Abstract
Background
Resting sympathetic hyperactivity and impaired parasympathetic reactivation after exercise have been described in patients with heart failure (HF). However, the association of these autonomic changes in patients with HF and sarcopenia is unknown.
Objective
The aim of this study was to evaluate the impact of autonomic modulation on sarcopenia in male patients with HF.
Methods
We enrolled 116 male patients with HF and left ventricular ejection fraction < 40%. All patients underwent a maximal cardiopulmonary exercise testing. Maximal heart rate was recorded and delta heart rate recovery (∆HRR) was assessed at 1st and 2nd minutes after exercise. Muscle sympathetic nerve activity (MSNA) was recorded by microneurography. Dual-energy X-ray absorptiometry was used to measure body composition and sarcopenia was defined by the sum of appendicular lean muscle mass (ALM) divided by height in meters squared and handgrip strength.
Results
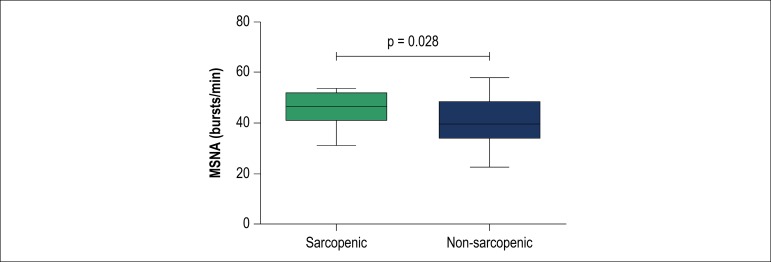
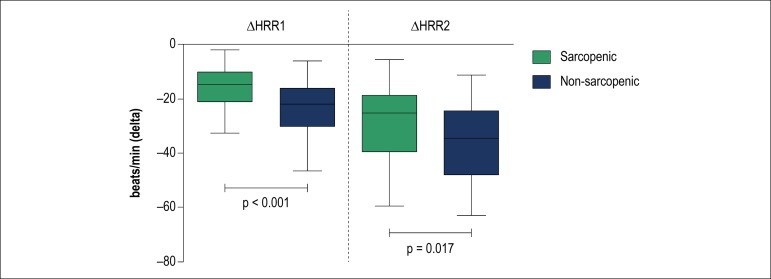
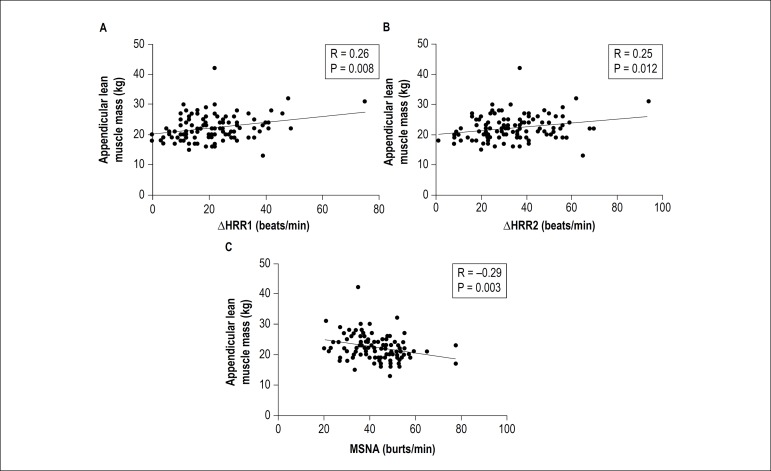
Sarcopenia was identified in 33 patients (28%). Patients with sarcopenia had higher MSNA than those without (47 [41-52] vs. 40 [34-48] bursts/min, p = 0.028). Sarcopenic patients showed lower ∆HRR at 1st (15 [10-21] vs. 22 [16-30] beats/min, p < 0.001) and 2nd min (25 [19-39] vs. 35 [24-48] beats/min, p = 0.017) than non-sarcopenic. There was a positive correlation between ALM and ∆HRR at 1st (r = 0.26, p = 0.008) and 2nd min (r = 0.25, p = 0.012). We observed a negative correlation between ALM and MSNA (r = -0.29, p = 0.003).
Conclusion
Sympatho-vagal imbalance seems to be associated with sarcopenia in male patients with HF. These results highlight the importance of a therapeutic approach in patients with muscle wasting and increased peripheral sympathetic outflow.
