Abstract
Purpose
Postoperative radiotherapy (RT) can improve survival for T1-2N1 breast cancer. However, there exists a concern whether BCS plus RT has the same or a superior therapeutic effect as that of mastectomy. In this study, we aimed to compare the long-term results between RT after BCS and postmastectomy RT in stage T1-2N1M0 breast cancer.
Patients and Methods
Totally 1816 pathological stage T1-2N1M0 breast cancer patients were analyzed. The propensity score matching (PSM) method was used to select 196 pairs of patients between BCS and mastectomy receiving postoperative RT. Five-year locoregional relapse (LRR), locoregional relapse-free survival (LRFS), distant metastasis (DM), distant metastasis-free survival (DMFS), disease-free survival (DFS), breast cancer-specific survival (BCSS) were analyzed as endpoints.
Results
In the whole group, significant differences were observed in all endpoints (P<0.05) between the no-RT and RT groups. For patients receiving mastectomy, DM, DMFS, DFS and BCSS rates had no differences between the two groups. For patients without RT in the multivariable analysis, the molecular subtype was associated with each endpoint (P<0.05). Age, primary tumor site, tumor size, and LVI status were significantly associated with DM. The analysis of 196 pairs of patients selected by PSM showed that BCS plus RT resulted in a significantly lower 5-year DM rate (P=0.015) and superior survival in terms of the 5-year DMFS (P=0.046), DFS (P=0.049) and BCSS (P=0.024) compared with mastectomy.
Conclusions
Postoperative radiotherapy remarkably improved survival in T1-2N1M0 breast cancer but not in the mastectomy subgroup, except for LRR and LRFS. Patients with BCS plus RT had better survival compared with those with postmastectomy radiation in terms of DM, DMFS, DFS and BCSS.
Keywords: breast cancer, stage T1-2N1M0, radiotherapy, breast-conserving surgery, mastectomy
Introduction
In the era of modern surgical and enhanced systemic therapy, postoperative radiotherapy (RT) applied to early breast cancer with one to three positive lymph nodes (LNs) had been strongly recommended as a relative indication with evidence accumulated.1,2 Two randomized studies (MA.20 and EORTC 22922 trials) published in 2015 demonstrated improved survival of early breast cancer patients with one to three positive LNs.3,4 The EBCTCG meta-analysis in 2014 concluded that approximately one breast cancer death was avoided in the 20 years after RT for every 1.5 recurrences of any type avoided during the first 10 years after RT, which indicated that RT reduced death by decreasing distant metastasis (DM) and not only locoregional recurrence (LRR).5 For early-stage breast cancer, RT may improve survival by affecting the distant micrometastasis environment, which was reported in a recent study.6
Since the census announced by the National Institutes of Health in 1990 recommended breast conserving surgery (BCS) as a selection for early breast cancer, the population receiving BCS has been growing.7 At present, approximately 60% of patients with early breast cancer undergo BCS.8–10 There exists a concern whether BCS plus RT has the same or a superior therapeutic effect as that of mastectomy. Previous studies have shown better survival in patients undergoing BCS plus RT compared with mastectomy for early breast cancer.11–16 Although these studies confirmed the positive outcomes of BCS plus RT, few of them considered RT when comparing survival outcomes between BCS and mastectomy for early breast cancer with one to three positive LNs. The value that RT played in BCS compared with mastectomy was not clear. In our study, we retrospectively analyzed pathological stage T1-2N1M0 patients treated with or without RT and aimed to compare the long-term results of BCS plus RT compared with postmastectomy radiotherapy (PMRT).
Patients and methods
Patients
We reviewed 2301 female patients diagnosed with primary invasive, pathological stage T1-2N1M0 breast cancer according to the 7th edition of International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) breast cancer staging system17,18 between April 1998 and December 2016 from our center, excluding those with bilateral breast cancer and receiving neoadjuvant chemotherapy. Patients with undefined molecular subtype or the absence of radiotherapy-related information were excluded. In total, 1816 patients were included in the study.
Treatment
Patients included in the study underwent mastectomy or breast conserving surgery with axillary LN dissection or sentinel LN biopsy. Most of them received systematic therapy as adjuvant chemotherapy, endocrine treatment, and Trastuzumab for human epidermal growth factor receptor-2 (Her-2)-positive tumor. RT of the chestwall and regional nodes was applied to postmastectomy patients with a dose prescription of 50 Gy in 25 fractions. Patients receiving BCS underwent RT of the whole breast up to a median dose of 50 Gy (range, 48 to 50 Gy) with 1.8–2 Gy/fraction. The median dose of the tumor bed boost was 10 Gy (range, 10–16 Gy). Most patients received a three-dimensional conformal radiotherapy technique with opposed tangential beams to the chest wall or whole breast, infraclavicular and supraclavicular region, and tumor bed boost were treated with an anterior electron beam or mixed photon and electron beam.
