Abstract
Background: Chemotherapy is one of the available options for prostate cancer (PC). However, the acquisition of chemoresistance has become a major cause of chemotherapy failure. The long noncoding RNA DANCR is demonstrated to serve as an oncogene in various human cancers, including PC. However, the potential role of DANCR in docetaxel (DTX) resistance of PC and its underlying mechanism remains unclear.
Methods: The abundance of DANCR, miR-34a-5p, and JAG1 mRNA was examined by quantitative reverse transcription PCR. The Cell Counting Kit-8 (CCK8) was used to determine the 50% inhibitory concentration value. Cell viability was evaluated by CCK8 and colony-formation assays. Transwells were utilized to analyze cell migration and invasion ability. The protein levels of LRP, P-gp, MRP1, and JAG1 were measured by Western blot assay. The target relationship between DANCR and miR-34a-5p, as well as miR-34a-5p and JAG1, was demonstrated by dual-luciferase, RNA immunoprecipitation, and RNA pull-down analysis. Tumor xenograft was undertaken to confirm the effect of DANCR on DTX resistance in PC.
Results: DANCR and JAG1 were significantly upregulated, but miR-34a-5p was downregulated in DTX-resistant PC. Silencing of DANCR improved the DTX efficacy in DTX-resistant PC cells. DANCR served as a competing endogenous RNA of miR-34a-5p, leading to the derepression of miR-34a-5p target JAG1, which eventually triggered the resistance to DTX in DTX-tolerated PC.
Conclusion: The DANCR/miR-34a-5p axis enhanced DTX resistance of PC via targeting JAG1, providing a novel insight to improve chemotherapy for PC.
Keywords: DANCR, miR-34a-5p, JAG1, docetaxel resistance, prostate cancer
Introduction
Prostate cancer (PC) is the second most commonly diagnosed malignancy in men after lung cancer and remains one of the major causes of cancer-related death among men around the world.1 Although great advances have been achieved in early diagnosis and treatment, PC continues to pose a considerable public threat due to the substantial morbidity and mortality.2 Androgen deprivation therapy is a highly effective approach to control the development of PC, but most patients at the advanced stages ultimately evolve into castration-resistant PC, leading to treatment failure of androgen deprivation therapy.3 Docetaxel (DTX)-based chemotherapy regimens have exhibited modest survival benefits for patients with castration-resistant PC and applied as a standard treatment.4 However, the acquisition of DTX resistance following repetitive cycles of chemotherapy usually reduces the effectiveness of PC treatment.5 Thus, uncovering the underlying mechanism involved in the development of DTX resistance and developing novel strategies to overcome this resistance is imperative for PC treatment. With developments in the area of molecular biology and cell biology, numerous biomarkers responsible for the progression and chemoresistance of PC have been identified. As stated by Sanjay et al,6 overexpression of PTEN contributed to the apoptosis of PC cells by impeding the synthesis of oncogenic protein. Also, Varambally et al7 reported that a high EZH2 level was associated with the poor prognosis of PC patients, and knockdown of EZH2 by small interfering RNA resulted in the inhibition of cell proliferation in vitro. Moreover, CXCR4 inhibitor AMD3100 enhanced DTX cytotoxicity in PC, hinting that inhibition of CXCR4 might be a promising therapeutic avenue for PC patients with DTX resistance.5
Recently, the achievement of high-throughput sequencing technology has enhanced our understanding of the human transcriptome, revealing that only a small proportion of human genomes have the potential to encode proteins, and the majority of transcripts lack this function, named noncoding RNAs.8,9 Long noncodiong RNAs (lncRNAs) are a type of noncoding transcript larger than 200 nucleotides, which can modulate gene expression at epigenetic, transcription, and post-transcription levels.10,11 To date, numerous lncRNAs have been confirmed to be involved in the initiation and development of multiple malignancies, highlighting the vital roles of lncRNAs as diagnostic and therapeutic biomarkers in human cancers.12 Besides, there is increasing evidence that ectopic expression of some lncRNAs may regulate the chemotherapy resistance of many types of cancers, including PC. As presented by Gu et al,13 enforced abundance of lncRNA HOXD-AS1 was correlated with the malignant pathological phenotypes of PC patients, and enhanced cell proliferation and chemoresistance via recruiting WDR5.
The lncRNA anti-differentiation noncoding RNA (lncRNA DANCR) located on chromosome 4 is a promising cancer-related lncRNA. Aberrant expression of DANCR plays a significant role in tumorigenesis of various human cancers. Recently, emerging literature proves that DANCR can be a potential biomarker for the diagnosis and treatment of colorectal cancer patients.14 DANCR contributes to cell migration and invasion by silencing lncRNA-LET in gastric cancer.15 Moreover, DANCR knockdown degrades the stem-cell properties and results in the suppression of cell vitality and tumorigenesis in hepatocellular carcinoma.16 In PC, cell invasion ability is induced by DANCR through epigenetically suppressing TIMP2/3.17 However, the role of DANCR in DTX resistance of PC remains unclear.
In this study, we report a stimulatory effect of DANCR knockdown on the DTX sensitivity of PC. Furthermore, we identify for the first time that DANCR functioned as a competing endogenous RNA (ceRNA) of miR-34a-5p in PC, leading to the derepression of miR-34a-5p target JAG1. Thus, our findings provide a novel regulatory mechanism of the DANCR/miR-34a-5p/JAG1 pathway in the chemosensitivity of PC.
Materials and methods
Clinical samples
Thirty fresh PC specimens from patients who suffered from surgical resection at the Fourth Hospital of Hebei Medical University between June 2015 and July 2017 were collected following a standard protocol. All of them received DTX-based chemotherapy without any other preoperative treatment such as radiotherapy, immunotherapy, and hormone castration. PC patients were divided into DTX-sensitive (n=15) or DTX-resistant (n=15) groups depending on the outcomes following DTX-based chemotherapy. After surgery, PC specimens were instantly frozen in liquid nitrogen and preserved at −80 °C for subsequent analysis. These experiments were performed according to the Declaration of Helsinki Principles with the approval of the Research Ethics Committee of the Fourth Hospital of Hebei Medical University, and written informed consents were obtained from all participants.