Keywords: Heart Failure, Sarcopenia, Sympathetic Hyperactivity, Blunted Vagal Reactivation.
Introduction
Changes in body composition play an important role in the pathogenesis and progression of chronic heart failure (HF).1 Sarcopenia, which is characterized by a decrease in skeletal muscle mass and strength, affects 19.5% of ambulatory patients with HF,2 and is associated with several alterations such as impaired endothelial function, reduced 6-minute walking distance, and attenuated peak VO2.2,3 Although sarcopenia has been frequently described in elderly patients as a consequence of the ageing process, it can also be present in younger patients with HF.4
Resting sympathoexcitation is a hallmark in chronic HF.5 In addition, accumulated evidence shows that this autonomic dysregulation is highly associated with increased morbidity and mortality.5 In normal conditions, sympathetic nervous system exerts anabolic action via β2-adrenoceptors on skeletal muscle,6 but in experimental model of HF, the exacerbated sympathetic nervous activity contributes to downregulation of β2-adrenoceptors favoring skeletal muscle atrophy and weight loss.7
Reduced parasympathetic activity has also been reported in patients with HF.8,9 Binkley and colleagues 10 showed impaired parasympathetic activity in patients with HF evaluated by heart rate variability. Moreover, heart rate recovery (HRR), an important cardiac deceleration mechanism after maximum effort, can also be used to assess parasympathetic activity immediately after maximal exercise testing.11 Furthermore, HRR is an easy, low-cost, and clinical assessment of vagal reactivation, and provides additional prognostic information.12-14
Muscle sympathetic nerve activity (MSNA) and HRR, as measures of sympathetic and parasympathetic activity, respectively, have not been studied in sarcopenic patients with HF. Therefore, the aim of this study was to evaluate the impact of autonomic modulation assessed by MSNA (by microneurography technique) and HRR immediately after maximal exercise testing in patients with HF and sarcopenia.
Methods
Study population
Between May 1, 2016 and December 31, 2017, we prospectively enrolled 116 male outpatients with stable chronic HF. Inclusion criteria were: (1) age between 18 and 65 years old; (2) at least 1 year of HF diagnosis; (3) left ventricular ejection fraction (LVEF) lower than 40% measured by echocardiography; (4) non-ischemic and ischemic etiologies; (5) compensated HF with optimal medication for at least three months prior the study; and (6) New York Heart Association (NYHA) class I to IV.
Patients with autonomic diabetic neuropathy, chronic renal failure with haemodialysis, heart transplantation, pacemaker, muscular dystrophy (i.e. Duchenne muscular dystrophy), any hormonal treatment, history of cancer, ongoing infection, and myocardial infarction with percutaneous coronary intervention or revascularization up to 6 months prior to the study entry, were not included.
Muscle sympathetic nerve activity
MSNA was directly recorded from the peroneal nerve using the microneurography technique.15,16 Multiunit post-ganglionic muscle sympathetic nerve recordings were made using a tungsten microelectrode placed in the peroneal nerve near the fibular head. Nerve signals were amplified by a factor of 50,000 to 100,000 and band-pass filtered (700 to 2000 Hz). For recording and analysis, nerve activity was rectified and integrated (time constant 0.1 second) to obtain a mean voltage display of sympathetic nerve activity. MSNA was expressed as burst frequency (bursts per minute).
