Previvors are individuals who have a much greater predisposition to cancer than individuals in the general population but who have not yet developed the disease. This group comprises individuals with deleterious mutations, family histories of cancer, and other high-risk factors for cancer 1. Preventive strategies targeting previvors correspond to the earliest measure of cancer prevention 2,3. Interventions addressed to previvors are more efficient in the sense that a greater benefit can be observed per individual receiving the intervention 2. Ovarian cancer is the most lethal of all gynecological malignancies, and screening programs do not significantly decrease mortality from this disease, although previvors can currently be well identified 4-7. Women with germline pathogenic mutations of the BRCA1 and/or BRCA2 genes have a lifetime risk of ovarian cancer development that ranges from 20% to 65% 8-10. For these ovarian cancer previvors, the concept of taking action to avoid cancer is incipient, and well-structured strategies and programs are lacking 11,12.
The origin of precursor lesions and ovarian cancer
High-grade serous ovarian carcinoma (HGSC), the most frequent and aggressive histological type of ovarian cancer, originates in fimbrial cells that are secondarily implanted in the ovary 13. This type of carcinoma can start in the fallopian tubes and implant and grow in the ovary at an early stage, or it can originate in normal tubal cells implanted in the ovulatory wound during ovulation 13. The origin of cancer depends on local factors and/or host fragility 14,15. Women with mutations in the BRCA genes carry conditions that favor the development of HGSC in the tubal epithelium 15. It is therefore reasonable to consider that efforts to prevent ovarian cancer should focus on intervention in the fimbriae to prevent cell implantation in the ovaries, and thus, cancer development.
Factors that prevent fimbrial cell implantation in the ovary, such as the prolonged use of anovulatory contraceptives and salpingectomy for any reason, have been shown to significantly reduce the incidence of ovarian cancer 16,17. The implantation of fimbrial cells in the ovary begins with the onset of ovulation. The amount of ovulation occurring over a woman's lifetime is closely related to the incidence rate of ovarian cancer 18,19. It is reasonable to suppose that earlier interruptions of fimbrial cell implantation will have a greater benefit in the prevention of ovarian cancer.
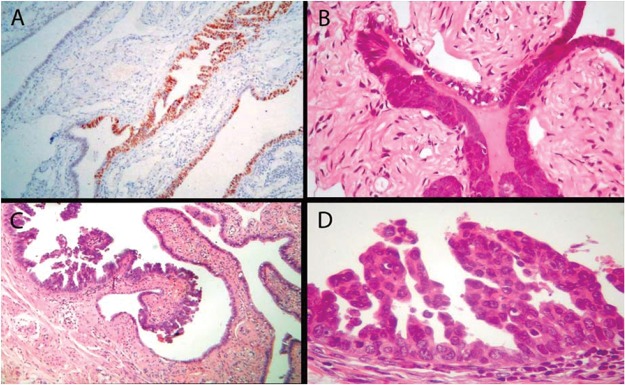
Precursor lesions and early serous carcinoma were first identified in specimens from prophylactic salpingo-oophorectomies performed in high-risk patients 20,21. Examples of these precursors, which precede the onset of invasive ovarian cancer by several years, are the p53 protein signature, atypia in hyperplasic epithelium, and serous tubal intraepithelial carcinoma (STIC) 21 (Figure 1). Precursor cancer is a definable pathological state that progresses to cancer and can be targeted to prevent cancer progression 2. The HGSC precursor, termed the p53 signature, precedes invasive ovarian carcinoma by decades, and STIC precedes carcinoma by at least 6 years 21-23. Therefore, acting on these precursors seems to be a good strategy to prevent HGSC, but this strategy should be implemented before the age at which prophylactic surgery has to been performed.
Figure 1.
Spectrum of tubal fimbrial lesions. (A) Epithelial cells expressing p53 (p53 signature), (B) hyperplastic tubal epithelium with atypia, (C) and (D) STIC.
Ovarian cancer in previvors in the genetic testing era
Many factors have contributed to the increased identification of ovarian cancer previvors, including a) the advent and popularization of genetic testing, b) increased interest in the identification of ovarian carcinoma survivors with pathogenic mutations who are candidates for targeted therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, and c) the identification of the relatives of these patients who also carry pathogenic mutations 24-26. The major question is, what measures should we take with these ovarian cancer previvors beyond the recommended prophylactic salpingo-oophorectomy, which has undesirable consequences?
