clin Endocrinol (Oxf) 1990;33:365–73. doi: 10.1111/j.1365-2265.1990.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 5.Hughes E, Fedorkow D, Collins J, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev. 2003:CD000155. doi: 10.1002/14651858.CD000155. [DOI] [PubMed] [Google Scholar]
- 6.Rein DT, Breidenbach M, Curiel DT. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006;2:137–43. doi: 10.2217/14796694.2.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raki M, Rein DT, Kanerva A, Hemminki A. Gene transfer approaches for gynecological diseases. Mol Ther. 2006;14:154–63. doi: 10.1016/j.ymthe.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–15. [PubMed] [Google Scholar]
- 9.McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6:45–55. doi: 10.1093/humupd/6.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–90. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- 11.Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M. Vascular endothelial growth factor A and C gene expression in endometriosis. Hum Pathol. 2004;35:1369–75. doi: 10.1016/j.humpath.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Hull ML, Charnock-Jones DS, Chan CL, Bruner-Tran KL, Osteen KG, Tom BD, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–99. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- 13.Machado DE, Abrao MS, Berardo PT, Takiya CM, Nasciutti LE. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil Steril. 2008;90:148–55. doi: 10.1016/j.fertnstert.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 14.Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–9. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- 15.Takayama K, Reynolds PN, Adachi Y, Kaliberova L, Uchino J, Nakanishi Y, et al. Vascular endothelial growth factor promoter-based conditionally replicative adenoviruses for pan-carcinoma application. Cancer Gene Ther. 2007;14:105–16. doi: 10.1038/sj.cgt.7700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, et al. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–95. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- 17.Park ACP, Ferin M, Xiao E, Zeitoun K. Inhibition of endometriosis development in Rhesus monkeys by blocking VEGF receptor: a novel treatment for endometriosis. Fertility Sterility. 2004:82. [Google Scholar]
- 18.Dabrosin C, Gyorffy S, Margetts P, Ross C, Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–18. doi: 10.1016/S0002-9440(10)64251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rein DT, Breidenbach M, Kirby TO, Han T, Siegal GP, Bauerschmitz GJ, et al. A fiber-modified, secretory leukoprotease inhibitor promoter-based conditionally replicating adenovirus for treatment of ovarian cancer. Clin Cancer Res. 2005;11:1327–35. [PubMed] [Google Scholar]
- 20.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyorffy S, Palmer K, Gauldie J. Adenoviral vector expressing murine angiostatin inhibits a model of breast cancer metastatic growth in the lungs of mice. Am J Pathol. 2001;159:1137–47. doi: 10.1016/S0002-9440(10)61790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyorffy S, Palmer K, Podor TJ, Hitt M, Gauldie J. Combined treatment of a murine breast cancer model with type 5 adenovirus vectors expressing murine angiostatin and IL-12: a role for combined anti-angiogenesis and immunotherapy. J Immunol. 2001;166:6212–7. doi: 10.4049/jimmunol.166.10.6212. [DOI] [PubMed] [Google Scholar]
- 23.Fortin M, Lepine M, Merlen Y, Thibeault I, Rancourt C, Gosselin D, et al. Quantitative assessment of human endometriotic tissue maintenance and regression in a noninvasive mouse model of endometriosis. Mol Ther. 2004;9:540–7. doi: 10.1016/j.ymthe.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Othman EE, Salama S, Ismail N, Al-Hendy A. Toward gene therapy of endometriosis: adenovirus-mediated delivery of dominant negative estrogen receptor genes inhibits cell proliferation, reduces cytokine production, and induces apoptosis of endometriotic cells. Fertil Steril. 2007;88:462–71. doi: 10.1016/j.fertnstert.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–7. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]