Maximal cardiopulmonary exercise test
All patients underwent symptom-limited cardiopulmonary exercise test (Vmax Encore 29 System; VIASYS Healthcare Inc., Palm Springs, California, USA) performed on a cycle ergometer (Ergometer 800S; SensorMedics, Yorba Linda, California, USA), using a ramp protocol with workload increments of 5 or 10 Watts per minute. Oxygen consumption (VO2) and carbon dioxide output (VCO2) were measured by means of gas exchange on a breath-by-breath basis and expressed as 30-s averages. The patients were initially monitored for 2 minutes at rest when seated on the ergometer; then they were instructed to pedal at a pace of 60-70 rpm and the completion of the test occurred when, in spite of verbal encouragement, the patient reached maximal volitional fatigue. A respiratory exchange ratio (RER) higher than 1.10 was reached for all patients. Heart rate (HR) was monitored continuously at rest, during the test and recovery phase, using a 12-lead digital electrocardiogram (CardioSoft 6.51 ECG/CAM-14, GE Medical Systems Information Technologies, Wisconsin, USA).17
After achieving peak workload, the patients continued to pedal at 10 watts for 2 minutes, followed by 4 minutes seated on the ergometer, this 6-min period was considered the recovery phase. Delta (∆) HRR was calculated by subtracting the HR values at 1st (∆HRR1) and 2nd (∆HRR2) minutes of the recovery phase from the peak HR.12
Body composition and muscle strength
Body composition measurements - total lean and fat mass - were performed using dual-energy X-ray absorptiometry (DXA) (Lunar iDXA; GE Medical Systems Lunar, Madison, USA). Then, skeletal muscle mass index (SMI) was calculated as the sum of appendicular lean muscle mass of both arms and legs divided by height in meters squared.18
After adjusting handle position, muscle strength was assessed by handgrip dynamometer (Model J00105; Jamar Hydraulic Hand Dynamometer) using the dominant hand in a supinated position with elbow flexed at 90º. There was 1-min rest interval between efforts and the maximum value of three attempts was used.19
Sarcopenia was defined as SMI and muscle strength lower than 7.26 kg/m2 and 30 kg, respectively.20
Laboratory Measurements
Blood samples were drawn in the morning after 12h overnight fasting. The laboratory tests included B-type natriuretic peptide (BNP; pg/mL) plasma level, serum sodium (mEq/L), serum potassium (mEq/L), creatinine (mg/dL), haemoglobin level (g/dL), high-sensitivity C-reactive protein (hs-CRP; mg/L), lipid profile (triglyceride, total cholesterol, high-density lipoprotein, and low-density lipoprotein; mg/dL), and fasting glucose (mg/dL).
Statistical analysis
Data are presented as mean ± standard deviation and median with lower and upper quartile (95%CI). One-sample Kolmogorov-Smirnov test was used to evaluate the distribution normality of the studied variables. Student's t-test and Mann-Whitney U test were used to compare parametric and nonparametric variables, respectively. Chi-square test and Spearman's correlation were used as appropriate. The Statistical Package for the Social Sciences version 23 (SPSS Inc., Chicago, Illinois, USA) was used to perform all the statistical analysis. P value lower than 0.05 was considered statistically significant.
Results
Clinical-demographic data
We prospectively enrolled 116 male patients (Table 1) with stable chronic HF, 33 of whom were identified to have sarcopenia (28%). Patients with sarcopenia were older, had higher BNP concentration, and lower hemoglobin compared with patients without sarcopenia. No difference was found between sarcopenic and non-sarcopenic patients regarding the dosage of β-blocker medication (20 ± 9.6 vs. 23 ± 10.5 mg b.i.d., p = 0.39; respectively) and medication in general (Table 1).
Table 1.