Prophylactic salpingo-oophorectomy is currently recommended for women with deleterious BRCA1 mutations at 35-40 years of age (or at any time after childbearing is completed). For those with deleterious BRCA2 mutations, this surgery is recommended at 40-45 years of age 27,28. However, the performance of prophylactic surgery at the recommended age confers protection in approximately 80-90% of cases 29-31. In addition, a considerable number of women already present with occult lesions at the time of surgery, and the disease may develop in the peritoneum. Hidden serous carcinoma or STIC has been detected in 1-17% of surgical specimens obtained during such surgeries 32,33.
In a prospective study that followed 5,783 women with a BRCA1 or BRCA2 mutation for 5.6 years, 186 ovarian, fallopian, and peritoneal cancers were observed in addition to the 46 occult cancers observed at the time of salpingo-oophorectomy and the 32 peritoneal cancers observed after oophorectomy 34. In this study, the specimens from the risk reduction surgeries performed in the patients with deleterious BRCA1 mutations, the detection frequency of occult carcinoma varied with age (1.5% at <40 years old, 3.8% at 40-49 years old, and over 7% at >50 years old, reaching 12% at 60-64 years old) 34. The occurrence rate of peritoneal carcinoma after salpingo-oophorectomy in patients with deleterious BRCA1/BRCA2 mutations ranged from 0.8% to 1.8% 30,35-37. Moreover, most women do not undergo prophylactic salpingo-oophorectomy at the recommended age; approximately only 17% undergo surgery before the age of 40 years old 38.
Prophylactic salpingo-oophorectomy has a great impact on women's quality of life, as it results in premature menopause, vasomotor symptoms, sexual dysfunction, cardiovascular disease, osteoporosis, cognitive deficits, and an increased risk of premature death 39-46. These undesired effects are the main barriers to patients' adherence to these prophylactic procedures, even in the face of the great risk of ovarian cancer 47,48.
Prophylactic salpingectomy with delayed oophorectomy
An alternative to prophylactic salpingo-oophorectomy, which does not result in early menopause, is prophylactic salpingectomy with delayed oophorectomy. The theoretical benefit of this procedure is based on the expectation that early removal of the fallopian tubes prevents the implantation of fimbrial cells in the ovulatory wound. Thus, early salpingectomy provides greater protection than late salpingectomy.
Bilateral salpingectomy with delayed oophorectomy is less damaging to women than salpingo-oophorectomy; consequently, this procedure has a greater the chance to be accepted by women 9,49-53. A prospective, nonrandomized, pilot study with 43 premenopausal patients with a BRCA mutation was conducted, and 19 (44%) patients chose salpingectomy with delayed oophorectomy, 12 (28%) patients chose salpingo-oophorectomy, and 12 (28%) patients chose to be screened only 52. The patients who underwent salpingectomy were satisfied with their choice and had decreased worry and anxiety about cancer after the surgery. In a qualitative study performed with 39 BRCA1/2 mutation carriers and 23 health professionals using explorative interviews, the maintenance of ovarian function with the delay of the negative effects of early menopause and infertility was considered a facilitator influencing the choice to undergo salpingectomy with delayed oophorectomy instead of salpingo-oophorectomy by both patients and treating professionals 50. On the other hand, the seriousness of ovarian cancer and the lack of strong evidence for the new strategy worries professionals and patients 50,51. However, although there are no large prospective randomized studies indicating increased safety and a reduced risk of ovarian cancer with salpingectomy compared to the safety and risk with oophorectomy, we have some promising evidence supporting this concept. A simulation model was developed to estimate the costs and benefits of the following three risk-reducing strategies in BRCA mutation carriers: bilateral salpingo-oophorectomy at 40 years old, bilateral salpingectomy at 40 years old, and bilateral salpingectomy at 40 years old followed by bilateral oophorectomy at 50 years old 54. Although bilateral salpingo-oophorectomy was associated with the greatest risk reduction for ovarian cancer, when quality of life was included in the model, bilateral salpingectomy with delayed oophorectomy proved to be an acceptable alternative for those unwilling to undergo the first procedure 54.
The proportion of patients who choose delayed oophorectomy suggests that patient accrual for a clinical trial of prophylactic salpingectomy with delayed oophorectomy is possible.
