Abstract
Background
Fibromyalgia (FM) is a centralized pain state that until recently has been shrouded in mystery and questionable as a disease entity in the eyes of many physicians, who considered it purely psychogenic. Fibromyalgia is now thought of as a discrete diagnosis with a clustering of symptoms characterized by central nervous system pain amplification along with anergia, memory loss, disturbances of mood, and sleep disruption. The condition is present in approximately 2% to 8% of the population.
Material/Methods
We review the link between inflammatory mechanisms and FM from a neuropsychiatric perspective.
Results
Recent studies are pointing to a neuroinflammatory etiology that may open up more effective treatment strategies in the future.
Conclusions
Better conceptualization of FM may also elucidate a neuropsychiatric understanding of how nociception, dysthymia, and suicidality co-develop and feed off one another.
MeSH Keywords: Central Nervous System, Fibromyalgia, Neuroimmunomodulation, Neuropsychiatry
Background
Fibromyalgia (FM) is a disorder characterized by aberrant central afferent processing [1]. Patients with FM complain of multifocal pain, fatigue, insomnia, and cognitive dysfunction. Pain is the predominant finding; allodynia and hyperalgesia are common. Severe fatigue, impaired cognition, and nonrestorative sleep are also found, along with a host of other somatic complaints [2,3]. These patients also present with mood changes, decreases in libido, and changes in social and occupational functioning [3,4]. Its pathophysiology is not fully understood. Past research that included neuroimaging studies have suggested alterations in neurotransmitters and altered peripheral and central pain processing [1]. A dampening of the so-called diffuse noxious inhibitory control (DNIC) mechanism may predispose to pain sensitization [5].
In 1990, the American College of Rheumatology (ACR) established research diagnostic criteria for FM [6]. Their criteria included a history of chronic and widespread pain, along with 11 or more out of 18 areas of point tenderness. Qualifying for chronic widespread pain required pain in the left side and the right side of the body; pain above the waist; and pain below the waist. In addition, axial skeletal pain had to be present. Pain duration needed to be for 3 months or more. For tender points to be considered positive, the patient had to perceive the palpation with pressures of 4 kg/cm2 or less to be painful [7].
It became increasingly clear with time that focusing on tender points was not helpful. In 2010, a multicenter study of 829 previously diagnosed FM patients and controls focused on a widespread pain index (WPI) as a measure of the number of painful body regions [8]. Based on physician physical and interview examinations and statistical analyses, a case definition of FM was developed. This led to new preliminary ACR diagnostic criteria, and a symptom severity (SS) scale. It turned out that the most important diagnostic variables were WPI and categorical scales for cognitive symptoms, nonrestorative sleep, fatigue, and number of somatic symptoms. The SS scale consisted of the summed categorical scales. By combining the SS scale and the WPI, a new case definition of fibromyalgia consisting of a WPI ≥7 plus a SS ≥5 was suggested [7]. In 2016, the ACR revised the criteria further (Table 1) [9].
Table 1.
Fibromyalgia criteria – 2016 revision.
| Fibromyalgia Criteria – 2016 revision | |
| A patient satisfies modified 2016 fibromyalgia criteria if the following 3 conditions are met: | |
| 1) Widespread pain index (WPI) ≥7 and symptom severity scale (SSS) score ≥5 OR WPI of 4–6 and SSS score ≥9 | |
| 2) Generalized pain, defined as pain in at least 4 of 5 regions, must be present. Jaw, chest, and abdominal pain are not included in generalized pain definition | |
| 3) Symptoms have been generally present for at least 3 months | |
| A diagnosis of fibromyalgia is valid, irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses. | |
| Widespread pain index (WPI) | |
| Note the number of areas in which the patient has had pain over the last week. In how many areas has the patient had pain? (Score range 0–19) | |
| Region 1 (Left upper region) | Left jaw*, left shoulder girdle, left upper arm, left lower arm |
| Region 2 (Right upper region) | Right jaw*, right shoulder girdle, right upper arm, right lower arm |
| Region 3 (Left lower region) | Left hip (buttock, trochanter), left upper leg, left lower leg |
| Region 4 (Right lower region) | Right hip (buttock, trochanter), right upper leg, right lower leg |
| Region 5 (Axial region) | Neck, upper back, lower back, chest*, abdomen* |
| Symptom severity scale (SSS) | |
| – Fatigue | |
| – Waking unrefreshed | |
| – Cognitive symptoms | |
| For the each of the 3 symptoms above, indicate the level: | |
| 0) No problem | |
| 1) Slight or mild problems, generally mild or intermittent | |
| 2) Moderate, considerable problems, often present, and/or at a moderate level | |
| 3) Severe: pervasive, continuous, life-disturbing problems | |
| The symptom severity scale (SSS) score is the sum of the severity scores of the 3 symptoms (fatigue, waking unrefreshed, and cognitive symptoms) (0–9) plus the sum (0–3) of the number of the following symptoms the patient has been bothered by that occurred during the previous 6 months: | |
| 1) Headaches (0–1) | |
| 2) Pain or cramps in lower abdomen (0–1) | |
| 3) Depression (0–1) | |
| The final symptom severity score is between 0 and 12. | |
| Fibromyalgia severity scale (FS) | |
| The fibromyalgia severity (FS) scale is the sum of the WPI and SSS. | |
The FS scale is also known as the polysymptomatic distress (PSD) scale.
Not included in generalized pain definition.
Table adapted from Wolfe, 2016 [9].
