Abstract
Introduction
This article describes the development and initial clinical testing of an innovative home-based treatment for upper extremity hemiplegia that integrates contralaterally controlled functional electrical stimulation with hand therapy video games.
Methods
We explored the ability of seven participants with moderate-to-severe hand impairment to self-administer 12 weeks of contralaterally controlled functional electrical stimulation video game therapy at home for 10 h/week and in-lab with a therapist for four h/week. Clinical suitability was assessed by device usage logs, qualitative surveys, and clinical motor and cognitive outcomes.
Results
Three participants completed the study with > 95% compliance and four did not. Factors linked to incompletion included development of trigger finger in the non-paretic hand, acceptance of a new full-time job, residence relocation, and persistence of drowsiness from anti-spasticity medication. Those who completed the treatment perceived qualitative benefits and experienced gains in motor and cognitive outcomes.
Conclusion
Individuals with moderate-to-severe chronic post-stroke upper extremity hemiplegia can self-administer contralaterally controlled functional electrical stimulation video game therapy for up to 90 min/day at home. We also identified social and physiological factors that may preclude its use for daily home treatment. Further studies are warranted and are in progress to estimate treatment effect and optimal dose of this intervention.
Keywords: Electrical stimulation, neurorehabilitation, virtual reality, occupational therapy, stroke rehabilitation, assistive technology
Introduction
Upper limb hemiparesis is highly persistent after stroke. After six months of conventional care, two out of three people cannot use the paretic hand for activities of daily living.1 Conventional occupational therapy, which delivers 30–60 repetitions per session, may not provide sufficient practice to improve motor relearning, as hundreds of repetitions per day are likely needed for cortical change.2 Therapists assign home programs to increase practice between clinic visits, but poor adherence due to impairment severity and low motivation causes inactivity at home.3 There is a need for therapies that can be self-administered independent of chronicity and severity to motivate paretic limb use during times when it would otherwise be idle.
Existing interventions for hemiplegia have been difficult to adapt for self-administration or home use. Specifically, constraint-induced movement therapy is effective for delivering high dose and improving hand function, but it is not tolerable for people with greater disability and are unable to use only the paretic hand for hours of task practice.4 Commercially available assistive rehabilitation robots are effective across a wider range of impairment, but adapting them for home use is an area of need.5 To make them more suitable for home use, researchers are increasing portability,6 providing assistance during physical task practice,7 and increasing engagement using video game features like rhythm following8 and multiplayer interaction.9 Neuromuscular electrical stimulation (NMES) techniques are effective for improving muscle strength and fatigue resistance,10 but they have been difficult to integrate with skilled task practice, which is important for motor relearning.11 Devices that trigger stimulation using cyclic programs, tilt sensors, or EMG fully open the hand, but do not assist graded hand opening, which is needed for finer motor skill practice.
Contralaterally controlled functional electrical stimulation (CCFES) therapy was developed to maximize paretic hand participation during skilled task practice in the clinic and at home. CCFES closely links motor intent with execution12 by transcutaneously stimulating the paretic hand open to match the opening of the unimpaired hand. It enables people with hemiplegia to use opening/closing of the unimpaired hand to control the amount of assistance to their paretic hand while practicing functional tasks. Although significant improvements in dexterity, impairment, and activity limitation measures were shown with CCFES,13–16 there is an opportunity to further improve outcomes by increasing the motor relearning qualities of the home sessions, which comprised 70% of total treatment hours in prior studies. Participants in our prior studies self-administered repetitive CCFES-assisted hand opening for 2 h/day, which delivers a high dose, but is suboptimal for motor relearning because this is not goal-oriented or skill-requiring and does not require concentration or motor planning, which are qualities that support task-dependent neuroplasticity.17
We developed hand therapy video games that work in concert with CCFES because the motor learning features of both may be complimentary and increase the treatment’s motor-relearning potential. Video games designed around principles associated with effective motor learning (goal-directed movement, performance feedback,18 difficulty shaped to skill,19 reduced compensatory movements,20 and increased motivation21) have benefited motor and cognitive function outcomes22 – potentially by recruiting cortical regions of executive control or planning.23 Our approach is novel because other NMES modalities are applied as adjuncts separately from functional task practice24 or the stimulation parameters are controlled by a therapist during virtual reality task practice.25 This intervention may improve motor outcomes in the lab and at home by delivering a high dose of practice not possible with unassisted paretic limb movement. CCFES can assist movement beyond volitional ability while individuals practice skill-requiring, goal-oriented tasks required by the hand therapy video games. Additionally, the hand therapy video games can provide performance feedback, engagement, and appropriate difficulty to motivate repetitive practice and improvement of performance. However, it is unknown if people with chronic hemiplegia can successfully self-administer CCFES video game therapy at home or what factors may affect their participation.
This article describes the integration and initial clinical use of CCFES with video games with CCFES as a cohesive therapy in the lab and at home. The goal of this proof-of-concept study was to examine the suitability of a 12-week treatment of the intervention for a small sample of people with chronic hemiplegia (>12 months) and changes to motor outcomes and cognition.