Follow-up
The duration of patient follow-up was calculated from the date of surgery to either the day of death or the day of the last examination. Patients without recent examination records were followed-up via telephone calls. Five-year local and regional LNs recurrence, distant metastasis, and survival status were recorded. Locoregional relapse (LRR), locoregional relapse-free survival (LRFS), distant metastasis (DM), distant metastasis-free survival (DMFS), disease-free survival (DFS), breast cancer-specific survival (BCSS) were analyzed as the endpoints. LRR was defined as any recurrence within the ipsilateral chest wall or ipsilateral regional LNs, including the axillary, internal mammary infraclavicular and supraclavicular nodes, confirmed by histology or cytology. LRFS was calculated from the date of surgery to the date of LRR, death due to any cause, or the last follow-up. DM was defined as any relapse in distant sites, and DMFS was measured from the date of surgery to the date of DM or death or the last follow-up. DFS were defined as the time from the date of surgery until any recurrence (LRR or DM) or death from any cause. The calculated endpoint of BCSS was the date of death from breast cancer or last follow-up visit.
Statistical analysis
Statistical Product and Service Solution version 22.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. The Chi-square test was used to compare categorical variables of baseline characteristics. Survival rates were estimated using the Kaplan-Meier (K-M) method. The log-rank test was used for univariable analysis to identify significant independent prognostic factors. Multivariable analyses were performed including the significant factors in univariable analysis with the Cox proportional hazards model to calculate hazard ratios (HRs), 95% confidence intervals (CIs). Two-tailed P-values<0.05 were considered statistically significant. The propensity score matching (PSM) method19,20 was used to match the patients between the two groups (BCS plus RT and PMRT) in a 1:1 ratio by logistic regression considering their clinical and pathological features including age, primary tumor site, menopausal status, tumor size, number of positive axillary LNs, lymphovascular invasion (LVI) status, histological grade and molecular subtype.
Ethics statement
This retrospective study was approved by the Institutional Review Board (IRB) of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University. The written consent was not required by the IRB because of the retrospective property. We promise to protect the confidentiality of patients and the study was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
Among the 1816 patients analyzed in the study, 1406 and 410 patients underwent mastectomy and BCS, respectively. After surgery, 1040 patients received no RT, while 776 patients underwent RT (397 and 379 patients underwent mastectomy and BCS, respectively). In total, 1726 and 1198 patients received adjuvant chemotherapy and endocrine treatment, respectively. The median patient age was 47 y (range 22–87 y). The clinical and pathological characteristics of the no-RT and RT groups are listed in Table 1.
Table 1.
Characteristics of 1816 pathologically staged T1-2N1M0 breast cancer with or without postoperative radiotherapy
| Characteristics | No-RT (n=1040) | RT (n=776) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age (years) | ||||
| ≤40 | 201 | 19.3 | 203 | 26.2 |
| >40 | 839 | 80.7 | 573 | 73.8 |
| Primary tumor site | ||||
| OQ | 730 | 70.2 | 558 | 71.9 |
| IQ/CQ | 281 | 27.0 | 180 | 23.2 |
| Unknown | 29 | 2.8 | 38 | 4.9 |
| Menopausal status | ||||
| Premenopausal/perimenopause | 570 | 52.5 | 515 | 66.4 |
| Postmenopausal | 470 | 45.2 | 261 | 42.7 |
| Type of surgery | ||||
