Abstract
Objective:
The aim of the present study was to determine the effects of intravenous (IV) and intracoronary administration of Vitamin C on the incidence of periprocedural myocardial injury in patients undergoing primary percutaneous coronary intervention (PCI).
Methods:
In this prospective, double-blind, randomized clinical trial, that was conducted in Tehran Heart Center, Iran, between October 2016 and March 2017, 252 patients undergoing primary PCI were enrolled to receive either 3 g of IV Vitamin C before PCI and 100 mg of intracoronary Vitamin C during PCI in addition to the routine treatment (n = 126) or just the routine treatment (n = 126). Cardiac biomarkers were measured before and then 6 and 12 h postprocedurally. We determined the occurrence of contrast-induced acute kidney injury (CI-AKI), according to the levels of serum creatinine, neutrophil gelatinase-associated lipocalin, and platelet activation biomarker (P-selectin) in a subset of 119 patients before and 6 h after PCI.
Findings:
In the patients who received Vitamin C, the serum levels of troponin T after 12 h and creatine kinase-MB after 6 h were significantly lower than those in the placebo group (P = 0.003 andP = 0.00, respectively). CI-AKI occurred in 6 (4.7%) patients in the study group and 8 (6.3%) patients in the control group; there was no significant reduction in CI-AKI in the study group. In addition, the two groups were statically similar as regards the changes in the level of P-selectin.
Conclusion:
In primary PCI patients, the prophylactic use of IV and intracoronary Vitamin C can confer additional clinical benefits such as cardioprotection.
KEYWORDS: Contrast-induced acute kidney injury, creatine kinase-MB, primary percutaneous coronary intervention, troponin T, Vitamin C
INTRODUCTION
Periprocedural myocardial injury (PMI) is a common complication that occurs after percutaneous coronary intervention (PCI). In patients with PCI-related myocardial injury, there is a rise in cardiac troponin, which is a more sensitive marker than creatine kinase-MB (CK-MB) for minor degrees of myocardial damage. In 2007, the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force presented a bespoke definition for PCI-associated myocardial injury as an elevation in cardiac troponin values more than fivefold; the upper limit of normal (ULN) in patients with normal baseline values (≤99th percentile ULN) or a rise in cardiac troponin values ≥20% if the baseline values are elevated and stable.[1]
Elevations in cardiac enzymes can be due to several factors, including distal embolization or thrombi, side-branch occlusions, slow-flow or no-reflow phenomena, epicardial or microvascular spasms, and disruption of collateral flow.[1] Angiographic collateral flow is evaluated using the Rentrop collateral score: 0 means no filling of collateral vessels, 1 means filling of collateral vessels without any epicardial filling, 2 means partial epicardial filling by collateral vessels, and 3 means complete epicardial filling by collateral vessels.[2]
Coronary interventions and ischemia, followed by reperfusion, often trigger a harmful cascade.[3] Increased vascular levels of reactive oxygen species in some procedures (e.g., elective PCI) induce an increase in F2-isoprostane formation,[4] and elective PCI increases the level of 8-iso-prostaglandin F2-alpha, which is formed nonenzymatically through a free-radical catalyzed attack on esterified arachidonates.[5]
Exogenous antioxidant agents confer protection against reperfusion, caused oxidative stress. Vitamin C or ascorbic acid, a water-soluble antioxidant vitamin, can effectively scavenge superoxides and other reactive oxygen species such as superoxide dismutase (SOD) and nicotinamide adenine dinucleotide phosphate oxidase; a major source of superoxide which also prevents the oxidation of tetrahydrobiopterin and increases endothelial nitric oxide synthase enzyme activity.[3]
The preprocedural intravenous (IV) infusion of Vitamin C has a significant role in the reduction of myocardial reperfusion injury and improvement in microcirculation in PCI-treated patients.[4,6]
Contrast-induced acute kidney injury (CI-AKI) is one of the complications of contrast media. A recent systematic review showed that prophylactic administration of ascorbic acid plus saline may reduce the risk of CI-AKI.[7] A previous investigation reported that the level of plasma neutrophil gelatinase-associated lipocalin (NGAL), as an early sensitive biomarker, was able to predict AKI after PCI insofar, as there was a significant rise in serum NGAL level at 2, 4, and 8 postprocedural hours.[8]
A granule membrane protein of platelets and endothelial cells, P-selectin induces platelet activation, which enhances platelet aggregation and thrombus formation. There is established evidence of P-selectin expression in coronary artery disease and higher expression in patients with acute coronary syndromes.[9] It has been indicated that Vitamin C interferes with P-selectin translocation to the endothelial cell surface in response to radicals. In patients with aortic stenosis, there was a reduction in soluble P-selectin in the Vitamin C group.[10]
Despite all the previous research into the effects of Vitamin C, its role in the prevention of PMI in a large population has yet to be fully studied. Accordingly, we decided to assess the effects of Vitamin C on the level of PMI biomarkers following acute myocardial infarction and PCI.
