Abstract
Objective
To compare the clinical characteristics of a group of men and women with rheumatoid arthritis (RA) and determine the differences between genders.
Materials and Methods
A descriptive and comparative cross-sectional study was developed with a group of 50 men and a control group of 50 women with RA, from a rheumatology center in the city of Guayaquil, Ecuador. Data collected included clinical manifestations, comorbidities, treatment, and disease activity. Clinical and activity differences between sexes were analyzed.
Results
Women were more devoted to housework (66%), while men consumed more tobacco (34%) and alcohol (38%). Fatigue (60%), loss of appetite (54%), and weight loss (44%) were more common in women. No differences were found in comorbidities or treatment. Women had higher values of DAS-28 (3.4 vs 2.5), HAQ-DI (1.1 vs 0.4), ESR (33.0 vs 23.2), painful joints (8 vs 3), swollen joints (6 vs 2), and overall physician assessment (3 vs 2).
Conclusion
The results are similar to other publications that establish that women have a more aggressive disease with greater activity of the disease and disability.
1. Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease that is characterized by a progressive and disabling course [1]. The prevalence of RA is 0.5-1%, with a woman to man ratio of 3:1 [2]. It is 4 to 5 times higher in women under 50 years, but after 60 years the ratio becomes approximately 2 to 1 [3]. In Latin America, the prevalence of this disease is high with a ratio of 5.2 women per man in Colombia [4], 5.2 to 1 in Argentina [5], and 5.5 to 1 in Cuba [6].
When studying the influence of gender on the clinical course of the disease, the results have been contradictory. Some studies report a less favorable state in men [7], while other authors claim the opposite [8–11].
Lesuis et al. [12], Sokka et al. [13], and Hallert et al. [14] have shown that women have greater functional disability, disease activity, and pain than men. It has also been found that being a man is a predictor of remission [8, 9] and that women have greater work disability in terms of loss of work days and productivity [15]. The aim of this study is to describe and compare the clinical and serological characteristics of a group of men with RA compared to a control group of women.
2. Materials and Methods
A cross-sectional study was carried out in patients with preestablished diagnosis of RA from a rheumatology center in the city of Guayaquil. The time period was 6 months from June 2016 to December of the same year.
2.1. Characteristics of the Population
A group of 50 men and a control group of 50 women were randomly chosen. Only patients over 18 years of age were included. Patients with other connective tissue diseases and those who did not wish to participate in the study were excluded. After informed consent was obtained, information was collected about demographic data, habits, clinical manifestations, comorbidities, treatment, painful and swollen joint count, visual analogue scale for pain (VAS), and physician assessment. Alcohol consumption was defined as consuming 15 drinks or more per week in men and 8 drinks or more per week in women. A smoker was defined as a person who currently smokes cigarettes or who has smoked 100 cigarettes in his lifetime.
In addition, patients filled the Health Assessment Questionnaire Disability Index (HAQ-DI) to assess functional capacity and the Patient Health Questionnaire-9 (PHQ-9) for depression.
The laboratory data from the patient's last clinical history was accessed, from which we obtained C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), and anti-Cyclic Citrullinated Peptide (anti-CCP). With these data, the DAS-28 disease activity index was calculated.
According to the latest statistics from the National Institute of Statistics and Census (INEC) [16], the population of Ecuador exceeds 17 million inhabitants, with 50.4% being women, with an index of 98.2 men for every 100 women. 71.9% of the population define themselves as mestizos. 62.8% live in urban areas. The unemployment rate in the Ecuadorian population is 4.4%, adequate employment 41.1%, and underemployment 18.3%. As for the employees, 58.7% are men and 41.3% are women. As for the unemployed, 48.2% are men and 51.8% are women. Illiteracy in women is about 7.7%, 1.9 percentage points higher than male illiteracy. More than 900,000 Ecuadorians consume alcohol: 89.7% are men and 10.3% are women; 431 500 Ecuadorians smoke: 85.5% are men and 14.5% are women. The prevalence of RA in Ecuador is 0.9%.
