Abstract
GCN5L1 regulates protein acetylation and mitochondrial energy metabolism in diverse cell types. In the heart, loss of GCN5L1 sensitizes the myocardium to injury from exposure to nutritional excess and ischemia/reperfusion injury. This phenotype is associated with the reduced acetylation of metabolic enzymes and elevated mitochondrial reactive oxygen species (ROS) generation, although the direct molecular targets of GCN5L1 remain largely unknown. In this study, we sought to determine the mechanism by which GCN5L1 impacts energy substrate utilization and mitochondrial health. We find that hypoxia and reoxygenation (H/R) leads to a reduction in cell viability and Akt phosphorylation in GCN5L1 knockdown AC16 cardiomyocytes, in parallel with elevated glucose utilization and impaired fatty acid use. We demonstrate that glycolysis is uncoupled from glucose oxidation under normoxic conditions in GCN5L1 depleted cells. We show that GCN5L1 directly binds to the Akt-activating mTORC2 component Rictor, and that loss of Rictor acetylation is evident in GCN5L1 knockdown cells. Finally, we show that restoring Rictor acetylation in GCN5L1 depleted cells reduces mitochondrial ROS generation and increases cell survival in response to H/R. These studies suggest that GCN5L1 may play a central role in energy substrate metabolism and cell survival via regulation of Akt/mTORC2 signaling.
Keywords: GCN5L1, Akt, Rictor, Glycolysis, Hypoxia, Heart
INTRODUCTION
Mitochondrial dysfunction in cardiac myocytes leads to impaired energy production and reactive oxygen species (ROS) generation, resulting in tissue damage, cardiac dysfunction, and heart failure.1-4 In the healthy myocardium, fuel substrate utilization is tightly controlled. However, dysregulation occurs in injured or failing heart tissue, where glycolysis is uncoupled from glucose oxidation and fatty acid oxidation can be upregulated.5-8 The mechanisms by which substrate utilization may become dysregulated under conditions of cardiac injury are therefore of great therapeutic interest.
GCN5L1 is a recently described, mitochondrially-enriched protein that regulates extranuclear protein acetylation, metabolism, and mitochondrial biogenesis.9,10 Loss of GCN5L1 expression in the liver is associated with ROS generation and metabolic alterations, such as changes in hepatic energy metabolism.11 Our laboratory has recently shown that GCN5L1 is upregulated in response to a high fat diet in cardiac myocytes, and promotes the activation of proteins involved in fatty acid oxidation.12 Furthermore, we demonstrated that GCN5L1 expression is downregulated by myocardial infarction (MI) and hypoxia/reoxygenation (H/R) in cardiac cells, is necessary for the recovery of ex vivo hearts after ischemia/reperfusion injury, and reduces ROS generation after hypoxia/reoxygenation in cardiomyocytes.13 Despite these findings, the mechanisms by which GCN5L1 protects against H/R are not fully understood.
We demonstrate here that loss of GCN5L1 results in decreased Akt activation/GSK3β inactivation in cardiomyoctyes exposed to hypoxia, which accompanies a switch from fatty acid to glucose utilization. We demonstrate that this switch is associated with an increase in the expression of glycolytic enzymes and a reduction in glucose-driven oxidative respiration. Finally, we identify the mTORC2 component Rictor as a target of acetylation by GCN5L1, and demonstrate that restoring Rictor activity, via the expression of an acetylated Rictor mimetic, reduces mitochondrial ROS generation and increases survival in GCN5L1 knockdown cells subject to H/R. These results indicate that GCN5L1 contributes to pro-survival signaling and the preservation of normally coupled glycolysis and glucose oxidation after H/R.
MATERIALS AND METHODS
Mouse generation and housing
C57BL/6J wildtype mice were obtained from The Jackson Laboratory, and were used in accordance with protocols approved by the University of Pittsburgh IACUC. Experimental procedures were carried out in approved animal facilities at the University of Pittsburgh.
Cell culture and transfection
AC16 cells were obtained from Millipore. These cells are derived from the fusion of primary adult ventricular cardiomyocytes with SV40 fibroblasts,14 and exhibit protein expression and metabolic characteristics similar to primary human cardiomyocytes.14-16 Cells were cultured in DMEM (ThermoFisher) containing 25 mM glucose and supplemented with 10% Fetal Bovine Serum (ThermoFisher) and Antibiotic-Antimycotic (ThermoFisher). Lentiviral particles were used to transduce cells at a multiplicity of infection (MOI) of 10 with scrambled control or GCN5L1 shRNAs (Sigma-Aldrich), followed by puromycin selection. Knockdown of GCN5L1 was confirmed using RT-qPCR and western blot. Rictor mutation experiments were conducted using control plasmid pUC19, Myc-tagged Rictor plasmid gifted from David Sabatini (Addgene plasmid #11367),17 and Rictor mutant plasmids targeting lysines K1116, K1119, and K1125 (3KQ and 3KR) kindly gifted from Paul S. Mischel.18 Plasmids were transfected into AC16 cells using Lipofectamine 3000, and cells were used for subsequent experiments after 48 hours.
Hypoxia–Reoxygenation (H/R) studies
After plating and culturing AC16 cells generated as described above for 24 hours, media was replaced with Esumi Buffer: 137 mM NaCl, 12 mM KCl, 0.5 mM MgCl2, 0.9 mM CaCl2, 20 mM HEPES; 20 mM 2-deoxy-D-glucose (2-DG), pH 6.2 (hypoxia). Cells were then immediately transferred to a hypoxia chamber (1% O2, 5% CO2, 94% N2) for indicated time frames, followed reoxygenation via replacement of the media with normoxic buffer, and further incubation for specified time frames under normal atmospheric oxygen. Controls were simultaneously cultured in fresh media (DMEM containing 25 mM glucose and supplemented with 10% Fetal Bovine Serum and Antibiotic-Antimycotic, as indicated above) under normoxic conditions for the same duration. To evaluate H/R-induced signaling prior to mortality, cells were subjected to 1 hour of hypoxia followed by 30 minutes of reoxygenation.