Fibromyalgia is a complex disorder and it requires a multidisciplinary approach to treatment. The treatment approach to FM nowadays emphasizes self-care education of the patient combined with cognitive behavioral therapy (CBT), exercise, and pharmacotherapy. In terms of medications, antidepressants (tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors [SNRIs] (duloxetine and milnacipran)), and α2-δ ligands (gabapentin and pregabalin) have shown some efficacy in reducing pain complaints. Dopamine agonists (pramipexole), tramadol, and other opioids, and cannabinoids (nabilone) have been tried, with anecdotal success [2]. Further evidence-based trials using complementary treatments are needed [10], and treatment advances will be reliant on a deeper understanding of the pathophysiology of FM. Neuroinflammation offers a research approach that may provide an organizing focus for future studies. Despite increasing research interest in FM, recent reviews have been broad in scope, focusing on neuroinflammatory findings in several chronic pain disorders. Accordingly, this review examines the clinical and preclinical data linking neuroinflammation to FM, the proposed neurobiologic mechanisms underlying this association from a neuropsychiatry perspective, and directions for future research.
Microglial Activation in FM
The neuroimmune system is involved in structural brain development, neurobehavioral function, aging, and neurodegeneration. There is research suggesting that neuroinflammation is prominent in the FM central sensitization syndrome [11,12]. Elevated cerebrospinal fluid (CSF) cytokine levels have been reported in FM, and chemokines have also been implicated [11–15]. However, no study has provided direct evidence of CNS glial activation in FM.
Albrecht et al. (2019) conducted a positron emission tomography (PET) study using the ligand [11C] PBR28, which binds to a protein that is upregulated in activated microglia and astrocytes, called the translocator protein (TSPO). The researchers combined datasets that were collected independently at 2 sites (Massachusetts General Hospital [MGH] and Karolinska Institute [KI]). Thirty-one FM patients and 27 healthy controls (HC) were studied using [11C] PBR28 PET. In an important sub-study, the researchers at the KI looked a smaller sample of 11 FM and 11 HC subjects in an attempt to clarify the relative contributions of microglia and astrocytes to FM. These subjects were PET scanned at KI with another ligand, called [11C]-L-deprenyl-D2, which is assumed to primarily signal astrocytic activity [16].
PET markers were compared across groups, and differences were compared against clinical variables. Translocator protein density by distribution volume (TSPO VT) is increased in activated microglia; an indication of neuroinflammation. The FM subjects, compared to HC subjects, showed widespread cortical elevations and no decreases in [11C] PBR28 distribution volume (VT), and standardized uptake value (SUV) images calculated by normalizing images by injected dose/body weight. A voxel-based morphometry analysis showed brain areas with significantly higher SUVR in FM patients. In these same regions there was generally higher VT. The regions of interest cited were the dorsolateral and dorsomedial prefrontal cortex (dlPFC and dmPFC), primary sensory and motor cortices (S1/M1), precuneus, posterior cingulate cortex (PCC), supplementary motor area (SMA), and superior parietal lobule (SPL), as well as the anterior midcingulate (aMCC), posterior MCC (pMCC), and frontoinsular anterior insular (AI) cortex. There were no regions where SUVR was significantly higher in HCs [16].
In essence, microglial activation was most pronounced in the mediolateral walls of the frontal and parietal lobes, with elevated [11C] PBR28 VT and SUVR correlated spatially and in magnitude. In contrast, there were no areas with significant group differences in [11C]-L-deprenyl-D2 signal, suggesting that astrocytic stimulation was not prominent in FM subjects. In a provocative if exploratory set of findings, FM subject ratings of their fatigue were associated with higher [11C] PBR28 SUVR in the aMCC and pMCC (both p<0.03) [16].
Microglial activation appears to stimulate TSPO elevation in the regions mentioned above, and thus appears to be key in FM pathophysiology. This raises the possibility that modulating microglial activation will open a possible therapeutic niche in FM. Larger studies will be needed to clarify whether astrocytes contribute to FM pathophysiology and whether they too would need modulation.
Fatigue is a classic symptom in FM. It is therefore notable that in chronic fatigue syndrome (CFS), the binding of 11C-(R)-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline-carbox-amide (11C-(R)-PK11195), as a ligand of PET for a translocator protein, is significantly elevated in the cingulate cortex, as well as in the hippocampus, amygdala, thalamus, midbrain, and pons of CFS subjects compared to healthy controls [17].
Microglia, first described a century ago, are key neuroimmune cells and have 3 essential functions: a sentinel function involved in constant sensing of environmental changes, a housekeeping function that promotes neuronal well-being and normal operation, and a defense function necessary for responding to threats and providing neuroprotection. Microglia use a menu of gene expression options to perform these tasks. In response to specific stimuli, microglia also have the capacity to damage and kill neurons. Injury to neurons in Alzheimer’s, Parkinson’s, Huntington’s, and prion diseases, as well as in amyotrophic lateral sclerosis, frontotemporal dementia, and chronic traumatic encephalopathy, results from disruption of the sentinel or housekeeping functions and dysregulation of the defense function, leading to neuroinflammation [18]. Pathways associated with such injury include several sensing and housekeeping pathways such as the Trem2, Cx3cr1, and progranulin pathways, which act as immune checkpoints to keep the microglial inflammatory response under control, and the scavenger receptor pathways, which promote clearance of injurious materials. Peripheral interference from systemic inflammation or the gut microbiome can also alter progression of such injury. Initiation or exacerbation of neurodegeneration results from an imbalance between these microglial functions; correcting such an imbalance may be a potential mode for therapy [18].
