Abstract
Epac (exchange protein activated by cyclic-AMP) 2 is a direct target of 3'−5'-cyclic adenosine monophosphate (cAMP) and is involved in cAMP-mediated signal transduction through activation of the Ras-like small GTPase Rap. Crystallographic analyses revealed that activation of Epac2 by cAMP is accompanied by dynamic structural changes. Epac2 is expressed mainly in brain, neuroendocrine and endocrine tissues, and is involved in diverse cellular functions in the tissues. In this review, we summarize the structure and function of Epac2. We also discuss the physiological and pathophysiological roles of Epac2, and the possibility of Epac2 as a therapeutic target.
Keywords: Epac2, cAMP, Rap
1. Introduction
3'−5'-cyclic adenosine monophosphate (cAMP), which is one of the major second messengers derived from adenosine triphosphate (ATP) by adenylate cyclase following various extracellular stimulations, regulates many biological processes. Initially, it was considered that cAMP exerts its actions through activation of cAMP-dependent protein kinase A (PKA) or interaction with cyclic nucleotide binding ion channels (Beavo and Brunton, 2002).
In 1998, a novel cAMP target protein cAMP-GEF, also called Epac (hereafter referred to as Epac) was identified by two independent groups using computational database search (de Rooij et al., 1998) or differential display screen (Kawasaki et al., 1998). While PKA transduces cAMP signaling by direct phosphorylation of target proteins, Epac-mediated signaling depends mainly on its activating effect on the small GTPases Rap, Rap1 and Rap2. (de Rooij et al., 1998; Kawasaki et al., 1998; de Rooij et al., 2000; Gloerich and Bos, 2011). The active forms of Rap interact specifically with their effector proteins and activate downstream targets to control divergent biological processes (Frische and Zwartkruis, 2010).
This review briefly summarizes the structure, function, intracellular signaling, and physiological and pathophysiological roles of Epac, in particular of the Epac2 isoform, and also mentions the potential of Epac2 as a drug target for treatment of diseases.
2. Structure and function of Epac2
There are two isoforms of Epac, Epac1 and Epac2, which are coded by Rapgef3 and Rapgef4 genes, respectively. The coding region of Rapgef4 gene comprises 31 exons and 30 introns located on chromosome 2q31. Rapgef3 mRNA is ubiquitously expressed, with high levels in thyroid, kidney, ovary, skeletal muscle, and heart, and with relatively low levels in brain. The expression of Rapgef4 mRNA is more restricted and is predominant in brain and neuroendocrine and endocrine tissues (de Rooij et al., 1998; Kawasaki et al., 1998; Ozaki et al., 2000).
Epac2 is a multi-domain protein with molecular weight of ~116 kDa consisting of two parts, the regulatory and catalytic regions. The amino-terminal regulatory region contains two cyclic nucleotide-binding domains (cNBD-A and cNBD-B) and a DEP (Dishevelled, Egl-10, and Pleckstrin) domain (Bos, 2006). Epac1 has a similar domain organization to Epac2, but has only one cNBD in the regulatory region. cNBD-A of Epac2 has lower binding affinity to cAMP compared with cNBD-B (de Rooij et al., 2000). It is proposed that cNBD-A determines the subcellular localization (Niimura et al., 2009) and that the DEP domain is responsible for its membrane association and altering the localization of Epac1 to the plasma membrane (Qiao et al., 2002; Consonni et al., 2012). The carboxy-terminal catalytic region consists of a CDC25 homology domain (CDC25-HD), a Ras exchange motif (REM), and a Ras association (RA) domain (Bos, 2006). The REM is thought to be involved in both stabilization of CDC25-HD and intramolecular interaction with the second cNBD. Interaction of a RA domain of Epac2 with an active GTP-bound form of Ras may control the localization of Epac2 and regulate the spatio-temporal activation of Rap (Li et al., 2006).
Three isoforms of Epac2, Epac2A, Epac2B, and Epac2C, produced by alternative promoter usage and differential splicing, have been identified. Epac2A, named Epac2 originally, is expressed mainly in brain and neuroendocrine and endocrine tissues such as pituitary and pancreatic islets as mentioned above. Epac2B, which lacks the cNBD-A domain, is similar to Epac1 in domain structure, and is expressed mainly in adrenal gland (Niimura et al., 2009). Epac2C, which lacks both a cNBD-A and a DEP domain, is expressed predominantly in liver (Ueno et al., 2001) (Fig. 1A). The tissue-specific expression of the Epac2 isoforms is regulated by DNA methylation of alternative promoters (Hoivik et al., 2013). Physiological roles of Epac2B in adrenal grand and Epac2C in liver have not been fully elucidated. However, Epac2C is likely to control bile acid-stimulated canalicular formation in the liver (Fu et al., 2011).
Figure 1. Domain structure of Epacs and mechanism of activation of Epac2.