Cell culture
Human PC cell lines DU145 and PC3 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, People's Republic of China). DU145 cells were cultured in minimal essential medium in the presence of 10% FBS. PC3 cells were cultivated in specific Ham’s F-12K medium (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Gibco). All cells were maintained at 37 °C in a humidified chamber containing 5% CO2 at 37 °C. DTX-resistant PC cell lines DU145/DTX and PC3/DTX were generated by continuous exposure with stepwise increased doses of DTX (Sigma-Aldrich Co., St Louis, MO, USA) for a period of 6 months. DU145/DTX and PC3/DTX cells were preserved in a 5 nmol/L final concentration of DTX to maintain their resistance.
Plasmids and transfection
Small interfering RNA targeting DANCR (si-DANCR) or JAG1 (si-JAG1), miR-34a-5p mimics (miR-34a-5p), miR-34a-5p inhibitor (anti-miR-34a-5p), DANCR-overexpression plasmid pcDNA-DANCR, and negative control were provided by GenePharma Co., Ltd (Shanghai, People's Republic of China). When the cells reached 80% confluence, oligonucleotides or plasmids were transiently transfected into DU145/DTX and PC3/DTX using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific). Forty-eight hours thereafter, cells were harvested for subsequent analysis.
Quantitative reverse-transcription PCR (qRT-PCR)
Total RNA in tumor tissues and cell lines was isolated using RNAiso Plus reagent (TaKaRa, Dalian, People's Republic of China) following the manufacturer’s instructions. The complementary DNA (cDNA) for DANCR and JAG1 were obtained using the M-MLV Reverse Transcriptase Kit (Thermo Fisher Scientific), and cDNA for miR-34a-5p was synthesized using the TaqMan® miRNA reverse transcription kit (Thermo Fisher Scientific). The RT primer sequence for miR-34a-5p was 5ʹ-CTCAACTGGTGTCGTGGAGTCGGCAATTCAG TTGAGAACAACCA-3ʹ. To determine relative gene expression, sequence-specific PCR primers for DANCR, miR-34a-5p, or JAG1 were mixed with SYBR Green PCR Master Mix (Thermo Fisher Scientific) and qRT-PCR analysis was done on the Applied Biosystems 7500 Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific). The results were analyzed using the 2–ΔΔCt method with β-actin or U6 small RNA as the internal control, respectively. qRT-PCR primers were as follows: DANCR, 5ʹ-GCCACTATGTAGAGGGTTTC-3ʹ (forward) and 5ʹ-ACCTGCGCTAAGAACTGAGG-3ʹ (reverse); miR-34a-5p, 5ʹ-CTGGGAGGTGGCAGTGTCTTAGC-3ʹ (forward) and 5ʹ-TCAACTGGTGTCGT GGAGTCGG-3ʹ (reverse); JAG1, 5ʹ-CTCATCAGCGGTGTCTCAAC-3ʹ (forward) and 5ʹ-GGCACACACACTTAAATCCG-3ʹ (reverse); β-actin, 5ʹ-GGCATCGTGAT GGACTCCG-3ʹ (forward) and 5ʹ-GCTGGAAGGTGGACAGCGA-3ʹ (reverse); and U6, 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ (forward) and 5ʹ-CGCTTCACGAATT TGCGTGTCAT-3ʹ (reverse).
Cell proliferation and cytotoxic assays
For cytotoxic analysis, cells treated with different doses of DTX (2, 5, 10, 20, 50, and 100 nmol/L) (Sigma-Aldrich Co.) were seeded into 96-well plates and cultured in 100 μL medium for 48 h. Then, 10 µL Cell Counting Kit-8 (CCK-8) reagent (Beyotime, Shanghai, People's Republic of China) was added into the medium and incubated at 37 °C for another 2 h and the absorbance at 450 nm was measured on a Microplate Reader (Thermo Fisher Scientific). The cell growth inhibition rate (IR) was calculated following the formula: IR=(1–OD values of control group/OD values of drug group)×100%. This was followed by estimation of the 50% inhibitory concentration (IC50) value.
For cell proliferation analysis, transfected DTX-resistant PC cells in 100 μL medium were seeded into 96-well plates and incubated for 0–96 h as described above. The absorbance at 450 nm was determined on a Microplate Reader (Thermo Fisher Scientific).
Colony-forming assay
Transfected PC3/DTX and DU145/DTX cells were seeded into 6-well plates at a density of 500 cells per well. Following incubation at 37 °C, 5% CO2 for 2 weeks, visible colonies were fixed and stained with 0.1% crystal violet (Sigma-Aldrich Co.), and the number of colonies was counted under an inverted microscope (Olympus, Tokyo, Japan).
Cell migration and invasion assay
Cell migration and invasion ability were assessed using the Transwell assay. For the migration assay, PC3/DTX and DU145/DTX cells transfected with different oligonucleotides or plasmids were resuspended in serum-free medium and seeded into the upper chamber (Corning Incorporated, Corning, NY, USA). Complete medium containing 10% FBS was added to the bottom chamber as a chemoattractant. After incubation at 37 °C for 16 h, the nonmigratory cells on the upper surface were removed and cells that migrated to the basal side of the membrane were fixed and stained with 0.5% crystal violet. Finally, cells in random five visual fields were counted under a microscope (Olympus). For invasion assay, the upper chambers were precoated with matrigel (BD Biosciences), followed by evaluation of the invasion ability as described above.