Measurements of mitochondrial Ca2+ uptake
Mitochondrial calcium uptake and permeability transition pore opening was measured fluorimetrically in the presence of the Ca2+-sensitive probe Calcium-Green 5N (0.1 μM) using digitonin-permeabilized cells, based on methods that have been previously described.19-23 For the assays, 1×106 cells were harvested, washed and resuspended in an intracellular-like buffer (125 mM KCl, 2 mM potassium phosphate, 1 mM MgCl2, and 20 mM Hepes, pH 7.4). Digitonin (0.002%) and respiratory complex I substrates (5 mM pyruvate/malate) were then added. Experiments were carried out at 30 °C using a microplate reader (FLUOstar Omega, BMG Labtech). After 3-5 min of incubation, successive pulses of 10 μM CaCl2 were added to the samples until spontaneous release of Ca2+ caused an increase in fluorescence, indicating mitochondrial permeability transition pore opening.
Survival and growth assays
Live cell counts were obtained using 96-well plates using Cell Counting Kit-8 kit (CCK-8, Sigma) according to the manufacturer’s instructions. Briefly, cells were washed in PBS and incubated in CCK-8 reagent added to serum-free media (DMEM containing 25mM glucose and Antibiotic-Antimycotic) for 1 hour, after which the absorbance at 450 nm was measured. For hypoxia studies, absorbances were normalized to simultaneously cultured normoxia controls, and survival was determined as a percentage of live cells in hypoxia wells relative to normoxia wells. For growth rate measurements, cells were seeded at equal densities and cultured overnight in media containing no substrate, glucose (25 mM) only, or BSA conjugated palmitate (100 μM) only. Growth was normalized to a simultaneously-seeded plate where CCK-8 media was introduced immediately, and analyzed after 1 hour.
Seahorse XF analysis
GCN5L1 knockdown cells and control cells generated as described above were plated overnight in a 96-well Seahorse XF96 extracellular analyzer plate at a density of 5,000 cells per well. At 80-90% confluence, respiration and extracellular acidification were analyzed as previously described.24 Briefly, oligomycin (1 μM), FCCP (1 μM), and antimycin A (1 μM) were administered in sequence. Respiration (taken as Oxygen Consumption Rate, OCR) and Extracellular Acidification Rate, ECAR, for each well were measured in three times in sequence after each injection, yielding measures of the following: baseline respiration and ECAR, ATPase driven respiration, maximal respiration, glycolytic reserve, spare capacity for oxygen consumption, and proton leak.
Glucose uptake and Glucose-6-phosphate content
Glucose uptake was measured in live cells in a 96-well plate using a Glucose Uptake Cell-Based Assay Kit (Cayman) according to the manufacturer’s instructions. Briefly, cells seeded overnight in 96-well plates were incubated with 200 μg/mL 2-NBDG (a fluorescent deoxyglucose analog) in glucose-free media for 4 hours, washed, and fluorescence was measured. Control cells were co-incubated in apigenin, a GLUT1 inhibitor. Results were normalized to cell densities quantitated using CCK-8 as described above. Glucose-6-phosphate concentrations were measured in cell lysates from cells using a Glucose-6-Phosphate (G-6-P) Fluorometric Assay (Cayman) according to the manufacturer’s instructions. Briefly, equal numbers of collected cells that had been cultured overnight in glucose-containing media, as described above, were lysed in assay buffer and deproteinated with MPA. Supernatants were incubated with G-6-PDH to catalyze the oxidation of G-6-P to 6-phospho-D-gluconate, and the concomitantly reduced NAPDH was measured using a fluorometric detector. A standard curve of known G-6-P concentrations was simultaneously generated, and linear regression was used to determine absolute G-6-P concentrations in lysates.
SIRT3 activity assay
Cells were lysed in 1% CHAPS buffer free from deacetylase inhibitors, and supernatants were tested for SIRT3 activity using the FLUOR DE LYS SIRT3 fluorometric drug discovery assay kit (Enzo) according to the manufacturer’s instructions.
RT-qPCR
RNA was extracted from cells using RNEasy kit (Qiagen). cDNA was generated with 500 ng-1 μg of RNA using Maxima Reverse Transcriptase (ThermoFisher). Quantitative PCR (qPCR) was performed using SYBR-Green (ThermoFisher) with primers for Gcn5l1 (Cat. QT00016002), Sirt3 (QT00091490) and Gapdh (Cat. QT00079247) (Qiagen).
Immunoblotting
Cells or cardiac tissues were lysed in 1% CHAPS buffer. A μLITE analyzer (BioDrop) was used to determine protein concentrations, and equal amounts were run on an SDS-PAGE gel. Protein was transferred to nitrocellulose membranes, which were blocked using Odyssey blocking buffer, and incubated in primary antibodies overnight (GAPDH, Cell Signaling, Cat. 2118 [14C10], rabbit mAb, 1:1000; Akt, Cell Signaling, Cat. 4685 [11E7], rabbit mAb, 1:1000; p-Akt [Ser473], Cell Signaling, Cat. 4051 [587F11], mouse mAb, 1:1000; GSK3β, Cell Signaling, Cat. 12456 [D5C5Z], rabbit mAb, 1:1000; p-GSK3β [Ser9], Cell Signaling, Cat. 5558 [D85E12], rabbit mAb 1:1000 [Ser9]; Rictor, Cell Signaling, Cat. 2114 [53A2], rabbit mAb 1:500; GCN5L1 1:250, rabbit pAb generated as previously described9), followed by incubation at room temperature with fluorescent secondary antibodies for 1 hour (800 nm anti-rabbit, 700 nm anti-mouse, LiCor). Bands were visualized using an Odyssey Imager and quantitated using Image Studio Lite v 5.2 (LiCor).