Demographic and clinical characteristics of the study population
| Variables | All patients (n = 116) | Patients with sarcopenia (n = 33) | Patients without sarcopenia (n = 83) | P value |
|---|---|---|---|---|
| Age (y) | 55 ± 9 | 59 ± 6 | 54 ± 9 | 0.002 |
| Weight (kg) | 71.1 ± 14.4 | 59.4 ± 7.4 | 75.8 ± 13.8 | < 0.001 |
| Height (m) | 1.67 ± 0.07 | 1.66 ± 0.07 | 1.67 ± 0.07 | 0.401 |
| BMI (kg/m2) | 25.5 ± 4.5 | 21.6 ± 2.5 | 27.1 ± 4.2 | < 0.001 |
| Aetiology (Ischaemic/non-ischaemic) | 30/86 | 8/25 | 22/61 | 1.000 |
| NYHA class (I/II/III/IV) | 40/41/28/7 | 9/11/11/2 | 31/30/17/5 | 0.500 |
| LVEF (%) | 28 ± 8 | 26 ± 7 | 29 ± 8 | 0.124 |
| BNP (pg/mL) | 773 ± 877 | 1159 ± 924 | 621 ± 816 | 0.006 |
| Sodium (mEq/L) | 139 ± 3 | 138 ± 4 | 139 ± 3 | 0.383 |
| Potassium (mEq/L) | 4.6 ± 0.4 | 4.6 ± 0.3 | 4.6 ± 0.4 | 0.535 |
| Creatinine (mg/dL) | 1.24 ± 0.39 | 1.27 ± 0.47 | 1.23 ± 0.35 | 0.568 |
| Haemoglobin (g/dL) | 13.9 ± 1.7 | 13.3 ± 1.6 | 14.1 ± 1.7 | 0.022 |
| hs-CRP (mg/L) | 8.96 ± 16.0 | 12.4 ± 13.6 | 7.6 ± 16.7 | 0.147 |
| Triglyceride (mg/dL) | 118 ± 68 | 96 ± 38 | 127 ± 75 | 0.031 |
| Cholesterol (mg/dL) | 170 ± 45 | 159 ± 37 | 174 ± 48 | 0.111 |
| HDL (mg/dL) | 44 ± 15 | 47 ± 16 | 44 ± 14 | 0.306 |
| LDL (mg/dL) | 103 ± 35 | 95 ± 25 | 106 ± 38 | 0.155 |
| Fasting glucose (mg/dL) | 108 ± 21 | 106 ± 24 | 109 ± 20 | 0.510 |
| Medication | ||||
| β-blocker | 33 (100) | 78 (94) | 0.319 | |
| Statins | 18 (55) | 49 (59) | 0.682 | |
| ACEI/ARB | 31 (94) | 76 (92) | 1.000 | |
| Diuretics | 26 (79) | 62 (75) | 0.811 | |
| Anticoagulants | 12 (36) | 32 (39) | 1.000 | |
| Hydralazine | 6 (18) | 18 (22) | 0.802 | |
| Isosorbide | 6 (18) | 18 (22) | 0.802 | |
| Spironolactone | 24 (73) | 58 (70) | 0.824 |
Data are presented as mean ± SD or %. P value referred to Student's t-test and Chi-square test for medication. ACEI, angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; BMI: body mass index; BNP: B-type natriuretic peptide; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association.
Muscle sympathetic nerve activity, heart rate recovery and functional capacity
Patients with sarcopenia had higher MSNA (Figure 1) and lower ∆HRR1 and ∆HRR2 (Figure 2) when compared with non-sarcopenic patients. There was no statistical difference in resting HR and peak HR between sarcopenic and non-sarcopenic patients.
Figure 1.
Muscle sympathetic nerve activity (MSNA) in bursts/min. Values are presented as medians with lower and upper quartiles (CI 95%). Note that sarcopenic patients showed an increase of 18% in MSNA.
Figure 2.
Delta heart rate recovery at 1st (∆HRR1) and 2nd (∆HRR2) minutes immediately after maximal exercise testing. Values are presented as medians with lower and upper quartiles (CI 95%). Note that sarcopenic patients showed a lower HRR at 1st (47% difference) and 2nd minutes (40% difference).
Spearman’s correlation showed a positive correlation between appendicular lean muscle mass and ∆HRR1 and ∆HRR2 (Figures 3A and 3B, respectively). In addition, we observed a negative correlation between appendicular lean muscle mass and MSNA (Figure 3C).
Figure 3.
(A) Spearman’s correlation between appendicular lean muscle mass and delta heart rate recovery at 1st minute (∆HRR1). (B) Spearman’s correlation between appendicular lean muscle mass and delta heart rate recovery at 2nd minute (∆HRR2). (C) Spearman’s correlation between appendicular lean muscle mass and muscle sympathetic nerve activity (MSNA).
Absolute VO2peak, relative VO2peak, and peak workload were significantly lower in patients with sarcopenia than those without. Sarcopenic patients also showed higher ventilatory equivalent for carbon dioxide (VE/VCO2) slope and dead space to tidal volume (VD/VTpeak) than non-sarcopenic patients, whereas VEpeak was lower in patients with sarcopenia than those without (Table 2).
Table 2.