Western blot assay
Cells washed with cold PBS were lysed in RIPA buffer containing 1% protease inhibitor (Sigma-Aldrich Co.) for protein extraction, followed by the detection of protein concentration using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) in accordance with the manufacturer’s procedures. Then, 30 μg of protein was divided by SDS-PAGE gel and blotted onto PVDF membranes (EMD Millipore, Billerica, MA, USA). Next, the membranes were blocked with 5% nonfat milk powder and incubated with primary antibodies against LRP, MDR1, MRP1, JAG1, and β-actin (Abcam, Cambridge, MA, USA). After washing with Tris-buffered saline–Tween 20 buffer, the membranes were further incubated with horseradish peroxidase-conjugated secondary antibody (Abcam). Protein blots were visualized using enhanced chemiluminescence (Beyotime) and quantified using Image Lab software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Dual-luciferase assay
The putative binding regions of DANCR and miR-34a-5p or miR-34a-5p and JAG1 were predicted by bioinformatics analysis. Partial sequences of DANCR containing wild-type or mutant binding sites of miR-34a-5p were cloned into psiCHECK™-2 plasmid (Promega Corporation, Fitchberg, WI, USA) to generate the wild-type plasmid (DANCR-WT) or mutant-type plasmid (DANCR-MUT). Similarly, wild-type or mutant plasmid JAG 3ʹUTR-WT or JAG 3ʹUTR-MUT was also constructed as the same approach. Afterward, luciferase reporter plasmid was transfected into DU145/DTX and PC3/DTX cells together with miR-NC or miR-34a-5p. About 48 h after transfection, cells were harvested and luciferase activity was assessed by the Dual-Luciferase Reporter Assay System (Promega Corporation).
RNA immunoprecipitation (RIP)
RIP analysis was conducted in DU145/DTX and PC3/DTX cells using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (EMD Millipore) following the manufacturer’s instructions. In brief, DU145/DTX and PC3/DTX cells were transfected with miR-34a-5p or miR-NC for 48 h. Then, cells were lysed in RIP buffer and cell extraction was incubated with ProteinA/G magnetic beads bounded with primary antibodies against Ago2 (Abcam) or IgG (Abcam). The protein and DNA in the immunoprecipitant complex were removed, and enrichment levels of DANCR were measured by qRT-PCR.
RNA pull-down
The interaction between DANCR and miR-34a-5p was examined using the Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific) in line with the manufacturer’s protocols. Biotin-labeled wild-type DANCR (Bio-DANCR WT) containing the putative miR-34a-5p binding sites and the biotin-labeled mutant DANCR (Bio-DANCR MUT) designed to disrupt the base-pairing between DANCR and miR-34a-5p were mixed with protein extracts of DU145/DTX and PC3/DTX cells and M-280 streptavidin-coated magnetic beads (Invitrogen). The beads were subsequently washed with ice-cold lysis buffer, low-salt buffer, and high-salt buffer. Following that, bound RNAs were purified from the RNA–protein complex and miR-34a-5p expression was determined by qRT-PCR.
Tumor xenograft
BALB/c nude mice (6 weeks old, male) were purchased from Vital River Laboratory Animal Technology (Beijing, People's Republic of China) and housed in specific pathogen-free conditions with a 12-h light/dark cycle. 6×106 PC3/DTX cells were transfected with si-NC or si-DANCR and then subcutaneously injected into the nude mice. Tumors were measured every 5 days and the volume was calculated using the formula: volume (mm3) = (length×width2)/2. At the end of implantation, tumors were harvested and weighed. This study was performed according to the Guidelines for Care and Use of Laboratory Animal with the approval of the Ethics Committee of Animal Research of the Fourth Hospital of Hebei Medical University.
Statistical analysis
All experiments in the present study were repeated in triplicate. Statistical differences in groups were analyzed by Student’s t test or one-way ANOVA using SPSS 20 software (IBM Corporation, Armonk, NY, USA) and results were displayed as the mean±SD. p<0.05 was regarded as statistically significant.
Results
DANCR was upregulated in DTX-resistant PC tissues and cells
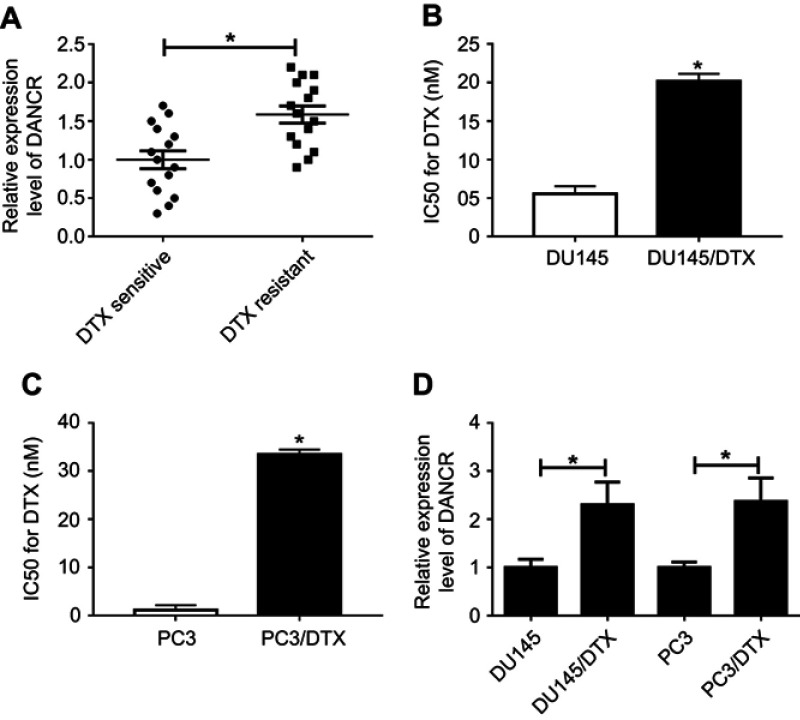
Here, an elevated abundance of DANCR was observed in DTX-resistant PC tissues by qRT-PCR assay (Figure 1A). To further demonstrate whether DANCR was associated with drug sensitivity, DTX-resistant PC cell lines DU-145/DTX and PC3/DTX were established and DTX resistance was evaluated by the IC50 value of DTX using the CCK-8 assay. Results showed that the IC50 value for DTX in DU-145/PTX cells was 20.14 nmol/L, which is higher than that in DU-145 cells at 5.56 nmol/L (Figure 1B). Likewise, the IC50 value for DTX in PC3/PTX cells was 33.48 nmol/L, which is markedly higher than that in PC3 cells at 1.16 nmol/L (Figure 1C), indicating the successful construction of DTX-resistant PC cell lines DU-145/DTX and PC3/DTX. Next, qRT-PCR assay further revealed that DANCR expression in DU-145/DTX and PC3/DTX cells was strikingly higher than that in DU-145 and PC3 cells (Figure 1D). These findings indicated that abnormal expression of DANCR might play a key role in the DTX resistance of PC.