| Mastectomy | 1009 | 97.0 | 397 | 51.2 |
| BCS | 31 | 3.0 | 379 | 48.8 |
| Tumor size | ||||
| ≤3 cm | 809 | 77.8 | 648 | 83.5 |
| >3 cm | 231 | 22.2 | 128 | 16.5 |
| Number of positive axillary LNs | ||||
| 1–2 | 905 | 87.0 | 625 | 80.5 |
| 3 | 135 | 13.0 | 151 | 19.5 |
| Histological grade | ||||
| I/II | 679 | 65.3 | 448 | 57.7 |
| III | 292 | 28.1 | 310 | 39.9 |
| Unknown | 69 | 6.6 | 18 | 2.3 |
| LVI status | ||||
| No | 842 | 81 | 462 | 59.5 |
| Yes | 198 | 19 | 314 | 40.5 |
| Molecular subtype | ||||
| Luminal A | 249 | 23.9 | 191 | 24.6 |
| Luminal B/Her-2 - | 372 | 35.8 | 297 | 38.3 |
| Luminal B/Her-2+ | 164 | 15.8 | 148 | 19.1 |
| Her-2 enriched | 118 | 11.3 | 51 | 6.6 |
| TNBC | 137 | 13.2 | 89 | 11.5 |
Abbreviations: RT, radiotherapy; OQ, outer quadrant; IQ, inner quadrant; CQ, central quadrant; BCS, breast-conserving surgery; LN, lymph node; LVI, lymphovascular invasion; Her-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
Survival analysis in no-RT versus RT
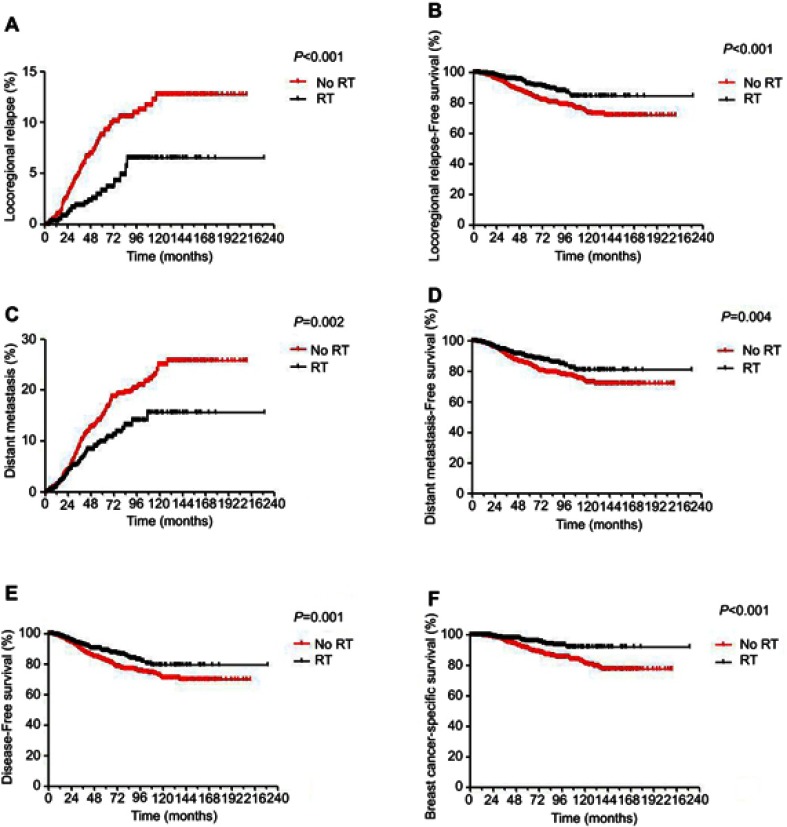
The median follow-up time was 4.7 years (range, 0–19 years). There was a total of 166 deaths, including 145 patients who died of breast cancer, 16 patients who died of a disease other than breast cancer, and 2 patients who died of a secondary tumor. In total, 111 patients experienced LRR, and 238 patients underwent DM. Significant differences were observed in 5-year LRR rate (P<0.001), 5-year LRFS (P<0.001), 5-year DM rate (P=0.002), 5-year DMFS (P=0.004), 5-year DFS (P=0.001) and 5-year BCSS (P<0.001) when comparing no-RT versus RT in the whole group (Figure S1). For patients receiving mastectomy, the 5-year LRR rate was significantly lower (4.2% vs 8.9%, P=0.008), while the 5-year LRFS was significantly better (89% vs 85.3%, P=0.012) in the RT group than in the no-RT group. However, no significant differences were observed in DM, DMFS, DFS and BCSS between the two groups (Table 2).
Table 2.
Survival analysis for 1406 T1-2N1M0 breast cancer underwent mastectomy
| n | LRR | P-value | DM | P-value | LRFS | P-value | DMFS | P-value | DFS | P-value | BCSS | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.791 | 0.012 | 0.591 | 0.325 | 0.084 | ||||||||
| No- RT | 1009 | 8.9 | 14.8 | 85.3 | 84.5 | 82.5 | 91.3 | ||||||
| RT | 397 | 4.2 | 14.8 | 89.0 | 85.0 | 84.2 | 92.8 |
Note: P-values were calculated using the unadjusted log-rank test.
Abbreviations: RT, radiotherapy; LRR, locoregional relapse; DM, distant metastasis; LRFS, locoregional relapse-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; BCSS, breast cancer-specific survival.
Prognostic predictor in the no-RT group
Univariable analysis and multivariable analyses were conducted in 1040 patients without RT. Clinical and pathological features such as age (≤40 vs >40 years), primary tumor site, menopausal status, tumor size (≤3 vs >3 cm), number of positive axillary LNs (No.1/2 vs No.3), LVI status, histological grade and molecular subtype were included in univariable analysis as prognostic factors (Table 3). Age, primary tumor site, tumor size, LVI status, and molecular subtype were significantly associated with 5-year LRFS, DM, DMFS DFS and BCSS (P<0.05). Multivariable analysis incorporated the predictive factors from the univariable analysis and showed that the molecular subtype was associated with each end point. In addition, age (P=0.009) and primary tumor site (P<0.001) were independent prognostic factors for LRR. Age (P=0.013), primary tumor site (P=0.013), tumor size (P<0.001), and LVI status (P=0.001) were significantly associated with DM. For BCSS, statistically significant differences were observed in age, tumor size, and LVI status (P<0.05). The results of the adjusted multivariable analysis are shown in Table 4.