Cardiopulmonary, body composition and strength variables of the patients
| Variables | All patients (n = 116) | Patients with sarcopenia (n = 33) | Patients without sarcopenia (n = 83) | P value |
|---|---|---|---|---|
| Absolute VO2peak (L/min) | 1.43 ± 0.48 | 1.09 ± 0.31 | 1.58 ± 0.47 | < 0.001 |
| Relative VO2peak (ml/(kg/min)) | 20.1 ± 6.3 | 18.3 ± 5.3 | 21.0 ± 6.5 | 0.036 |
| Peak workload (Watts) | 103 ± 47 | 77 ± 27 | 115 ± 51 | < 0.001 |
| Resting HR (beats/min) | 72 ± 13 | 75 ± 16 | 71 ± 12 | 0.254 |
| HRpeak (beats/min) | 134 ± 27 | 130 ± 27 | 135 ± 28 | 0.323 |
| VE/VCO2 slope | 35 ± 7 | 37 ± 8 | 33 ± 7 | 0.015 |
| VD/VTpeak | 0.17 ± 0.02 | 0.19 ± 0.04 | 0.16 ± 0.04 | < 0.001 |
| VEpeak (L/min) | 62.5 ± 18.3 | 53.5 ± 14.1 | 66.4 ± 18.4 | < 0.001 |
| Body composition and strength | ||||
| Total LM (kg) | 49.6 ± 8.4 | 43.0 ± 5.2 | 52.5 ± 8.0 | < 0.001 |
| ALM (kg) | 22.2 ± 4.3 | 18.3 ± 2.3 | 23.9 ± 3.8 | < 0.001 |
| SMI (kg/m2) | 7.97 ± 1.21 | 6.63 ± 0.58 | 8.54 ± 0.92 | < 0.001 |
| Fat mass (kg) | 18.2 ± 8.5 | 12.9 ± 4.9 | 20.5 ± 8.8 | < 0.001 |
| Fat (%) | 26 ± 8 | 22 ± 7 | 27 ± 8 | < 0.001 |
| Handgrip strength (kg) | 33 ± 8 | 26 ± 3 | 36 ± 8 | < 0.001 |
Data are presented as mean ± standard deviation or %. P value referred to Student's t-test. ALM: appendicular lean muscle mass; HR: heart rate; LM: lean mass; SMI: skeletal muscle mass index; VE: ventilation; VE/VCO2: ventilatory equivalent for carbon dioxide; VD/VT: dead space to tidal volume; VO2: oxygen consumption.
Body composition and muscle strength characteristics
Body mass index was lower in sarcopenic patients when compared with non-sarcopenic, with a significant reduction in appendicular lean muscle mass, total lean mass, fat mass, and fat percentage (Table 2). SMI and muscle strength assessed by handgrip dynamometer were also lower in patients with sarcopenia compared with those without sarcopenia.
Discussion
The main and new findings of this study are that sarcopenic patients with HF have increased resting MSNA and blunted vagal reactivation after maximal exercise testing when compared with patients without sarcopenia. Moreover, the appendicular lean muscle mass seems to be associated with higher MSNA and blunted HRR. Additionally, as previously demonstrated,2 we also confirmed the reduction in exercise tolerance (decreased peak VO2 and peak workload) in patients with HF and muscle wasting.
HF is a complex disease associated with several comorbidities. One of the major co-morbidities observed in patients with advanced chronic HF is sarcopenia, which is associated with poor prognosis.21 Although the aetiology of sarcopenia is multifactorial, several mechanisms have been suggested to explain this decrease in muscle mass in patients with HF, such as increased inflammatory profile,22 increased oxidative stress,23 overactivation of ubiquitin-proteasome system,24 and increased C-terminal agrin fragment (CAF).25 These alterations, acting independently or in combination, may lead to excessive muscle protein degradation and reduced muscle protein synthesis.
Besides the mechanisms mentioned above, exacerbated sympathetic nerve activity seems to be an important pathophysiological feature in HF leading to the loss of skeletal muscle.6 In an experimental model of HF, Bacurau and colleagues 6 showed that sympathetic hyperactivity contributes to the development of skeletal myopathy by changing muscle morphology.6 β2-adrenoceptors play a key role in regulating skeletal muscle mass in both anabolic and catabolic state.26 However, chronic sympathetic hyperactivity may be toxic to skeletal muscle,27 which favors weight loss and sarcopenia in patients with HF. Moreover, increased sympathetic outflow is associated with higher chance of arrhythmias,28 and adverse remodeling of the heart.29
Interestingly, pharmacological treatment of HF is focused on blocking sympathetic activity, mainly by using cardio-selective and non-selective β-blockers. 30 Treatment with β-blockers can increase total body fat mass and total body fat content in patients with HF, without apparent improvement in muscle mass.30,31 In this study, we did not observe differences between groups in β-blocker treatment and dosage. In this context, future randomized clinical trials are required to assess the real impact of β-blocker therapy on skeletal muscle mass in patients with HF.