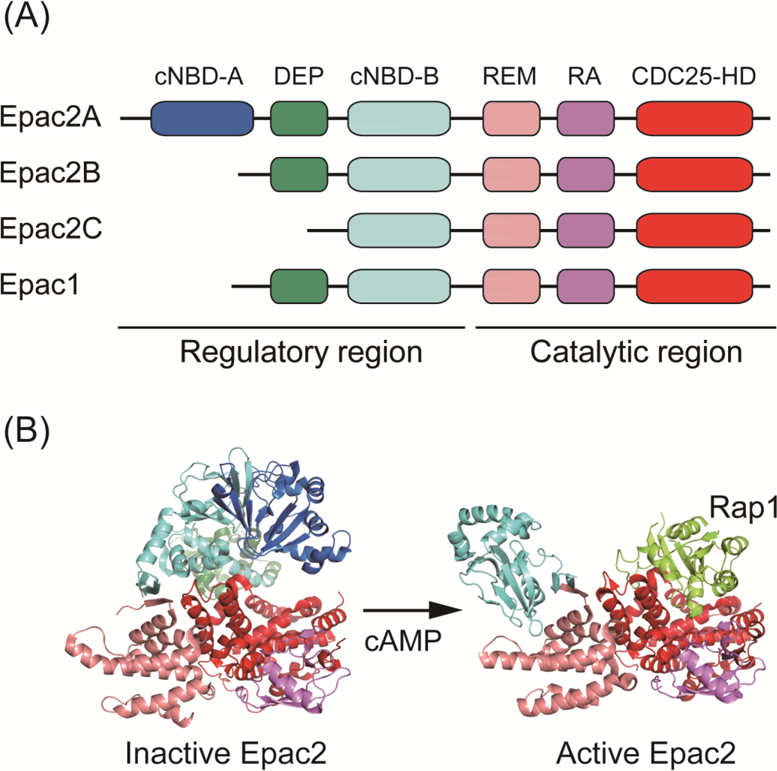
(A) Domain structure of Epac1 and Epac2. Epacs have an amino-terminal regulatory region and a carboxy-terminal catalytic region. The regulatory region contains one or two cyclic nucleotide binding domains (cNBD) and a DEP (Dishevelled, Egl-10, and Pleckstrin) domain. The catalytic region contains a REM (Ras exchange motif), a RA (Ras association) domain and a CDC25-HD (CDC25 homology domain).
(B) Crystal structures of inactive-form (PDB code 2BYV) and active-form (in complex with Rap1B and cAMP analog; PDB code 3CF6) of Epac2. In the inactive-form of Epac2, the Ras-binding region in CDC25-HD is masked by the regulatory region. The binding of cAMP to cNBD-B induces a conformational change, enabling access by Rap to the catalytic region.
The major function of Epac2 is guanine-nucleotide exchange for Rap1 and Rap2. Small GTPases cycle between an inactive GDP-bound form and an active GTP-bound form. They are tightly regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which are responsible for stimulation of GTP loading and catalysis of GTP hydrolysis, respectively. CDC25-HD of Epac2 interacts with GDP-bound Rap1, and its subsequent activation by exchange of GDP for GTP stimulates downstream signaling through interaction with its specific effector proteins. Well-known functions of Rap are regulation of actin cytoskeletal dynamics such as integrin-mediated cell adhesion, cadherin-mediated formation of cell junctions and potentiation of insulin secretion in pancreatic β-cells (Shibasaki et al., 2007; Roscioni et al., 2008; Frische and Zwartkruis, 2010).
Crystallographic analyses have advanced our understanding of the mechanism underlying cAMP-induced activation of Epac2 at the molecular level (Rehmann et al., 2006; Rehmann et al., 2008). In the crystal structure of the full length of Epac2 in the absence of cAMP, a catalytic region is masked by a regulatory region forming auto-inhibited conformation (Fig. 1B). cNBD-B interacts with this catalytic region both by ion bonds with REM and by forming beta strand-like secondary structures with REM and CDC25-HD, which are called ionic latch and switchboard, respectively. Thus, in auto-inhibited form, a regulatory region of Epac2 prevents Rap1 from accessing the catalytic region and therefore sterically inhibits the activation of Rap1 (Rehmann et al., 2006). Analysis of the crystal structure of the ternary complex of Epac2, cAMP analog, and Rap1B implies a mechanism of Epac2 activation as follows. The binding of cAMP to cNBD-B induces a dynamic conformational change that forms an open-conformation in which the catalytic region is rotated about 90° to the side and thus is translated closer to cNBD-B. Interaction of the cAMP analog with both cNBD-B and a REM ensures stabilization of the open conformation. This dynamic conformational change allows the Rap1-interacting region in the GEF domain to be exposed at its surface, thereby inducing GEF activity toward Rap1 (Rehmann et al., 2008) (Fig. 1B).
3. Roles of Epac2 in pancreatic islets
3.1. Insulin secretion from pancreatic β-cells
Type 2 diabetes is becoming a greater global health problem; the number of patients is rapidly increasing in both developed and developing countries. Type 2 diabetes is associated with impaired insulin secretion from pancreatic β-cells and impaired insulin action in peripheral target tissues. Insulin secretion from pancreatic β-cells is regulated by various factors. Although glucose is physiologically the most important regulator of insulin secretion (Henquin, 2000), hormones, neurotransmitters, and fatty acids also are required for normal regulation of insulin secretion mainly through G-protein-coupled receptors (Ahren, 2009; Seino et al., 2011). Among them, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), gut hormones called incretins, which are secreted from enteroendocrine cells following meal ingestion, are known to stimulate insulin secretion in a glucose-dependent manner (Drucker, 2006; Nauck, 2009; Seino et al., 2010). GLP-1 and GIP bind to their specific receptors, the GLP-1 receptor and GIP receptor, respectively (Thorens, 1992; Usdin et al., 1993; Yamada et al., 1995). As these incretin receptors are coupled to Gs-protein, their activation increases the intracellular cAMP concentration. Thus, cAMP-signaling plays a crucial role in insulin secretion from pancreatic β-cells to maintain glucose homeostasis. The action of cAMP in pancreatic β-cells had long been thought to be mediated through activation of PKA (Montague and Howell, 1972). However, cAMP stimulates exocytosis of insulin granules from a readily releasable pool (RRP), and it was found that this effect is unaffected by PKA inhibition, suggesting an alternative pathway by which cAMP enables insulin potentiation distinct from that of the PKA-dependent pathway (Renstrom et al., 1997). In yeast two-hybrid screening for a novel target protein for cAMP using the sulfonylurea receptor SUR1, the regulatory subunit of the pancreatic β-cell ATP-sensitive potassium (KATP) channel as bait, the cAMP-binding protein cAMP-GEFII (Epac2) was identified in mouse insulin-secreting MIN6 cells (Ozaki et al., 2000).