2.2. Statistical Analysis
The statistical program SPSS V. 22 was used to analyze the data and calculate frequencies, percentages, means, standard deviations, minimum, and maximum. To compare the ordinal data, the chi square test was used, and to compare means, the ANOVA coefficient. The statistical significance used was 0.05 with a reliability of 95%.
3. Results
The mean age of the men was 49 years and of women 47 years. The majority of the patients were mestizos. Regarding marital status, 76% of men and 60% of women were married. The majority came from urban areas, 90% men and 86% women. 72% of the men worked while 66% of the women performed housework (p<0.05). Likewise, smoking was more common in men (34% vs 8% p <0.05), as was alcohol consumption (30% vs 0% p <0.05). Table 1 shows the demographic characteristics of both groups.
Table 1.
Demographics.
| Men (N = 50) | Women (N = 50) | p | |
|---|---|---|---|
| Mean age | 49 ± 13 | 47 ± 13 | N.S |
|
| |||
| Ethnicity | |||
|
| |||
| White | 1 (2%) | 1 (2%) | N.S |
|
| |||
| Mestizo | 47 (94%) | 49 (98%) | N.S |
|
| |||
| Afro-Ecuadorian | 2 (4%) | - | N.S |
|
| |||
| Marital status | |||
|
| |||
| Single | 6 (12%) | 1 (2%) | N.S |
|
| |||
| Married | 38 (76%) | 35 (70%) | N.S |
|
| |||
| Divorced | 5 (10%) | 10 (20%) | N.S |
|
| |||
| Widow | 1 (2%) | 4 (8%) | N.S |
|
| |||
| Area | |||
|
| |||
| Urban | 45 (90%) | 43 (86%) | N.S |
|
| |||
| Rural | 5 (10%) | 7 (14%) | N.S |
|
| |||
| Occupation | |||
|
| |||
| Work | 36 (72%) | 15 (30%) | ≤0.001 |
|
| |||
| No work | 14 (28%) | 2 (4%) | ≤0.001 |
|
| |||
| Household chores | - | 33 (66%) | ≤0.001 |
|
| |||
| Habits | |||
|
| |||
| Smoking | 17 (34%) | 4 (8%) | ≤0.001 |
|
| |||
| Alcohol | 19 (38%) | - | ≤0.001 |
N.S: nonsignificant.
Regarding the characteristics of the disease, the average age of onset was 40 years for both groups. There was no significant difference in the delay until the visit with the specialist: 24 ±57 months in men and 32± 46 months in women. The rheumatoid factor was positive in 90% of men and 96% women, and anti-CCP in 84% men and 86% women.
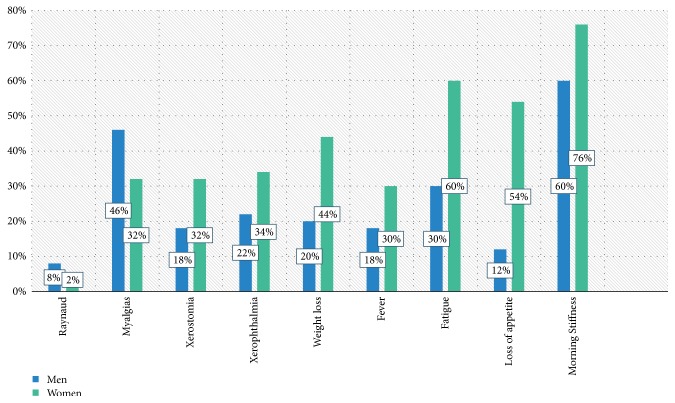
The disease began in the majority of patients insidiously, 56% men and 52% women, with symmetrical involvement of 82% men and 74% women. Regarding extra-articular manifestations (Figure 1), women had greater fatigue (60% vs. 30% p=0.003), weight loss (44% vs. 20% p=0.010), and loss of appetite (54% vs. 12% p≤0.001).
Figure 1.
Clinical manifestations.