Figure 1.
DANCR was upregulated in DTX-resistant PC tissues and cell lines. (A) DANCR expression in DTX-sensitive or DTX-resistant PC tissues was examined by qRT-PCR assay. (B and C) The IC50 value for DTX was assessed by CCK-8 assay. (D) The abundance of DANCR in DTX-sensitive or DTX-resistant PC cells was detected by qRT-PCR. *p<0.05.
Abbreviations: CCK-8, Cell Counting Kit-8; DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; IC50, 50% inhibitory concentration; PC, prostate cancer; qRT-PCR, quantitative reverse transcription PCR.
DNACR knockdown enhanced sensitivity to docetaxel in DTX-resistant PC cells
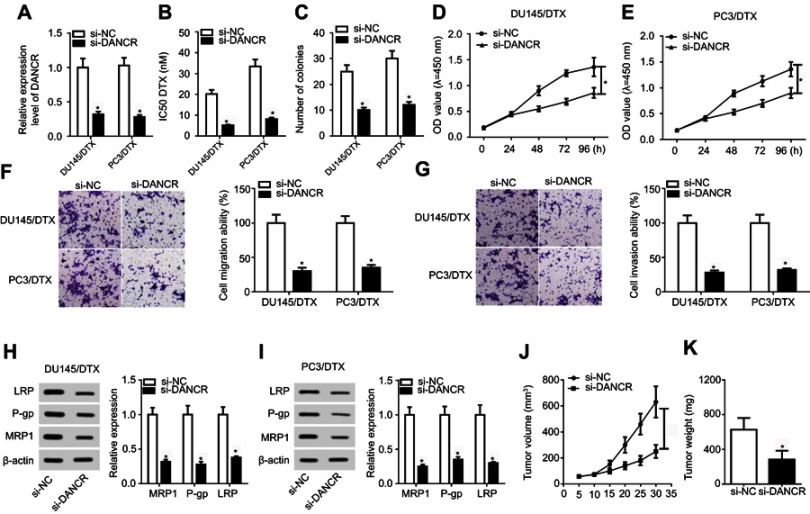
We first confirmed that DANCR was underexpressed in si-DANCR-transfected DU-145/DTX and PC3/DTX cells compared with negative control (Figure 2A), indicating that si-DANCR can be used for the following loss-of-function analyses. The results of the CCK-8 assay revealed that silencing of DANCR improved the DTX sensitivity of DTX-resistant PC cells, as demonstrated by the decreased IC50 value in DU-145/DTX and PC3/DTX cells compared with the si-NC group (Figure 2B). Moreover, transfection of si-DANCR in DU-145/DTX and PC3/DTX cells resulted in reduced colony number (Figure 2C) and decreased cell viability (Figure 2D and E), migration (Figure 2F), and invasion (Figure 2G), which were detected by colony-formation, CCK-8, or Transwell analyses. The protein levels of multidrug resistance associated protein MRP1, multidrug resistance 1 gene (MDR1)-coded protein P-gp, and lung resistance-related protein LRP obviously declined in DU-145/DTX and PC3/DTX cells after transfection with si-DANCR (Figure 2H and I). To further verify the effect of DANCR on drug sensitivity of DTX-resistant PC in vivo, PC3/DTX cells transfected with si-NC or sh-DANCR were subcutaneously vaccinated into BABL/c nude mice. As presented in Figure 2J and K, knockdown of DANCR significantly blocked tumor growth including the tumor volume and weight. Collectively, DANCR depletion impeded tumor progression and enhanced the sensitivity of DTX-resistant PC to DTX.
Figure 2.
DANCR knockdown improved docetaxel sensitivity of DTX-resistant PC cells. DU145/DTX and PC3/DTX cells were transfected with si-NC or si-DANCR. (A) The expression level of DANCR was determined by qRT-PCR. (B) The IC50 value for DTX was calculated by CCK-8 assay. (C) The colony number was evaluated by colony-forming assay. (D and E) The CCK-8 assay was performed to detect cell proliferation. (F and G) Transwell analysis was performed for the measurement of cell migration and invasion ability. (H and I) The expression of drug-resistance associated proteins LRP, P-gp, and MRP1 was examined by Western blot assay. (J) Tumor volume was detected every week after injection. (K) Tumor weight was investigated at the end point of 35 days. *p<0.05.
Abbreviations: CCK-8, Cell Counting Kit-8; DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; IC50, 50% inhibitory concentration; PC, prostate cancer; qRT-PCR, quantitative reverse transcription PCR; si-DANCR, small interfering RNA targeting DANCR.
DANCR directly targeted miR-34a-5p and decreased its expression in PC
By performing bioinformatics analysis using DIANA-LncBase online software, we observed the existence of putative binding sites between DANCR and miR-34a-5p (Figure 3A). Next, we further verified the true interaction between DANCR and miR-34a-5p. The luciferase reporter assay showed that introduction of miR-34a-5p reduced the luciferase activity of DANCR WT reporter in DU145/DTX and PC3/DTX cells, whereas no significant change was observed for DANCR MUT reporter (Figure 3B and C). The results of the RIP assay revealed that enrichment of DANCR in the Ago2 immunoprecipitation complex was obviously enhanced in DU145/DTX and PC3/DTX cells after transfection with miR-34a-5p, while IgG failed to show any efficacy of enrichment (Figure 3D). Moreover, RNA pull-down analysis disclosed that Bio-DANCR WT could pull down miR-34a-5p from the RNA–protein complex, but Bio-DANCR MUT was devoid of this enrichment ability, supporting that miR-34a-5p was a bona fide DANCR-targeting miRNA (Figure 3E and F). After that, we performed qRT-PCR analysis to examine the expression of miR-34a-5p in DTX-resistant PC cells. As a result, the expression of miR-34a-5p was markedly decreased in DU145/DTX and PC3/DTX cells compared to control groups (Figure 3G). Then, the effect of DANCR on miR-34a-5p expression was investigated by qRT-PCR assay. As a result, transfection of pcDNA-DANCR suppressed miR-34a-5p expression in DU145/DTX and PC3/DTX cells. Inversely, knockdown of DANCR by transfection of si-DANCR increased the miR-34a-5p level (Figure 3H and I). Altogether, these data concluded that DANCR negatively regulated miR-34a-5p expression via targeting this miRNA.