Glucose-induced insulin secretion (GIIS) occurs in a biphasic manner, a first phase of prompt, marked, and transient increase followed by a second phase of moderate and sustained increase (Curry et al., 1968). The first and second phases are associated with exocytosis of insulin granules from the RRP and a reserve pool (RP), respectively (Henquin, 2000; Barg et al., 2002; Bratanova-Tochkova et al., 2002). Although the precise mechanisms of biphasic secretion are not completely unraveled, the first phase is most likely evoked primarily by entry of Ca2+ into the β-cells; the second phase is sustained by Ca2+ and various metabolic signals generated by glucose-metabolism (Seino et al., 2011). In addition, F-actin remodeling is involved in the second phase of insulin secretion (Wang et al., 2007; Uenishi et al., 2013).
To determine the role of Epac2 in insulin secretion, global Rapgef4−/−mice were generated (Shibasaki et al., 2007). Rapgef4−/−mice are fertile, and there are no apparent abnormalities in general appearance or behavior (the mice are available on request to the corresponding author). TIRFM analysis of isolated β-cells from Rapgef4−/−mice indicated that the Epac2/Rap1signal is involved mainly in augmentation of the first phase of insulin secretion (Shibasaki et al., 2007). A biosimulation study suggested that the Epac2/Rap1signaling augments insulin secretion by increasing the size of the RRP and recruiting insulin granules from the RRP to the plasma membrane (Shibasaki et al., 2007). In Epac2-mediated exocytosis of insulin granules, Epac2 forms a complex with Rim2 (Ozaki et al., 2000; Kashima et al., 2001) that functions as scaffold for a variety of proteins involved in exocytosis. In pancreatic β-cells, Rim2 is localized in both plasma membrane and insulin granules, and determines the docking and priming states of exocytosis (Shibasaki et al., 2004; Yasuda et al., 2010). Epac2 also interacts with Piccolo, a possible Ca2+ sensor (Fujimoto et al., 2002), which increases the stability of the Epac2-Rim2 complex. Intricate interactions among Epac2, Rim2, Piccolo, and Rab3 play many important roles in cAMP-regulated insulin granule exocytosis (Shibasaki et al., 2004). On the other hand, a recent study of Rapgef3−/−mice has shown that Epac1 also is involved in insulin secretion, but primarily in GIIS rather than in cAMP-potentiated insulin secretion (Kai et al., 2013). However, since Epac1 is expressed at a low level in pancreatic islets compared to Epac2, the physiological role of Epac1 remains to be clarified.
It is well accepted that changes in the intracellular Ca2+ concentration play a pivotal role in the regulation of insulin granule exocytosis. In this regard, Epac2 was shown to regulate cytosolic Ca2+ dynamics in its potentiating effects on insulin secretion. This might involve phospholipase C-ε (PLC-ε), an isoform of the PLC family, which links Epac2 and various channel activities involved in cytosolic Ca2+ levels including those of the KATP channel, ryanodine receptor (RyR), and inositol 1,4,5-triphosphate (IP3) receptor. Indeed in PLC-ε knockout (Plce−/−) mice, Epac2-activated insulin potentiation was severely inhibited (Dzhura et al., 2011). PLC-ε has a domain structure containing two RA domains at its carboxy-terminus (Kelley et al., 2001), and PLC-ε is activated by the association of the active form of Rap to a RA domain (Schmidt et al., 2001; Song et al., 2002; Dzhura et al., 2010). It was shown that Epac2 modulates the activity of the KATP channels in pancreatic β-cells (Kang et al., 2006). The mechanism of this phenomenon was inferred from the finding that phosphatidylinositol 4,5-bisphosphate (PIP2) activates the KATP channel and decreases its sensitivity toward ATP (Baukrowitz et al., 1998). Thus, Epac/Rap-mediated hydrolysis of PIP2 localized around the KATP channel by PLC-ε, which hydrolyzes PIP2 to generate IP3, might increase the sensitivity of the channel to ATP, thereby inhibiting the channel (Holz et al., 2006).
GLP-1 is known to mobilize Ca2+ from endoplasmic reticulum (ER) to cytosol in pancreatic β-cells (Gromada et al., 1995; Holz et al., 1999). In this process, Epac2 is involved in RyR-mediated Ca2+ mobilization (Kang et al., 2001; Kang et al., 2003). Although the precise mechanism of RyR-mediated Ca2+ mobilization remains to be elucidated, these findings suggest that Epac2 is involved in Ca2+-induced Ca2+ release from ER. In addition, it has been suggested that activation of PLC-ε by Epac2 stimulates IP3 receptor-mediated Ca2+ mobilization (Schmidt et al., 2001).
Epac2 was also reported to regulate glucose metabolism. GLP-1 may increase the activity of glucokinase, the rate-limiting enzyme in glycolysis, through Epac2-Rim2-Rab3 signaling (Park et al., 2012). Ca2+ mobilization by GLP-1 has been reported to stimulate mitochondrial ATP synthesis in MIN6 cells (Tsuboi et al., 2003).