Immunoprecipitation
For GCN5L1:Rictor interaction assays, whole heart tissue lysates (0.5 mg) from WT C5L7BL/6J mice were generated as above and incubated overnight at 4 °C in primary antibody against control IgG or GCN5L1 (1:200). Lysates were then incubated in a 1:1 mixture of Protein A and Protein G beads for 4 hours at 4 °C, and washed thoroughly in PBS. Bound proteins were eluted into reducing SDS lysis buffer, and immunoblotting proceeded as described above. For Rictor acetylation assays, AC16 cells were lysed as described above in the presence of 100 μM Trichostatin A and 5 mM nicotinamide to inhibit deacetylase activity. Lysates (0.5 mg) were incubated overnight in primary antibody against acetyl-lysine (1:200, Cell Signaling). Lysates were then incubated in a 1:1 mixture of Protein A and Protein G beads, and washed 3 times. Acetylated residues were eluted into reducing buffer containing SDS, and immunoblotting proceeded as described above.
Mitochondrial ROS measurement
GCN5L1 knockdown cells transfected with Rictor mutants as described above were seeded in a 96-well plate, washed with PBS and incubated with MitoSOX Red (5 μM, ThermoFisher) at 37 °C for 10 minutes. Cells were thoroughly washed again, and fluorescence (ex: 510 nm, em: 580 nm) was measured. Cells were then incubated with MitoTracker green (50 nM, ThermoFisher) at 37 °C for 10 minutes, washed, and fluorescence (ex: 490 nm, em: 516 nm) was measured again. Mitochondrial ROS values were calculated as the ratio of the MitoSOX signal normalized to the MitoTracker signal.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7. Student’s t-tests were used for simple comparisons between groups. One-way Analyses of Variance (ANOVA) were used to compare more than two groups, followed by post-hoc Student’s t-tests. For studies examining multiple timepoints (i.e. H/R survival studies) a 2-way ANOVA was used with post-hoc Sidak’s multiple comparisons tests. A P value <0.05 was considered significant. Sample numbers are listed in the figure legends. All data are represented as the mean ± SEM.
RESULTS
Silencing of GCN5L1 reduces Akt signaling under conditions of hypoxia/reoxygenation
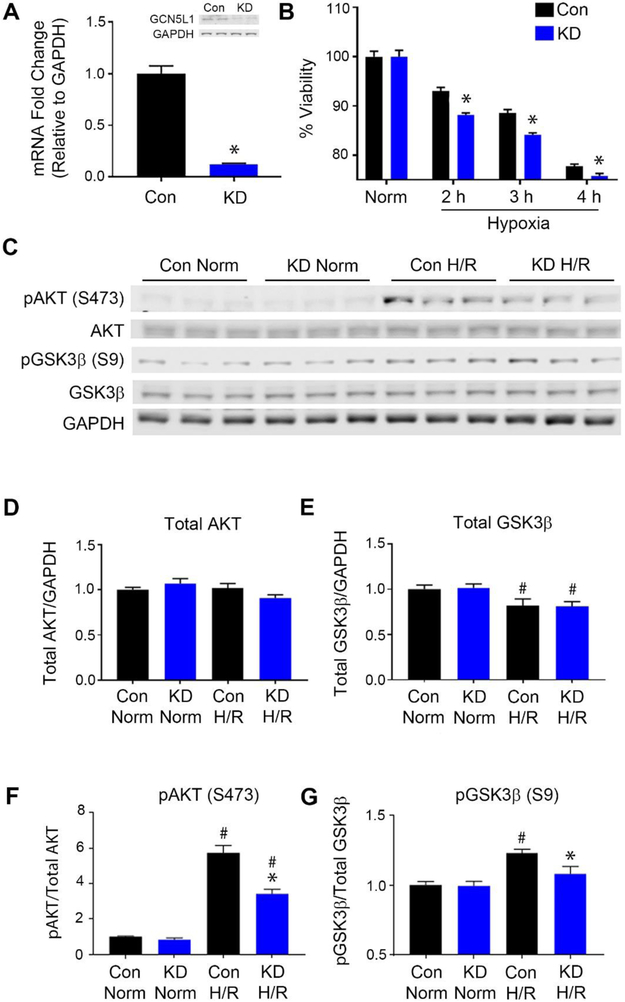
AC16 cells transduced with lentiviral particles containing shRNA to silence GCN5L1 expression as previously described13 were used to examine the pro-survival signaling of GCN5L1 (Figure 1A). No differences in SIRT3 expression were observed (Supplemental Figure 1). Knockdown cells were subjected to 2-4 hours of hypoxia followed by 1 hour of reoxygenation. Cells with diminished GCN5L1 expression (GCN5L1 KD) had markedly reduced survival rates after reoxygenation for all the hypoxia timepoints measured (Figure 1B). A survival defect after one hour of reoxygenation suggested that a rapid, non-genomic signaling event may account for this effect, and we therefore examined the activation of known cardioprotective kinases.
Figure 1: GCN5L1 knockdown decreases AKT and GSK3β activation.