Table 3.
Summary of prognostic factor univariate analysis in 1040 pathologically staged T1-2N1M0 breast cancer without postoperative radiotherapy
| Factors | n | LRR | P-value | DM | P-value | LRFS | P-value | DMFS | P-value | DFS | P-value | BCSS | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.005 | 0.01 | 0.041 | 0.047 | 0.008 | 0.05 | |||||||
| ≤40 | 201 | 15.5 | 20.4 | 79.2 | 79.6 | 74.8 | 89.2 | ||||||
| >40 | 839 | 7.0 | 13.6 | 86.5 | 85.4 | 84.2 | 91.5 | ||||||
| Primary tumor site | 0.000 | 0.013 | 0.141 | 0.054 | 0.074 | 0.269 | |||||||
| OQ | 730 | 5.6 | 11.5 | 87.7 | 87.6 | 85.5 | 92.2 | ||||||
| IQ/CQ | 281 | 13.5 | 19.3 | 82.1 | 80.4 | 78.8 | 88.5 | ||||||
| Menopausal status | 0.568 | 0.176 | 0.578 | 0.876 | 0.879 | 0.555 | |||||||
| Premenopause/perimenopause | 570 | 9.9 | 16.5 | 84.8 | 83.5 | 80.9 | 90.8 | ||||||
| Postmenopause | 470 | 7.4 | 13.1 | 85.2 | 85.2 | 83.8 | 91.3 | ||||||
| Tumor size | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||
| ≤3 cm | 809 | 7.2 | 11.9 | 88.2 | 87.5 | 85.5 | 92.7 | ||||||
| >3 cm | 231 | 14.7 | 25.9 | 74.0 | 72.8 | 70.6 | 85.1 | ||||||
| Number of positive axillary LNs | 0.399 | 0.525 | 0.361 | 0.413 | 0.354 | 0.884 | |||||||
| 1–2 | 905 | 8.5 | 14.9 | 85.3 | 84.5 | 82.6 | 91.1 | ||||||
| 3 | 135 | 10.3 | 15.6 | 83.2 | 82.8 | 80.3 | 90.4 | ||||||
| Histological grade | 0.454 | 0.109 | 0.141 | 0.052 | 0.119 | 0.273 | |||||||
| I/II | 679 | 7.4 | 13.3 | 87.2 | 86.2 | 84.5 | 92.0 | ||||||
| III | 292 | 10.6 | 18.0 | 81.6 | 80.9 | 79.0 | 89.3 | ||||||
| LVI status | 0.088 | 0.000 | 0.000 | 0.000 | 0.000 | 0.01 | |||||||
| No | 842 | 8.3 | 12.8 | 86.5 | 86.6 | 84.5 | 91.9 | ||||||
| Yes | 198 | 12.6 | 27.6 | 76.5 | 70.5 | 69.3 | 86.6 | ||||||
| Molecular subtype | 0.000 | 0.009 | 0.000 | 0.006 | 0.001 | 0.001 | |||||||
| Luminal A | 249 | 3.3 | 90.8 | 92.4 | 90.4 | 89.9 | 94.6 | ||||||
| Luminal B/Her-2 - | 372 | 7.2 | 87.2 | 86.7 | 86.2 | 84.4 | 93.7 | ||||||
| Luminal B/Her-2+ | 164 | 9.6 | 82.0 | 84.2 | 82.0 | 79.2 | 90.1 | ||||||
| Her-2 enriched | 118 | 18.1 | 78.0 | 74.0 | 78.0 | 73.4 | 82.8 | ||||||
| TNBC | 137 | 14.8 | 77.7 | 76.8 | 75.4 | 73.0 | 84.7 |
Note: P-values were calculated using the unadjusted log-rank test.
Abbreviations: RT, radiotherapy; OQ, outer quadrant; IQ, inner quadrant; CQ, central quadrant; LN, lymph node; LVI, lymphovascular invasion; Her-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer; LRR, locoregional relapse; DM, distant metastasis; LRFS, locoregional relapse-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; BCSS, breast cancer-specific survival.
Table 4.