METHODS
The present study was conducted as a prospective, single-center, randomized, double-blind trial in Tehran Heart Center affiliated with Tehran University of Medical Sciences, Tehran, Iran (Registration No. IRCT 201606048698N19 at http://www.irct.ir, a primary registry in the WHO's registry network setup). In total, 252 patients with acute ST-elevation myocardial infarction (STEMI) admitted to our hospital (Tehran Heart Center) between October 2016 and March 2017 and scheduled for primary PCI were evaluated. The inclusion criteria were comprised patients (men or women) between 18 and 80 years of age, ischemic discomfort lasting longer than 20 min and shorter than 6 h, a diagnosis of acute myocardial infarction based on clinical presentations and electrocardiographic findings of new ST elevations ≥2 mm (≥0.2 mV) in men and ≥1.5 mm (≥0.15 mV) in women in leads V2–V3 and/or ≥1 mm (≥0.1 mV) in the other two contiguous limb leads, and undergoing PCI within 120 min of admission.
The key exclusion criteria included patients with cardiac arrest, ventricular fibrillation, or cardiogenic shock requiring chest compressions or cardiopulmonary resuscitation; patients with evidence of coronary collaterals (the Rentrop grade = 2–3) to the region at risk on initial coronary angiography (at the time of admission); patients with impaired renal function (creatinine >3.0 mg/dL); patients on N-acetylcysteine; patients with known medically unmanageable allergies to aspirin, clopidogrel, heparin, contrast media, or stainless steel; patients with current warfarin therapy; life expectancy shorter than 12 months; and recipients of heart transplantation.
The study protocol was approved by the Local Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.PSRC.REC.1396.2529), and informed written consent was obtained from all the participants.
All the patients who met the eligibility criteria were randomized using permuted randomization block in a 1:1 ratio in a study group of 126 patients to receive a prophylactic infusion of Vitamin C and in a control group of 126 patients. The study group, in addition to the routine treatment as well as general hydration with ≤500 mL normal saline (within 6 h) at a normal rate (0.5 or 1 mL/kg/h), initially received 3 g of Vitamin C (Vitamin C 500 mg/5 mL; Osvah Pharmaceutical Co., Tehran, Iran) diluted in 50 mL of 0.9% saline infused slowly through IV drips at a rate of 0.5 g/min in the emergency department before angioplasty and stenting. Afterward in the catheterization laboratory after stenting, the study group was given 100 mg of intracoronary Vitamin C. The control group administered the same volume of a saline solution (NaCl) both for IV and intracoronary administration.
Catheterization was done through the radial or mainly femoral artery. Then, a hemostatic puncture closing device was used. The choice of the type of contrast media was left to the discretion of the interventional cardiologist in the catheterization laboratory. Two types of iso-osmolar nonionic contrast media in different volumes were used in the coronary angioplasty procedures: iodixanol (Visipaque 320, GE Healthcare, Norway) and iopromide (Ultravist 300, Bayer Schering Pharma, Germany).
We took blood samples before and subsequently 6 and 12 h after PCI to measure the level of CK-MB and high-sensitive Troponin T. The assay fulfills the guidelines of American College of Cardiology/European Society of Cardiology/Academy of the American Association for Clinical Chemistry in achieving <10% coefficient of variation at the 99 percentile upper reference limit of the reference population. Normal limits were <3.77 ng/mL for CK-MB and <24 ng/L for high-sensitive troponin T. In addition, we measured the levels of NGAL and P-selectin in a subset of 119 patients by collecting blood samples before and after 6 h following PCI.
A PMI event after PCI was the primary end-point of the current study. An increase in the level of CK-MB and troponin T was defined as a PMI event. The second end-point was the occurrence of CI-AKI, defined as an increase in the level of NGAL within 6 h of exposure to the contrast media. The level of P-selectin was measured at each study time point for an assessment of platelet aggregation.