Neurodegenerative diseases cause progressive loss of cognitive and/or motor function and pose major challenges for societies with rapidly aging populations [19]. Microglial activation, for example, has been implicated in Alzheimer’s disease (AD) pathogenesis. In a recent study, the TSPO tracer [(11)C] PBR28 was used as a marker for microglial activation in the 5XFAD mouse model of AD [20]. Following intravenous administration of [(11)C] PBR28 in 6-month-old 5XFAD mice and in wild-type controls, PET scans with the ligand were obtained. In addition, autoradiography with [(3)H] PBR28 was carried out in the same subjects to more clearly establish the distribution of the ligand, and immunohistochemistry was performed to evaluate TSPO co-localization with microglia. Results showed brain uptake of [(11)C] PBR28 in 5XFAD mice was higher than that of control mice, and [(3)H] PBR28 binding was enhanced in the same areas of the cortex and hippocampus where there was staining of microglial Iba-1 and amyloid deposits. The researchers stressed that co-localization of TSPO was with microglia and not with astrocytes [20].
Human genetics studies have shown that disease-causing rare mutations and risk-associated common alleles overlap in different neurodegenerative disorders. Shared pathological mechanisms include defective protein quality-control and degradation pathways, dysfunctional mitochondrial homeostasis, and lack of mitonuclear collaboration, stress granules, and maladaptive innate immune responses [19]. To the list of neurodegenerative neuropsychiatric diseases associated with microglial-activated neuroinflammation we can now add FM.
Comorbid Depression in FM
Depression is a common comorbid condition in FM and a major contributor to poor quality of life and disability [21]. However, depression can be difficult to assess in patients with FM due to overlapping somatic symptoms. Almost all diagnostic survey instruments struggle with criteria contamination bias due to somatic symptoms of FM patients with chronic pain and fatigue. There is some evidence that symptom overlap reflects a common etiology in the brain. Regardless, many studies establish that even sub-threshold mood dysfunction can worsen pain and socioeconomic functioning in FM patients. There is a need for careful depression screening and proper management in FM. Several studies have used the Structured Clinical Interview (SCID), as it is currently the gold standard instrument to detect psychiatric disorders comorbidities in FM [22,23]. Veltri et al. (2012) recommend using the Mood Spectrum Self-Report [MOODS-SR] for subsyndromal phenomenology in FM. It has been validated and used also in patients with other medical diseases. The MOODS-SR can be useful in screening FM patients because it permits recognition of sub-threshold mood symptoms, with minimal contamination by somatic conditions [21].
Major depressive disorder (MDD) is associated with elevated peripheral inflammatory markers. The neuroinflammatory hypothesis of MDD is supported by several main findings. First, in humans and animals, activation of the immune system causes sickness syndrome behaviors that present during a major depressive episode (MDE), such as low mood, anhedonia, anorexia, and weight loss. Second, peripheral inflammatory markers are often found in MDD. Third, neuroinflammatory disorders are associated with high rates of major depressive episodes (MDEs) [21].
However, to date scanty evidence for CNS inflammation during MDE has existed limiting the neuroinflammatory hypothesis. A recent PET study looked at TSPO VT in the prefrontal cortex (PFC), ACC, and insula in patients with an MDE secondary to MDD [24]. In MDE subjects (n=20), TSPO VT was significantly elevated by 26% in the PFC, 32% in the ACC, and 33% in the insula in patients with MDE vs. controls (n=20). Furthermore, In MDE subjects, higher TSPO VT in the ACC correlated significantly with higher depression severity [24].
This finding was corroborated in another TSPO study that used [11C] (R)-PK11195 PET to compare TSPO availability in the ACC, PFC, and insula in 14 medication-free patients with MDE of at least moderate severity and 13 matched healthy control subjects [25]. The researchers confirmed evidence for increased TSPO activity in the ACC during a moderate to severe MDE, suggesting that predominantly microglial activation in the ACC is involved in MDEs [25].
In this same study, TSPO was found to be significantly elevated in MDE subjects with suicidal thinking compared with those without it. This signaling of localized microglial activation was most significant in the ACC and insula. This finding is reminiscent of the voxel-based morphometry meta-analysis of 6 major psychiatric disorders, including MDD, which showed that structural changes were commonly found in dACC and anterior insula (AI) [26]. The triangulation of FM, MDD, and suicidality based in neuroinflammation in the ACC-MCC region needs further clarification but may hold clues to pathophysiology and therapeutics in the future.
Suicidality and FM
Studies suggest that FM is associated with a high prevalence of suicidality. In one such recent study of 383 FM patients, researchers found that 48% of the patients reported suicidal ideation; 39.7% reported passive suicidal ideation, and 8.3% reported active suicidal ideation [27]. Suicidal ideation was correlated with depression, anxiety, sleep quality, and global mental health. Interestingly, weak associations were observed between suicidal ideation and both pain and general physical health. Moreover, in a survey study of 180 FM patients, 16.7% reported 1 to 3 suicide attempts [28]. FM is also associated with an increased rate of mortality from suicide. In fact, FM is associated with several vulnerabilities connected to an increased risk of suicidal behaviors: being female and suffering from chronic pain, psychological distress, and sleep disturbances [29]. However, the literature to date relating suicidal risks and FM has been meager.
Two recent studies contribute to the literature. The first sought to estimate the prevalence of suicidal ideation and the risk of suicide in a sample of FM patients (n=44) compared with healthy subjects (n=50) and with of patients with chronic low back pain (n=32) [30]. The authors explored the relevance of pain intensity, depression, and sleep quality as risk factors suicidal ideation. Suicidal ideation was prominent among patients with FM (P<0.0001), low among those with low back pain, and absent in healthy controls. The risk of suicide, measured with the Plutchik Suicide Risk Scale, was also higher among patients with FM than in patients with low back pain or in controls (P<0.0001). The likelihood for suicidal ideation and the risk of suicide were higher among patients with FM (odds ratios of 26.9 and 48.0, respectively) than in patients with low back pain (odds ratios 4.6 and 4.7, respectively). Comorbid depression was the only factor associated with suicidal ideation or the risk of suicide [30].