Methods
Participants
We enrolled seven participants (Table 1), who provided written informed consent to this work, which was approved and conducted in accordance to the ethical standards of the Institutional Review Board of the MetroHealth System (Cleveland, Ohio, USA). Recruitment occurred over 18 months at a rate of approximately one participant every two months. The goal was to recruit at least one participant representing each of the following categories of impairment: moderate, moderately-severe, and severe. Individuals were recruited from those who participated in our prior home-based FES studies without video games. The rationale was that if individuals familiar with FES are not able to tolerate the added complexity of self-administering hand therapy video game sessions, then those without FES experience might have greater difficulty.
Table 1.
Participant demographics.
| ID | Sex | Age (years) | Years post stroke | Dominant hand | Paretic hand | Education (years) | Stroke type |
|---|---|---|---|---|---|---|---|
| 1 | F | 67 | 11.2 | R | R | 12 | Ischemic cortical |
| 2 | M | 52 | 3.3 | R | L | 12 | Ischemic thrombotic cortical |
| 3 | M | 40 | 1.6 | R | R | 13 | Intracerebral hemorrhagic subcortical |
| 4 | M | 68 | 2 | L | L | 16 | Subcortical-right lacunar infarct in basal ganglia, corona radiata |
| 5 | F | 44 | 2.7 | R | R | 12 | Ischemic thrombotic subcortical |
| 6 | M | 77 | 7.3 | R | R | 14 | Left frontal lobe infarct |
| 7 | F | 68 | 2.7 | L | R | 15 | Pontine infarct |
Inclusion criteria included age 18–80 who were >6 months after their first stroke, ability to recall 2 of 3 words after 30 min, upper extremity hand section of the Fugl-Meyer Motor Assessment (F-M)26 score ≤11 of 14 (moderate to severe impairment), no concomitant occupational therapy, Medical Research Council scale ≤4/5 for paretic finger extensors, ability to follow three-stage commands, adequate active movement to position the paretic hand for table-top task practice, availability of caregivers to assist as-needed, intact skin on the paretic arm, tolerance of surface NMES for full paretic hand opening without pain, and full volitional opening/closing of non-paretic hand.
Exclusion criteria were any co-existing neurological conditions involving the upper limbs, severely impaired cognition or communication, uncontrolled seizure disorder, history of cardiac arrhythmias with hemodynamic instability, any implanted electronic medical device, any upper extremity intramuscular Botox injections within three months, insensate upper limb, uncompensated hemi-neglect, and severe upper limb pain.
Treatment regimen
Twelve weeks of treatment occurred in the lab and in the participant’s home. The treatment combined hand therapy video games with components that were efficacious in prior CCFES studies, which were repetitive hand opening (to facilitate full range-of-motion movement) and therapist-guided task practice (to transfer motor skills to function). In lab, participants used CCFES with 45 min of video games and 45 min of therapist-guided functional task practice. Functional tasks began with learning to coordinate CCFES assistance with volitional effort during repetitive hand opening. This progressed to practicing activities of daily living requiring hand opening, which were shaped to participant ability. Examples included using cups, containers, utensils, doorknobs, clothing, etc. At home, participants self-administered 10 45-min CCFES-assisted sessions per week, which included two 15-min game sessions and one 15-min session of repetitive hand opening exercise (6 s open, 8 s rest, 45 repetitions per session). To achieve 10 home sessions per week, 2 were assigned every day participants did not come to the lab, 1 was assigned for every lab session day, and participants were required to skip 2 sessions per week as a break. We aimed for 126 h of training per participant, which is 78 h (62%) of video games, 30 h (24%) of repetitive hand opening, and 18 h (14%) functional task practice. Actual durations varied due to adherence and rest during training sessions.
Integration of CCFES with video games
The investigational stimulator provided biphasic current-controlled transcutaneous stimulation of 40 mA at 35 Hz. Stimulation intensity was adjusted using pulse durations from 0 to 250 µs and controlled by a kinematic sensor glove worn on the non-paretic hand.
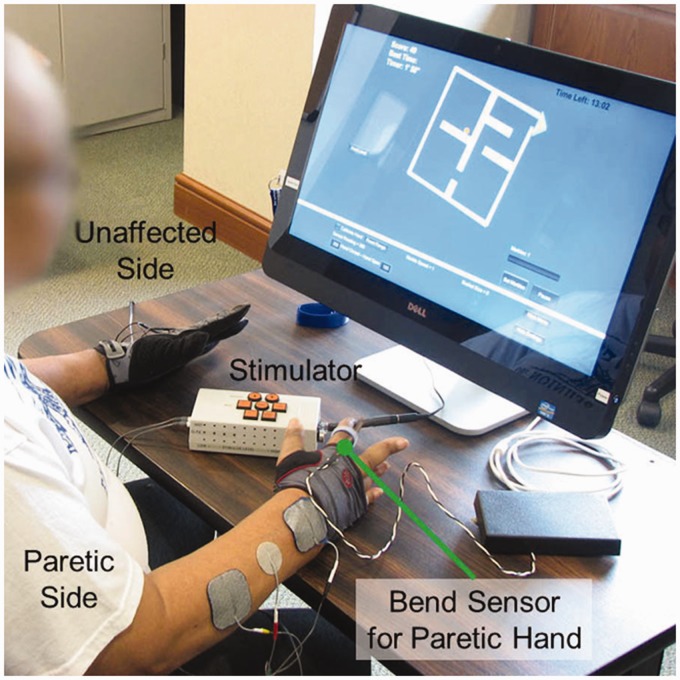
All games were programmed using Unity 3D (Unity Technologies, San Francisco, CA) and controlled by paretic hand opening/closing. The games run on a 24″ touchscreen Windows 8 (Microsoft Corp., Redmond, WA) PC (Figure 1, Dell Inspiron 2330, Dell Corp., Round Rock, TX). Game input from the paretic hand was sensed by a bend sensor (Images SI, Inc., Staten Island, NY) that was attached to a fingerless mitten (Handana Corp., Austin, TX), which was easy to don even when the fingers are not extended. The bend sensor was secured to the dorsal aspect of the finger with least residual function using Velcro rings. The bend sensor plugs into a USB analog-to-digital converter (Phidgets 8/8/8 Interface Kit) sampling at 1 kHz.