3.2. Glucagon secretion from pancreatic α-cells
Glucagon, which is secreted from pancreatic α-cells, also has an essential role in glucose-homeostasis: it stimulates glycogenolysis and gluconeogenesis in liver. Glucagon secretion from α-cells is regulated by a variety of factors including nutrients such as glucose and amino acids, hormones, and neurotransmitters (Quesada et al., 2008; Sandoval and D’Alessio, 2015). Adrenaline stimulates glucagon secretion by L-type Ca2+ channel-dependent exocytosis through activation of Epac2 as well as PKA via a large increase in intracellular cAMP levels by binding to the Gs-coupled β-adrenergic receptors on α-cells (De Marinis et al., 2010). On the other hand, GLP-1 was reported to exert an inhibitory effect on glucagon secretion (De Marinis et al., 2010). However, whether or not this is a direct effect on α-cells or an indirect effect through somatostatin secretion from δ-cells is inconclusive (de Heer et al., 2008).
4. Roles of Epac2 in CNS
It is well known that cAMP regulates the molecular mechanisms of a wide variety of neuronal processes through an Epac-dependent pathway as well as a PKA-dependent pathway. In brain, both Epac1 and Epac2 are expressed (Kawasaki et al., 1998). Consistent with the observation that Epac2 is expressed abundantly in neuronal cells (Kawasaki et al., 1998), diverse roles of Epac2 in brain have been pointed out regarding neurotransmitter release, neuronal differentiation, neurite growth, memory, learning and pathophysiological roles in some brain disorders such as Alzheimer’s disease and autism (Schmidt et al., 2013).
4.1. Neurotransmitter release
Epacs were shown to be involved in the enhancement of neurotransmitter release in glutamatergic synapses from calyx of Held and in the crayfish neuromuscular junction. (Sakaba and Neher, 2001; Zhong and Zucker, 2005; Gekel and Neher, 2008). Interaction of Epac2 with Rim1, which is an isoform of the Rim family and is expressed predominantly in brain, may participate in regulated exocytosis of synaptic vesicles in neuronal cells as well as in large dense core granules in pancreatic β-cells (Ozaki et al., 2000). A recent study of Rapgef4−/−mice demonstrated the involvement of Epac2 in cAMP-dependent potentiation of neurotransmission through maintenance of the RRP at mossy fiber synapses in the hippocampus during sustained transmission or after tetanus-induced mossy fiber-long-term potentiation (LTP) (Fernandes et al., 2015).
4.2. Neural development and remodeling
Epac has roles in development of brain including neurite growth and neuronal differentiation. Initial observations showed that Epac-specific agonist induces neurite outgrowth in rat pheochromocytoma PC12 cells (Christensen et al., 2003). In an additional study using PC12 cells, it was shown that the Epac signal extended the duration of PKA-dependent extracellular signal-regulated kinase (ERK) 1/2 activation, and thereby converted cAMP from a proliferative into a neurite outgrowth-promoting signal (Kiermayer et al., 2005). Epac also has a role in mechanisms of cAMP-regulated axon growth and guidance, and in mediating axon regeneration in adult mammalian CNS tissue. (Murray and Shewan, 2008).
Growing evidence supports isoform-specific roles of Epac2 in these processes. It was suggested recently that while the cAMP sensor Rapgef2 is involved in signaling to ERK to mediate neuritogenesis, Epac2 can induce the growth arrest involved in the process of differentiation through signaling to the p38 mitogen activated protein kinase (MAPK) (Emery et al., 2014). In spine synapses, Epac2 induces synapse remodeling by altering synapse shrinkage and turnover, and also causes functional depression of spiny synapses through forming a molecular complex with postsynaptic adhesion molecule neuroligin 3, which is responsible for membrane translocation of Epac2 and consequent activation of Rap (Woolfrey et al., 2009; Penzes et al., 2011).
4.3. Higher brain function
The Epac-specific activator 8-pCPT-2’-O-Me-cAMP (8-pCPT) enhances maintenance of LTP in CA1 of mouse hippocampal slices through ERK signaling (Gelinas et al., 2008). In hippocampal CA1 excitatory synapses, Epac mediates pituitary adenylate cyclase-activating peptide-dependent long-term depression (LTD) through activation of p38-MAPK, mobilization of Ca2+, and protein synthesis (Ster et al., 2009). These findings suggest that Epac is likely to have important roles in synaptic plasticity, thus controlling higher brain functions such as memory and learning. Intrahippocampal injection of 8-pCPT has been reported to improve fear memory retrieval in contextual fear conditioning but not its acquisition and consolidation (Ostroveanu et al., 2010). This study also suggested that Epac2 is involved in the time-limited memory retrieval (Ostroveanu et al., 2010). In murine primary cortical neurons and HT-4 cells, Epac2 enhanced phosphorylation of PKB/Akt, which is known to support neuronal survival and memory processes through Rap1 activation. In these processes, AKAP150 regulates phosphorylation of PKB/Akt by controlling two cAMP pathways, the Epac2-dependent and PKA-dependent pathways (Nijholt et al., 2008). The double knockout of Rapgef3 and Rapgef4 in forebrain of mice impaired LTP, spatial learning, and social interaction. These impairments were mediated by microRNA miR-124 transcription and zinc finger protein Zif268 translation (Yang et al., 2012). In addition, Epac2 depletion was found to induce impairments in social interaction, ultrasonic vocalization and cortical structure. In these mice, abnormal columnar organization occurred in the anterior cingulate cortex, the region related to socially-driven interactions, and dendric spine motility and density on cortical neurons were reduced (Srivastava et al., 2012a).