Figure 3.
DANCR directly targeted miR-34a-5p. (A) The putative binding sites of DANCR in miR-34a-5p were predicted using DIANA-LncBase software. (B and C) Luciferase activity of wild-type or mutant DANCR in DU145/DTX and PC3/DTX cells following miR-NC or miR-34a-5p transfection was determined by luciferase reporter assay. (D) The correlation between DANCR and miR-34a-5p in DU145/DTX and PC3/DTX cells was detected by RNA immunoprecipitation (RIP) assay. (E and F) The direct interaction between DANCR and miR-34a-5p was assessed by RNA pull-down assay. (G) The miR-34a-5p level in DTX-sensitive or DTX-resistant PC cells was detected by qRT-PCR. (H and I) The expression of DANCR and miR-34a-5p in DU145/DTX and PC3/DTX cells, which were initially transfected with pcDNA, pcDNA-DANCR, si-NC, or si-DANCR, was determined by qRT-PCR assay. *p<0.05.
Abbreviations: DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; MUT, mutant; PC, prostate cancer; qRT-PCR, quantitative reverse transcription PCR; si-DANCR, small interfering RNA targeting DANCR; WT, wild type.
DANCR overturned miR-34a-5p-mediated docetaxel sensitivity in DTX-tolerated PC cells
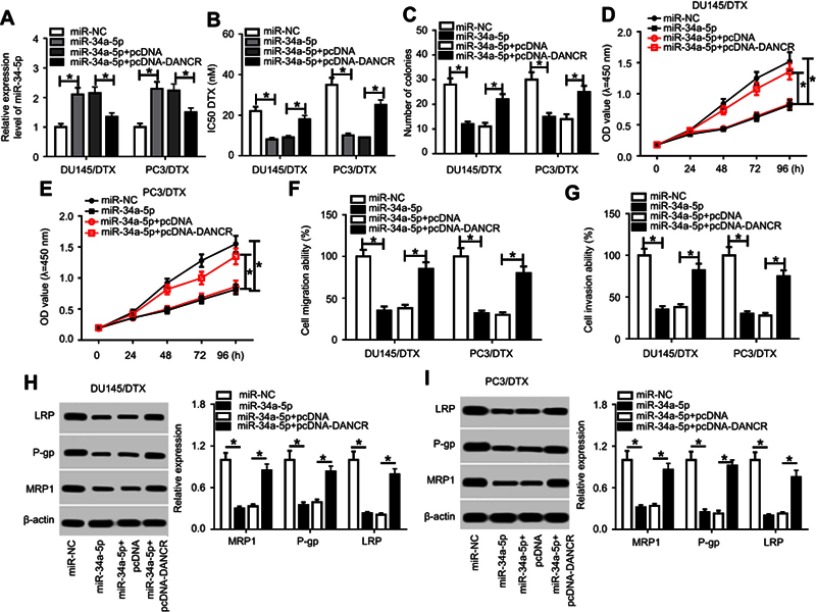
To validate the modulation of the DANCR/miR-34a-5p axis in the chemosensitivity of DTX-tolerated PC cells, rescue experiments were carried out through transfection of miR-NC, miR-34a-5p, miR-34a-5p+pcDNA, or miR-34a-5p+pcDNA-DANCR into DU145/DTX and PC3/DTX cells. The results of the qRT-PCR assay revealed that transfection of miR-34a-5p mimics significantly stimulated miR-34a-5p expression, which was reversed by pcDNA-DANCR transfection (Figure 4A). Moreover, forced expression of miR-34a-5p led to a marked decrease of the IC50 value for DTX in DU145/DTX and PC3/DTX cells. However, re-expression of DANCR strikingly abolished the miR-34a-5p-mediated reduction in IC50 value (Figure 4B). Meanwhile, we also found that exogenous expression of miR-34a-5p suppressed colony formation (Figure 4C), cell proliferation (Figure 4D and E), migration (Figure 4F), and invasion (Figure 4G), as well as downregulating drug-resistant associated proteins P-gp, MRP1, and LRP (Figure 4H and I) in DU145/DTX and PC3/DTX cells. However, restoration of DANCR abrogated miR-34a-5p-mediated cell phenotypes and protein expression in DTX-resistant PC cells (Figure 4C–I). Taken together, these data indicated that DANCR attenuated sensitivity to DTX via repressing miR-34a-5p in DTX-resistant PC cells.
Figure 4.
DANCR weakened the stimulatory effect of miR-34a-5p on docetaxel sensitivity of DTX-tolerated PC cells. DU145/DTX and PC3/DTX cells were transfected with miR-NC, miR-34a-5p, miR-34a-5p+pcDNA, or miR-34a-5p+pcDNA-DANCR, followed by detection of miR-34a-5p expression (A), IC50 value for DTX (B), colony number (C), cell proliferation (D and E), migration (F), invasion (G), and drug-resistance-related proteins LRP, P-gp, and MRP1 expression (H and I). *p<0.05.
Abbreviations: DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; IC50, 50% inhibitory concentration; PC, prostate cancer.