Previous studies showed that HRR has an important prognostic value in the general population 12 and in patients with HF.32 In addition, HRR is a very simple and easy way to indirectly evaluate the reactivation of the parasympathetic nervous system immediately after maximum effort in cardiopulmonary exercise testing.11 Several investigators showed that the kinetic of HRR in a 6-min recovery period was reduced in patients with HF 33 and this reduction seems to be independent of b-adrenergic blocker therapy.34 Ushijima et al.35 showed an association between norepinephrine and HRR in patients with myocardial infarction, arguing that increased sympathetic excitation at maximum exercise may suppress the parasympathetic reactivation leading to HRR attenuation.35
Taken together, the sympathovagal impairment in patients with HF is associated with poor outcome, and this autonomic imbalance may worsen the loss of muscle mass in these patients. In fact, we showed greater MSNA and lower decrease in HRR at 1st and 2nd minutes post-exercise in sarcopenic patients with HF. Furthermore, reduced appendicular lean muscle mass was correlated with lower HRR1 (r = 0.26), HRR2 (r = 0.25) and greater MSNA (r = -0.29).
We recognize limitations in our study. The present study included only male patients, so we are not able to generalize these results to female patients with HF. Further studies are necessary to investigate the influence of sarcopenia on gender-related differences. The date when HF was diagnosed was not available in patients’ medical records, and to compensate for this missing information, we included only patients with at least one year of diagnosis. We assessed parasympathetic activity using the HRR as a marker of vagal reactivation. Although our study has a clinical applicability, more studies using HR variability should clarify the role of cardiac autonomic control on sarcopenia in patients with HF.
Conclusion
Sympatho-vagal imbalance seems to be associated with sarcopenia in male patients with HF. These results highlight the importance of a therapeutic approach in patients with muscle wasting and increased peripheral sympathetic outflow.
Funding Statement
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2015/22814-5). Fonseca GWP was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 148758/2016-9); Dos Santos MR by FAPESP (2016/24306-0); Negrão CE by FAPESP (2015/22814-5). All foundations are from São Paulo-SP, Brazil.
Footnotes
Sources of Funding
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2015/22814-5). Fonseca GWP was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 148758/2016-9); Dos Santos MR by FAPESP (2016/24306-0); Negrão CE by FAPESP (2015/22814-5). All foundations are from São Paulo-SP, Brazil.
Study Association
This article is part of the thesis of Doctoral submitted by Guilherme Wesley Peixoto da Fonseca, from Universidade Federal de São Paulo.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the CAPPesq under the protocol number 0892/07. All the procedures in this study were in accordance with the 1975 Helsinki Declaration, updated in 2013. Informed consent was obtained from all participants included in the study.
Author contributions
Conception and design of the research: Fonseca GWP, dos Santos MR, Alves MJNN; Acquisition of data: Fonseca GWP, dos Santos MR, Souza FR, Costa MJA, Takayama L, Pereira RMR, Alves MJNN; Analysis and interpretation of the data: Fonseca GWP, dos Santos MR, Costa MJA,Souza FR, Pereira RMR, Negrão CE, Alves MJNN; Statistical analysis: Fonseca GWP, dos Santos MR; Obtaining financing: Negrão CE, Alves MJNN; Writing of the manuscript: Fonseca GWP, Souza FR, Alves MJNN; Critical revision of the manuscript for intellectual content: dos Santos MR, von Haehling S, Pereira RMR, Negrão CE, Anker SD, Alves MJNN.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14(6):323–341. doi: 10.1038/nrcardio.2017.51. [DOI] [PubMed] [Google Scholar]
- 2.Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34(7):512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 3.Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J, et al. Sarcopenia and Endothelial Function in Patients With Chronic Heart Failure: Results From the Studies Investigating Comorbidities Aggravating Heart Failure (SICA-HF) J Am Med Dir Assoc. 2017;18(3):240–245. doi: 10.1016/j.jamda.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, et al. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle. 2017;8(4):542–548. doi: 10.1002/jcsm.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135(3):302–307. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 6.Bacurau AV, Jardim MA, Ferreira JC, Bechara LR, Bueno CR Jr, Alba-Loureiro TC, et al. Sympathetic hyperactivity differentially affects skeletal muscle mass in developing heart failure: role of exercise training. J Appl Physiol (1985) 2009;106(5):1631–1640. doi: 10.1152/japplphysiol.91067.2008. [DOI] [PubMed] [Google Scholar]