Summary of prognostic factor multivariate analysis in 1040 pathologically staged T1-2N1M0 breast cancer without postoperative radiotherapy
| HR | 95% CI | P-value | ||
|---|---|---|---|---|
| LRR | Age >40 y | 0.529 | 0.33–0.85 | 0.009 |
| IQ/CQ | 2.275 | 1.40–3.25 | <0.001 | |
| Molecular subtype | 0.001 | |||
| DM | Age >40 y | 0.645 | 0.46–0.91 | 0.013 |
| IQ/CQ | 1.505 | 1.09–2.08 | 0.013 | |
| Tumor size >3 cm | 2.144 | 1.54–2.98 | <0.001 | |
| Lymphovascular invasion | 2.033 | 1.39–2.98 | <0.001 | |
| Molecular subtype | 0.039 | |||
| LRFS | Tumor size >3 cm | 1.95 | 1.41–2.70 | <0.001 |
| Molecular subtype | 0.001 | |||
| DMFS | Molecular subtype | 1.372 | 1.00–1.88 | 0.05 |
| Tumor size >3 cm | 2.081 | 1.51–2.86 | <0.001 | |
| Lymphovascular invasion | 2.155 | 1.50–3.10 | <0.001 | |
| Molecular subtype | 0.025 | |||
| DFS | Age >40 y | 0.673 | 0.49–0.93 | 0.16 |
| Tumor size >3 cm | 1.915 | 1.41–2.60 | <0.001 | |
| Lymphovascular invasion | 1.996 | 1.41–2.83 | <0.001 | |
| Molecular subtype | 0.011 | |||
| BCSS | Age >40 y | 0.658 | 0.44–1.00 | 0.047 |
| Tumor size >3 cm | 2.030 | 1.38–2.99 | <0.001 | |
| Lymphovascular invasion | 1.755 | 1.07–2.88 | 0.026 | |
| Molecular subtype | 0.006 |
Note: P-values were calculated using an adjusted Cox proportional hazards model with forward conditional method.
Abbreviations: IQ, inner quadrant; CQ, central quadrant; LRR, locoregional relapse; DM, distant metastasis; LRFS, locoregional relapse-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; BCSS, breast cancer specific survival.
BCS plus RT versus postmastectomy radiotherapy after PSM
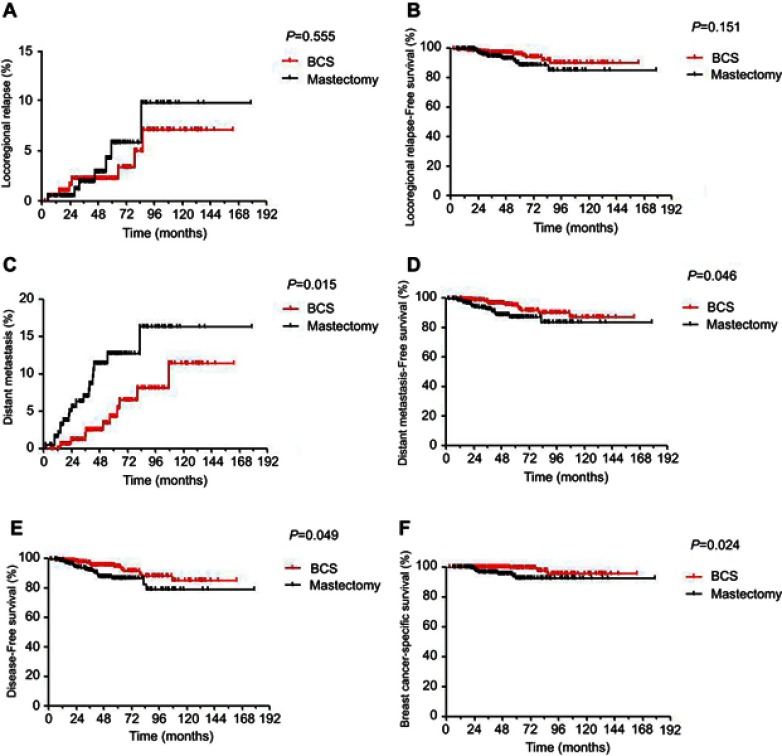
A total of 196 pairs of patients were selected by PSM from the 776 original patients receiving RT. The features between the two groups were compared before and after PSM (Table S1). In the PSM cohort, the median follow-up time was 4.3 years (range, 0–15 years). Analysis showed that BCS plus RT resulted in a significantly lower 5-year DM rate (4.3% vs 14.1%, P=0.015) and superior survival in terms of 5-year DMFS (94.6% vs 86.8%, P=0.046), DFS (94.3% vs 86.2%, P=0.049) and BCSS (97.9% vs 91.9%, P=0.024) compared with PMRT. However, no significant differences were observed in LRR and LRFS. The survival analysis is shown in Figure 1.
Table S1.