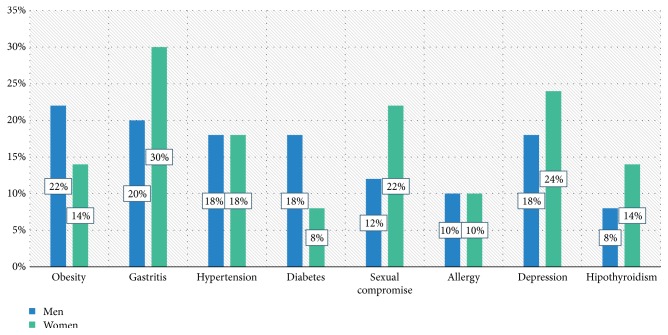
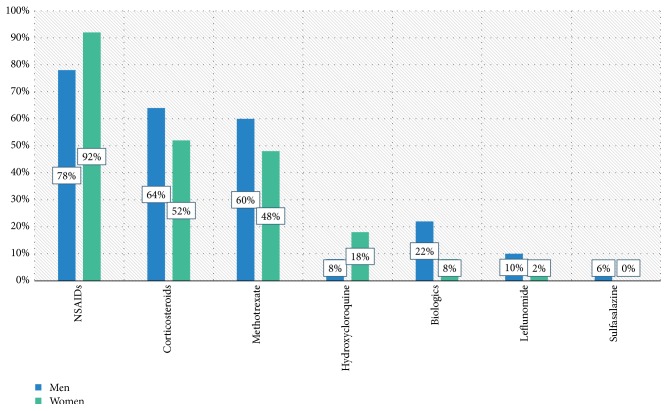
There were no significant differences in comorbidities (Figure 2) or in the treatment (Figure 3).
Figure 2.
Comorbidities.
Figure 3.
Treatment.
Men showed less disease activity in terms of physician's assessment (2 in men vs 3 in women), painful joint count (3 in men vs 8 in women), swollen joint count (2 in men vs 6 in women), ESR (23.2 mm/h in men vs 33.0 mm/h in women), DAS-28 (2.5 in men vs 3.4 in women), and HAQ-DI (0.37 in men vs 1.12 in women) (p <0.05) (Table 2). Likewise, the prevalence of functional disability and severe disability was lower in the group of men. No significant differences were found in VAS of pain (3 vs 4), CRP (13 mg / L vs 29 mg / L), or PHQ-9 (5 vs 4).
Table 2.
Markers of disease activity.
| Men (N = 50) | Women (N = 50) | p | |
|---|---|---|---|
| Physician assessment | 2 [0-8] | 3 [0-9] | 0.047 |
|
| |||
| VAS pain | 3 [0-8] | 4 [0-9] | N.S |
|
| |||
| Painful joint count | 3 [0-24] | 8 [0-28] | ≤0.001 |
|
| |||
| Swollen joint count | 2 [0-12] | 6 [0-26] | ≤0.001 |
|
| |||
| ESR (mm/h) | 23.2 [1-68] | 33.0 [19-70] | 0.046 |
|
| |||
| CRP (mg/L) | 13.0 [2-57] | 29.4 [1-112] | N.S |
|
| |||
| PHQ-9 | 5 [0-19] | 4 [0-17] | N.S |
|
| |||
| DAS-28 | 2.5 [0, 9-5, 8] | 3.4 [0, 9-7] | 0.011 |
|
| |||
| (i) Remission | 27 (54%) | 19 (38%) | NS |
|
| |||
| (ii) Low activity | 8 (16%) | 6 (12%) | NS |
|
| |||
| (iii) Moderate Activity | 14 (28%) | 13 (26%) | NS |
|
| |||
| (iv) High activity | 1 (2%) | 12 (24%) | ≤0.001 |
|
| |||
| HAQ-DI | 0.37 [0-3] | 1.12 [0-3] | ≤0.001 |
|
| |||
| (i) Functional disability | 6 (12%) | 18 (36%) | 0.005 |
|
| |||
| (ii) Severe disability | 1 (2%) | 11 (22%) | 0.002 |
N.S: nonsignificant.
4. Discussion
When comparing the group of men with RA with the control group of women, significant differences were found regarding habits, clinical manifestations, and disease activity.