The second study assessed 117 women with FM. Patients with presence vs. absence of suicidal ideation were compared with respect to sleep problems (Pittsburgh Sleep Quality Index), depression (Beck Depression Inventory [BDI]), health-related quality of life (SF-36 and Fibromyalgia Impact Questionnaire), the core symptoms of FM (visual analogue scales), and algometry of tender points [31]. The prevalence of suicidal ideation among FM patients was 32.5%. Significant differences between patients with vs. those without suicidal ideas emerged mainly for the various indices of depression. Patients with suicidal ideation also reported higher levels of anxiety, more dysfunction due to sleepiness, and more limitations due to emotional and physical problems. Logistic regression analysis revealed that cognitive depression symptoms reflected in the BDI Self-Blame cluster are more closely related to suicidal ideation [31].
These studies raise the question of a double-hit phenomenon. Perhaps the combined neuroinflammatory burden in the paralimbic cortex of patients with both FM and MDD renders them at high risk for suicidal ideation. The presence of suicidal ideation in FM patients is closely related to comorbid depression and to a higher impact of the disease in daily life.
Attachment Styles, Pain, and FM
MacLean (1990) described a central neurobehavioral system called the mammalian behavioral triad which provided mammals with an evolutionary survival advantage [32]. This triad consisted of a consistently found infant separation or isolation cry, maternal-parental nurturance, and social play. Using ablation as well as morphine infusions in a series of animal studies involving primates and other mammals, he and his colleagues were able to locate the ACC as the key node in this attachment network. MacLean, in this research, embellished the Attachment Theory of John Bowlby with comparative neuroanatomical data [33,34]. Attachment theory seeks to categorically assess how an individual perceives and experiences interpersonal relationships.
The Relationship Questionnaire developed by Bartholomew and Horowitz (1991) is often used to identify a subject’s predominant adult attachment style [35]. Bartholomew and Horowitz categorized 4 sub-types of adult attachment style: “secure” attachment style, which was characterized by a positive model of self and other in a relationship, and 3 insecure styles: “fearful” marked by a negative model of self and other, “preoccupied” with a negative model of self but a positive model of other, and “dismissing” with a positive model of self and negative model of other [35].
Other researchers use the Attachment Style Questionnaire (ASQ), which assesses anxious and avoidant dimensions of attachment [36]. Anxious attachment is characterized by an intense need for acceptance, leading to vigilance to cues that signal possible rejection. Avoidant attachment is marked by distress caused by intimacy, leading to avoidant strategies to modulate interpersonal relationships. These different styles will impact responses to social rejection. An fMRI study examined how the extent to which people exhibit the anxious and avoidant attachment dimensions correlated with brain activation using a social exclusion simulation [37]. Those with anxious attachment were found to show heightened activity in the dorsal ACC, also known as the MCC, and AI, areas known to be associated with rejection-related distress, and as mentioned above, with microglial activation in FM. Those with avoidant attachment showed less activity in the dorsal ACC and AI.
Social bonding is a hard-wired basic human need, and since secure attachment enjoys such a strong evolutionary selection bias, lack of attachment is extraordinarily painful. Not experiencing a sense of belonging (social rejection) is a most painful emotional experience [38]. Physical pain regulation and social pain regulation are both mediated in the ACC/AI paralimbic region, predominantly on the right. A set of central nervous system structures (ACC-MCC, thalamus, insula, and periaqueductal gray [PAG]) consistently respond to nociceptive stimuli causing pain. Activation of this so-called pain matrix or pain signature has been related to perceived pain intensity, both within and between individuals [39,40] (Figure 1). There is a linear correlation between resting ACC-PAG connectivity and the intensity of pain [41].
Figure 1.
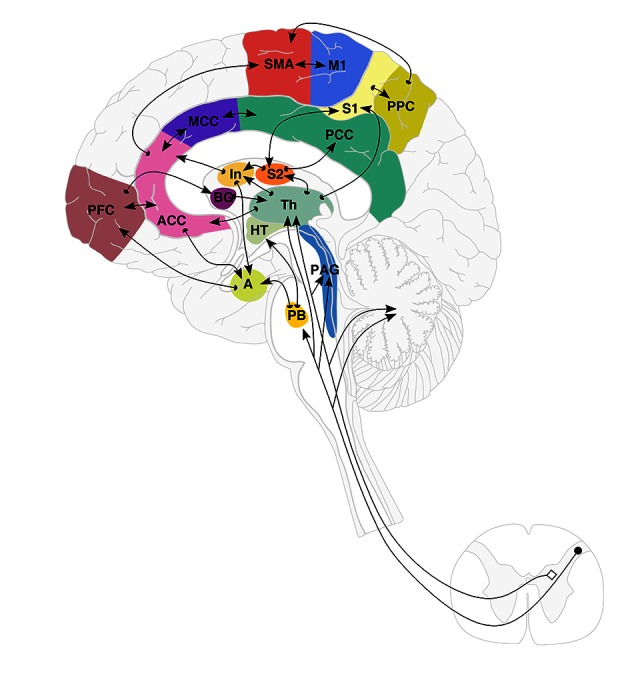
Schematic representation of Brain Connectome and the Pain Matrix (adapted from Apkarian 2005 and Price 2000 [39,40]). Cortical and sub-cortical pain networks and pathways involved in pain perception. Locations of brain regions involved in pain perception are shown in a schematic drawing showing the regions, their inter-connectivity, and afferent pathways. There are 15 areas in this matrix: anterior cingulate (ACC), medial cingulate (MCC), insula (In), thalamus (Th), prefrontal cortex (PFC), primary and secondary somatosensory cortices (S1, S2), primary and supplementary motor cortices (M1 and SMA), posterior parietal cortex (PPC), posterior cingulate (PCC), basal ganglia (BG), hypothalamus (HT), amygdala (A), parabrachial nuclei (PB), and periaqueductal gray (PAG).