The statistical analyses were performed using SPSS software, version 20.0 (IBM, USA). The normal distribution of the data was checked using the Kolmogorov–Smirnov test. The nonnormally distributed variables were log transformed before the analysis. The Chi-square test was employed to compare the patients' characteristics, as the discrete variables, between the two groups. The continuous variables were analyzed using the independent samples t-test, as appropriate. Interventional study data were also analyzed for the assessment of the effect of treatment on blood levels of troponin T and CK-MB, performing a repeated-measure ANOVA with between-subject factor (treatment group) and within-subject factor (time).
P < 0.05 was considered as statistically significant.
RESULTS
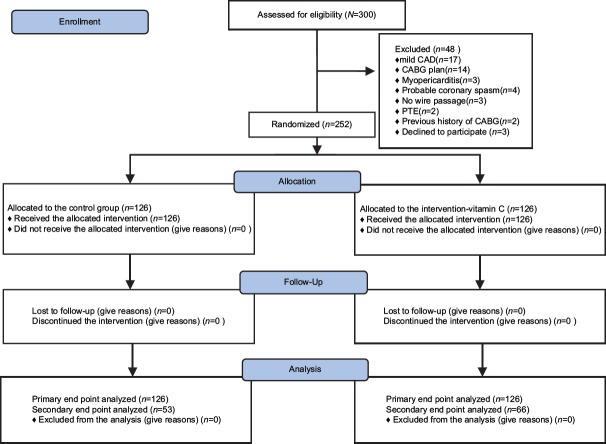
A total of 300 patients were enrolled in the present trial. Of the 300 patients, we excluded 48 because they had mild coronary artery disease (17/48 [35.41%]), coronary artery bypass grafting (CABG) surgery plan (14/48 [29.16%]), myopericarditis (3/48 [6.25%]), probable coronary spasm (4/48 [8.33%]), no wire passage (3/48 [6.25%]), pulmonary embolism (2/48 [4.16%]), a previous history of CABG (2/48 [4.16%]), or declined to participate in the study (3/48 [6.25%]). The remaining 252 patients were randomly assigned to receive either the routine treatment plus Vitamin C (n = 126) or only the routine treatment (n = 126) [Figure 1].
Figure 1.
Consort flowchart of the study. CAD = Coronary artery disease, CABG = Coronary artery bypass grafting, PTE = Pulmonary thromboembolism
The clinical characteristics, including age, sex, heart disease risk factors, and concomitant medications, were similar in the two groups [Table 1]. The type of the contrast media and the mean volume of the contrast media were not significantly different between the two groups, and nor was there a significant difference between the groups with respect to the baseline renal function [Table 1]. The angiographic parameters, consisting of the thrombolysis in myocardial infarction (TIMI) flow, target vessels, number of the vessels, and types of the stents used in the procedures, were similar in both groups too.
Table 1.
Patients’ characteristics of Vitamin C and control groups
| Characteristics | Control group (n=126) | Vitamin C group (n=126) | P |
|---|---|---|---|
| Age (years) | 57.18±10.4 | 58.64±10.41 | 0.26* |
| Sex (male) n (%) | 104 (82.5) | 97 (76.9) | 0.27** |
| Presence of cardiovascular risk factors | |||
| Diabetes mellitus, n (%) | 42 (33.33) | 50 (39.68) | 0.29** |
| Hypertension, n (%) | 52 (41.26) | 56 (44.44) | 0.61** |
| Smoking, n (%) | 50 (39.68) | 53 (42.06) | 0.70** |
| Hyperlipidemia, n (%) | 21 (16.66) | 29 (23.01) | 0.20** |
| Family history, n (%) | 24 (19) | 13 (10.3) | 0.05** |
| Ischemic heart disease, n (%) | 5 (3.96) | 15 (11.90) | 0.02** |
| Volume of infused contrast media (ml) | 228±69 | 227±63 | 0.52* |
| Types of iodine-based contrast media | |||
| Ultravist, n (%) | 6 (4.76) | 6 (4.76) | 1** |
| Visipaque, n (%) | 120 (95.23) | 120 (95.23) | |
| Ejection fraction (%) | 41.37±7.77 | 43.00±6.83 | 0.09* |
| Serum creatinine (mg/dL) | 0.93±0.2 | 0.94±0.23 | 0.63* |
| Number of diseased vessels (n) | |||
| One vessel | 58 | 43 | 0.13** |
| Two vessels | 45 | 50 | |
| Three vessels | 23 | 33 |