Figure 1.
Participant using contralaterally controlled functional electrical stimulation (CCFES) to assist paretic right hand opening during video game movement training.
Four CCFES-compatible games were iteratively developed and refined after play-testing by physiatrists, occupational therapists, and individuals with chronic stroke. Each game was designed to: (1) target paretic hand opening/closing motor skill, (2) have adjustable difficulty suitable for a range of impairment, (3) provide intuitive operation and presentation, (4) accurately link hand motion to game objects so that only targeted skills result in successful gameplay.27 Difficulty was adjusted to be challenging without causing frustration, which has been shown to benefit motor relearning after stroke.19
Hand therapy video games
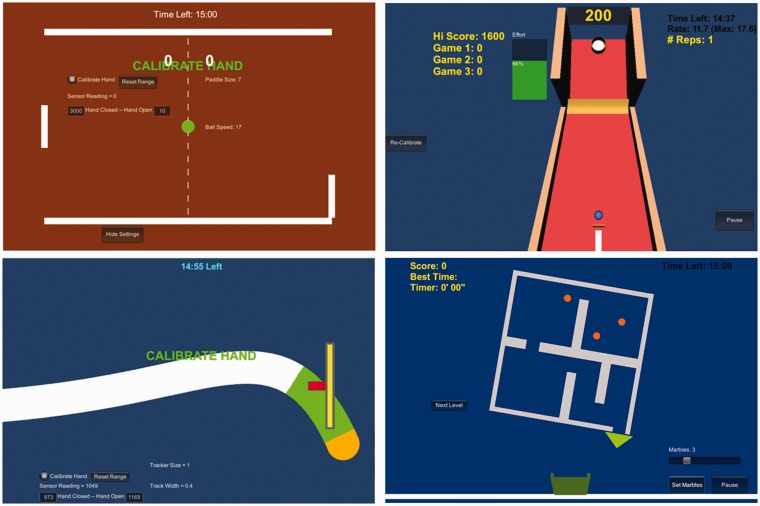
In Paddle Ball (Figure 2 upper left), the opening/closing of the paretic hand controlled a paddle’s vertical position in order to bounce a ball past the computer opponent’s paddle, which requires motor planning and graded hand opening. Difficulty was adjusted by changing paddle size to maintain a 75% hit/miss ratio. Ball speed was increased as the hand muscles became more fatigue resistant and opponent difficulty was increased as the participant’s skill improved.
Figure 2.
Screen shots from Paddle Ball (upper left), Skee Ball (upper right), Sound Tracker (lower left), and Marble Maze (lower right).
In Skee Ball (Figure 2 upper right), a ball was launched with distance proportional to the opening speed of the paretic hand. The target moved up or down the board, which required different hand opening speeds to hit. Difficulty was adjusted by changing the target ring size to maintain a 75% hit/miss ratio.
In Sound Tracker (Figure 2 lower left), the participant controlled a cursor’s vertical movement by opening and closing their paretic hand to trace a moving visual path generated by eight different songs. This requires continuous, graded finger movements, which were precision-demanding and challenged motor-learning processes that are important for motor recovery.3,28 Difficulty was adjusted by changing path and cursor widths so the participant could complete one song’s path (about 3 min) without resting.
In Marble Maze (Figure 2 lower right), the participant rotated a maze to guide marbles to fall out into a bucket at the bottom of the screen. Maze rotation was proportional to the degree of paretic hand opening. This game required sustained hand opening at graded amounts to allow marbles to roll through the maze. Difficulty was adjusted by increasing the number of concurrent marbles after a maze was completed within a target time, which required divided attention and path planning of multiple moving marbles. Once a participant completed a maze with 10 concurrent marbles, a more complex maze was presented to maintain engagement.
All games were calibrated to the paretic hand’s CCFES-assisted range of motion. Game difficulty was set by the treating therapist on a lab computer and stored on a USB drive that duplicated the settings to the home computer and logged usage and performance (play duration, scores, game statistics, and the number of hand opening repetitions).
Outcome measures
Adherence was quantified by comparing what therapists assigned with usage data logged by the video games and the stimulator, which reported the number of finger flexion/extension repetitions (half the number of finger velocity direction changes). Activity duration was defined as the amount of time spent attempting or performing video games, repetitive hand opening, or functional task practice, excluding setup or rest.