Those with insecure adult attachment styles appear to experience greater pain than people with secure attachment. Davies et al. (2009) did a large, population-based, cross-sectional study to examine whether subjects with chronic widespread pain (CWP) were more likely to report insecure attachment [42]. Subjects (n=2509; 59.2% female) with CWP were indeed more likely to report an insecure attachment style – preoccupied (relative risk [RR] 2.6), dismissing (RR 1.9) or fearful attachment style (RR 1.4) – than those free of pain. In the CWP subjects, insecure attachment style was associated with number of pain sites and degree of pain-related disability, but pain intensity was not significantly associated [42]. This research track may help us understand the existential challenge FM patients face.
Alexithymia (α=lack, lexis=word, thymos=mood or emotion) is a construct first developed by Sifneos in 1972, denoting both a cognitive and affective disruption characterized by difficulty in identifying and describing subjective feelings, characterized by trouble differentiating between feelings derived from physical sensations of emotional arousal, limited imaginative processes, and an externally-oriented cognitive style [43–45]. Alexithymia has been associated with FM [46], and alexithymic FM patients seem to experience a more profound impact of disease, reporting severe pain and poor quality of life (QoL) compared to non-alexithymic FM patients [47,48]. Studies have identified this personality trait as an amplifier of physical sensations that could lead to misinterpretation of sensations as symptoms of a medical disease [49,50]. Moreover, the deficits found in alexithymia have been associated with increased negative affect states such as depression and anxiety [51,52], along with chronic sympathetic stress-related hyperarousal in FM [53]. In functional neurological disorders (FND), a fearful insecure attachment style has been associated with alexithymia, depression, and anxiety, along with self-reported adverse life event burden [54]. Indeed, in FND, childhood abuse, alexithymia, and depression independently predict fearful attachment. In a study of somatic symptom disorder, alexithymia appeared to mediate the association of anxious insecure attachment with somatic symptom severity [55].
Recently, Peñacoba et al. (2017) examined the interrelationship of attachment styles and pain intensity in the context of certain emotional variables (anxiety, depression, and alexithymia) in a cohort of FM patients (n=146) and in healthy control (HC) subjects (n=122) [56]. The researchers measured attachment style, pain intensity, anxiety, depression, and alexithymia. The FM subjects had lower percentages of secure attachment style (69.9% FM vs. 86% HC). They also had higher avoidant attachment (19.8% FM vs. 7.4% HC) and higher anxious-ambivalent attachment (10.3% FM vs. 6.6% HC) (p=.007). In addition, the FM subjects showed significantly higher scores in 2 of the insecure attachment factors (p=.020) and lower scores on the secure attachment factor (p=.008) in comparison with HC subjects.
Higher alexithymia scores were noted in female subjects showing anxious-ambivalent and avoidant attachment styles in comparison to subjects showing a secure attachment style [56]. The FM patients with higher anxiety (p=.005) had more prominent anxious attachment styles compared to those with secure attachment style [56]. However, it is interesting that no significant relationship emerged between attachment style and FM pain intensity, as was also the case in the study of CWP [42]. In this FM study, higher scores for anxiety and depression were associated with fearful attachment [57]. The authors argue for an Attachment-Diathesis Model of Chronic Pain that would highlight the potential value of insecure attachment styles as predictors of the experience of mood dysfunction and pain in FM patients, and they call for further study.
Given the importance of disconnectedness in the drift into suicidality, it is surprising that there has been relatively little research examining the relationship of attachment styles to suicidal ideation and attempts in adults. Considering the association of suicidality and of insecure attachment in FM patients, it would be helpful to examine this relationship in more depth. One study investigated the relationship of adult attachment style and mental disorders in the National Comorbidity Survey Replication (N=5692, aged >18 years). Multiple logistic regression analyses and adjustments for confounding variables were used to examine these relationships [58]. The researchers found that insecure attachment styles were indeed associated with elevated reporting of suicidal ideation and attempts, as well as with the mental disorder categories studied. Secure attachment styles, on the other hand, were associated with fewer reports of suicidal ideation and attempts, and fewer signs of any anxiety disorder [58]. This dovetails with a recent study of adult attachment styles using the Experiences in Close Relationships-Revised (ECR-RD12) scale. In this study of patients with suicidal ideation, 85% of those endorsing suicidal ideation had insecure attachment compared to 63% in those without suicidal ideation. The odds ratio for suicidal ideation patients with insecure attachment was 3.33 (CI=1.10–10.04) [59]. Additional variables associated with suicidal ideation were depressive symptomatology, living alone (especially in men), and obesity (especially in women) [59].