Recent genetic studies suggested a role of Epac2 in the pathophysiological mechanism of autism. In 2003, rare coding mutations in Epac2 were identified in patients with autism (Bacchelli et al., 2003). Overexpression of this mutated form of Epac2 reduced the basal dendrite complexity in cortical pyramidal neurons and disrupted the interaction between Epac2 and Ras, suggesting that Epac2 enables crosstalk between Ras and Rap signaling and takes part in the regulation of basal dendrite complexity in cortical neurons (Srivastava et al., 2012b).
5. Functions of Epac2 in heart
In heart, stimulation of β-adrenergic receptors, which is coupled with Gs-protein, increases the intracellular cAMP levels. cAMP has physiological roles in cardiac functions including cardiac contractility, relaxation, heart rate and automaticity (Bers, 2008). Although these stimulations are necessary for part of normal adaptation to increase in physiologic demand, chronic elevation of cAMP due to sustained β-adrenergic stimulation has been associated with hypertrophy, arrhythmia and eventually development of heart failure (El-Armouche and Eschenhagen, 2009). These phenotypes might be due to over-activation of Epac as well as PKA.
In heart, Epac1 is predominantly expressed compared with Epac2 (Kawasaki et al., 1998; Metrich et al., 2008). Epac1 but not Epac2 is increased in pressure overload-induced hypertrophy (Ulucan et al., 2007) and knockdown of Epac1 inhibits β-adrenergic receptor-induced hypertrophy (Metrich et al., 2008). Epac1-mediated hypertrophy involves activation of hypertrophic transcription regulators such as NFAT (nuclear factor of activated T-cells) (Morel et al., 2005; Metrich et al., 2008) and MEF2 (Metrich et al., 2010; Pereira et al., 2012) through activation of several kinds of small GTPases such as Rac, Rap2B and H-Ras with PLC, phosphatase calcineurin and CaMKII. In contrast, Epac2 is more likely to be involved in enhancing susceptibility to arrhythmia in mice. It was reported that the Epac activator, 8-pCPT, caused ventricular tachycardia through the Ca2+/CaMKII pathway (Hothi et al., 2008). Using Rapgef3−/−mice, Rapgef4−/−mice, and Rapgef3−/−; Rapgef4−/−mice, it was shown that Epac2 but not Epac1 participates in the arrhythmogenic effect through CaMKII-dependent diastolic sarcoplasmic reticulum (SR) Ca2+ release; this process involves the β1-adrenargic receptor, Epac2, CaMKIIδ, and phosphorylation of the S2814 residue of RyR2 (Pereira et al., 2013). Furthermore, a study using fluorescent Epac2 ligand (Φ-O-Me-cAMP) showed distinct distributions between Epac1 and Epac2 in mice myocytes. Epac2 is localized along T-tubules while Epac1 is localized around the nucleus, supporting a role for Epac2 in arrhythmogenic SR Ca2+ leak in mice (Pereira et al., 2015). Whether or not Epac2 contributes to the progression of arrhythmia in human is unknown.
Epac2 also participates in atrial natriuretic peptide (ANP) secretion by heart in mice. The GLP-1 receptor was found to be expressed at cardiac atria, and activation of the receptor increased the plasma ANP concentration, which contributes to the antihypertensive effect through vascular smooth muscle relaxation and natriuresis in kidney. In this process, Epac2 is shown to link activation of the GLP-1 receptor and ANP secretion through PLC-dependent signals in cardiomyocytes (Kim et al., 2013).
6. Epac2 as a potential therapeutic target
Epac is considered to be a promising drug target for various diseases (Parnell et al., 2015). Epac2 as well as PKA has an important role in cAMP-mediated insulin potentiation. Epac2 agonist is expected to stimulate insulin secretion in a glucose-dependent manner, so it would have low risk of hypoglycemia clinically, as is key for incretin-based anti-diabetic therapies. Epac2 agonists might therefore have benefits for treatment of type 2 diabetes with impaired insulin secretion from pancreatic β-cells.
So far, Epac-specific agonists have been developed based on modification of cAMP. The important discovery of the Epac-specific cAMP analog 8-pCPT (Enserink et al., 2002) and its membrane-permeable derivative 8-pCPT-AM (Vliem et al., 2008) deepened our understanding of Epac-dependent and PKA-independent actions of cAMP signaling. However, because of its non-selectivity toward Epac isoforms, application to clinical use of these analogs is not possible due to side effects, on cardiac function for example. Indeed, sustained activation of cAMP signaling caused Epac1-dependent hypertrophy and fibrosis in heart in mice, as described above. A novel cAMP-analog Sp-8-BnT-cAMPS, which displays a great selectivity to Epac2 at cell level, has recently been developed (Schwede et al., 2015). It will be useful in further studies of Epac2.