A. Validation of the GCN5L1 knockdown by qPCR and western blot. N = 3 B. Survival rates of the GCN5L1 knockdown cells after 2, 3 or 4 hours of hypoxia, followed by 1 hour of reoxygenation.N = 48 wells per genotype and condition C. Representative immunoblots for pAKT, AKT, pGSK3β, and GSK3β after 1 hour of hypoxia and 30 minutes of reoxygenation. D-E. No difference is observed in total AKT protein expression, while GSK3β levels were reduced uniformly in both groups after H/R. F-G. Increased AKT phosphorylation H/R is significantly reduced in KD cells. GSK3β phosphorylation induced by H/R is diminished in KD cells compared to controls. N = 6 samples per genotype and condidtion. Norm = Normoxia, H/R = Hypoxia/reoxygenation. *p<0.05 vs. control cells, #p<0.05 vs. normoxia
We subsequently found that the pro-survival kinase Akt exhibited reduced phosphorylation after H/R injury in GCN5L1 KD cells relative to control cells (Figure 1C, 1F). Several reports suggest that the pro-survival activity of Akt in ischemia is mediated through phosphorylation and inactivation of GSK3β.25,26 We therefore probed whether phosphorylation of GSK3β was altered in GCN5L1 knockdown. As with Akt, no difference between genotypes was observed under normoxia. After hypoxia and reoxygenation, levels of total GSK3β decreased equally in both genotypes. However, the phosphorylation of GSK3β was significantly attenuated in GCN5L1 KD cells after H/R (Figure 1C, 1F, 1G). Combined, these data suggest that loss of GCN5L1 impacts cell survival in response to oxidative stress via reduced Akt pathway signaling.
GCN5L1 knockdown increases glucose utilization and reduces fatty acid utilization
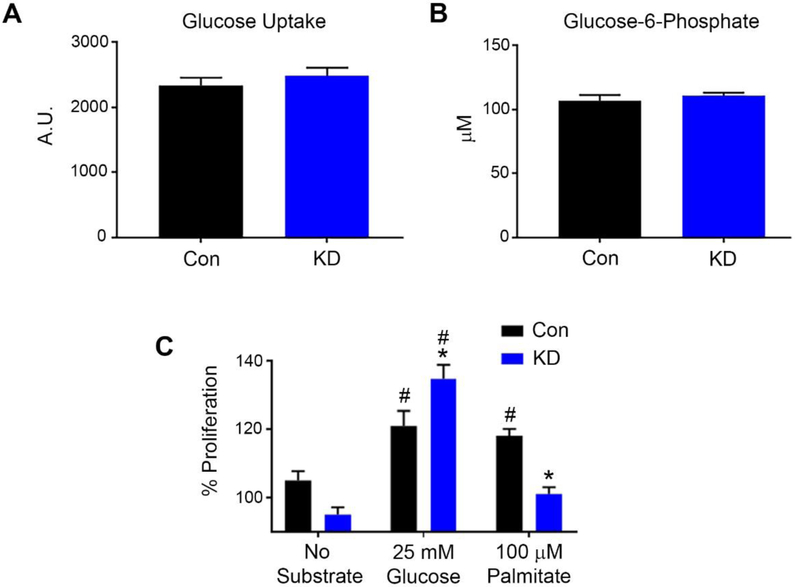
Akt activation triggers glucose uptake into cardiomyocytes, and the protective activity of Akt/GSK3β after ischemia is mediated in part through the regulation of glucose transport into the cell.27,28 We therefore investigated whether glucose transport is reduced in knockdown cells. Surprisingly, both the rate of glucose uptake (Figure 2A) and intracellular glucose-6-phosphate content (Figure 2B) were unchanged in GCN5L1 knockdown cells. We next examined the ability of the AC16 cells to utilize glucose or fatty acid substrates. We found that GCN5L1 cells thrived when glucose was present in the media, but were unable to proliferate to the same degree as control cells when only palmitate was available as a substrate (Figure 2C). These data suggest that the growth of GCN5L1 knockdown cells is less dependent on fatty acid oxidation, and more dependent on glucose availability.
Figure 2: Loss of GCN5L1 promotes glucose utilization over fatty acid utilization, without changing glucose entry.
A. Glucose uptake amounts (N=35 wells per genotype), and B. G-6-P levels (N=5 samples per genotype) are unchanged by GCN5L1 knockdown. C. Knockdown cells proliferate with glucose, but are unable to proliferate in palmitate-containing media. N=16 wells per genotype and treatment. *p<0.05 vs. control cells, #p<0.05 vs. no substrate.
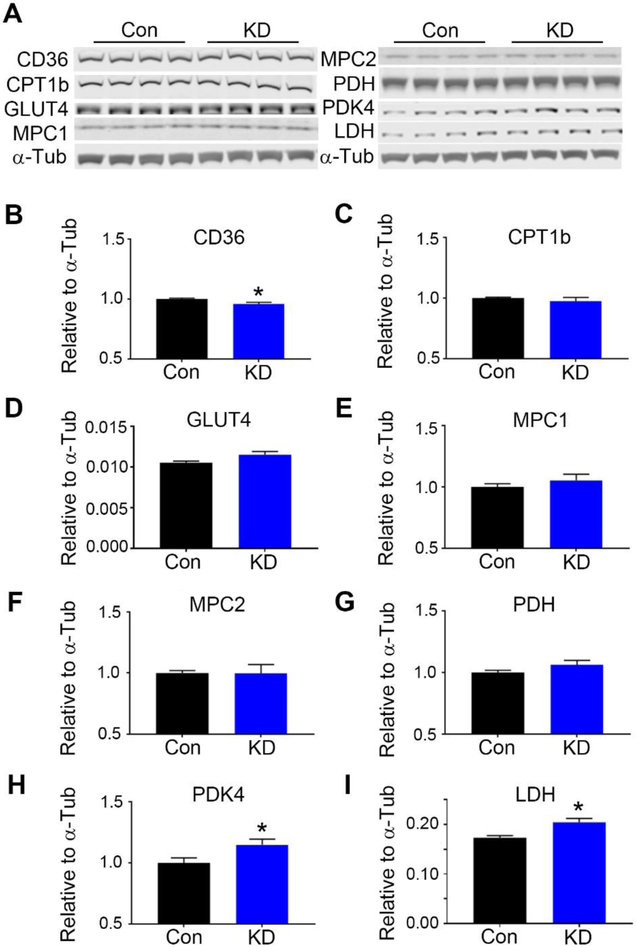
Akt has also been reported to mitigate reperfusion injury via the regulation of fatty acid oxidation and glycolytic enzymes such as PDK4 and CPT1β, via activation of PPARα.29 We therefore examined the expression of these and other proteins involved in glycolysis, glucose oxidation, and fatty acid oxidation. We found that the fatty acid translocase CD36 displayed a small, but significant decrease in GCN5L1 knockdown cells (Figure 3A), while the glycolytic enzymes PDK4 and LDH were significantly upregulated (Figures 3H, 3I). No significant change was found in the expression of glucose transporter GLUT4, fatty acid oxidation enzyme CPT1β, mitochondrial glucose transporters MCP1 or MCP2, or the pyruvate utilization enzyme PDH (Figures 3D-3G).