*Resulted from twoindependent samples t-test, **Resulted from Chi-square test. Data presented as mean±SD, or n and percent of the patients. SD=Standard deviation
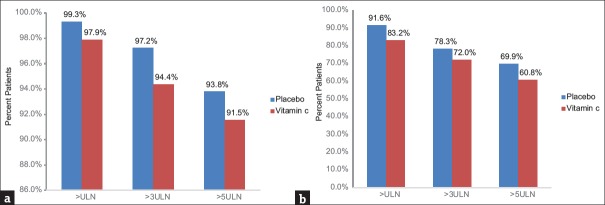
In the study of primary end-point, serum levels of troponin T after 12 h were significantly lower in the study group than in the control group (P = 0.003) [Figure 2]. An increase by fivefold, the ULN in the level of troponin T was significantly less frequent in the Vitamin C group (91.5% vs. 93.8%; P = 0.009), as was the rise by threefold, the ULN in the level of troponin T (94.4% vs. 97.2%; P = 0.078) [Figure 2]. The serum level of CK-MB after 6 h (P = 0.00) [Figure 2] and the levels of CK-MB more than fivefold and threefold the ULN [Figure 3] were also significantly reduced by Vitamin C treatment compared with the control group (60.8% vs. 69.9%; P = 0.031 and 72% vs. 78.2%; P = 0.088, respectively).
Figure 2.
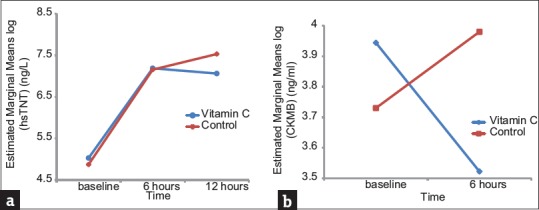
Changes in high-sensitive cardiac troponin T (a) and creatine kinase-MB (b) before and after primary percutaneous coronary intervention
Figure 3.
Percentages of the patients in the Vitamin C group and the control group defined by the range of troponin T (a) CK-MB (b) ULN = Upper limit of normal, CK-MB = Creatine kinase-MB
The frequency of CI-AKI was 5.5%, 4.7% (n = 6) in the Vitamin C group and 6.3% (n = 8) in the control group based on the definition of CI-AKI as a 25% rise in the level of serum creatinine 48 h after contrast exposure. There were no significant differences in CI-AKI between the two groups. The level of serum creatinine was decreased from 0.94 to 0.93 mg/dL (P = 0.011) in the study group, whereas it was increased from 0.93 to 0.95 mg/dL (P = 0.001) in the control group within 48 h of the contrast media administration. Of the 252 patients, 119 patients (66 in the Vitamin C group and 53 in the control group) were randomly assigned to determine the occurrence of CI-AKI by measuring the plasma level of NGAL. There were no significant differences between the two groups regarding the baseline characteristics. The change in the level of NGAL within 6 h was statistically similar in the two groups (P = 0.36).
The serum level of P-selectin after the procedure was decreased in both groups within 6 h; Vitamin C supplementation made no significant difference in the level of P-selectin (P = 0.65).
DISCUSSION
In the present study, a novel strategy was applied for intracoronary plus IV administration of the antioxidant ascorbic acid in patients with STEMI who were undergoing primary PCI within a limited time to achieve the appropriate concentration of Vitamin C for scavenging free radicals. Our findings demonstrated that ascorbic acid reduced the incidence of PMI and conferred cardioprotective effects.
PMI occurs as a serious PCI complication and ranges widely, from minor elevations in cardiac biomarkers to large myocardial injuries.
Prasad et al.[11] reported that only an elevation in cardiac troponin T, as a sensitive and specific marker of myonecrosis, was correlated with higher rates of death and myocardial injury.
However, any slight but statistically significant increase in CK-MB after PCI is clinically important as it can raise the risk of death.[12] Therefore, we evaluated the possible effects of Vitamin C administration on the level of these biomarkers to assess its role on PMI.