Preimplantation genetic diagnosis (PGD)
Women with pathogenic mutations related to ovarian cancer have a high risk of developing ovarian cancer and may also transmit these mutations to their offspring. Current genetic testing enables the identification of pathogenic germline mutations in women of any age and in embryos.
Preimplantation genetic diagnosis (PGD) is considered to be an acceptable intervention to prevent the transmission of deleterious genetic conditions to the next generation, although much ethical controversy surrounds the consideration of which deleterious conditions should be deemed serious enough to justify such an intervention 55-57. PGD decisions are complex, as reflected in a study conducted in Israel with 70 women with deleterious BRCA1/2 mutations, for whom the possibility of preventing the transmission of these mutations through PGD and IVF was offered at no cost. Only 25.7% of patients accepted the proposal, and acceptance had no relationship to age or religious beliefs 58.
The combined performance of early prophylactic salpingectomy, oocyte uptake, IVF, and, when acceptable, PGD, would have many advantages over oophorectomy, at least from a theoretical point of view. First, it would not only preserve ovarian function but also enable pregnancy with oocyte uptake and IVF. The need for IVF, in turn, presents a great opportunity for PGD. Considering that precursors (the p53 signature and STIC) predate ovarian cancer by decades or years, this strategy could provide an opportunity for early intervention. Moreover, salpingectomy could even be performed in young women with pathogenic mutations before they first ovulate. This strategy, combined with oocyte uptake, IVF, and PGD, would allow the implantation of healthy embryos in mothers at a reduced risk of ovarian cancer while preventing infertility due to premature ovarian failure and/or early menopause 59,60. Women adhering to this strategy would have more time for oocyte capture and the growth of healthy embryos for future implantation than would those expecting through natural pregnancies.
Consequences of the proposal and discussion
When considered alone, many concepts, such as prophylactic salpingo-oophorectomy, prophylactic salpingectomy with delayed oophorectomy, oocyte uptake, IVF, PGD, ovarian failure, and premature menopause, may be difficult for laypeople to understand. A strategy combining all of these concepts, named “the maximal effort to prevent ovarian cancer while preserving the ovaries,” should be proposed to high-risk patients and might be better accepted by a large number of women.
This strategy could be tested in a randomized, global study that addresses all aspects of this complex issue from the perspectives of oncology, human reproduction, genetics, legality, medical ethics, and relevant educational, cultural, psychosocial, and religious aspects.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Dean M, Scherr CL, Clements M, Koruo R, Martinez J, Ross A. “When information is not enough”: A model for understanding BRCA-positive previvors’ information needs regarding hereditary breast and ovarian cancer risk. Patient Educ Couns. 2017;100((9)):1738–43. doi: 10.1016/j.pec.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Wacholder S. Precursors in cancer epidemiology: aligning definition and function. Cancer Epidemiol Biomarkers Prev. 2013;22((4)):521–7. doi: 10.1158/1055-9965.EPI-13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, et al. The Case for a Pre-Cancer Genome Atlas (PCGA) Cancer Prev Res (Phila) 2016;9((2)):119–24. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn N. Ovarian cancer--a challenging disease. Gynecol Oncol. 1996;63((1)):10–3. doi: 10.1006/gyno.1996.0269. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn N, Bast R, Knapp R, Nilsson B, Zurawski V, Jr, Sjövall K. Long-term follow-up of the Stockholm screening study on ovarian cancer. Gynecol Oncol. 2000;79((3)):466–70. doi: 10.1006/gyno.2000.5983. [DOI] [PubMed] [Google Scholar]