Summary of characteristics distribution in BCS or mastectomy underwent postoperative radiotherapy before and after PSM
| Characteristics | BCS n=379 | Mastectomy n=397 | P-value | BCS n=196 | Mastectomy n=196 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| Before PSM | After PSM | |||||||||
| Age (years) | 0.315 | 1.000 | ||||||||
| ≤40 | 93 | 24.5 | 110 | 27.7 | 47 | 24.0 | 47 | 24.0 | ||
| >40 | 286 | 75.5 | 287 | 72.3 | 149 | 76.0 | 149 | 76.0 | ||
| Primary tumor site | 0.001 | 1.000 | ||||||||
| OQ | 287 | 75.7 | 271 | 68.3 | 153 | 78.1 | 153 | 78.1 | ||
| IQ/CQ | 84 | 22.2 | 96 | 24.2 | 40 | 20.4 | 40 | 20.4 | ||
| Unknown | 8 | 2.1 | 30 | 7.5 | 3 | 1.5 | 3 | 1.5 | ||
| Menopausal status | 0.057 | 1.000 | ||||||||
| Premenopausal/perimenopause | 239 | 63.1 | 276 | 69.5 | 138 | 70.4 | 138 | 70.4 | ||
| Postmenopausal | 140 | 36.9 | 121 | 30.5 | 58 | 29.6 | 58 | 29.6 | ||
| Tumor size | 0.000 | 1.000 | ||||||||
| ≤3 cm | 348 | 91.8 | 300 | 83.5 | 243 | 88.7 | 243 | 88.7 | ||
| >3 cm | 31 | 8.2 | 97 | 16.5 | 31 | 11.3 | 31 | 11.3 | ||
| Number of positive axillary LNs | 0.000 | 1.000 | ||||||||
| 1–2 | 328 | 86.5 | 297 | 74.8 | 170 | 86.7 | 170 | 86.7 | ||
| 3 | 51 | 13.5 | 100 | 25.2 | 26 | 13.3 | 26 | 13.3 | ||
| Histological grade | 0.568 | 1.000 | ||||||||
| I/II | 226 | 59.6 | 222 | 55.9 | 119 | 60.7 | 119 | 60.7 | ||
| III | 145 | 38.3 | 165 | 41.6 | 75 | 38.3 | 75 | 38.3 | ||
| Unknown | 8 | 2.1 | 10 | 2.5 | 2 | 2.9 | 2 | 2.9 | ||
| Lymphovascular invasion status | 0.433 | 1.000 | ||||||||
| No | 231 | 60.9 | 231 | 58.2 | 166 | 60.6 | 166 | 60.6 | ||
| Yes | 148 | 39.1 | 166 | 41.8 | 108 | 39.4 | 108 | 39.4 | ||
| Molecular subtype | 0.000 | 1.000 | ||||||||
| Luminal A | 118 | 31.1 | 73 | 18.4 | 52 | 26.5 | 52 | 26.5 | ||
| Luminal B/Her-2 - | 154 | 40.6 | 143 | 36.0 | 82 | 41.8 | 82 | 41.8 | ||
| Luminal B/Her-2+ | 66 | 17.4 | 82 | 20.7 | 38 | 19.4 | 38 | 19.4 | ||
| Her-2 enriched | 12 | 3.2 | 39 | 9.8 | 5 | 2.6 | 5 | 2.6 | ||
| TNBC | 29 | 7.7 | 60 | 15.1 | 19 | 9.7 | 19 | 9.7 | ||
Note: P-values were calculated using chi-square test.
Abbreviations: BCS, breast conserving surgery; PSM, propensity score match; OQ, outer quadrant; IQ, inner quadrant; CQ, central quadrant; LN, lymph node; LVI, lymphovascular invasion; Her-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
Figure 1.
Kaplan-Meier survival curves for 196 pairs of BCS/mastectomy underwent postoperative radiotherapy. (A) Locoregional relapse. (B) Distant metastasis. (C) Distant metastasis-free survival. (D) Distant metastasis-free survival. (E) Progression-free survival survival. (F) Breast cancer-specific survival. P-values were calculated using an unadjusted log-rank test.
Discussion
In our study, although significant differences were observed between the RT group and the no-RT group at all endpoints, the subgroup analysis for mastectomy comparing RT with no-RT showed that RT improved the LRR rate (P=0.008) and LRFS (P=0.012) but not the DM, DMFS, DFS and BCSS rates. We can presume that BCS plus RT primarily contributed to the enhancement of survival. Therefore, we conducted a comparison of BCS plus RT with PMRT to evaluate the effect of RT in the two types of operations. To balance the confounders, our study matched all the clinical and pathological features by a ratio of 1:1.
The results of two randomized trials (MA.20 and EORTC 22922 trials) published in 2015 showed improved DFS and DMFS with regional nodal irradiation, with the EORTC 22922 trial additionally showing improved BCSS.3,4 Our study showed a lower 5-year LRR rate (3.2% vs 8.8%), DM rate (9.9% vs 15%) and improved LRFS (91.9% vs 85%), DMFS (89.4% vs 84.2%), DFS (88.7% vs 82.2%), BCSS (95.6% vs 91%) in those treated with RT compared with those not treated with RT in the whole cohort, which was consistent with the studies above.