Numerous studies have demonstrated the role of oxidative stress in cardiac reperfusion injury. Reperfusion of the ischemic myocardium in STEMI patients is also associated with the release of 4-hydroxy-2-nonenal, which is produced by lipid peroxidation in cells. Furthermore, the antioxidant activity of SOD is significantly decreased among patients with impaired tissue perfusion (myocardial blush grade <2).[13]
Basili et al.[4] reported that plasma levels of 8-hydroxy-2'-deoxyguanosine and 8-iso-prostaglandin F2-alpha, as the indices of oxidative stress, were significantly increased in their PCI group, while they were significantly reduced in their Vitamin C-treated group.
Ascorbic acid is a wide range of free radical scavengers, a powerful antioxidant, and acts as a co-antioxidant too. The rapid release of free radicals has been observed after the reperfusion of the ischemic myocardium in experimental models, and in primary PCI, in patients with acute STEMI, which is known to play a potential role in the creation of oxidative stress. Vitamin C plasma concentrations as a primary circulatory antioxidant decreased ≥50% on the 1st day after cardiac arrest. Furthermore, it reduced in many other critically ill patients such as sepsis, hemorrhage, myocardial infarction, or traumatic brain injury. The infusion of Vitamin C attenuates the production of superoxide and the pathophysiological cascade of disadvantageous heart effects such as acute myocardial infarction, reperfusion injury in PCI, and postoperative atrial fibrillation.[3,14] To reach these positive outcomes on oxidative stress, we administered Vitamin C both intravenously and intracoronary. However, we did not evaluate the biomarkers of oxidative stress in this study.
Few clinical studies have investigated IV Vitamin C during PCI for PMI. As was mentioned before, recent studies by Basili et al.[4] have shown that the infusion of 1 g of Vitamin C 1 h before elective PCI will scavenge oxygen radicals and improve microcirculatory perfusion.[4] However, only 56 patients were studied in their research, while the sample size used in the present research was larger, that is, 252 patients.
In a study by Wang et al.,[6] the infusion of 3 g of Vitamin C within 6 h before elective PCI significantly decreased the incidence of PMI. The authors postulated that this finding might have been in consequence of the potential role of oxidative stress and decreased levels of plasma 8-hydroxy-2'-deoxyguanosine (a marker of oxidative stress). Hence, we used 3 g dose of Vitamin C administered intravenously before primary PCI.
Only one study by Ramos et al., which was done on 99 patients, estimated the effect of Vitamin C administered just before PCI for acute myocardial infarction on oxidative stress. It was shown that administrating intravenously high doses of ascorbic acid, 320 mmol/l during 3 h, and then receiving an oral doses for 84 continuous days after PCI resulted in a significant increase in plasma (ferric-reducing ability of plasma) as the total antioxidant capacity of plasma, but did not affect the infarct size, ejection fraction, and heart biomarkers between the groups.[15]
The participants of the study were patients with STEMI who underwent primary PCI. These patients have a high level of oxidative stress.[3,13,14] To reach a high concentration of Vitamin C and fight oxygen free radicals, intracoronary plus IV was administered.
Supraphysiological blood concentrations of Vitamin C are needed to scavenge superoxide radicals. In a few studies on Vitamin C pharmacokinetics, it has been shown that the infusion of high doses of Vitamin C can provide the peak concentrations needed for the induction of antioxidant effects without any adverse effects.[16,17] We chose the dose of Vitamin C in our study in accordance with the abovementioned studies as well as that by Mak et al.,[18] on the intracoronary administration of Vitamin C which is sufficient in vitro to compete with O2. and prevent its participation in a reaction.
Although PCI promotes the return of blood flow to the epicardium, the rise in cardiac enzymes tends to continue due to the occurrence of PMI. Fewer patients in our Vitamin C-allocated group experienced a rise in their level of CK-MB and a rise in their level of troponin T threefold or fivefold the ULN 12 h post-PCI. It could be associated with decreased long-term mortality.[11,12]
As the previous studies have shown,[4,6,15] the reason may be the antioxidant effects of Vitamin C and improvement in microvascular perfusion. Nonetheless, the exact mechanism for the prevention of PMI is still far from clear.
Sadat et al.[19] and Xu et al.[7] conducted a systematic review with a meta-analysis of relevant randomized controlled trials and reported that Vitamin C had effective nephroprotection impacts against CI-AKI. In a study by Khaledifar et al.,[20] ascorbic acid was added to normal saline infusions, but it failed to show beneficial effects in terms of CI-AKI prevention.