It was shown that sulfonylurea drugs, widely used for the treatment of type 2 diabetes, are potent selective activators for Epac2 as assessed by a FRET (fluorescence resonance energy transfer) system, by which activation and subsequent dynamic structural change of Epac2 can be monitored (Zhang et al., 2009). However, other groups failed to detect the direct activation of Epac2 by sulfonylureas using cell free system (Tsalkova et al., 2011; Rehmann, 2012). Using molecular docking simulation, site-directed mutagenesis, Epac2A-FRET biosensor, and direct sulfonylurea-binding experiments, the amino acid residues Cys105, Gly114, Ser116 and His124 in the cNBD-A of Epac2A that interact with sulfonylureas have recently been identified (Takahashi et al., 2013). Binding of sulfonylureas to Epac2A depends on the concentration of cAMP and the structures of the drugs. Sulfonylureas and cAMP cooperatively activate Epac2A through binding to cNBD-A and cNBD-B, respectively. As the plasma membrane is a crucial factor in Epac2A’s activation of Rap1 (Liu et al., 2008), activation of Epac2A by sulfonylureas may require the cellular environment. Thus, it is considered that sulfonylureas except for gliclazide activate Epac2/Rap1 signaling in concert with cAMP signaling in its augmenting effect on insulin secretion (Takahashi et al., 2015). Since sulfonylureas have no effects on activation of Epac1 or PKA, these findings may provide a clue to the development of Epac2-specific agonists independent of the structure of cAMP.
7. Conclusion
Since the discovery of Epac proteins in 1998, accumulating studies have shed light on PKA-independent cAMP action in various cell types and tissues. Other than in the pancreatic islets, brain, and heart summarized in this review, Epac has been revealed to function in a wide range of organs and cell types including vasculature, lung, kidney, endometrium, osteoclasts, and inflammatory cells. Despite such advances, a great number of issues remain to be elucidated, including the isoform-specific molecular and physiological functions and spatio-temporal regulation of Epacs in each organ. Generation of tissue-specific genetically-modified animals and development of isoform-selective agonist or antagonist will lead not only to the further understanding of isoform-specific functions but also the discovery of novel drugs for treating various diseases.
Figure 2.
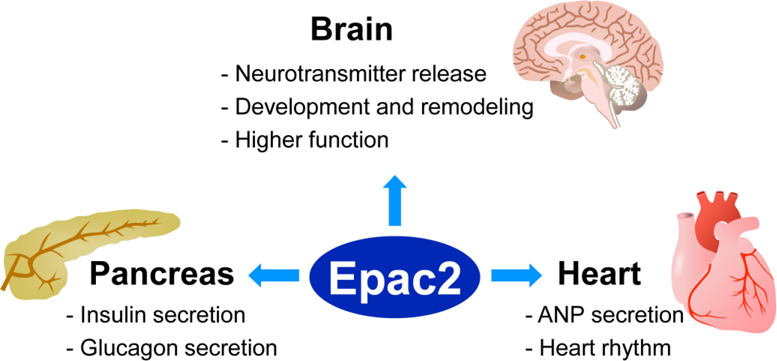
Functional roles of Epac2 in pancreas, brain, and heart.
See text for details.
Highlights.
Exchange protein directly activated by cAMP 2 (EPAC2) is encoded by Rapgef4 gene.
Epac2 functions as a guanine-exchange factor regulated by cAMP.
Epac2 has diverse roles in cellular functions of pancreatic islets, central nervous system and heart.
Epac2 is a potential therapeutic target for treatment of type 2 diabetes.
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM089820 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. We would like to thank members of our laboratory for their contributions to our studies cited in this review. The studies in our laboratory were supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sport, Science and Technology, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Ahren B, 2009. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8, 369–85. [DOI] [PubMed] [Google Scholar]
- Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP and Maestrini E, 2003. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry 8, 916–24. [DOI] [PubMed] [Google Scholar]
- Barg S, Eliasson L, Renstrom E and Rorsman P, 2002. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes 51 Suppl 1, S74–82. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP and Fakler B, 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282, 1141–4. [DOI] [PubMed] [Google Scholar]
- Beavo JA and Brunton LL, 2002. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol 3, 710–8. [DOI] [PubMed] [Google Scholar]
- Bers DM, 2008. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70, 23–49. [DOI] [PubMed] [Google Scholar]
- Bos JL, 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31, 680–6. [DOI] [PubMed] [Google Scholar]
- Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu YJ, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H and Sharp GW, 2002. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 51 Suppl 1, S83–90. [DOI] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG and Doskeland SO, 2003. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278, 35394–402. [DOI] [PubMed] [Google Scholar]
- Consonni SV, Gloerich M, Spanjaard E and Bos JL, 2012. cAMP regulates DEP domain-mediated binding of the guanine nucleotide exchange factor Epac1 to phosphatidic acid at the plasma membrane. Proc Natl Acad Sci U S A 109, 3814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry DL, Bennett LL and Grodsky GM, 1968. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 83, 572–84. [DOI] [PubMed] [Google Scholar]
- de Heer J, Rasmussen C, Coy DH and Holst JJ, 2008. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51, 2263–70. [DOI] [PubMed] [Google Scholar]
- De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren AH, Shibasaki T, Gribble F, Renstrom E, Seino S, Eliasson L and Rorsman P, 2010. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N-and L-type Ca2+ channel-dependent exocytosis. Cell Metab 11, 543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A and Bos JL, 2000. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275, 20829–36. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A and Bos JL, 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–7. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, 2006. The biology of incretin hormones. Cell Metab 3, 153–65. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Chepurny OG, Kelley GG, Leech CA, Roe MW, Dzhura E, Afshari P, Malik S, Rindler MJ, Xu X, Lu Y, Smrcka AV and Holz GG, 2010. Epac2-dependent mobilization of intracellular Ca(2)+ by glucagon-like peptide-1 receptor agonist exendin-4 is disrupted in beta-cells of phospholipase C-epsilon knockout mice. J Physiol 588, 4871–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, Lu Y, Schwede F, Genieser HG, Smrcka AV and Holz GG, 2011. Phospholipase C-epsilon links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets 3, 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Armouche A and Eschenhagen T, 2009. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 14, 225–41. [DOI] [PubMed] [Google Scholar]