JAG1 was downregulated in DTX-resistant PC cells and targeted by miR-34a-5p
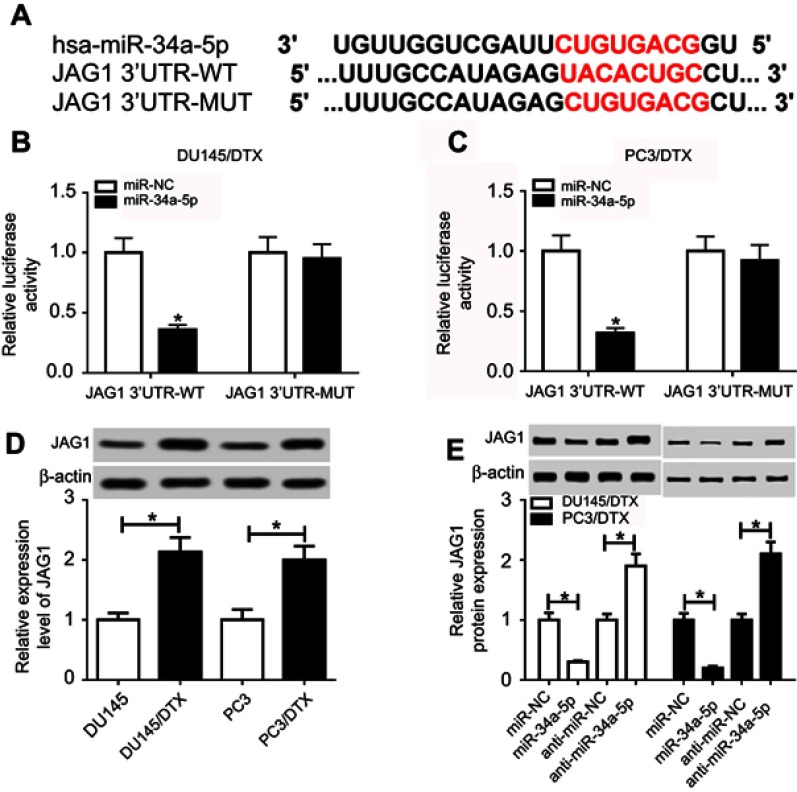
miRTarBase and TargetScan software was applied to determine the downstream targets of miR-34a-5p associated with the chemoresistance of human cancers. As a result, JAG1 was screened out as a putative target of miR-34a-5p due to its involvement in drug resistance (Figure 5A). Afterward, the luciferase assay was performed to verify the interaction between miR-34a-5p and JAG1. The results showed that overexpression of miR-34a-5p impaired the luciferase activity of JAG1 3ʹUTR-WT reporter in DU145/DTX and PC3/DTX cells, while the luciferase activity of JAG1 3ʹUTR-MUT reporter had no significant change (Figure 5B and C). The following Western blot assay showed that JAG1 protein expression in DU145/DTX and PC3/DTX cells was higher than that in DU145 and PC3 cells (Figure 5D). Moreover, miR-34a-5p supplement inhibited, while miR-34a-5p knockdown promoted, the protein expression of JAG1 in DU145/DTX and PC3/DTX cells (Figure 5E). These data suggested that miR-34a-5p negatively modulated JAG1 expression via targeted binding.
Figure 5.
JAG1 was targeted by miR-34a-5p. (A) The putative binding regions of miR-34a-5p within the 3ʹUTR of JAG1 were predicted using miRTarBase and TargetScan online software. (B and C) Luciferase activity of JAG1 3ʹUTR-WT or JAG1 3ʹUTR-MUT reporter in DU145/DTX and PC3/DTX cells transfected with miR-NC or miR-34a-5p. (D and E) The protein levels of JAG1 in nontransfected or transfected DU145/DTX and PC3/DTX cells. *p<0.05.
Abbreviations: DTX, docetaxel; MUT, mutant; WT, wild type.
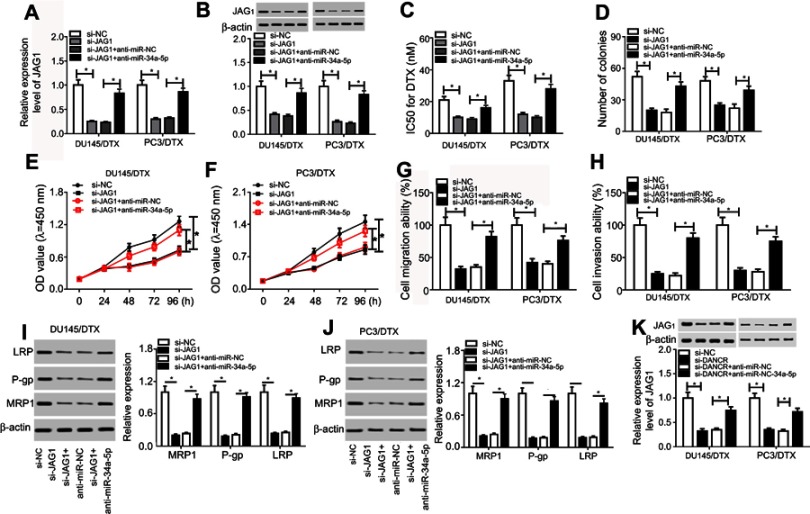
DANCR knockdown enhanced docetaxel sensitivity of DTX-resistant PC cells via repressing JAG1 via targeting miR-34a-5p
To clarify the correlation between miR-34a-5p and JAG1 in drug sensitivity of DTX-resistant PC cells, rescue experiments were carried out by transfection of si-NC, si-JAG1, si-JAG1+anti-miR-NC, or si-JAG1+anti-miR-34a-5p into DU145/DTX and PC3/DTX cells. Obviously reduced abundances of JAG1 at mRNA and protein levels were observed in DU145/DTX and PC3/DTX cells after transfected with si-JAG1, while anti-miR-34a-5p introduction reversed the inhibitory effect of si-JAG1 on JAG1 expression (Figure 6A and B). Furthermore, JAG1 knockdown enhanced the sensitivity of DTX-tolerated PC cells to DTX, demonstrated by the decreased IC50 value (Figure 6C) and colony number (Figure 6D), inhibited cell proliferation (Figure 6E and F), migration (Figure 6G), and invasion (Figure 6H), and low expression of drug-resistant associated proteins P-gp, MRP1, and LRP (Figure 6I and J) in DU145/DTX and PC3/DTX cells. However, inhibition of miR-34a-5p established si-JAG1-mediated drug sensitivity in DTX-resistant PC cells (Figure 4C–J). To explore whether DANCR should be responsible for DTX resistance via modulation of the miR-34a-5p/JAG1 axis, si-DANCR was transfected into DU145/DTX and PC3/DTX cells along with anti-miR-NC or anti-miR-34a-5p, followed by detection of the JAG level. As a result, knockout of DNACR significantly degraded JAG1 expression, which was restored by miR-34a-5p inhibition (Figure 6K). Altogether, our findings indicated that the DANCR/miR-34a-5p/JAG1 axis played a regulatory role in the DTX resistance of DTX-resistant PC cells.