Brueck et al.[21] administered 500 mg of ascorbic acid at 24 h and 1 h before elective cardiac catheterization and reported that it did not prevent CI-AKI in their patients with chronic kidney disease. However, ascorbic acid as compared with saline hydration alone decreased the rate of CI-AKI in patients with serum creatinine levels below 1.4 mg/dL.
Recently, Rollins et al.[22] in a murine model of CI-AKI observed that ascorbic acid reduced the urinary NGAL-to-creatinine ratio, which is indicative of the frequency and severity of the renal injury.
Due to the conflicting results and lack of studies done in a primary setting in the available literature, we conducted the present study to evaluate the effects of ascorbic acid on the occurrence of CI-AKI in patients undergoing primary coronary angioplasty. In our study, the rate of CI-AKI occurrence was 4.6% totally, 3.9% (n = 6) in the Vitamin C-treated group and 5.2% (n = 8) in the control group based on the definition of CI-AKI, as at least a 25% relative increase in the baseline serum creatinine level in unselected patients. In our study, almost 90% of the study population had normal serum creatinine levels (<1.2 mg/dL).
Our findings were in line with the Brueck et al.[21] and Khaledifar et al.[20] studies which showed that Vitamin C infusion on the occurrence of CI-AKI has no more beneficial effect than hydration with sodium chloride; however, they used a lower dose of ascorbic acid and studied patients with chronically impaired renal function.
In the current study, baseline serum creatinine, hydration, and the use of iso-osmolar nonionic contrast media, which play a role in the occurrence of CI-AKI, were similar in the two groups. Further, the concentration of serum creatinine was measured within 48 h of exposure. We observed a statically nonsignificant decline in the mean serum creatinine concentration in the Vitamin C group, and a statically nonsignificant rise in the mean serum creatinine concentration in the control group. However, these changes, albeit nonsignificant, may significantly modify the clinical outcome.
Nephrotoxic and ischemic injuries such as those caused by iodinated contrast media lead to the accumulation of NGAL in cortical tubules of human kidney, blood, and urine. Thus, whole-blood NGAL can predict CI-AKI in patients undergoing PCI with good sensitivity and specificity.[8,23] We measured the level of NGAL in 119 patients and found no significant change in the two study groups; therefore, NGAL failed to predict CI-AKI, which has been reported in some other studies as well.[24] Here, it might be because of our small sample.
P-selectin adheres certain leukocytes and platelets to the endothelium. Stohlawetz et al.[25] showed that soluble P-selectin was a sensitive marker for initial platelet activation and reported a rise in the level of soluble P-selectin in different cardiovascular diseases such as coronary artery disease, hypertension, and atrial fibrillation. Stellos et al.[26] assessed the data from 667 patients with symptomatic coronary artery disease that underwent coronary intervention and concluded that an increased level of platelet P-selectin was correlated with a rise in the level of markers of myocardial necrosis (troponin T and CK-MB), particularly in those with STEMI. Thus, P-selectin was selected to examine platelet aggregation.
The administration of antioxidants appears to decrease P-selectin levels. In this regard, Tahir et al.[10] demonstrated a reduction in the level of serum P-selectin among patients with chronic degenerative aortic stenosis in their Vitamin C group.
Basili et al. study has shown that anoxia-reoxygenation increases reactive oxygen species and platelet production of TxB2 and isoprostanes which were inhibited by Vitamin C. Therefore, the effect of Vitamin C on platelet aggregation was measured by P-selectin.[27]
In the present study, conducted on STEMI patients, we found that while the level of P-selectin was high, as was expected, in both Vitamin C-treated and control groups before the procedure because of myocardial damage, platelet activation, and the formation of thrombosis in the onset of acute coronary syndrome, it was decreased after angioplasty. Despite the impact of Vitamin C on platelet function and P-selectin,[28] the difference between the two groups failed to constitute statistical significance, and Vitamin C supplementation has no additional effect on reducing platelet aggregation. In our subgroup analysis, the two groups were similar with regard to TIMI flow and thrombosis grade.
Our study could have been plausible by doing a larger trial, and with a larger sample size, we could reach a firm conclusion regarding the effects of Vitamin C on PMI, thrombosis formation, and CI-AKI. Furthermore, we could have assessed the infarct size plus a functional determination by cardiac magnetic resonance too. Moreover, we failed to determine the optimal dose and duration of Vitamin C infusion intravenously before PCI and intracoronarily during PCI. In addition, had we performed a long-term follow-up of our study population, our evaluation of the impact of Vitamin C on clinical outcomes would have been more robust.