- Emery AC, Eiden MV and Eiden LE, 2014. Separate cyclic AMP sensors for neuritogenesis, growth arrest, and survival of neuroendocrine cells. J Biol Chem 289, 10126–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL and Bos JL, 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4, 901–6. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Riordan S, Nomura T, Remmers CL, Kraniotis S, Marshall JJ, Kukreja L, Vassar R and Contractor A, 2015. Epac2 Mediates cAMP-Dependent Potentiation of Neurotransmission in the Hippocampus. J Neurosci 35, 6544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frische EW and Zwartkruis FJ, 2010. Rap1, a mercenary among the Ras-like GTPases. Dev Biol 340, 1–9. [DOI] [PubMed] [Google Scholar]
- Fu D, Wakabayashi Y, Lippincott-Schwartz J and Arias IM, 2011. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci U S A 108, 1403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T and Seino S, 2002. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem 277, 50497–502. [DOI] [PubMed] [Google Scholar]
- Gekel I and Neher E, 2008. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci 28, 7991–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ and Nguyen PV, 2008. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem 15, 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M and Bos JL, 2011. Regulating Rap small G-proteins in time and space. Trends Cell Biol 21, 615–23. [DOI] [PubMed] [Google Scholar]
- Gromada J, Dissing S, Bokvist K, Renstrom E, Frokjaer-Jensen J, Wulff BS and Rorsman P, 1995. Glucagon-like peptide I increases cytoplasmic calcium in insulin-secreting beta TC3-cells by enhancement of intracellular calcium mobilization. Diabetes 44, 767–74. [DOI] [PubMed] [Google Scholar]
- Henquin JC, 2000. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 1751–60. [DOI] [PubMed] [Google Scholar]
- Hoivik EA, Witsoe SL, Bergheim IR, Xu Y, Jakobsson I, Tengholm A, Doskeland SO and Bakke M, 2013. DNA methylation of alternative promoters directs tissue specific expression of Epac2 isoforms. PLoS One 8, e67925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW and Chepurny OG, 2006. Cell physiology of cAMP sensor Epac. J Physiol 577, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M and Habener JF, 1999. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37). J Biol Chem 274, 14147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothi SS, Gurung IS, Heathcote JC, Zhang Y, Booth SW, Skepper JN, Grace AA and Huang CL, 2008. Epac activation, altered calcium homeostasis and ventricular arrhythmogenesis in the murine heart. Pflugers Arch 457, 253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, Yeung PK, Tam S, Wang J, Lam KS, Vanhoutte PM, Bos JL, Chung SS, Xu A and Chung SK, 2013. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop beta-cell dysfunction and metabolic syndrome. FASEB J 27, 4122–35. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG and Holz GG, 2001. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol 536, 375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA and Holz GG, 2006. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol 573, 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG and Holz GG, 2003. Epac-selective cAMP analog 8-pCPT-2’-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278, 8279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H and Seino S, 2001. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276, 46046–53. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE and Graybiel AM, 1998. A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–9. [DOI] [PubMed] [Google Scholar]
- Kelley GG, Reks SE, Ondrako JM and Smrcka AV, 2001. Phospholipase C(epsilon): a novel Ras effector. EMBO J 20, 743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S and Piiper A, 2005. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell 16, 5639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA and Drucker DJ, 2013. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 19, 567–75. [DOI] [PubMed] [Google Scholar]
- Li Y, Asuri S, Rebhun JF, Castro AF, Paranavitana NC and Quilliam LA, 2006. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J Biol Chem 281, 2506–14. [DOI] [PubMed] [Google Scholar]
- Liu C, Takahashi M, Li Y, Song S, Dillon TJ, Shinde U and Stork PJ, 2008. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol 28, 7109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrich M, Laurent AC, Breckler M, Duquesnes N, Hmitou I, Courillau D, Blondeau JP, Crozatier B, Lezoualc’h F and Morel E, 2010. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell Signal 22, 1459–68. [DOI] [PubMed] [Google Scholar]
- Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E and Lezoualc’h F, 2008. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 102, 959–65. [DOI] [PubMed] [Google Scholar]
- Montague W and Howell SL, 1972. The mode of action of adenosine 3’:5’-cyclic monophosphate in mammalian islets of Langerhans. Preparation and properties of islet-cell protein phosphokinase. Biochem J 129, 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G and Lezoualc’h F, 2005. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res 97, 1296–304. [DOI] [PubMed] [Google Scholar]
- Murray AJ and Shewan DA, 2008. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci 38, 578–88. [DOI] [PubMed] [Google Scholar]
- Nauck MA, 2009. Unraveling the science of incretin biology. Am J Med 122, S3–S10. [DOI] [PubMed] [Google Scholar]
- Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T and Seino S, 2009. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol 219, 652–8. [DOI] [PubMed] [Google Scholar]
- Nijholt IM, Dolga AM, Ostroveanu A, Luiten PG, Schmidt M and Eisel UL, 2008. Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal 20, 1715–24. [DOI] [PubMed] [Google Scholar]
- Ostroveanu A, van der Zee EA, Eisel UL, Schmidt M and Nijholt IM, 2010. Exchange protein activated by cyclic AMP 2 (Epac2) plays a specific and time-limited role in memory retrieval. Hippocampus 20, 1018–26. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y and Seino S, 2000. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2, 805–11. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim SJ, Park SH, Son DG, Bae JH, Kim HK, Han J and Song DK, 2012. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic beta-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology 153, 574–82. [DOI] [PubMed] [Google Scholar]