The previous studies included clinical and pathological features in the analysis and showed that age <40 years, and presence of LVI, histological grade III were risk factors significantly associated with increased rates of LRR for early breast cancer.21–25 Our study analyzed not only locoregional control but also distant metastasis and death. Multivariable analysis in our study showed that age (P=0.009) and primary tumor site (P<0.001) were significantly associated with LRR. Age (P=0.013), primary tumor site (P=0.013), tumor size (P<0.001), and LVI status (P=0.001) were independent prognostic factors for DM. Significant differences were observed in age, tumor size, and LVI status (P<0.05) for BCSS. In the past, clinical pathologic features were combined to evaluate RT indications. In recent years, molecular biology information has been increasingly widely used to construct genetic models that predict the risk of recurrence and metastasis, such as the EPclin and RecurIndex models.26–28
A serious retrospective studies demonstrated positive survival outcomes in patients with BCS plus RT compared with mastectomy.11–16 Few of these studies have explored whether BCS still exceeds mastectomy in the case of RT. A study published in 2014 compared BCT (lumpectomy followed by radiotherapy) and PMRT before and after PSM, showing that BCT resulted in superior BCSS.11 However, the study did not compare other survival endpoints, such as locoregional control and DM, and therefore, the manner in which RT contributed to the survival benefit was uncertain. In our study, we used the PSM method to equilibrate confounders and compared LRR, DM, LRFS, DMFS, DFS and BCSS. The analysis indicated that BCT had significantly lower DM rates, and gained superior DMFS, DFS and BCSS. It is possibly assumed that RT decreased the risk of death by reducing DM.
In the era of modern surgical and enhanced systemic therapy, the MA.20 and EORTC 22922 trials3,4 were conducted to explore the benefit of regional irradiation in early breast cancer. Both trials showed that regional RT resulted in a greater DM survival benefit than locoregional survival benefit, which indicated that RT did not only act on the local tumor. The EBCTCG meta-analysis in 2011 and 20145,29 indicated that postoperative radiotherapy is likely to improve survival both by reducing LRR and DM. The survival benefit was more significant in pathological stage N1 compared with N2-3 tumors. This is possibly because a larger tumor burden and higher metastatic risk restricted the benefit RT provided, while a lower tumor burden in N1 helped to improve survival.
A series of studies reported that in metastatic malignancies, such as melanoma and lung cancer, radiotherapy combined with immunotherapy showed an objective response within the radiation field as well as non-irradiated metastatic targets, such as skin, lymph nodes, liver, bone and lung through immune activation in tumor microenvironment and residual tumor burden.30–33 A study conducted in the Netherlands showed superior survival after BCS plus RT compared with mastectomy for early breast cancer with 10-year long-term follow-up.15 The outcome of the study indicated that the preserved breast can serve as a tumor microenvironment to facilitate the effect of radiotherapy for better survival. Furthermore, two recent studies confirmed that tumor microenvironments with different expression levels of fibroblasts and macrophages can influence the treatment effect.34,35 A recently published study6 confirmed that circulating tumor cell (CTC) status is predictive of radiotherapeutic benefit in early-stage breast cancer. The results showed that CTC status was associated with the benefit of RT among BCS but not with the benefit of RT among mastectomy in terms of LRFS, DFS and overall survival (OS). This finding indicated that the benefit of RT among CTC-positive patients may be limited to those who underwent BCS, perhaps owing to the higher burden of residual local disease in these patients. The evidence above indicates that the tumor microenvironment and low tumor burden help RT improve survival in BCS compared with mastectomy.
The study had several limitations. First, selection bias existed due to the retrospective nature of our study. Second, the number of HER-2 enriched subtype was rare (2.6%) in each group after PSM, which was not consistent with the real distribution pattern and may not objectively reflect the benefits that RT confer in the BCS group. Third, after PSM, the large-scale cohort of different molecular subtypes restricted us in conducting subgroup analyses to determine the benefits for each subtype. Finally, the prognosis is favorable for early breast cancer, and the majority of previous studies followed up for 10 years or even longer to perceive the effect of treatment. We only assessed 5-year survival because the median follow-up time was not long enough and longer investigations are needed.
In conclusion, our study showed that postoperative radiotherapy contributed to significant improvements in each 5-year survival endpoint for stage T1-2N1M0 breast cancer. In the mastectomy subgroup, statistically significant improvement was observed only in LRR and LRFS. Superior survival was shown after BCS plus RT compared with PMRT in terms of 5-year DM, DMFS, DFS and BCSS. These findings deserve further investigation to demonstrate the factors contributing to this effect.