- 6.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306((17)):1865–73. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387((10022)):945–56. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daum H, Peretz T, Laufer N. BRCA mutations and reproduction. Fertil Steril. 2018;109((1)):33–8. doi: 10.1016/j.fertnstert.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Long Roche KC, Abu-Rustum NR, Nourmoussavi M, Zivanovic O. Risk-reducing salpingectomy: Let us be opportunistic. Cancer. 2017;123((10)):1714–20. doi: 10.1002/cncr.30528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105((11)):812–22. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 11.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317((23)):2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 12.Streff H, Profato J, Ye Y, Nebgen D, Peterson SK, Singletary C, et al. Cancer Incidence in First- and Second-Degree Relatives of BRCA1 and BRCA2 Mutation Carriers. Oncologist. 2016;21((7)):869–74. doi: 10.1634/theoncologist.2015-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42((7)):918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho JP, Carvalho FM. Is Chlamydia-infected tubal fimbria the origin of ovarian cancer? Med Hypotheses. 2008;71((5)):690–3. doi: 10.1016/j.mehy.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Crum C, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5((1)):35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev. 2017;26((1)):55–62. doi: 10.1097/CEJ.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 17.Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107((2)):dju410. doi: 10.1093/jnci/dju410. pii. [DOI] [PubMed] [Google Scholar]
- 18.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2((7716)):163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 19.Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn. 2013;5((4)):292–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto M, Crum CP, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol Oncol. 2008;111((2)):226–32. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8((1)):1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu RC, Wang P, Lin SF, Zhang M, Song Q, Chu T, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248((1)):41–50. doi: 10.1002/path.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soong TR, Kolin DL, Teschan NJ, Crum CP. Back to the Future? The Fallopian Tube, Precursor Escape and a Dualistic Model of High-Grade Serous Carcinogenesis. Cancers (Basel) 2018;10((12)):E468. doi: 10.3390/cancers10120468. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winters S, Martin C, Murphy D, Shokar NK. Breast Cancer Epidemiology, Prevention, and Screening. Prog Mol Biol Transl Sci. 2017;151:1–32. doi: 10.1016/bs.pmbts.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Wallace AJ. New challenges for BRCA testing: a view from the diagnostic laboratory. Eur J Hum Genet. 2016;24((Suppl 1)):S10–8. doi: 10.1038/ejhg.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eccles DM, Balmaãa J, Clune J, Ehlken B, Gohlke A, Hirst C, et al. Selecting Patients with Ovarian Cancer for Germline BRCA Mutation Testing: Findings from Guidelines and a Systematic Literature Review. Adv Ther. 2016;33((2)):129–50. doi: 10.1007/s12325-016-0281-1. [DOI] [PubMed] [Google Scholar]
- 27.Tucker PE, Cohen PA. Review Article: Sexuality and Risk-Reducing Salpingo-oophorectomy. Int J Gynecol Cancer. 2017;27((4)):847–52. doi: 10.1097/IGC.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 28.Tschernichovsky R, Goodman A. Risk-Reducing Strategies for Ovarian Cancer in BRCA Mutation Carriers: A Balancing Act. Oncologist. 2017;22((4)):450–9. doi: 10.1634/theoncologist.2016-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296((2)):185–92. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 30.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101((2)):80–7. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menkiszak J, Chudecka-Głaz A, Gronwald J, Cymbaluk-Płoska A, Celewicz A, Świniarska M, et al. Prophylactic salpingo-oophorectomy in BRCA1 mutation carriers and postoperative incidence of peritoneal and breast cancers. J Ovarian Res. 2016;9:11. doi: 10.1186/s13048-016-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25((25)):3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 33.Mingels MJ, Roelofsen T, van der Laak JA, de Hullu JA, van Ham MA, Massuger LF, et al. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol Oncol. 2012;127((1)):88–93. doi: 10.1016/j.ygyno.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32((15)):1547–53. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304((9)):967–75. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebbeck TR. Prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers. Eur J Cancer. 2002;38((Suppl 6)):S15–7. doi: 10.1016/S0959-8049(02)00269-1. [DOI] [PubMed] [Google Scholar]
- 37.Powell CB. Risk reducing salpingo-oophorectomy for BRCA mutation carriers: twenty years later. Gynecol Oncol. 2014;132((2)):261–3. doi: 10.1016/j.ygyno.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Garcia C, Wendt J, Lyon L, Jones J, Littell RD, Armstrong MA, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014;132((2)):428–33. doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121((1)):163–8. doi: 10.1016/j.ygyno.2010.12.326. [DOI] [PubMed] [Google Scholar]