- Parnell E, Palmer TM and Yarwood SJ, 2015. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci 36, 203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Woolfrey KM and Srivastava DP, 2011. Epac2-mediated dendritic spine remodeling: implications for disease. Mol Cell Neurosci 46, 368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J and Bers DM, 2013. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation 127, 913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Rehmann H, Lao DH, Erickson JR, Bossuyt J, Chen J and Bers DM, 2015. Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes. Proc Natl Acad Sci U S A 112, 3991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Ruiz-Hurtado G, Morel E, Laurent AC, Metrich M, Dominguez-Rodriguez A, Lauton-Santos S, Lucas A, Benitah JP, Bers DM, Lezoualc’h F and Gomez AM, 2012. Epac enhances excitation-transcription coupling in cardiac myocytes. J Mol Cell Cardiol 52, 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Mei FC, Popov VL, Vergara LA and Cheng X, 2002. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem 277, 26581–6. [DOI] [PubMed] [Google Scholar]
- Quesada I, Tuduri E, Ripoll C and Nadal A, 2008. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 199, 5–19. [DOI] [PubMed] [Google Scholar]
- Rehmann H, 2012. Epac2: a sulfonylurea receptor? Biochem Soc Trans 40, 6–10. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O and Bos JL, 2008. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 455, 124–7. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Das J, Knipscheer P, Wittinghofer A and Bos JL, 2006. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 439, 625–8. [DOI] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L and Rorsman P, 1997. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic β-cells. J Physiol 502 ( Pt 1), 105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioni SS, Elzinga CR and Schmidt M, 2008. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol 377, 345–57. [DOI] [PubMed] [Google Scholar]
- Sakaba T and Neher E, 2001. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc Natl Acad Sci U S A 98, 331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA and D’Alessio DA, 2015. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 95, 513–48. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dekker FJ and Maarsingh H, 2013. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 65, 670–709. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW and Jakobs KH, 2001. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol 3, 1020–4. [DOI] [PubMed] [Google Scholar]
- Schwede F, Bertinetti D, Langerijs CN, Hadders MA, Wienk H, Ellenbroek JH, de Koning EJ, Bos JL, Herberg FW, Genieser HG, Janssen RA and Rehmann H, 2015. Structure-guided design of selective Epac1 and Epac2 agonists. PLoS Biol 13, e1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T and Minami K, 2011. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest 121, 2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Fukushima M and Yabe D, 2010. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig 1, 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y and Seino S, 2004. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem 279, 7956–61. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J and Seino S, 2007. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A 104, 19333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Satoh T, Edamatsu H, Wu D, Tadano M, Gao X and Kataoka T, 2002. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C epsilon. Oncogene 21, 8105–13. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Jones KA, Woolfrey KM, Burgdorf J, Russell TA, Kalmbach A, Lee H, Yang C, Bradberry MM, Wokosin D, Moskal JR, Casanova MF, Waters J and Penzes P, 2012a. Social, communication, and cortical structural impairments in Epac2-deficient mice. J Neurosci 32, 11864–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Anderson CT, Smith KR, Russell TA, Lee H, Yasvoina MV, Wokosin DL, Ozdinler PH, Shepherd GM and Penzes P, 2012b. An autism-associated variant of Epac2 reveals a role for Ras/Epac2 signaling in controlling basal dendrite maintenance in mice. PLoS Biol 10, e1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J and Fagni L, 2009. Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol 587, 101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Shibasaki T, Park JH, Hidaka S, Takahashi T, Ono A, Song DK and Seino S, 2015. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes 64, 1262–72. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shibasaki T, Takahashi H, Sugawara K, Ono A, Inoue N, Furuya T and Seino S, 2013. Antidiabetic sulfonylureas and cAMP cooperatively activate Epac2A. Sci Signal 6, ra94. [DOI] [PubMed] [Google Scholar]
- Thorens B, 1992. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A 89, 8641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalkova T, Gribenko AV and Cheng X, 2011. Exchange protein directly activated by cyclic AMP isoform 2 is not a direct target of sulfonylurea drugs. Assay Drug Dev Technol 9, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP and Rutter GA, 2003. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J 369, 287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenishi E, Shibasaki T, Takahashi H, Seki C, Hamaguchi H, Yasuda T, Tatebe M, Oiso Y, Takenawa T and Seino S, 2013. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J Biol Chem 288, 25851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H and Seino S, 2001. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics 78, 91–8. [DOI] [PubMed] [Google Scholar]
- Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S and Ishikawa Y, 2007. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol 293, H1662–72. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Mezey E, Button DC, Brownstein MJ and Bonner TI, 1993. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133, 2861–70. [DOI] [PubMed] [Google Scholar]
- Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL and Rehmann H, 2008. 8-pCPT-2’-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem 9, 2052–4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oh E and Thurmond DC, 2007. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem 282, 9536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M and Penzes P, 2009. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci 12, 1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Hayami T, Nakamura K, Kaisaki PJ, Someya Y, Wang CZ, Seino S and Seino Y, 1995. Human gastric inhibitory polypeptide receptor: cloning of the gene (GIPR) and cDNA. Genomics 29, 773–6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Xu X, Tian Q, Zhang J, Qian K, Wang YX, Petralia RS, Tu W, Zhu LQ, Wang JZ and Lu Y, 2012. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron 73, 774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T and Seino S, 2010. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab 12, 117–29. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T and Seino S, 2009. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325, 607–10. [DOI] [PubMed] [Google Scholar]
- Zhong N and Zucker RS, 2005. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci 25, 208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