- 7.Kim YS, Sainz RD, Summers RJ, Molenaar P. Cimaterol reduces beta-adrenergic receptor density in rat skeletal muscles. J Anim Sci. 1992;70(1):115–122. doi: 10.2527/1992.701115x. [DOI] [PubMed] [Google Scholar]
- 8.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989;64(18):1162–1167. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- 9.De Jong MJ, Randall DC. Heart rate variability analysis in the assessment of autonomic function in heart failure. J Cardiovasc Nurs. 2005;20(3):186–195. doi: 10.1097/00005082-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991;18(2):464–472. doi: 10.1016/0735-1097(91)90602-6. [DOI] [PubMed] [Google Scholar]
- 11.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38(7):1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 12.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 13.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151(4):851 e7–851 13. doi: 10.1016/j.ahj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104(16):1911–1916. [PubMed] [Google Scholar]
- 15.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 16.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84(1):82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 17.Dos Santos MR, Sayegh AL, Bacurau AV, Arap MA, Brum PC, Pereira RM, et al. Effect of Exercise Training and Testosterone Replacement on Skeletal Muscle Wasting in Patients With Heart Failure With Testosterone Deficiency. Mayo Clin Proc. 2016;91(5):575–586. doi: 10.1016/j.mayocp.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 19.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26(2):118–122. doi: 10.1016/j.ejim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111(8):996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 23.Bechara LR, Moreira JB, Jannig PR, Voltarelli VA, Dourado PM, Vasconcelos AR, et al. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int J Cardiol. 2014;175(3):499–507. doi: 10.1016/j.ijcard.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285(4):C806–C812. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- 25.Steinbeck L, Ebner N, Valentova M, Bekfani T, Elsner S, Dahinden P, et al. Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) Eur J Heart Fail. 2015;17(12):1283–1293. doi: 10.1002/ejhf.400. [DOI] [PubMed] [Google Scholar]
- 26.Kim YS, Sainz RD. Beta-adrenergic agonists and hypertrophy of skeletal muscles. Life Sci. 1992;50(6):397–407. doi: 10.1016/0024-3205(92)90374-x. [DOI] [PubMed] [Google Scholar]
- 27.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113(6):739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volders PG. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm. 2010;7(12):1900–1906. doi: 10.1016/j.hrthm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Machackova J, Sanganalmath SK, Barta J, Dhalla KS, Dhalla NS. Amelioration of cardiac remodeling in congestive heart failure by beta-adrenoceptor blockade is associated with depression in sympathetic activity. Cardiovasc Toxicol. 2010;10(1):9–16. doi: 10.1007/s12012-009-9058-y. [DOI] [PubMed] [Google Scholar]
- 30.Lainscak M, Keber I, Anker SD. Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol. 2006;106(3):319–322. doi: 10.1016/j.ijcard.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 31.Cvan Trobec K, Grabnar I, Kerec Kos M, Vovk T, Trontelj J, Anker SD, et al. Bisoprolol pharmacokinetics and body composition in patients with chronic heart failure: a longitudinal study. Eur J Clin Pharmacol. 2016;72(7):813–822. doi: 10.1007/s00228-016-2041-1. [DOI] [PubMed] [Google Scholar]
- 32.Nanas S, Anastasiou-Nana M, Dimopoulos S, Sakellariou D, Alexopoulos G, Kapsimalakou S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol. 2006;110(3):393–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Myers J, Hadley D, Oswald U, Bruner K, Kottman W, Hsu L, et al. Effects of exercise training on heart rate recovery in patients with chronic heart failure. Am Heart J. 2007;153(6):1056–1063. doi: 10.1016/j.ahj.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Racine N, Blanchet M, Ducharme A, Marquis J, Boucher JM, Juneau M, et al. Decreased heart rate recovery after exercise in patients with congestive heart failure: effect of beta-blocker therapy. J Card Fail. 2003;9(4):296–302. doi: 10.1054/jcaf.2003.47. [DOI] [PubMed] [Google Scholar]
- 35.Ushijima A, Fukuma N, Kato Y, Aisu N, Mizuno K. Sympathetic excitation during exercise as a cause of attenuated heart rate recovery in patients with myocardial infarction. J Nippon Med Sch. 2009;76(2):76–83. doi: 10.1272/jnms.76.76. [DOI] [PubMed] [Google Scholar]