In summary, the results of the present prospective randomized study showed that the prophylactic infusion of Vitamin C, as a safe, well-tolerated, and inexpensive antioxidant, appeared to have decreased PCI-related PMI by reducing the level of troponin T and CK-MB, as predictors of mortality, as well as the level of serum creatinine.
AUTHORS' CONTRIBUTION
Negar Shafaei Bajestani participated in literature search, clinical studies, data collection, data analysis/interpretation, and manuscript preparation and finalization. Azita Hajhossein Talasaz contributed in proposing ideas, study design, definition of intellectual content, data analysis/interpretation, and manuscript editing and finalizing. Mojtaba Salarifar, Hamidreza Pourhosseini, and Farshad Sadri participated in sample collection, patient management, and manuscript editing and finalizing. Arash Jalali contributed in statistical analysis and manuscript finalizing. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study is a Clinical Pharmacy Residency thesis supported by Tehran University of Medical Sciences, and the authors sincerely thank the research nurses for their assistance in preprocedural data gathering in Tehran Heart Center.
REFERENCES
- 1.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–64. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 2.Elias J, Hoebers LP, van Dongen IM, Claessen BE, Henriques JP. Impact of collateral circulation on survival in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention with a concomitant chronic total occlusion. JACC Cardiovasc Interv. 2017;10:906–14. doi: 10.1016/j.jcin.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo R, Prieto JC, Castillo R. Cardioprotection against ischaemia/reperfusion by Vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinical applications. Clin Sci (Lond) 2013;124:1–5. doi: 10.1042/CS20110663. [DOI] [PubMed] [Google Scholar]
- 4.Basili S, Tanzilli G, Mangieri E, Raparelli V, Di Santo S, Pignatelli P, et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: Relationship with oxidative stress markers. JACC Cardiovasc Interv. 2010;3:221–9. doi: 10.1016/j.jcin.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Berg K, Wiseth R, Bjerve K, Brurok H, Gunnes S, Skarra S, et al. Oxidative stress and myocardial damage during elective percutaneous coronary interventions and coronary angiography. A comparison of blood-borne isoprostane and troponin release. Free Radic Res. 2004;38:517–25. doi: 10.1080/10715760410001688339. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZJ, Hu WK, Liu YY, Shi DM, Cheng WJ, Guo YH, et al. The effect of intravenous Vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol. 2014;30:96–101. doi: 10.1016/j.cjca.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Zheng X, Liang B, Gao J, Gu Z. Vitamins for prevention of contrast-induced acute kidney injury: A systematic review and trial sequential analysis. Am J Cardiovasc Drugs. 2018;18:373–86. doi: 10.1007/s40256-018-0274-3. [DOI] [PubMed] [Google Scholar]
- 8.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Poniatowski B, Pawlak K, et al. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: Are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol. 2008;127:290–1. doi: 10.1016/j.ijcard.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 9.George R, Bhatt A, Narayani J, Thulaseedharan JV, Sivadasanpillai H, Tharakan JA. Enhanced P-selectin expression on platelet-a marker of platelet activation, in young patients with angiographically proven coronary artery disease. Mol Cell Biochem. 2016;419:125–33. doi: 10.1007/s11010-016-2756-4. [DOI] [PubMed] [Google Scholar]
- 10.Tahir M, Foley B, Pate G, Crean P, Moore D, McCarroll N, et al. Impact of Vitamin E and C supplementation on serum adhesion molecules in chronic degenerative aortic stenosis: A randomized controlled trial. Am Heart J. 2005;150:302–6. doi: 10.1016/j.ahj.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Prasad A, Singh M, Lerman A, Lennon RJ, Holmes DR, Jr, Rihal CS. Isolated elevation in troponin T after percutaneous coronary intervention is associated with higher long-term mortality. J Am Coll Cardiol. 2006;48:1765–70. doi: 10.1016/j.jacc.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 12.Hanna EB, Hennebry TA. Periprocedural myocardial infarction: Review and classification. Clin Cardiol. 2010;33:476–83. doi: 10.1002/clc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaminski K, Bonda T, Wojtkowska I, Dobrzycki S, Kralisz P, Nowak K, et al. Oxidative stress and antioxidative defense parameters early after reperfusion therapy for acute myocardial infarction. Acute Card Care. 2008;10:121–6. doi: 10.1080/17482940701744334. [DOI] [PubMed] [Google Scholar]