Qualitative reports were provided by participants in daily dairies and an exit interview. The study PI (Fu) conducted the interview, which consisted of questions related to impressions of therapeutic effect, impression of dose, ability to self-administer home sessions, ability to operate study devices, and impressions about the hand therapy video games. Participants reported issues related to home treatment or device use in daily diaries. Exit interviews recorded participant opinions about perceived efficacy, acceptance of the intervention, dose, and device use.
Hand impairment and function were assessed in the lab by an occupational therapist (who was different from the treating therapist, but was not blinded) on six occasions: twice at baseline (1 week apart), mid-treatment (6 weeks), end of treatment (12 weeks), one-month follow-up (16 weeks), and two months follow-up (20 weeks). Outcome measures included the upper extremity F-M (max score of 66)26 and arm motor ability test (AMAT) (max score of 5).29
Cognition was also assessed to explore how cognitive function may influence therapeutic effect or be influenced by the hand therapy video games. Cognitive function was assessed at baseline, end of treatment, and two-month follow-up (to minimize learning effects) using the reading subtest from the Wide Range Achievement Test – Fourth Ed. (to estimate premorbid cognitive ability),30 Digit Span subtest from the Wechsler Adult Intelligence Scale – Third Ed. (attention),31 Symbol Digit Modalities Test (processing speed),32 Judgment of Line Orientation (visuospatial perception),33 the Hopkins Verbal Learning Test (episodic memory),34 and the Tower Test subtest from the Delis–Kaplan Executive Functioning Systems (executive function).35
Results
Participants who did not complete the protocol
Participants 1, 2, and 5 withdrew before mid-treatment and participant 3 was withdrawn from analysis because the participant did not complete home sessions on a daily basis as instructed. Although this participant completed 52% of home sessions, most were done the day before lab visits, often after midnight. In the exit interview, he reported that it was difficult to complete home sessions due to drowsiness, which caused him to sleep most of the day and was attributed to tizanidine use (prescribed for muscle spasticity).
Participant 1 developed stenosing tenosynovitis (trigger finger) in the non-paretic hand at three weeks of treatment, which was resolved. When this adverse event occurred, she had perfect adherence, but also revealed to us a prior diagnosis of osteoarthritis in the same hand. This was the first participant enrolled in the study and was assigned the full dose of 90 min/day of home sessions on day 1. For subsequent participants, we gradually increased the duration of home sessions up to the full dose during the first two weeks. We also instructed subsequent participants to gently open/close their non-paretic hand and avoid unnecessary strain.
Participants 2 and 5 withdrew because they became unable to attend twice weekly lab visits. Participant 2 had perfect adherence before he withdrew during treatment week 5 after obtaining a full-time job and participant 5 withdrew before treatment started to relocate her residence. Participant 2 did not return for his mid-treatment assessment.
Participants who did not complete the protocol also did not require caregivers to assist with the home sessions. Participants 1 and 5 lived alone, participant 3 lived with his three-year-old child, and participant 2 lived with his spouse and teenage son.
Participants who completed the protocol
The three participants who completed the protocol represented individuals with severe impairment (participant 4 was unable to volitionally open the paretic hand), moderate impairment (participant 6 was able to open the hand despite extensor weakness), and moderately severe impairment (participant 7 had limited hand opening due to flexor tone).
Participant 4’s caregiver was a spouse who accompanied him to all lab visits. Participant 6 lived alone and did not have a caregiver. Participant 7 had a spouse, but the spouse did not attend any of the lab visits or help with the home sessions.
Participant 4 (severe impairment)
Video game assignment
Participant 4 learned a new game during the first lab visit of each week and was assigned the game for home use after he demonstrated proficiency in the lab. This gradually increased the duration of each home session by 15 min per week so that the full 45-minute dose was assigned by week 4. Participant 4 was able to learn and play the games with minimal frustration and pauses when they were introduced in the order of Paddle Ball, Sound Tracker, Skee Ball, and then Marble Maze.
Qualitative reports
Participant 4 reported in exit interviews that he wished the intervention was part of his original treatment after stroke. He perceived improvements in paretic hand opening, closing, and use at treatment end. Furthermore, he believed that he would benefit from continued device use. Although he thought that two 45-min sessions per day of home use was just right, he would have preferred three games per session, but could not tolerate that amount due to his severity. At home, he initially needed assistance from a caregiver for electrode placement, but became independent operating the stimulation device, electrodes, and touchscreen PC at the end of the first week. He felt that Marble Maze and Paddle Ball were the most entertaining and felt the level of challenge for all the games to be appropriate. Participant 4 found Sound Tracker to become boring over time due to the limited variety of music tracks. Moreover, unlike the other games, Participant 4 became discouraged if he missed any part of the track in Sound Tracker, even if his overall accuracy was over 95%. He thought the treatment duration of 12 weeks was the limit for his ability to commit consistently, since he traveled almost every weekend to visit and participate in his grandchildren’s sporting events. Being able to take the computer and CCFES device with him helped him adhere to the assigned home sessions.