Microbiome, Fenestrated Endothelium, and FM
It is now more accepted that there are inflammatory CNS reactions that occur in response to aberrant neuronal activity [60]. As reviewed above, there is evidence that this is the case in FM. While it is increasingly recognized that FM has a neurogenic neuroinflammatory pathophysiology ignited by psychosocial stress-related mood changes and physical and emotional pain leading to a CNS sensitization syndrome, it has been argued that small intestine bacterial overgrowth (SIBO), a form of gastrointestinal microbial dysbiosis, also plays a role in FM [61–63]. In a small study, Pimentel et al. (2004) presented laboratory evidence of SIBO in 100% of FM subjects (n=42). The severities of the SIBO and the FM were positively correlated [64]. When SIBO is treated with antibiotics, FM improves in proportion to the efficacy of the antimicrobial [65]. Patients with FM are noted to have intestinal hyperpermeability and mitochondrial dysfunction and oxidative stress, which are noted in FM and in gastrointestinal microbial dysbiosis [66].
Research into the role of fenestrated endothelium in the circumventricular regions in linking up the FM etiopathogenetic factors of neurogenic neuroinflammation and gastrointestinal microbial dysbiosis is sorely needed. This is especially important with regard to the organum vasculosum of the lamina terminalis, given its proximity to the ACC and AI [67]. Stress can lead to neuroinflammation, as well as a peripheral immune response, through fenestrated endothelium in the circumventricular medial temporal lobe region or through vagal paraganglia, with the gut being increasingly recognized as the source. The organum vasculosum as an antigenic reservoir, given its proximity to the ACC, may play a special role in ACC inflammation, which, as we have seen, is a core feature of FM.
The association between early-life stress, including psychologically traumatic experiences such as childhood abuse or neglect, and alterations in pain processing later in life, has been widely investigated [68, 69]. Although the neurobiological mechanisms that mediate this association are still unknown, there is evidence that changes in several factors, including the hypothalamic-pituitary-adrenal (HPA) axis, monoamines, endogenous opioids, endocannabinoids, inflammatory mediators, and epigenetic mechanisms, are involved [2,70, 7]. In terms of inflammatory mediators, animal models support a dysregulated immune system theory following early-life stress, with initial suppression of inflammatory markers with shift to a pro-inflammatory state when insensitivity at the immune cell glucocorticoid receptor later in life emerges [70,72]. The initial suppression of inflammatory markers may prime the microglia, enhancing susceptibility to inflammatory disease, resulting in a protracted and excessive response to noxious stimulation in later life [73,74].
The fact that a microglial activation-based innate immune response preferentially attacks the brain across several neural networks in FM speaks to the widespread effects of the illness. Thus, we see regions of interest that show significant activation changes that include the dlPFC and dmPFC (task positive central executive network), the S1/M1 primary sensory and motor cortices, precuneus and PCC (default mode network), and the aMCC, pMCC, AI, SMA, and SPL (combined salience and fronto-cingulo-parietal networks). We hypothesize that the special transmodal ACC-AI-MCC-SMA paralimbic zone is particularly vulnerable as part of the FM innate inflammatory response, and as such, the diverse effects associated with FM will include widespread pain complaints, fatigue, depressed mood, fear of attachment loss, and suicidal ideation, all of which have been associated with significant neuroinflammatory findings and/or activation changes in this region on functional neuroimaging and PET scanning [75].
Conclusions
Recent research, some of which is reviewed in this paper, supports a network neuroinflammation model of FM, an often-misunderstood chronic stress-related condition that carries with it a high clinical burden of disease, with both elevated morbidity from pain and fatigue and mortality from the increased relative risk of depression and suicidality. Therapeutic strategies aimed at immunomodulation in the transmodal ACC-AI-MCC-SMA paralimbic zone may offer future benefits for FM and other neuropsychiatric conditions marked by a paralimbic innate neuroinflammatory response syndrome.
Footnotes
Source of support: Self financing
Conflicts of interest
The authors have no relevant funding or conflicts of interest to report. Gregory Fricchione serves as editor of Medical Science Monitor: Basic Research.
References
- 1.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–29. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancassiani F, Machado S, Ruggiero V, et al. The management of fibromyalgia from a psychosomatic perspective: An overview. Int Rev Psychiatry. 2017;29:473–88. doi: 10.1080/09540261.2017.1320982. [DOI] [PubMed] [Google Scholar]
- 3.Clauw DJ. Fibromyalgia: A clinical review. JAMA. 2014;311:1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Wilcke T, Diers M. New insights into the pathophysiology and treatment of fibromyalgia. Biomedicines. 2017;5(2) doi: 10.3390/biomedicines5020022. pii: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien AT, Deitos A, Trinanes Pego Y, et al. Defective endogenous pain modulation in fibromyalgia: A meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain. 2018;19:819–36. doi: 10.1016/j.jpain.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 7.Smith HS, Harris R, Clauw D. Fibromyalgia: An afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011;14:E217–45. [PubMed] [Google Scholar]
- 8.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46:319–29. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Sumpton JE, Moulin DE. Fibromyalgia. Handb Clin Neurol. 2014;119:513–27. doi: 10.1016/B978-0-7020-4086-3.00033-3. [DOI] [PubMed] [Google Scholar]