- 40.Finch A, Narod SA. Quality of life and health status after prophylactic salpingo-oophorectomy in women who carry a BRCA mutation: A review. Maturitas. 2011;70((3)):261–5. doi: 10.1016/j.maturitas.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Svejme O, Ahlborg HG, Nilsson J, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG. 2012;119((7)):810–6. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- 42.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5((3-4)):257–60. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14((3)):111–6. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arts-de Jong M, Maas AH, Massuger LF, Hoogerbrugge N, de Hullu JA. BRCA1/2 mutation carriers are potentially at higher cardiovascular risk. Crit Rev Oncol Hematol. 2014;91((2)):159–71. doi: 10.1016/j.critrevonc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Lipschutz DI. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;122((2 Pt 1)):395–6. doi: 10.1097/AOG.0b013e31829d4376. [DOI] [PubMed] [Google Scholar]
- 46.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121((4)):709–16. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antill Y, Reynolds J, Young MA, Kirk J, Tucker K, Bogtstra T, et al. Risk-reducing surgery in women with familial susceptibility for breast and/or ovarian cancer. Eur J Cancer. 2006;42((5)):621–8. doi: 10.1016/j.ejca.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 48.De LeeuwJR, van Vliet MJ, Ausems MG. Predictors of choosing life-long screening or prophylactic surgery in women at high and moderate risk for breast and ovarian cancer. Fam Cancer. 2008;7((4)):347–59. doi: 10.1007/s10689-008-9189-5. [DOI] [PubMed] [Google Scholar]
- 49.Harmsen MG, IntHout J, Arts-de Jong M, Hoogerbrugge N, Massuger LF, Hermens RP, et al. Salpingectomy With Delayed Oophorectomy in BRCA1/2 Mutation Carriers: Estimating Ovarian Cancer Risk. Obstet Gynecol. 2016;127((6)):1054–63. doi: 10.1097/AOG.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 50.Arts-de Jong M, Harmsen MG, Hoogerbrugge N, Massuger LF, Hermens RP, de Hullu JA. Risk-reducing salpingectomy with delayed oophorectomy in BRCA1/2 mutation carriers: patients’ and professionals’ perspectives. Gynecol Oncol. 2015;136((2)):305–10. doi: 10.1016/j.ygyno.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Harmsen MG, Arts-de Jong M, Hoogerbrugge N, Maas AH, Prins JB, Bulten J, et al. Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers (TUBA study): a prospective non-randomised multicentre study. BMC Cancer. 2015;15:593. doi: 10.1186/s12885-015-1597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nebgen DR, Hurteau J, Holman LL, Bradford A, Munsell MF, Soletsky BR, et al. Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: A pilot study in women with BRCA1/2 mutations. Gynecol Oncol. 2018;150((1)):79–84. doi: 10.1016/j.ygyno.2018.04.564. [DOI] [PubMed] [Google Scholar]
- 53.Dilley SE, Straughn JM, Leath CA 3rd The Evolution of and Evidence for Opportunistic Salpingectomy. Obstet Gynecol. 2017;130((4)):814–24. doi: 10.1097/AOG.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 54.Kwon JS, Tinker A, Pansegrau G, McAlpine J, Housty M, McCullum M, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121((1)):14–24. doi: 10.1097/AOG.0b013e3182783c2f. [DOI] [PubMed] [Google Scholar]
- 55.Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344((6268)):768–70. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 56.Chen HF, Chen SU, Ma GC, Hsieh ST, Tsai HD, Yang YS, et al. Preimplantation genetic diagnosis and screening: Current status and future challenges. J Formos Med Assoc. 2018;117((2)):94–100. doi: 10.1016/j.jfma.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Cimadomo D, Capalbo A, Ubaldi FM, Scarica C, Palagiano A, Canipari R, et al. The Impact of Biopsy on Human Embryo Developmental Potential during Preimplantation Genetic Diagnosis. Biomed Res Int. 2016;2016:7193075. doi: 10.1155/2016/7193075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mor P, Brennenstuhl S, Metcalfe KA. Uptake of Preimplantation Genetic Diagnosis in Female BRCA1 and BRCA2 Mutation Carriers. J Genet Couns. 2018;27((6)):1386–94. doi: 10.1007/s10897-018-0264-2. [DOI] [PubMed] [Google Scholar]
- 59.de la Noval BD. Potential implications on female fertility and reproductive lifespan in BRCA germline mutation women. Arch Gynecol Obstet. 2016;294((5)):1099–103. doi: 10.1007/s00404-016-4187-6. [DOI] [PubMed] [Google Scholar]
- 60.Lambertini M, Goldrat O, Toss A, Azim HA, Jr, Peccatori FA, Ignatiadis M, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev. 2017;59:61–70. doi: 10.1016/j.ctrv.2017.07.001. [DOI] [PubMed] [Google Scholar]