Adherence and content of motor practice
Participant 4 completed 99% of the assigned twice-daily home video game and repetitive opening exercise treatment sessions (Table 2). He logged 107 h of total motor practice, 65% of which were video games, 28% repetitive hand opening exercise, and 7% functional task practice. Two missed sessions (out of 220) occurred due to out-of-state travel, though he took the device with him for shorter visits to see grandchildren. The number of repetitions per h was 631 for video games, 72.4 for functional tasks, and 178 for repetitive hand opening exercises.
Table 2.
Participant adherence.
| Video games |
Repetitive hand opening |
|||||
|---|---|---|---|---|---|---|
| ID | Hours, % of total | Sessions assigned/done | Hours, % of total | Sessions assigned/done | Functional task practice (h) | Total motor practice (h) |
| 4 | 69.9 (65) | 218/220 | 29.8 (28) | 118/119 | 7.2 (7%) | 106.9 |
| 6 | 72.3 (64) | 233/225 | 28.3 (25) | 113/113 | 13 (11%) | 113.6 |
| 7 | 98.7 (73) | 320/334 | 27.5 (20) | 104/110 | 8.2 (6%) | 134.4 |
Motor impairment
Upper extremity F-M score for Participant 4 peaked at 31 points (of 66 possible) at treatment end, which was a 3-points increase from baseline (Figure 3(a)). However, mid-treatment, one-month, and two-months follow-up F-M scores were all lower than or equal to baselines.
Figure 3.
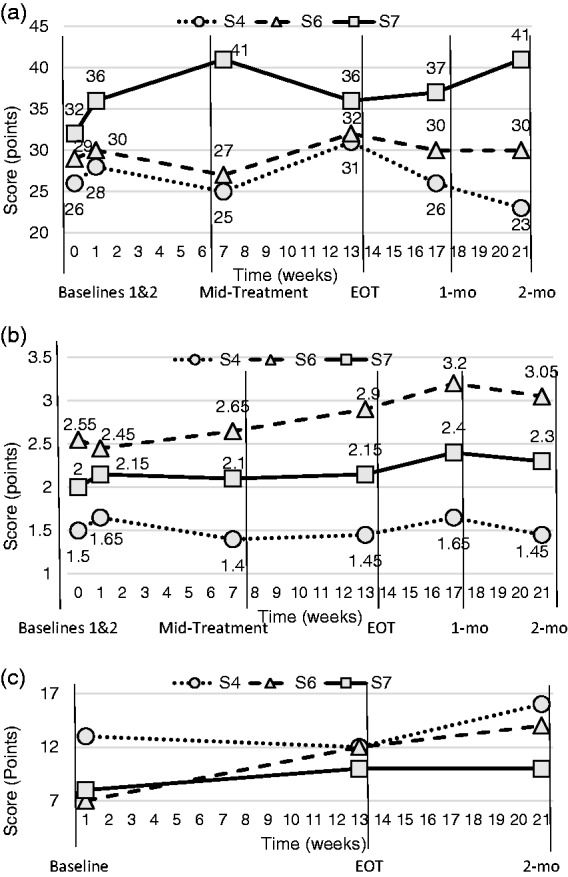
Assessment outcomes for (a) Fugl-Meyer upper extremity (impairment), (b) Arm motor abilities test (function) and (c) Delis–Kaplan towers test (cognitive executive function).
Motor function
Arm Motor Ability Test scores for Participant 4 did not increase past the baseline of 1.65 points for the entire protocol (Figure 3(b)). The minimum of 1.4 points occurred at mid-treatment.
Cognitive function
The Towers Test of executive function was the only cognitive outcome measure on which participants exhibited improvements that exceeded expected practice effects related to prior test exposure. Participant 4’s total achievement score of 12 points at treatment end was not higher than baseline of 13 points, but it increased to 16 at two months’ follow-up (Figure 3(c)).
Participant 6 (moderate impairment)
Video game assignment
The treating therapist used the same assignment strategy used for Participant 4, which was tolerated well.
Qualitative reports
Participant 6 also wished this treatment was part of his conventional care and found the treatment frequency and duration to be just right. He self-administered home sessions without caregiver assistance by the end of week 1 and perceived improved paretic hand opening, closing, and use at treatment end, which he believed would continue to improve if he continued to receive the treatment. Additionally, he found all the games engaging and appropriately difficult except for Sound Tracker, which was too easy and unengaging. As a retiree who lived alone, he reported that the social interaction with study staff during lab visits and the engagement of the games motivated him to maintain adherence.
Adherence and content of motor practice
Participant 6 had 100% adherence to both video games and repetitive hand opening exercise and received 114 h of motor practice, 64% of which were video games, 25% repetitive hand opening exercise, and 11% functional task practice (Table 2). The number of repetitions per hour was 949 for video games, 86.5 for functional tasks, and 180 for repetitive hand opening exercises.
Motor impairment
Participants 6’s F-M score peaked at 32 points at treatment end, which was a 2-points increase over baseline 2 (Figure 3(a)). The lowest score of 27 points occurred at mid-treatment and both one and two-months follow-up scores returned to the baseline 2 value of 30 points.
Motor function
Participant 6’s AMAT score peaked to 3.2 points at one-month follow-up, which was an increase of 0.75 points from baseline 2 (Figure 3(b)). At two-month follow-up, his score of 3.05 points was higher than his score of 2.9 points at end of treatment.