Smoking was more common in men, as in the study by Krishnan, Sokka, and Hannonen [17]. Voulgari et al. [18] found no difference in weight loss between genders while in this study women had greater weight loss. The prevalence of fatigue was higher in women, similar to the study by Sokka et al. [13]. On the other hand, there was no difference in the presence of dry symptoms, unlike the study by Weyand et al. [7].
In the study by Hallert et al. [14] 33% of patients presented some comorbidity, without differences between sexes. In the present study, no differences were found regarding comorbidities. In contrast, Albrecht [19] found that depression, fibromyalgia, and hypothyroidism were more frequent in women while cardiovascular diseases and diabetes were more frequent in men.
Similar to this study, Sokka et al. [13] found that measures of disease activity were higher in women than in men, including swollen joint count, tender joint count, ESR, VAS for physician global estimate, pain, and patient global status. Other studies have found higher DAS28 remission rates [8, 9] and treatment responses in men [11].
van Vollenhoven [20] raises concern as to whether these differences between genders are due to an inherent difference in the biology of the disease, differences in treatment or response to the same treatment between men and women, subjective experience of the disease, or measurements of disease that are not sex neutral. Likewise, Sokka et al. [13] suggested that most of the gender differences can originate from the measures of disease activity instead of the activity of the disease itself, since they found that among patients who had 0 to 1 swollen joint, women had higher values than men for all other subjective disease activity measures.
Women have higher ESR [21] and worse scores [22] in most of the questionnaires; therefore, the activity indexes for this group are usually worse. Leeb et al. [23] showed that the DAS28 values differed considerably according to sex and the perception of pain. Women are more likely to experience different types of recurrent pain and report higher scores [24]. Furthermore, it has been found that the pain threshold is lower in women and that they experience more physical pain than men for the same noxious stimulus [25].
When measuring disease severity in terms of structural damage, Gossec et al. [26] did not find differences in radiographic scores between genders. Another study [27] also found similar percentages of erosive disease in men and women.
It has been reported that female sex is a predictor of disability and that the progression of disability is three times faster in women [28]. In the present study, women had a higher HAQ-DI score and a higher prevalence of disability, as in the study by Häkkinen et al. [29], in which these differences were attributed to differences in muscle strength and pain score. As women have a lower muscular strength than men, the impact of RA in the functional capacity is greater in this group.
Sex hormones also play an important role in the differences between genders. The severity of RA correlates inversely with androgen levels, which is a possible explanation for the lower severity of the disease in men [30]. Testosterone interacts with the immune system suppressing the humoral and cellular response [31]. Other possible explanation according to Lesuis et al. [12] is that there is a possible subtreatment and a longer duration of the disease in women. Lard et al. [32] also reported a longer delay in treatment in women with early arthritis compared to men. We did not find any differences in treatment or delay until the visit to the specialist.
In this study, women presented with clinical characteristics and measures of disease activity more severe than men, which agrees with previous publications. It is not completely clear whether these differences are due to the intrinsic nature of the disease or to the instruments utilized to assess the disease severity. Also, different gender coping mechanisms can influence this outcome. Since sex differences influence therapeutic goals and response to treatment, these must be considered in the individual therapeutic approach of each patient. In Ecuador there are no other studies comparing the characteristics of RA between men and women; however, there is another descriptive study of patients with RA that included 353 women and 37 men, with similar epidemiological characteristics [33].
5. Conclusion
It is evident that women have greater activity of the disease than men, represented in poorer quality of life and associated comorbidities such as depression. Due to this, it is important to implement screening tests and perform multidisciplinary and integral management. When considering gender differences, one has to take into account the fact that disease activity measures themselves may be influenced by gender.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
The study was approved by the Ethics and Teaching Committee of the Centro de Reumatología y Rehabilitación (CERER) with a registration No. 002/2015; Folio 01: Book of Acts No. 1.