- 11.Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain – interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J Neuroimmunol. 2015;280:49–55. doi: 10.1016/j.jneuroim.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montague K, Malcangio M. The therapeutic potential of targeting chemokine signalling in the treatment of chronic pain. J Neurochem. 2017;141:520–31. doi: 10.1111/jnc.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backryd E, Tanum L, Lind AL, et al. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J Pain Res. 2017;10:515–25. doi: 10.2147/JPR.S128508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadetoff D, Lampa J, Westman M, et al. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol. 2012;242:33–38. doi: 10.1016/j.jneuroim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Puma C, Danik M, Quirion R, et al. The chemokine interleukin-8 acutely reduces Ca(2+) currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J Neurochem. 2001;78:960–71. doi: 10.1046/j.1471-4159.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht DS, Forsberg A, Sandstrom A, et al. Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation. Brain Behav Immun. 2019;75:72–83. doi: 10.1016/j.bbi.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatomi Y, Mizuno K, Ishii A, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An (1)(1)C-(R)-PK11195 PET study. J Nucl Med. 2014;55:945–50. doi: 10.2967/jnumed.113.131045. [DOI] [PubMed] [Google Scholar]
- 18.Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan L, Cookson MR, Petrucelli L, La Spada AR. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21:1300–9. doi: 10.1038/s41593-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei N, Tang SP, Ashworth S, et al. In vivo imaging of microglial activation by positron emission tomography with [(11)C]PBR28 in the 5XFAD model of Alzheimer’s disease. Glia. 2016;64:993–1006. doi: 10.1002/glia.22978. [DOI] [PubMed] [Google Scholar]
- 21.Veltri A, Scarpellini P, Piccinni A, et al. Methodological approach to depressive symptoms in fibromyalgia patients. Clin Exp Rheumatol. 2012;30:136–42. [PubMed] [Google Scholar]
- 22.Carta M, Ruggiero V, Sancassiani F, et al. The use of antidepressants in the long-term treatment should not improve the impact of fibromyalgia on quality of life. Clin Pract Epidemiol Ment Health. 2013;9:120–24. doi: 10.2174/1745017901309010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carta MG, Moro MF, Pinna FL, et al. The impact of fibromyalgia syndrome and the role of comorbidity with mood and post-traumatic stress disorder in worsening the quality of life. Int J Soc Psychiatry. 2018;64:647–55. doi: 10.1177/0020764018795211. [DOI] [PubMed] [Google Scholar]
- 24.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry. 2015;72:268–75. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes SE, Hinz R, Conen S, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: A positron emission tomography study. Biol Psychiatry. 2018;83:61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calandre EP, Navajas-Rojas MA, Ballesteros J, et al. Suicidal ideation in patients with fibromyalgia: A cross-sectional study. Pain Pract. 2015;15:168–74. doi: 10.1111/papr.12164. [DOI] [PubMed] [Google Scholar]
- 28.Calandre EP, Vilchez JS, Molina-Barea R, et al. Suicide attempts and risk of suicide in patients with fibromyalgia: A survey in Spanish patients. Rheumatology (Oxford) 2011;50:1889–93. doi: 10.1093/rheumatology/ker203. [DOI] [PubMed] [Google Scholar]
- 29.Lafuente-Castro CP, Ordonez-Carrasco JL, Garcia-Leiva JM, et al. Perceived burdensomeness, thwarted belongingness and suicidal ideation in patients with fibromyalgia and healthy subjects: A cross-sectional study. Rheumatol Int. 2018;38:1479–86. doi: 10.1007/s00296-018-4067-4. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Rodriguez I, Garcia-Leiva JM, Jimenez-Rodriguez BM, et al. Suicidal ideation and the risk of suicide in patients with fibromyalgia: A comparison with non-pain controls and patients suffering from low-back pain. Neuropsychiatr Dis Treat. 2014;10:625–30. doi: 10.2147/NDT.S57596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triñanes Y, Gonzalez-Villar A, Gomez-Perretta C, Carrillo-de-la-Pena MT. Suicidality in chronic pain: predictors of suicidal ideation in fibromyalgia. Pain Pract. 2015;15:323–32. doi: 10.1111/papr.12186. [DOI] [PubMed] [Google Scholar]
- 32.MacLean PD. The triune brain in evolution: Role in paleocerebral functions. Springer Science & Business Media; 1990. [DOI] [PubMed] [Google Scholar]
- 33.Bowlby J. Attachment and Loss. Vol I: Attachment. New York: Basic Books Classics; 1969. [Google Scholar]
- 34.Bowlby J. Attachment and Loss. Vol. II: Separation. New York: Basic Books; 1973. [Google Scholar]
- 35.Bartholomew K, Horowitz LM. Attachment styles among young adults: A test of a four-category model. J Pers Soc Psychol. 1991;61:226–44. doi: 10.1037//0022-3514.61.2.226. [DOI] [PubMed] [Google Scholar]
- 36.Feeney JA, Noller P, Hanrahan M. Assessing adult attachment Attachment in adults: Clinical and developmental perspectives. New York: Guilford Press; 1994. pp. 128–52. [Google Scholar]
- 37.DeWall CN, Masten CL, Powell C, et al. Do neural responses to rejection depend on attachment style? An fMRI study. Soc Cogn Affect Neurosci. 2012;7:184–92. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 41.Segerdahl AR, Themistocleous AC, Fido D, et al. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain. 2018;141:357–64. doi: 10.1093/brain/awx337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies KA, Macfarlane GJ, McBeth J, et al. Insecure attachment style is associated with chronic widespread pain. Pain. 2009;143:200–5. doi: 10.1016/j.pain.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sifneos PE. The prevalence of ‘alexithymic’characteristics in psychosomatic patients. Psychother Psychosom. 1973;22:255–62. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- 44.Taylor GJ. Alexithymia: Concept, measurement, and implications for treatment. Am J Psychiatry. 1984;141(6):725–32. doi: 10.1176/ajp.141.6.725. [DOI] [PubMed] [Google Scholar]