Cognitive function
Participant 6’s Tower Test score increased 7 points from 7 points at baseline to 14 points at two-month follow-up (Figure 3(c)).
Participant 7 (moderately severe impairment)
Video game assignment
The treating therapist used the same assignment strategy used for Participant 4, which was tolerated well.
Qualitative assessment
Participant 7 was able to place electrodes and self-administer the home sessions without caregiver help from day 1. She perceived improved paretic hand opening, closing, and use at the end of treatment. She also wished that the treatment had been part of her conventional care after her stroke. Although she felt that the lab treatment frequency and duration were just right, she would have preferred that the home sessions to last 1 h per day rather than two due to the treatment making her tired. She did not believe further participation would benefit her. Marble Maze was her favorite game, but she found all the games engaging and appropriately challenging. Participant 7 was retired and reported that a single 1-h home session per day would have been preferable to two 1-h sessions since she liked to cook a lot.
Adherence and content of motor practice
Participant 7 adhered to 96% of assigned video game sessions, 95% of repetitive hand opening exercises, and received 134 h of motor practice (73% of which were video games, 20% repetitive hand opening exercise, and 6% functional task practice, Table 2). The number of repetitions per hour was 916 for video games, 44.9 for functional tasks, and 170 for repetitive hand opening exercises. She developed positional vertigo (unrelated to the study) during week 5 of treatment, which resolved with vestibular therapy. Her primary care provider recommended that she halt study treatment for 7 days during week 6 of the protocol, while she received vestibular therapy, which caused her to miss 2 lab visits, 14 video game sessions, and 14 hand opening exercise sessions.
Motor impairment
Participant 7’s F-M score peaked to 41 points at mid-treatment, which increased 5 points from baseline 2 (Figure 3(a)). However, the score regressed to baseline 2’s 36 points by the end of treatment. At two-month follow-up her score had increased to 41, the peak at mid-treatment.
Motor function
AMAT score for Participant 7 at mid-treatment and treatment end did not exceed the baseline 2 score of 2.15 points, but one-month and two-month follow-up scores (2.4 points and 2.3 points, respectively) were increased over baselines (Figure 3(b)).
Cognitive function
Participant 7’s Tower Test score increased 2 points, from 8 points at baseline to 10 points at treatment end, which was maintained at follow-up (Figure 3(c)).
Discussion
This study demonstrated that individuals with varying severity of hemiplegia are able to self-administer CCFES integrated with video games at home for up to 90 min per day over 12 weeks. Even though four participants were not able to complete the protocol as instructed, much was learned from their experience.
Lessons learned
Factors that prevented our participants from completing the 2-h per day dose of this intervention include osteoarthritis or trigger finger of the non-paretic hand, full-time employment, and severity of anti-spasticity medication side-effects. Participant 3 reported drowsiness from tizanidine had limited ability to adhere to home treatment. Additionally, the frequency and duration of the intervention may need to be reduced to be more suitable for people with hemiplegia and full-time employment. Participant 2 prioritized employment over participation because he was unable to continue his prior career after stroke, but still wanted to financially support his teenage son. Furthermore, CCFES requires bilateral hand use, so highly repetitive use of the non-paretic hand may exacerbate prior osteoarthritis or trigger finger. Gradually increasing home treatment duration to prevent pain in these individuals needs to be further studied.
Order of game introduction
Although further study is also needed to determine if the order and frequency of game use has any effect, in this study, Paddle Ball was introduced first because it only requires episodic movements, which allows for rest between hits. Sound Tracker was introduced second because it requires continuous movement that does not allow for rest, but the control scheme is like Paddle Ball, which the participants became familiar with. Due to the greater stamina required by continuous movement, we assigned Sound Tracker in 7-min sessions for home use until participants were able to complete 15 min in the lab without needing to pause the game for rest. We also discovered a need to improve Sound Tracker to be more engaging, as both participants 1 and 2 found the game too easy and boring as the eight song selections lost their novelty. Skee Ball was assigned after participants were able to use CCFES to assist rapid hand opening without causing spasticity. Marble Maze was introduced last because it required strength to maintain specific degrees of hand opening.
Clinical outcomes
Although these case studies provide some indication that CCFES integrated with hand therapy video games may be more efficacious than CCFES alone, this requires further investigation, which is ongoing in a randomized clinical trial.36 Participants who completed the protocol experienced modest outcome improvements, some of which exceeded gains of participants with similar baseline impairment who received a comparable dose of CCFES therapy without video games in our previous randomized control trial (RCT).13 Participant 4 experienced the least change in hand function (AMAT) of the three, but he also had the most severe hand impairment and was unable to volitionally open the paretic hand. He did improve 3 points in F-M (from baseline 2 to treatment end), which was 25% greater than the average gain of 2.4 points by severely impaired participants > 2 years post-stroke who received a comparable dose of CCFES therapy without video games (repetitive hand opening and task practice only) in our previous randomized control trial (RCT).13 Despite this modest improvement in motor outcomes, he improved performance on the Towers Test of executive function, which requires participants to use the non-paretic hand to stack five rings onto three pegs in different configurations using the fewest moves possible. This could indicate that the treatment influences cognitive and motor processes independently.