Figure 6.
DANCR regulated docetaxel sensitivity of DTX-resistant PC cells via the miR-34a-5p/JAG1 axis. DU145/DTX and PC3/DTX cells were transfected with si-NC, si-JAG1, si-JAG1+anti-miR-NC, or si-JAG1+anti-miR-34a-5p, followed by the detection of JAG1 expression (A and B), IC50 value for DTX (C), colony number (D), cell proliferation (E and F), migration (G), invasion (H), and LRP, P-gp, and MRP1 proteins (I and J). (K) JAG1 expression in DU145/DTX and PC3/DTX cells following the transfection of si-NC, si-DANCR, si-DANCR+anti-miR-NC, or si-DANCR+anti-miR-34a-5p. *p<0.05.
Abbreviations: DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; IC50, 50% inhibitory concentration; PC, prostate cancer; si-DANCR, small interfering RNA targeting DANCR.
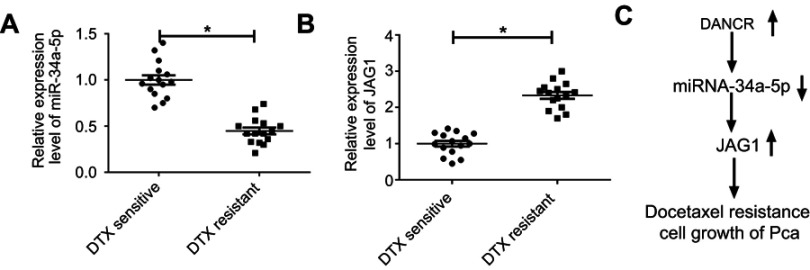
Ectopic expression of miR-34a-5p and JAG1 in DTX-resistant PC tissues
To explore the clinical relevance of DANCR, miR-34a-5p, and JAG1, we further determined the levels of miR-34a-5p and JAG1 in DTX-sensitive and DTX-resistant PC tissues by qRT-PCR assay. The results showed that miR-34a-5p expression was significantly downregulated and JAG1 was upregulated in DTX-resistant PC tissues compared to DTX-sensitive tissues (Figure 7A and B). Next, we presented a diagram to illustrate the mechanism of the DANCR/miR-34a-5p/and JAG1 axis in DTX resistance in PC (Figure 7C).
Figure 7.
miR-34a-5p was downregulated and JAG1 was upregulated in DTX-resistant PC tissues. (A and B) The levels of miR-34a-5p and JAG1 in DTX-sensitive and resistant PC tissues. (C) Schematic representation of DANCR/miR-34a-5p/JAG1 in the modulation of docetaxel resistance in PC. *p<0.05.
Abbreviations: DANCR, anti-differentiation noncoding RNA; DTX, docetaxel; PC, prostate cancer.
Discussion
DTX is considered a promising chemotherapeutic option for PC, but the emergence of DTX resistance has become a primary obstacle for chemotherapy. Growing evidence has demonstrated that misregulation of lncRNAs results in tumorigenesis and chemoresistance in several types of human cancers.12,18 Thereby, identification of a novel lncRNA associated with the chemoresistance of PC is of great significance for the development of lncRNA-targeted therapy. Here, we initially proposed the viewpoint that DANCR was highly expressed in DTX-resistant PC tumor tissues and cell lines. DANCR knockdown enhanced the sensitivity of DTX-resistant PC cells to DTX by suppressing JAG1 through serving as a ceRNA of miR-34a-5p.
Accumulated evidence has verified the regulatory role of DANCR in the progression and chemoresistance of various cancers. Abnormal expression of DANCR has been reported in several types of human malignancies, like osteosarcoma,19 lung cancer,20 and gastric cancer.15 Recently, emerging literature also revealed that DANCR attenuates the sensitivity of glioma to cisplatin via activation of the AXL/PI3K/Akt/NF-κB pathway.21 Consistent with the previous study, we observed an elevated abundance of DANCR in DTX-resistant PC tumor tissues and cell lines. Functionally, DANCR knockdown improved sensitivity to DTX in DTX-resistant PC in vitro and in vivo.
Numerous mechanisms have been validated in the interaction between lncRNA and miRNA in various aspects of tumor biology. The lncRNA may serve as a ceRNA to restrain the binding of miRNA and its target gene, eventually leading to the derepression of mRNA. In our study, we attempted to determine the mechanism of DANCR involved in DTX resistance of PC. As a result, miR-34a-5p was identified to be targeted by DANCR. Rescue experiments further verified that miR-34a-5p sensitized DTX-resistant PC to DTX, and the effect of miR-34a-5p was revered by DANCR overexpression. In agreement with our data, reliable evidence demonstrates that miR-34a-5p plays a central role in the regulation of chemotherapy in PC. As reported by Wen et al,22 miR-34a enhanced paclitaxel chemotherapy in paclitaxel-resistant PC cells. Moreover, miR-34a attenuated DTX resistance in prostate cancer through repressing RET.23
miRNAs are reported to degrade the expression of special target genes through binding their 3ʹUTR. One of our jobs is to identify the downstream target gene modulated by miR-34a-5p. As a result, JAG1 was affirmed as a potential target gene of miR-34a-5p. JAG1 is one of the canonical ligands that function prevailingly in the highly conserved Notch signaling pathway. Recently, JAG1 has been reported to induce activation of the Notch signaling pathway via physically interacting with Notch receptors, leading to the alteration of a series of cell processes, including cell differentiation, proliferation, survival, and apoptosis.24 An increasing number of studies show that JAG1 plays a vital role in the progression and chemoresistance of multiple kinds of cancers. High-level coexpression of JAG1 and surface receptor Notch1 was initially observed in breast cancer and signified a poor prognosis.25,26 Following research confirmed that silencing of JAG1 blocks the metastasis of colorectal cancer and osteosarcoma via inactivation of the Notch pathway.27,28 Moreover, upregulation of JAG1 oncogene is observed in nilotinib and imatinib-resistant myeloblastic leukemia cells.29 miR-26b and miR-128 contribute to the resistance of nasopharyngeal carcinoma or glioma to cisplatin by downregulating JAG1.30,31 Consistent with previous studies, we observed that knockdown of JAG1 enhanced the chemosensitivity of DTX-tolerated PC cells to DTX, and the biological function of JAG1 was rescued by miR-34a-5p inhibitor. Furthermore, silencing of DANCR repressed the expression of JAG1 by serving as a molecular sponge for miR-34a-5p.