Figure 3: GCN5L1 knockdown alters the expression of glycolytic and FAO proteins.
A. Representative immunoblots of the proteins measured. B. CD36 levels are significantly decreased in knockdown cells. C. CPT1b, D. GLUT4, E. MPC1, F. MPC2, and G. PDH remain unchanged. H. PDK4, and I. LDH are significantly upregulated in knockdown cells. N = 5 samples per genotype. *p<0.05 vs. control cells
Seahorse XF assays were used to further elucidate the metabolic profile resulting from GCN5L1 loss in proliferating cardiac AC16 cells. We found that GCN5L1 cells exhibited higher ECAR rates, suggesting increased glycolysis even under aerobic conditions, while showing a significantly reduced glycolytic reserve (Figure 4A, 4B). Additionally, we found that while cells with decreased GCN5L1 expression exhibited normal baseline respiration (OCR), maximal oxygen utilization was significantly dampened (Figures 4C, 4D). These data suggest that loss of GCN5L1 drives cardiac cells towards increased glucose utilization for ATP generation. However, a concurrent suppression of mitochondrial respiratory capacity results in the uncoupling of glycolysis from glucose oxidation, which likely results in a reduced energetic output.
Figure 4: GCN5L1 loss reduces aerobic respiration, and favors anaerobic respiration.
A. Basal respiration rate (OCR, oxygen consumption rate) is unchanged between control and knockdown cells. B. Maximal respiration is significantly impaired in KD cells. C. Basal ECAR (extracellular acidification rate) is elevated in KD cells, while D. Glycolytic reserve is diminished. N = 36-42 wells per genotype. *p<0.05 vs. control cells
GCN5L1 directly binds to Rictor, and loss of Rictor acetylation in GCN5L1 depleted cells limits recovery from ischemic injury
Finally, we attempted to find a mechanistic link between changes in pro-survival signaling, mitochondrial ROS generation and altered energy substrate metabolism observed in GCN5L1 knockdown cells. Akt may be phosphorylated at serine 473 by the mTOR Complex 2 (mTORC2).30 Rictor, a component of the complex, has been reported to be activated by acetylation.18,31 Futhermore, loss of Rictor activity has been shown to result in increased mitochondrial ROS generation and changes in glucose metabolism in several cell types.32,33 To test whether there was any direct interaction between these two proteins, we immunoprecipitated GCN5L1 from whole mouse cardiac tissue lysates, and found that there was direct binding between Rictor and GCN5L1 (Figure 5A, Supplemental Figure 2). To understand if the loss of GCN5L1 acetyltransferase activity impacts the acetylation of Rictor, we immunoprecipitated control and GCN5L1 knockdown AC16 cell lysates using anti-acetyl lysine antibodies and blotted for Rictor. Using this technique, we found that the acetylation status of Rictor was significantly decreased in GCN5L1 knockdown cells compared to wildtype after H/R (Figure 5B). We next examined whether restoring Rictor acetylation could rescue the deleterious phenotypes observed in GCN5L1 knockdown cells, by transfecting cells with Rictor plasmids containing point mutations to either mimic (3KQ) or preclude (3KR) lysine acetylation.18 Our laboratory and others have previously found that GCN5L1 impacts mitochondrial ROS generation.11,13 We found that while control-transfected GCN5L1 knockdown cells continued to produce elevated mitochondrial ROS in response to H/R, transfection with the acetylation-mimic 3KQ mutant decreased ROS production (Figure 5C). In contrast, acetylation-defective 3KR transfection does not result in a significant change in ROS production in GCN5L1 knockdown cells (Figure 5C). Finally, we hypothesized that the reduced mitochondrial ROS observed in GCN5L1 knockdown cells transfected with the acetylation-mimic 3KQ plasmid would rescue the loss of cell viability observed in response to H/R. As expected, restoring Rictor acetylation via 3KQ expression restored survival after 3 hours of hypoxia and 1 hour of reoxygenation to levels similar to wildtype cells, while this recovery was absent in vector-transfected controls or Rictor acetylation-deficient 3KR transfected cells (Figure 5D). Combined, these data suggest that loss of GCN5L1 limits the acetylation and activation of Rictor, which limits its pro-survival signaling capacity in response to hypoxic stress.
Figure 5: GCN5L1 reduces Rictor/mTORC2 acetylation.