Participant 6 exhibited the greatest improvements in both hand (AMAT) and executive function (Towers Test), which were maintained at both one and two-month follow-ups. The change in F-M of 3 points at treatment end did not exceed the clinically important difference of 5.25 for overall upper extremity function.37 The improvement in AMAT by 0.75 points (from baseline 2 to one-month follow-up) exceeds the clinically important difference of 0.4438 and the average gain of 0.1 made by moderately impaired participants > 2 years post-stroke in our previous CCFES RCT.13 Additionally, improvement of 7 points in the Towers Test (from baseline to two-month follow-up) exceeded the practice effect of 2.1 points.39 The improvement could be linked to him achieving the highest practice repetitions per hour of the three participants and/or his relatively greater baseline function. He also reported using the paretic hand more than before during activities of daily living at end of treatment.
Participant 7 also improved performance on motor and executive function tasks that were maintained at follow-ups. However, the 0.25-point AMAT improvement did not exceed the clinically important difference and the 2 points Towers Test increase could be attributed to practice effect. These case studies provide some indication that CCFES integrated with hand therapy video games may be more efficacious than CCFES alone and may widen the time post-stroke window in which patients may achieve significant benefit.
Limitations
“The modest motor outcome gains may not be sufficient to improve daily use of the participants’ paretic hand. Furthermore, inclusion criteria for this exploratory trial included individuals who may not be optimal responders to CCFES therapy. In work published after the current study began, we found evidence that individuals with moderate hand impairment (>10 degrees of wrist and finger extension) and <2 years post-stroke gained the most hand dexterity from CCFES without video games compared to cyclic stimulation.”
A source of potential bias in this study could stem from the lack of blinding of participants and study staff. Although the assessing therapist did not treat the participants, she was not blinded to the intervention. Additionally, all the participants in this study have been a part of at least one prior research study involving functional electrical stimulation alone, without video games, which may have biased them to be more familiar to the stimulation equipment. This study was also unable to determine the independent therapeutic effects of the intervention’s components (CCFES, hand therapy video games and functional task practice). Because of the added equipment and complexity of the hand therapy video games, it is important to estimate the independent therapeutic effect of hand therapy video games, which is underway in an ongoing randomized controlled trial that compares a group treated with CCFES alone or CCFES with hand therapy video games.36
Conclusion
This work demonstrated that some individuals with hand hemiplegia due to stroke are capable of self-administering 8–10 h/week of a relatively complex home therapy that integrates CCFES with video games, but severely impaired individuals may require caregiver support. Additionally, all participants expressed the desire for this intervention to have been a part of their original care because it was engaging, intuitive to operate, and gave them a sense of improved upper limb function. Furthermore, the two less-impaired participants benefitted most in hand function and executive function outcomes. These findings have implications for the development of other self-administered interventions and have informed the design of our ongoing randomized trials estimating this intervention’s treatment effect and dose.
Acknowledgements
The authors would like to acknowledge the contributions of Amy Friedl OTR/L as outcome assessor, Margaret Maloney RN as study nurse, Lisa Ferguson for cognitive assessment support, and study physician Lynne Sheffler MD.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Authors Knutson and Chae are inventors of CCFES (US Patent US8165685B1). Authors Fu, Knutson, and Chae were also inventors of hand therapy video games. Both CCFES and hand therapy video game technology were licensed by Case Western Reserve University to Synapse Biomedical Corp. (Oberlin, OH, USA) on 1 February 2019.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH/NCATS CTSA UL1TR000439 and KL2TR000440.
Guarantor
MJF
Contributorship
All authors conceived the principles of the methodology. MJF, MYH, RW and TH carried out the experiments. MJF, JC, JSK, and RB analyzed the results. MJF wrote the first draft of the paper and all authors reviewed and edited the manuscript and approved the final version of the manuscript.
Authors’ note
Data from this research study can be obtained by contacting the corresponding author, Michael Fu at mfu@metrohealth.org
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil 2009; 90: 1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingunn Kåringen I and Dysvik E. The elderly stroke patient’s long-term adherence to physiotherapy home exercises. Adv Physiother 2011; 13: 145–152.