- 45.Taylor GJ, Bagby RM, Parker JD. The alexithymia construct: A potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32:153–64. doi: 10.1016/s0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]
- 46.Di Tella M, Castelli L. Alexithymia and fibromyalgia: Clinical evidence. Front Psychol. 2013;4:909. doi: 10.3389/fpsyg.2013.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sancassiani F, Preti A, Cacace E, et al. Alexithymia and sense of coherence: Does their impact on fibromyalgia suggest new targets for therapy? Gen Hosp Psychiatry. 2018 doi: 10.1016/j.genhosppsych.2018.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Castelli L, Tesio V, Colonna F, et al. Alexithymia and psychological distress in fibromyalgia: prevalence and relation with quality of life. Clin Exp Rheumatol. 2012;30:70–77. [PubMed] [Google Scholar]
- 49.Lumley MA, Neely LC, Burger AJ. The assessment of alexithymia in medical settings: Implications for understanding and treating health problems. J Pers Assess. 2007;89:230–46. doi: 10.1080/00223890701629698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuzer V, Bulut SD, Bastug B, et al. Causal attributions and alexithymia in female patients with fibromyalgia or chronic low back pain. Nord J Psychiatry. 2011;65:138–44. doi: 10.3109/08039488.2010.522596. [DOI] [PubMed] [Google Scholar]
- 51.Steinweg DL, Dallas AP, Rea WS. Fibromyalgia: Unspeakable suffering, a prevalence study of alexithymia. Psychosomatics. 2011;52:255–62. doi: 10.1016/j.psym.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Sayar K, Gulec H, Topbas M. Alexithymia and anger in patients with fibromyalgia. Clin Rheumatol. 2004;23:441–48. doi: 10.1007/s10067-004-0918-3. [DOI] [PubMed] [Google Scholar]
- 53.Di Tella M, Ghiggia A, Tesio V, et al. Pain experience in Fibromyalgia syndrome: The role of alexithymia and psychological distress. J Affect Disord. 2017;208:87–93. doi: 10.1016/j.jad.2016.08.080. [DOI] [PubMed] [Google Scholar]
- 54.Williams B, Ospina JP, Jalilianhasanpour R, et al. Fearful attachment linked to childhood abuse, alexithymia, and depression in motor functional neurological disorders. J Neuropsychiatry Clin Neurosci. 2019;31:65–69. doi: 10.1176/appi.neuropsych.18040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riem MME, Doedee E, Broekhuizen-Dijksman SC, Beijer E. Attachment and medically unexplained somatic symptoms: The role of mentalization. Psychiatry Res. 2018;268:108–13. doi: 10.1016/j.psychres.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 56.Peñacoba C, Perez-Calvo S, Blanco S, Sanroman L. Attachment styles, pain intensity and emotional variables in women with fibromyalgia. Scand J Caring Sci. 2018;32(2):535–44. doi: 10.1111/scs.12477. [DOI] [PubMed] [Google Scholar]
- 57.Blanco S, Peñacoba C, Sanromán L, Pérez-Calvo S. Analysis of quantitative and qualitative measures of attachment in patients with fibromyalgia: The influence on nursing care AU – Blanco, Sheila. Int J Ment Health. 2018;47:50–63. [Google Scholar]
- 58.Palitsky D, Mota N, Afifi TO, et al. The association between adult attachment style, mental disorders, and suicidality: Findings from a population-based study. J Nerv Ment Dis. 2013;201:579–86. doi: 10.1097/NMD.0b013e31829829ab. [DOI] [PubMed] [Google Scholar]
- 59.Ruckert-Eheberg IM, Lukaschek K, Brenk-Franz K, et al. Association of adult attachment and suicidal ideation in primary care patients with multiple chronic conditions. J Affect Disord. 2018;246:121–25. doi: 10.1016/j.jad.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 60.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 61.Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17:577–91. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Littlejohn G. Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat Rev Rheumatol. 2015;11:639–48. doi: 10.1038/nrrheum.2015.100. [DOI] [PubMed] [Google Scholar]
- 63.Vasquez A. Neuroinflammation in fibromyalgia and CRPS is multifactorial. Nat Rev Rheumatol. 2016;12:242. doi: 10.1038/nrrheum.2016.25. [DOI] [PubMed] [Google Scholar]
- 64.Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450–52. doi: 10.1136/ard.2003.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallace DJ, Hallegua DS. Fibromyalgia: The gastrointestinal link. Curr Pain Headache Rep. 2004;8(5):364–68. doi: 10.1007/s11916-996-0009-z. [DOI] [PubMed] [Google Scholar]
- 66.Goebel A, Buhner S, Schedel R, et al. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology (Oxford) 2008;47:1223–27. doi: 10.1093/rheumatology/ken140. [DOI] [PubMed] [Google Scholar]
- 67.Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–86. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 68.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood?: A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 69.Sherman AL, Morris MC, Bruehl S, et al. Heightened temporal summation of pain in patients with functional gastrointestinal disorders and history of trauma. Ann Behav Med. 2015;49:785–92. doi: 10.1007/s12160-015-9712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017;95:1257–70. doi: 10.1002/jnr.23802. [DOI] [PubMed] [Google Scholar]
- 71.Maccari S, Krugers HJ, Morley-Fletcher S, et al. The consequences of early-life adversity: Neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–23. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- 72.Ganguly P, Brenhouse HC. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cogn Neurosci. 2015;11:18–30. doi: 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K-C, Wang S-J, Fan L-W, et al. Interleukin-1 receptor antagonist ameliorates neonatal lipopolysaccharide-induced long-lasting hyperalgesia in the adult rats. Toxicology. 2011;279:123–29. doi: 10.1016/j.tox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avitsur R, Maayan R, Weizman A. Neonatal stress modulates sickness behavior: Role for proinflammatory cytokines. J Neuroimmunol. 2013;257:59–66. doi: 10.1016/j.jneuroim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Mesulam MM. Principles of behavioral and cognitive neurology. Oxford University Press; 2000. [Google Scholar]