Acknowledgments
We thank the native English speaking scientists of American Journal Experts for editing our manuscript.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
References
- 1.Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. J Clin Oncol. 2016;34(36):4431–4442. doi: 10.1200/JCO.2016.69.1188 [DOI] [PubMed] [Google Scholar]
- 2.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012 [DOI] [PubMed] [Google Scholar]
- 3.Poortmans PM, Struikmans H, Bartelink H. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(19):1879–1880. doi: 10.1056/NEJMc1510505 [DOI] [PubMed] [Google Scholar]
- 4.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 5.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman CR, Seagle BL, Friedl T, et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. Jama Oncol. 2018;4(8):e180163. doi: 10.1001/jamaoncol.2017.4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH Consensus Development. Conference statement on the treatment of early-stage breast cancer. Oncology (Williston Park). 1991;5(2):120–124. [PubMed] [Google Scholar]
- 8.Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus development conference on the treatment of patients with early stage invasive breast carcinoma. Cancer Am Cancer Soc. 1999;86(4):628–637. [PubMed] [Google Scholar]
- 9.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–2690. doi: 10.1245/s10434-009-0635-x [DOI] [PubMed] [Google Scholar]
- 10.Lee MC, Rogers K, Griffith K, et al. Determinants of breast conservation rates: reasons for mastectomy at a comprehensive cancer center. Breast J. 2009;15(1):34–40. doi: 10.1111/j.1524-4741.2008.00668.x [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267–274. doi: 10.1001/jamasurg.2013.3049 [DOI] [PubMed] [Google Scholar]
- 12.Hofvind S, Holen A, Aas T, Roman M, Sebuodegard S, Akslen LA. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol. 2015;41(10):1417–1422. doi: 10.1016/j.ejso.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 13.Fisher S, Gao H, Yasui Y, Dabbs K, Winget M. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol. 2015;26(6):1161–1169. doi: 10.1093/annonc/mdv107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann-Johnsen OJ, Karesen R, Schlichting E, Nygard JF. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian Women primary operated between 1998 and 2008. Ann Surg Oncol. 2015;22(12):3836–3845. doi: 10.1245/s10434-015-4441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–1170. doi: 10.1016/S1470-2045(16)30067-5 [DOI] [PubMed] [Google Scholar]
- 16.Hartmann-Johnsen OJ, Karesen R, Schlichting E, Nygard JF. Better survival after breast-conserving therapy compared to mastectomy when axillary node status is positive in early-stage breast cancer: a registry-based follow-up study of 6387 Norwegian women participating in screening, primarily operated between 1998 and 2009. World J Surg Oncol. 2017;15(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union against cancer and the American joint committee on cancer. Cancer Am Cancer Soc. 2010;116(22):5336–5339. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2004;22(21):4247–4254. doi: 10.1200/JCO.2004.01.042 [DOI] [PubMed] [Google Scholar]
- 22.Tendulkar RD, Rehman S, Shukla ME, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1-3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83(5):e577–e581. doi: 10.1016/j.ijrobp.2012.01.076 [DOI] [PubMed] [Google Scholar]
- 23.Huang CJ, Hou MF, Chuang HY, et al. Comparison of clinical outcome of breast cancer patients with T1-2 tumor and one to three positive nodes with or without postmastectomy radiation therapy. Jpn J Clin Oncol. 2012;42(8):711–720. doi: 10.1093/jjco/hys080 [DOI] [PubMed] [Google Scholar]
- 24.McBride A, Allen P, Woodward W, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89(2):392–398. doi: 10.1016/j.ijrobp.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 25.Lai SF, Chen YH, Kuo WH, et al. Locoregional recurrence risk for postmastectomy breast cancer patients with T1-2 and one to three positive lymph nodes receiving modern systemic treatment without radiotherapy. Ann Surg Oncol. 2016;23(12):3860–3869. doi: 10.1245/s10434-016-5435-5 [DOI] [PubMed] [Google Scholar]
- 26.Buus R, Sestak I, Kronenwett R, et al. Comparison of endopredict and EPclin with oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108(11). doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng SH, Horng CF, Huang TT, et al. An eighteen-gene classifier predicts locoregional recurrence in post-mastectomy breast cancer patients. EBioMedicine. 2016;5:74–81. doi: 10.1016/j.ebiom.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng SH, Huang TT, Cheng YH, et al. Validation of the 18-gene classifier as a prognostic biomarker of distant metastasis in breast cancer. PLoS One. 2017;12(9):e184372. doi: 10.1371/journal.pone.0184372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–524. doi: 10.1038/nrclinonc.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su S, Chen J, Yao H, et al. CD10(+)GPR77(+) Cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172(4):841–856. doi: 10.1016/j.cell.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 35.Su S, Zhao J, Xing Y, et al. Immune checkpoint inhibition overcomes ADCP-Induced Immunosuppression by macrophages. Cell. 2018;175(2):442–457. doi: 10.1016/j.cell.2018.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.