Consent
The patients signed an informed consent form, which reflects the approval of the clinical study according to article 361 of the Political Constitution of the Republic of Ecuador, article 7 of the Organic Health Law, and articles 15-16 of the Code of Medical Ethics of Ecuador. Patient anonymity of data was respected throughout the entire investigative process, and it was explained to the participants that the data collected would be used solely for the study.
Conflicts of Interest
The authors declare no conflicts of interests.
Authors' Contributions
C. Ríos and G. Maldonado were responsible for study conception and design. G. Maldonado, M. Intriago, and J. Cardenas were responsible for data acquisition. G. Maldonado and M. Intriago conducted data analysis and interpretation. M. Intriago and G. Maldonado wrote the manuscript draft. C. Ríos and G. Maldonado provided critical revision.
References
- 1.Maldonado J. A. C. G. Reumatología, 1era, Edic, Azurras, 2010.
- 2.Myasoedova E., Crowson C. S., Kremers H. M., Therneau T. M., Gabriel S. E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis & Rheumatology. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kvien T. K., Uhlig T., Odegard S., Heiberg M. S. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Annals of the New York Academy of Sciences. 2006;1069(1):212–222. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- 4.Barragán-Martínez C., Amaya-Amaya J., Pineda-Tamayo R., et al. Gender differences in latin-american patients with rheumatoid arthritis. Gender Medicine. 2012;9(6):490.e5–510.e5. doi: 10.1016/j.genm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Spindler A., Bellomio V., Berman A., et al. Prevalence of rheumatoid arthritis in Tucumán, Argentina. The Journal of Rheumatology. 2002;29(6):1166–1170. [PubMed] [Google Scholar]
- 6.Reyes-Llerena G. A., Guibert-Toledano M., Penedo-Coello A., et al. Community-based study to estimate prevalence and burden of illness of rheumatic diseases in cuba: A COPCORD study. JCR: Journal of Clinical Rheumatology. 2009;15(2):51–55. doi: 10.1097/RHU.0b013e31819b61cb. [DOI] [PubMed] [Google Scholar]
- 7.Weyand C. M., Schmidt D., Wagner U., Goronzy J. J. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis & Rheumatology. 1998;41(5):817–822. doi: 10.1002/1529-0131(199805)41:5<817::AID-ART7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Mancarella L., Bobbio-Pallavicini F., Ceccarelli F., et al. Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-α blockers: The GISEA study. The Journal of Rheumatology. 2007;34(8):1670–1673. [PubMed] [Google Scholar]
- 9.Forslind K., Hafstrom I., Ahlmen M., Svensson B. Barfot study group: sex: a major predictor of remission in early rheumatoid arthritis? Annals of the Rheumatic Diseases. 2006;66(1):46–52. doi: 10.1136/ard.2006.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka H., Tanaka Y., Sekiguchi N., et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM) Modern Rheumatology. 2007;17(1):28–32. doi: 10.1007/s10165-006-0532-0. [DOI] [PubMed] [Google Scholar]
- 11.Hyrich K. L., Watson K. D., Silman A. J., Symmons D. P. M. British Society for Rheumatology Biologics Register, Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 12.Lesuis N., Befrits R., Nyberg F., van Vollenhoven R. F. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Medicine. 2012;10(1, article 82) doi: 10.1186/1741-7015-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokka T., Toloza S., Cutolo M., et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA Study. Arthritis Research & Therapy. 2009;11(1):1–12. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallert E., Thyberg I., Hass U., Skargren E., Skogh T. Comparison between women and men with recent onset rheumatoid arthritis of disease activity and functional ability over two years (the TIRA project) Annals of the Rheumatic Diseases. 2003;62(7):667–670. doi: 10.1136/ard.62.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puolakka K., Kautiainen H., Pekurinen M., et al. Monetary value of lost productivity over a five year follow up in early rheumatoid arthritis estimated on the basis of official register data on patients' sickness absence and gross income: Experience from the FIN-RACo trial. Annals of the Rheumatic Diseases. 2006;65(7):899–904. doi: 10.1136/ard.2005.045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villacís B., Carrillo D. Pais atrevido: la nueva cara sociodemografica del Ecuador. http://www.ecuadorencifras.gob.ec/wp-content/descargas/Libros/Economia/Nuevacarademograficadeecuador.pdf.