- 14.Spoelstra-de Man AM, Elbers PW, Oudemans-van Straaten HM. Making sense of early high-dose intravenous Vitamin C in ischemia/reperfusion injury. Crit Care. 2018;22:70. doi: 10.1186/s13054-018-1996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos C, Brito R, González-Montero J, Valls N, Gormaz JG, Prieto JC, et al. Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch Med Sci. 2017;13:558–67. doi: 10.5114/aoms.2016.59713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duconge J, Miranda-Massari JR, Gonzalez MJ, Jackson JA, Warnock W, Riordan NH. Pharmacokinetics of Vitamin C: Insights into the oral and intravenous administration of ascorbate. P R Health Sci J. 2008;27:7–19. [PubMed] [Google Scholar]
- 17.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak S, Overgaard CB, Newton GE. Effect of Vitamin C and L-NMMA on the inotropic response to dobutamine in patients with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2424–8. doi: 10.1152/ajpheart.00453.2005. [DOI] [PubMed] [Google Scholar]
- 19.Sadat U, Usman A, Gillard JH, Boyle JR. Does ascorbic acid protect against contrast-induced acute kidney injury in patients undergoing coronary angiography: A systematic review with meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;62:2167–75. doi: 10.1016/j.jacc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 20.Khaledifar A, Momeni A, Ebrahimi A, Kheiri S, Mokhtari A. Comparison of N-acetylcysteine, ascorbic acid, and normal saline effect in prevention of contrast-induced nephropathy. ARYA Atheroscler. 2015;11:228–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Brueck M, Cengiz H, Hoeltgen R, Wieczorek M, Boedeker RH, Scheibelhut C, et al. Usefulness of N-acetylcysteine or ascorbic acid versus placebo to prevent contrast-induced acute kidney injury in patients undergoing elective cardiac catheterization: A single-center, prospective, randomized, double-blind, placebo-controlled trial. J Invasive Cardiol. 2013;25:276–83. [PubMed] [Google Scholar]
- 22.Rollins K, Noorani A, Janeckova L, Jones T, Griffiths M, Baker MP, et al. Ascorbic acid ameliorates renal injury in a murine model of contrast-induced nephropathy. BMC Nephrol. 2017;18:101. doi: 10.1186/s12882-017-0498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alharazy SM, Kong N, Saidin R, Gafor AH, Maskon O, Mohd M, et al. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after coronary angiography. Angiology. 2014;65:216–23. doi: 10.1177/0003319712474947. [DOI] [PubMed] [Google Scholar]
- 24.Ribitsch W, Schilcher G, Quehenberger F, Pilz S, Portugaller RH, Truschnig-Wilders M, et al. Neutrophil gelatinase-associated lipocalin (NGAL) fails as an early predictor of contrast induced nephropathy in chronic kidney disease (ANTI-CI-AKI study) Sci Rep. 2017;7:41300. doi: 10.1038/srep41300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stohlawetz P, Hergovich N, Stiegler G, Eichler HG, Höcker P, Kapiotis S, et al. Differential induction of P-selectin expression on platelets by two cell separators during plateletpheresis and the effect of gender on the release of soluble P-selectin. Transfusion. 1998;38:24–30. doi: 10.1046/j.1537-2995.1998.38198141494.x. [DOI] [PubMed] [Google Scholar]
- 26.Stellos K, Bigalke B, Stakos D, Henkelmann N, Gawaz M. Platelet-bound P-selectin expression in patients with coronary artery disease: Impact on clinical presentation and myocardial necrosis, and effect of diabetes mellitus and anti-platelet medication. J Thromb Haemost. 2010;8:205–7. doi: 10.1111/j.1538-7836.2009.03659.x. [DOI] [PubMed] [Google Scholar]
- 27.Basili S, Pignatelli P, Tanzilli G, Mangieri E, Carnevale R, Nocella C, et al. Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: Effect in patients undergoing elective percutaneous coronary intervention. Arterioscler Thromb Vasc Biol. 2011;31:1766–71. doi: 10.1161/ATVBAHA.111.227959. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed BM, Sanford KW, Fisher BJ, Martin EJ, Contaifer D, Jr, Warncke UO, et al. Impact of high dose Vitamin C on platelet function. World J Crit Care Med. 2017;6:37–47. doi: 10.5492/wjccm.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]