Conclusion
Our study provides the first evidence that DANCR enhances the resistance of DTX-tolerated PC cells to DTX via the miR-34a-5p/JAG1 axis, elucidating the mechanism of how DANCR is involved in the DTX resistance of PC. These findings prompt that DANCR may be an effective therapeutic avenue for PC patients with DTX resistance.
Ethics approval and consent to participate
These experiments were performed according to the Declaration of Helsinki Principles with the approval of the Research Ethics Committee of the Fourth Hospital of Hebei Medical University, and written informed consents were obtained from all participants. This study was performed according to the Guidelines for Care and Use of Laboratory Animal with the approval of the Ethics Committee of Animal Research of the Fourth Hospital of Hebei Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2015;387(10013):70–82. doi: 10.1016/S0140-6736(14)61947-4 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Miren GA, Juanita C. Androgen deprivation therapy: minimizing exposure and mitigating side effects. J Natl Compr Canc Netw. 2012;10(9):1088–1095. [DOI] [PubMed] [Google Scholar]
- 4.Lombard AP, Liu C, Armstrong CM, et al. ABCB1 mediates cabazitaxel-docetaxel cross-resistance in advanced prostate cancer. Mol Cancer Ther. 2017;16(10):2257–2266. doi: 10.1158/1535-7163.MCT-17-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domanska UM, Timmer-Bosscha H, Nagengast WB, et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14(8):709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanjay S, Raghu A, Stuti B. Multifunctional antioxidant nanoliposome-mediated delivery of PTEN plasmids restore the expression of tumor suppressor protein and induce apoptosis in prostate cancer cells. J Biomed Mater Res. 2018;106(12):3152–3164. doi: 10.1002/jbm.a.36510 [DOI] [PubMed] [Google Scholar]
- 7.Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. doi: 10.1038/nature01075 [DOI] [PubMed] [Google Scholar]
- 8.Michelhaugh SK, Leonard L, Jason B, Hui J, Gregory K, Bannon MJ. Mining Affymetrix microarray data for long non-coding RNAs: altered expression in the nucleus accumbens of heroin abusers. J Neurochem. 2011;116(3):459–466. doi: 10.1111/j.1471-4159.2010.07126.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnsson P, Lipovich L, Dan G, Morris KV. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840(3):1063–1071. doi: 10.1016/j.bbagen.2013.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding Rnas in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145(2):178–181. doi: 10.1016/j.cell.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 13.Gu P, Chen X, Xie R, et al. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther. 2017;25(8):1959–1973. doi: 10.1016/j.ymthe.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Meng Z, Lei L, Jian L, Yu-Xin C. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2015;8(9):11480–11484. [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Z, Li H, Du B, et al. LncRNA DANCR promotes migration and invasion through suppression of lncRNA-LET in gastric cancer cells. Biosci Rep. 2017;37(6):BSR20171070. doi: 10.1042/BSR20171070 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Yuan SX, Wang J, Yang F, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63(2):499–511. doi: 10.1002/hep.27893 [DOI] [PubMed] [Google Scholar]
- 17.Jia J, Li F, Tang XS, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7(25):37868–37881. doi: 10.18632/oncotarget.9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol. 2016;37(12):1–11. doi: 10.1007/s13277-016-5448-5 [DOI] [PubMed] [Google Scholar]
- 19.Jiang N, Wang X, Xie X, et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55. doi: 10.1016/j.canlet.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Jiang M. The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed Pharmacother. 2018;103:94–100. doi: 10.1016/j.biopha.2018.03.053 [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Zhou G, Li M, et al. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem Int. 2018;118:233–241. doi: 10.1016/j.neuint.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 22.Wen D, Peng Y, Lin F, Singh RK, Mahato RI. Micellar delivery of miR-34a modulator rubone and paclitaxel in resistant prostate cancer. Cancer Res. 2017;77(12):3244–3254. doi: 10.1158/0008-5472.CAN-16-2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin T, Zhang G, Hui Z, Li Y, Zhu C. Long non-coding RNA NEAT1 contributes to docetaxel resistance of prostate cancer through inducing RET expression by sponging miR-34a. RSC Adv. 2017;7(68):42986–42996. doi: 10.1039/C7RA06107B [DOI] [Google Scholar]
- 24.Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016;22:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael R, Silvia O, Lynn C, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65(18):8530–8537. doi: 10.1158/0008-5472.CAN-05-1069 [DOI] [PubMed] [Google Scholar]
- 26.Dickson BC, Mulligan AM, Zhang H, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Modern Pathol. 2007;20(6):685–693. doi: 10.1038/modpathol.3800785 [DOI] [PubMed] [Google Scholar]
- 27.Jia C, Zhang H, Ying C, et al. miR-598 inhibits metastasis in colorectal cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell Res. 2017;352(1):104–112. doi: 10.1016/j.yexcr.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 28.Wen ZQ, Li XG, Zhang YJ, Ling ZH, Lin XJ. Osteosarcoma cell-intrinsic colony stimulating factor-1 receptor functions to promote tumor cell metastasis through JAG1 signaling. Am J Cancer Res. 2017;7(4):801–815. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TM, Ha SA, Kim HK, et al. Gene expression signatures associated with the in vitro resistance to two tyrosine kinase inhibitors, nilotinib and imatinib. Blood Cancer J. 2011;1(8):e32. doi: 10.1038/bcj.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Yin W, Zhang Z, Shi G. Down-regulation of miR-26b induces cisplatin resistance in nasopharyngeal carcinoma by repressing JAG1. FEBS Open Bio. 2016;6(12):1211–1219. doi: 10.1002/2211-5463.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi DY, Su Q, Zhang FC, et al. Effect of microRNA-128 on cisplatin resistance of glioma SHG-44 cells by targeting JAG1. J Cell Biochem. 2017;119(4):3162–3173. [DOI] [PubMed] [Google Scholar]