- 17.Krishnan E., Sokka T., Hannonen P. Smoking-gender interaction and risk for rheumatoid arthritis. Arthritis Research & Therapy. 2003;5(3):158–162. doi: 10.1186/ar750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voulgari P. V., Papadopoulos I. A., Alamanos Y., Katsaraki A., Drosos A. A. Early rheumatoid arthritis: does gender influence disease expression? Clinical and Experimental Rheumatology. 2004;22(2):165–170. [PubMed] [Google Scholar]
- 19.Albrecht K. Gender-specific differences in comorbidities of rheumatoid arthritis. Zeitschrift Für Rheumatologie. 2014;73(7):607–614. doi: 10.1007/s00393-014-1410-3. [DOI] [PubMed] [Google Scholar]
- 20.van Vollenhoven R. F. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Medicine. 2009:7–12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinant G. J., Knottnerus J. A., Van Wersch J. W. J. Discriminating ability of the erythrocyte sedimentation rate: a prospective study in general practice. British Journal of General Practice. 1991;41(350):365–370. [PMC free article] [PubMed] [Google Scholar]
- 22.Barsky A. J., Peekna H. M., Borus J. F. Somatic symptom reporting in women and men. Journal of General Internal Medicine. 2001;16(4):266–275. doi: 10.1046/j.1525-1497.2001.016004266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leeb B. F., Haindl P. M., Maktari A., Nothnagl T., Rintelen B. Disease activity score-28 values differ considerably depending on patient's pain perception and sex. The Journal of Rheumatology. 2007;34(12):2382–2387. [PubMed] [Google Scholar]
- 24.Bingefors K., Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain—a gender perspective. European Journal of Pain. 2004;8(5):435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Smith Y. R., Stohler C. S., Nichols T. E., Bueller J. A., Koeppe R. A., Zubieta J. K. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. The Journal of Neuroscience. 2006;26(21):5777–5785. doi: 10.1523/jneurosci.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gossec L., Baro-Riba J., Bozonnat M.-C., et al. Influence of sex on disease severity in patients with rheumatoid arthritis. The Journal of Rheumatology. 2005;32(8):1448–1451. [PubMed] [Google Scholar]
- 27.Tengstrand B., Ahlmén M., Hafström I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. The Journal of Rheumatology. 2004;31(2):214–222. [PubMed] [Google Scholar]
- 28.Karpouzas G. A., Dolatabadi S., Moran R., Li N., Nicassio P. M., Weisman M. H. Correlates and predictors of disability in vulnerable US Hispanics with rheumatoid arthritis. Arthritis Care & Research. 2012;64(9):1274–1281. doi: 10.1002/acr.21689. [DOI] [PubMed] [Google Scholar]
- 29.Häkkinen A., Kautiainen H., Hannonen P., Ylinen J., Mäkinen H., Sokka T. Muscle strength, pain, and disease activity explain individual subdimensions of the Health Assessment Questionnaire disability index, especially in women with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65(1):30–34. doi: 10.1136/ard.2004.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutolo M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Research & Therapy. 2009;11(5):126–129. doi: 10.1186/ar2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutolo M. Sex hormone adjuvant therapy in rheumatoid arthritis. Rheumatic Disease Clinics of North America. 2000;26(4):881–895. doi: 10.1016/S0889-857X(05)70174-5. [DOI] [PubMed] [Google Scholar]
- 32.Lard L. R., Visser H., Speyer I., et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. American Journal of Medicine. 2001;111(6):446–451. doi: 10.1016/S0002-9343(01)00872-5. [DOI] [PubMed] [Google Scholar]
- 33.Ríos Acosta C., Maldonado Vélez G., Paredes Ponce C., et al. Clinical and serological characteristics of Ecuadorian patients with rheumatoid arthritis. Open Access Rheumatology: Research and Reviews. 2017;9:117–122. doi: 10.2147/OARRR.S130217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.