A. Co-Immunoprecipitation of Rictor with GCN5L1 from wildtype mouse cardiac tissues. B. Immunoprecipitation of Rictor from AC16 cells using anti acetyl-lysine antibody. N = 5-6 samples per genotype and condition. C. Mitochondrial ROS production of GCN5L1 knockdown cells after transfection with vector (vec), pseudoacetylated Rictor (3KQ) or acetylation incompetent Rictor (3KR). N = 24 wells per transfection. D. Survival of wildtype and GCN5L1 knockdown cells after transfection with vector, 3KQ Rictor, or 3KR Rictor, subjected to 3 hours of hypoxia and 1 hour of reoxygenation. N = 20 wells per transfection and condition. Norm = Normoxia, H/R = Hypoxia/reoxygenation. *p<0.05 vs control.
DISCUSSION
The data presented here show for the first time that GCN5L1 promotes the activation of Akt, and its loss shifts the metabolic profile of cardiac cells from fatty acid oxidation towards non-oxidative glucose utilization. We demonstrate that CD36, involved in fatty acid uptake, is downregulated in GCN5L1 knockdown cells, while glycolytic enzymes LDH and PDK4 are upregulated. We further show that glucose-driven aerobic respiration is impaired. Finally, we demonstrate that the Akt activating mTORC2 complex is necessary for the pro-survival effects of GCN5L1, and that the exogenous expression of acetylated Rictor prevents GCN5L1 loss from causing further cell injury in response to H/R.
We have previously shown that GCN5L1 loss results in reduced survival in an ex vivo model of cardiac ischemia reperfusion injury, and reduces overall cardiac function.13 We demonstrate here that one of the mechanisms that may be impaired is mitochondrial function via Rictor/mTORC2/Akt signaling, and the loss of aerobic respiration. Akt plays an important role in survival signaling during cardiac I/R injury.30 Akt directly phosphorylates and inactivates GSK3β, which promotes pro-survival mitochondrial activity. We observed decreases in the phosphorylation of both Akt and GSK3β in GCN5L1 knockdown cells after H/R injury, suggesting that GCN5L1 is necessary for the activation of these protective pathways.
In addition, Akt may protect against I/R injury in part though modulation of glucose uptake into cardiomyocytes after ischemia.27,28 While glucose uptake, G-6-P content, and GLUT4 expression all remain unchanged in GCN5L1 knockdown cells, we observed an increase in glucose utilization concurrent with the upregulation of glycolytic enzymes PDK4 and LDH. We further found that loss of GCN5L1 is associated with a simultaneous increase in extracellular acidification rate and decrease in maximal oxygen consumption rate, suggesting an uncoupling of glycolysis from glucose oxidation under aerobic conditions. Efficient glucose oxidation has previously been established to be beneficial after reperfusion injury.6,7,34 PDK4 inhibits PDH, and thus acts as a rate-limiting enzyme that inhibits the oxidation of pyruvate. PDK4 expression has been reported by Li and colleagues to block glucose uptake and drive reperfusion injury.35 LDH catalyzes the interconversion of pyruvate to lactate, the end result of anaerobic glycolysis, and contributes to potentially deleterious intracellular acidosis.36,37 In addition to the upregulation of proteins associated with anaerobic glycolysis, we observed a modest decrease in CD36 fatty acid translocase. While CD36 loss by itself does not predispose the heart to reperfusion injury,38 its downregulation here – combined with the reduced proliferation of GCN5L1 knockdown cells provided with only with fatty acids as a fuel source – supports a potential overall switch to glucose-driven energy metabolism.
Akt phosphorylation is mediated by mTORC2, which can be directly activated by acetylation of its Rictor component.18,31 The expression of a mutant that mimics the acetylated form of Rictor rescued cells where GCN5L1 expression is significantly reduced from ischemic injury, suggesting that GCN5L1 protects cardiomyocytes from H/R injury through the promotion of Rictor acetylation. In addition, ROS are significantly reduced when acetylated Rictor is expressed, suggesting that the mTORC2/Akt/GSK3β axis lies upstream of ROS production. Rictor modulation has also been shown to directly impact mitochondrial respiration and calcium handling.39,40 While we did not observe any differences in mitochondrial calcium retention between wildtype and GCN5L1 knockdown cells (Supplemental Figure 3), it would seem clear that there are links between mitochondrial metabolism and Rictor that involve GCN5L1 activity in cardiac cells. The association of mTORC2 components with mitochondria or mitochondrial associated ER,39-41 along with the newly-identified cytosolic role of GCN5L142, position Rictor as a plausible target for GCN5L1. Future work will be required to identify the cellular location of this interaction, and functional significance, in cardiac cells during ischemic injury.
In addition to Akt, a number of protective kinases are known to be activated during H/R in cardiac cells, including PKC, CaMKII, PKA and MAPKs like ERK, which has been found to be regulated by GCN5L1 expression in other contexts.11,13,43-50 Because we are able to reverse the injury associated with GCN5L1 loss using Rictor acetylation mimetic mutants, we have evidence that Rictor/Akt is necessary for the observed effects of GCN5L1 in response to H/R. However, we have not ruled out a role for other signaling kinases associated with protection from H/R induced injury.
These findings suggest that GCN5L1 may represent a novel therapeutic target for the treatment of diseases associated with cardiac hypoxia, including coronary heart disease and myocardial infarction. The severity of these diseases has been linked to the breakdown of pro-survival signaling, including Akt/GSK3β. Promoting the activation of Rictor through acetylation by GCN5L1, perhaps through the development of a small molecule activator, presents a novel point of intervention that has yet to be tested.