- 4.Fabbrini S, Casati G, Bonaiuti D. Is CIMT a rehabilitative practice for everyone? Predictive factors and feasibility. Eur J Phys Rehabil Med 2014; 50: 505–514. [PubMed] [Google Scholar]
- 5.Laut J, Porfiri M, Raghavan P. The present and future of robotic technology in rehabilitation. Curr Phys Med Rehabil Rep 2016; 4: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunaway S, Dezsi DB, Perkins J, et al. Case report on the use of a custom myoelectric elbow-wrist-hand orthosis for the remediation of upper extremity paresis and loss of function in chronic stroke. Mil Med 2017; 182: e1963–e1968. [DOI] [PubMed] [Google Scholar]
- 7.Polygerinos P, Wang Z, Galloway KC, et al. Soft robotic glove for combined assistance and at-home rehabilitation. Robot Auton Syst 2015; 73: 135–143. [Google Scholar]
- 8.Taheri H, Rowe JB, Gardner D, et al. Robot-assisted Guitar Hero for finger rehabilitation after stroke. Conf Proc IEEE Eng Med Biol Soc 2012; 2012: 3911–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak D, Nagle A, Keller U, et al. Increasing motivation in robot-aided arm rehabilitation with competitive and cooperative gameplay. J Neuroeng Rehabil 2014; 11: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson JS, Fu MJ, Sheffler LR, et al. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys Med Rehabil Clin 2015; 26: 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve 2001; 24: 1000–1019. [DOI] [PubMed] [Google Scholar]
- 12.Rushton DN. Functional electrical stimulation and rehabilitation – an hypothesis. Med Eng Phys 2003; 25: 75–78. [DOI] [PubMed] [Google Scholar]
- 13.Knutson JS, Gunzler DD, Wilson RD, et al. Contralaterally controlled functional electrical stimulation improves hand dexterity in chronic hemiparesis. Stroke 2016; 47: 2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson JS, Harley MY, Hisel TZ, et al. Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early-phase randomized clinical trial in subacute stroke patients. Neurorehabil Neural Repair 2012; 26: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson JS, Hisel TZ, Harley MY, et al. A novel functional electrical stimulation treatment for recovery of hand function in hemiplegia: 12-week pilot study. Neurorehabil Neural Repair 2009; 23: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson JS, Harley MY, Hisel TZ, et al. Improving hand function in stroke survivors: a pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch Phys Med Rehabil 2007; 88: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci 2013; 7: 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian SK, Massie CL, Malcolm MP, et al. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabil Neural Repair 2010; 24: 113–124. [DOI] [PubMed] [Google Scholar]
- 19.da Silva Cameirão M, Bermúdez I, Badia S, Duarte E, et al. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system. Restor Neurol Neurosci 2011; 29: 287–298. [DOI] [PubMed] [Google Scholar]
- 20.Alankus G and Kelleher C. Reducing compensatory motions in video games for stroke rehabilitation. USA: ACM Press, p.2049.
- 21.Putrino D. Telerehabilitation and emerging virtual reality approaches to stroke rehabilitation. Curr Opin Neurol 2014; 27: 631–636. [DOI] [PubMed] [Google Scholar]
- 22.Gamito P, Oliveira J, Coelho C, et al. Cognitive training on stroke patients via virtual reality-based serious games. Disabil Rehabil 2017; 39: 385–388. [DOI] [PubMed] [Google Scholar]
- 23.Orihuela-Espina F, Fernández del Castillo I, Palafox L, et al. Neural reorganization accompanying upper limb motor rehabilitation from stroke with virtual reality-based gesture therapy. Top Stroke Rehabil 2013; 20: 197–209. [DOI] [PubMed] [Google Scholar]
- 24.Gharib NM, Aboumousa AM, Elowishy AA, et al. Efficacy of electrical stimulation as an adjunct to repetitive task practice therapy on skilled hand performance in hemiparetic stroke patients: a randomized controlled trial. Clin Rehabil 2015; 29: 355–364. DOI: 10.1177/0269215514544131. [DOI] [PubMed]
- 25.Lee SH, Lee J-Y, Kim M-Y, et al. Virtual reality rehabilitation with functional electrical stimulation improves upper extremity function in patients with chronic stroke: a pilot randomized controlled study. Arch Phys Med Rehabil 2018; 99: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 27.Lange B, Koenig S, Chang C-Y, et al. Designing informed game-based rehabilitation tasks leveraging advances in virtual reality. Disabil Rehabil 2012; 34: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt E, Nagpal A, Greer KH, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res 2007; 182: 435–447. [DOI] [PubMed] [Google Scholar]
- 29.Kopp B, Kunkel A, Flor H, et al. The arm motor ability test: reliability, validity, and sensitivity to change of an instrument for assessing disabilities in activities of daily living. Arch Phys Med Rehabil 1997; 78: 615–620. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson G, Robertson G. Wide range achievement test., 4th ed Lutz, FL: Psychological Assessment Resources, Inc., 2006. [Google Scholar]
- 31.Wechsler D. Wechsler adult intelligence scale., 3rd ed San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 32.Smith A. Symbol digit modalities test: manual. Los Angeles, CA: Psychological Services.
- 33.Benton AL (ed). Contributions to neuropsychological assessment: a clinical manual. 2nd ed. New York: Oxford University Press, 1994.
- 34.Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991; 5: 125–142. [Google Scholar]
- 35.Delis D, Kaplan E, Kramer JH. Delis-Kaplan executive function system, San Antonio, TX: NCS Pearson, Inc., 2001. [Google Scholar]
- 36.Contralaterally controlled functional electrical stimulation plus video games for hand therapy after stroke – NCT03058796. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03058796 (accessed 4 November 2018).
- 37.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92: 791–798. [DOI] [PubMed] [Google Scholar]
- 38.Fulk G, Martin R, Page SJ. Clinically important difference of the arm motor ability test in stroke survivors. Neurorehabil Neural Repair 2017; 31: 272–279. [DOI] [PubMed] [Google Scholar]
- 39.Delis D, Kaplan E, Kramer JH. Delis-Kaplan executive function system technical manual, San Antonio, TX: The Psychological Corporation, 2011. [Google Scholar]