CONCLUSION
In conclusion, our findings demonstrate that GCN5L1 plays a role in the coupling of glucose metabolism and oxidation in cardiac cells. We demonstrate a decrease in Akt/GSK3β signaling in the absence of GCN5L1, concurrent with a reduction in fatty acid utilization and substrate oxidation. We show that loss of GCN5L1 drives increased glycolytic enzyme expression, with elevated acidification rates under oxygen-replete conditions. Finally, we show a direct relationship between GCN5L1 and the mTORC2 component Rictor, and demonstrate that Rictor acetylation is necessary to prevent the observed effects of GCN5L1 deletion on the recovery of cardiomyocytes from hypoxia and reoxygenation.
Supplementary Material
Supplemental Figure 1: Loss of GCN5L1 expression does not affect SIRT3 expression or activity. There was no significant difference in A. Sirt3 gene expression or, B. enzymatic activity between control and GCN5L1 KD cells.
Supplemental Figure 2: Expanded membrane image of Rictor:GCN5L1 IP experiment (Figure 5A). A larger image of the IP image, showing the membrane from ~98 kDa (bottom) to over 300 kDa (top).
Supplemental Figure 3: Loss of GCN5L1 expression in AC16 cells does not affect mitochondrial calcium retention capacity. There was no significant difference in mitochondrial permeability transition pore opening (mPTP) between control and GCN5L1 KD cells following serial additions of calcium.
CLINICAL PERSPECTIVES.
Loss of GCN5L1 in cardiac cells increases their susceptibility to ischemic injury, however the mechanism is not fully understood.
In this study, we show that loss of GCN5L1 reduces pro-survival Akt signaling, lowers mitochondrial glucose utilization for energy generation, and promotes mTORC2 subunit Rictor-dependent increases in mitochondrial reactive oxygen species generation.
These findings link GCN5L1 and the Akt/mTORC2 signaling pathway in cardiac cells, and indicate that this system may be important in the cardioprotective events that mitigate ischemic injury in the human myocardium.
ACKNOWLEDGEMENTS
We thank Paul S. Mischel (UC San Diego) for the kind gift of the Rictor acetylation mutant expression vectors.
FUNDING SOURCES
This work was supported by NIH T32 Fellowship (HL110849) to J.R.M.; by AHA Postdoctoral Fellowship (17POST33670489) to D.T.; and, by a University of Pittsburgh HVI-VMI Innovator Award, ADA Innovative Basic Science Award (#1-17-IBS-197) and NIH grants (HL116728 and HL132917) to I.S.
REFERENCES
- 1.Brookes PS, Yoon Y, Robotham JL, Anders MW & Sheu S-S Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. - Cell Physiol. 287, C817–C833 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Alam MR, Baetz D & Ovize M Cyclophilin D and myocardial ischemia–reperfusion injury: A fresh perspective. J. Mol. Cell. Cardiol. 78, 80–89 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Rivas GJ & Torre-Amione G Abnormal mitochondrial function during ischemia reperfusion provides targets for pharmacological therapy. Methodist Debakey Cardiovasc. J. 5, 2–7 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Shintani-Ishida K, Inui M & Yoshida K-I Ischemia-reperfusion induces myocardial infarction through mitochondrial Ca2+ overload. J. Mol. Cell. Cardiol. 53, 233–239 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Lopaschuk GD & Stanley WC Glucose metabolism in the ischemic heart. Circulation 95, 313–5 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Ussher JR et al. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc. Res. 94, 359–369 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Masoud WGT et al. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc. Res. 101, 30–38 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Masoud WGT, Abo Al-Rob O, Yang Y, Lopaschuk GD & Clanachan AS Tolerance to ischaemic injury in remodelled mouse hearts: less ischaemic glycogenolysis and preserved metabolic efficiency. Cardiovasc. Res. 107, 499–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott I, Webster BR, Li JH & Sack MN Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem. J. 443, 655–661 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott I et al. GCN5-like Protein 1 (GCN5L1) Controls Mitochondrial Content through Coordinated Regulation of Mitochondrial Biogenesis and Mitophagy. J. Biol. Chem. 289, 2864–2872 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L et al. GCN5L1 modulates cross-talk between mitochondria and cell signaling to regulate FoxO1 stability and gluconeogenesis. Nat. Commun. 8, 523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapa D et al. Acetylation of mitochondrial proteins by GCN5L1 promotes enhanced fatty acid oxidation in the heart. Am. J. Physiol. Heart Circ. Physiol. 313, H265–H274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning JR et al. Cardiac-specific deletion of GCN5L1 restricts recovery from ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 129, 69–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson MM et al. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 39, 133–147 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Truong J, Mailloux RJ & Chan HM Impact of methylmercury exposure on mitochondrial energetics in AC16 and H9C2 cardiomyocytes. Toxicol. Vitr. 29, 953–961 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Li N et al. Atorvastatin reduces alcohol-induced endoplasmic reticulum stress in AC16 cardiomyocytes. 10.1080/14017431.2018.1516891 (2018). doi: 10.1080/14017431.2018.1516891 [DOI] [PubMed]
- 17.Dos D Sarbassov DD et al. Rictor, a Novel Binding Partner of mTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway that Regulates the Cytoskeleton. Curr. Biol. 14, 1296–1302 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Masui K et al. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc. Natl. Acad. Sci. U. S. A. 112, 9406–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy AN, Bredesent DE, Cortopassi G, Wang E & Fiskum G Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Neurobiology DC 93, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichas F, Jouaville LS & Mazat JP Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89, 1145–53 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Fontaine E, Eriksson O, Ichas F & Bernardi P Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation By electron flow through the respiratory chain complex i. J. Biol. Chem. 273, 12662–8 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Zhao D et al. Cardiac-derived CTRP9 protects against myocardial ischemia/reperfusion injury via calreticulin-dependent inhibition of apoptosis. Cell Death Dis. 9, 723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan X et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pride CK et al. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc. Res. 101, 57–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thirunavukkarasu M et al. Protective effects of Phyllanthus emblica against myocardial ischemia-reperfusion injury: the role of PI3-kinase/glycogen synthase kinase 3β/βcatenin pathway. J. Physiol. Biochem. 71, 623–633 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Park E-S et al. Cardioprotective effect of KR-33889, a novel PARP inhibitor, against oxidative stress-induced apoptosis in H9c2 cells and isolated rat hearts. Arch. Pharm. Res. 40, 640–654 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Ji L et al. AMPK-Regulated and Akt-Dependent Enhancement of Glucose Uptake Is Essential in Ischemic Preconditioning-Alleviated Reperfusion Injury. PLoS One 8, e69910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heywood SE et al. High-density lipoprotein delivered after myocardial infarction increases cardiac glucose uptake and function in mice. Sci. Transl. Med. 9, eaam6084 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Ravingerov%á T et al. PPAR-alpha activation as a preconditioning-like intervention in rats in vivo confers myocardial protection against acute ischaemia–reperfusion injury: involvement of PI3K–Akt. Can. J. Physiol. Pharmacol. 90, 1135–1144 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Volkers M et al. Mechanistic Target of Rapamycin Complex 2 Protects the Heart From Ischemic Damage. Circulation 128, 2132–2144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glidden EJ et al. Multiple site acetylation of Rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J. Biol. Chem. 287, 581–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tassone B et al. Rictor/mTORC2 deficiency enhances keratinocyte stress tolerance via mitohormesis. Cell Death Differ. 24, 731–746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinert M et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol. Metab. 3, 630–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyck JRB et al. Absence of Malonyl Coenzyme A Decarboxylase in Mice Increases Cardiac Glucose Oxidation and Protects the Heart From Ischemic Injury. Circulation 114, 1721–1728 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Li T et al. Glucose oxidation positively regulates glucose uptake and improves cardiac function recovery after myocardial reperfusion. Am. J. Physiol. Metab. 313, E577–E585 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Liu B, Clanachan AS, Schulz R & Lopaschuk GD Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ. Res. 79, 940–8 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Dennis SC, Gevers W & Opie LH Protons in ischemia: Where do they come from; Where do they go to? J. Mol. Cell. Cardiol. 23, 1077–1086 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Kuang M, Febbraio M, Wagg C, Lopaschuk GD & Dyck JRB Fatty Acid Translocase/CD36 Deficiency Does Not Energetically or Functionally Compromise Hearts Before or After Ischemia. Circulation 109, 1550–1557 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Schieke SM et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 281, 27643–52 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Tang L-L et al. Mitochondrial toxicity of perfluorooctane sulfonate in mouse embryonic stem cell-derived cardiomyocytes. Toxicology 382, 108–116 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Xu Z-H, Liu C-H, Hang J-B, Gao B-L & Hu J-A Rituximab effectively reverses Tyrosine kinase inhibitors (TKIs) resistance through inhibiting the accumulation of rictor on mitochondria-associated ER-membrane (MAM). Cancer Biomarkers 20, 581–588 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Wu K et al. GCN5L1 interacts with αTAT1 and RanBP2 to regulate hepatic α-tubulin acetylation and lysosome trafficking. J. Cell Sci. jcs.221036 (2018). doi: 10.1242/jcs.221036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saurin AT et al. Targeted disruption of the protein kinase {C} epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc. Res. 55, 672–680 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Hassouna A, Matata BM & Galinanes M PKC-epsilon is upstream and PKC-alpha is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am. J. Physiol. Physiol. 287, C1418–25 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Mattiazzi A, Mundina-Weilenmann C, Vittone L & Said M Phosphorylation of phospholamban in ischemia-reperfusion injury: functional role of Thr17 residue. Mol. Cell. Biochem. 263, 131–136 (2004). [PubMed] [Google Scholar]
- 46.T. A. N. Jr et al. Differential regulation of cardiac actomyosin {S}−1 {MgATPase} by protein kinase {C} isozyme-specific phosphorylation of specific sites in cardiac troponin {I} and its phosphorylation site mutants. Biochemistry 35, 14923–14931 (1996). [DOI] [PubMed] [Google Scholar]
- 47.Tong H, Bernstein D, Murphy E & Steenbergen C The role of β-Adrenergic Receptor Signaling in Cardioprotection. FASEB J (2005). doi: 10.1096/fj.04-3067fje [DOI] [PubMed] [Google Scholar]
- 48.House SL, Melhorn SJ, Newman G, Doetschman T & Jel JS The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2. Am. J. Physiol. Circ. Physiol. 293, H354–65 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Haq SE, Clerk A & Sugden PH Activation of mitogen-activated protein kinases (p38-MAPKs, SAPKs/JNKs and ERKs) by adenosine in the perfused rat heart. FEBS Lett. 434, 305–8 (1998). [DOI] [PubMed] [Google Scholar]
- 50.Dhingra S, Sharma AK, Singla DK & Singal PK p38 and ERK1/2 MAPKs mediate the interplay of TNF-α and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am. J. Physiol. Circ. Physiol. 293, H3524–H3531 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Loss of GCN5L1 expression does not affect SIRT3 expression or activity. There was no significant difference in A. Sirt3 gene expression or, B. enzymatic activity between control and GCN5L1 KD cells.
Supplemental Figure 2: Expanded membrane image of Rictor:GCN5L1 IP experiment (Figure 5A). A larger image of the IP image, showing the membrane from ~98 kDa (bottom) to over 300 kDa (top).
Supplemental Figure 3: Loss of GCN5L1 expression in AC16 cells does not affect mitochondrial calcium retention capacity. There was no significant difference in mitochondrial permeability transition pore opening (mPTP) between control and GCN5L1 KD cells following serial additions of calcium.