Abstract
Introduction
Local field potential (LFP) recordings from deep brain stimulator (DBS) leads provide insight into the pathophysiology of Parkinson’s disease (PD). We recorded LFP activity from DBS leads within the subthalamic nucleus (STN) in PD patients undergoing DBS surgery in order to identify reproducible pathophysiological signatures of the disease.
Methods
LFPs were recorded in 11 hemispheres from PD patients undergoing STN-DBS. Bipolar recordings were performed off medication for 2 minutes at rest and another 2 minutes with continuous repetitive opening-closing of the contralateral hand. Spectral analysis and bicoherence were performed and compared between the two testing conditions.
Results
In all hemispheres, predominance of the beta band frequency (13–30 Hz) was observed at rest and during movement. Beta peak energy was significantly (p<0.05) increased during movement compared to rest in 6 of 10 hemispheres. Significant beta bicoherence was observed at rest and during movement in 5 of 10 hemispheres. The most robust LFP recordings were observed at the DBS contact(s) independently chosen for programming in 9 of the 10 hemispheres.
Conclusions
In PD patients, beta activity that increases with repetitive movement may be a signature of the ‘off’ medication state. These findings provide new data on beta oscillatory activity during the Parkinsonian ‘off’ state that may help further define the LFP signatures of PD.
Keywords: local field potential, deep brain stimulation, beta, spectral analysis, bicoherence
Introduction
The acquisition and subsequent analysis of local field potentials (LFP) during deep brain stimulation (DBS) surgery has not only enhanced the understanding of the pathophysiology of Parkinson’s disease (PD), but has also provided a potential means for improving the efficacy of DBS as a treatment modality (3,21). A number of frequency bands, including alpha (8–12 Hz), beta (13–30 Hz), gamma (35–100 Hz) and high frequency (250–300 Hz) have been implicated in the movement-related changes seen in both the PD off-medication (‘off’) and on-medication (‘on’) state from DBS lead recordings within the subthalamic nucleus (STN) (1–4,8,12,19,21–23,29,30,32,34,35).
The correlation between Parkinsonian ‘off” state symptoms and excessive beta frequency oscillatory activity has been demonstrated in a number of studies, several of which have shown reduction in beta activity with administration of levodopa or DBS (4,16,17,23). Despite the growing body of literature on beta frequency oscillations in PD, few studies have focused on comparing beta band activity at rest and then subsequently during continuous repetitive task performance in the ‘off’ state. Androulidakis et. al. analyzed continuous repetitive movements of the finger over a 100 second block breaking the data into three segments and found a reduction in beta modulation over time with no change in the main beta frequency (2).
In the present study, we recorded raw STN LFP activity from PD subjects undergoing DBS in the ‘off’ state with the contralateral hand both at rest and during continuous repetitive movement. This type of continuous LFP analysis is different from prior studies whereby LFP activity was assessed temporally at isolated intervals either before, during or after repetitive movements (1). Our objective was to assess net changes in beta activity between rest and repetitive task conditions summed over a two-minute epoch using spectral analysis and bicoherence.
Methods
All data were collected after Institutional Review Board (15122701-IRB01) approval. Informed written consent was obtained from all subjects. The imaging, microelectrode recording (MER), and implant methods have been previously described (14,15). In brief, all patients underwent stereotactic frame-based placement of bilateral DBS leads using direct MRI-guided targeting as well as microelectrode recording for target refinement. Once the optimal track was identified for implantation, the DBS lead was inserted. Lead location was confirmed using intraoperative CT (O-arm, Medtronic, Minneapolis, MN) merged with preoperative MRI/CT images. An average number of 1.55 MER tracks were performed on each side (min=1, max=3). Once the leads were implanted, and prior to macrostimulation testing (6), the LFP recording testing protocol was performed.
All data were acquired on an Alpha-Omega Neuro Omega system (Alpha-Omega, Nazareth, Israel). All data was analyzed using a bipolar montage. In most cases STN LFPs were recorded from each lead in a bipolar electrode configuration (0–1, 1–2, 2–3). Data for the other electrode pair was recorded in a referential electrode configuration (1,2,3,4 to reference that was placed in the skin near the surgical opening). This referential data was then mathematically re-montaged into a bipolar montage in MATLAB® (Mathworks, Natick, MA). Given that new leads have 8 electrode channels all data is now recorded in a referential mode and re-montaged to include all the separate electrodes in a ring to get a similar data pattern for the 4 channel electrodes. The output of each lead was connected to a custom-made cable that converted the output of each individual lead at the end of the specific DBS lead manufacturers testing cable to differential amplifiers. The data from the first two hemispheres were converted to a differential signal within an in-house MATLAB (Mathworks, Natick, MA) subroutine. The data were sampled at a sample rate of 1375 Hz with a gain of 55,000. The high pass filter was set at 1 Hz and the low pass filter was set at 300 Hz. A 60 Hz line filter was used to reduce the line noise. The outer guide cannula for the lead acted as the iso-ground. Data were stored on the Neuro Omega system and then transferred to a personal computer for further analysis, which is described below. Post-surgery, the data were converted to the MATLAB format via an Alpha-Omega conversion routine and then imported into MATLAB. Only the 4 channels of raw LFP data were analyzed.
STN LFPs were collected under two conditions: with the contralateral hand at rest and during a continuous repetitive movement. In the resting condition, the subjects were asked to relax both arms at their sides. After obtaining a stable baseline signal, LFPs were then recorded continuously for 120 seconds. The data were visually reviewed in real time to identify signal artifact or excessive noise. If signal artifact or excessive noise was identified, the signal acquisition was halted and re-started. During the continuous movement phase of testing, subjects were instructed to perform a self-initiated hand opening-closing maneuver for 120 seconds at a speed that was comfortable for them. They were also encouraged to perform maximum amplitude open-close movements and were continuously observed during the recording period for consistency and uniformity of movement. Notation was also made of any tremor activity (none was noted). If the motor task was interrupted or inconsistent, signal acquisition was halted and restarted. During the 120 second period of data collection, the subjects were intermittently reminded to open the hand wide and close it tightly. Once again data were reviewed in real time for signal artifact and noise. If more than two seconds of data was deemed corrupted the trial was re-started.
All data were analyzed using custom made scripts and built-in functions of MATLAB. The time series, x(t), were broken into 100 epochs of 1024 points length. The mean of the data epoch was calculated and then subtracted from all points in the epoch. The fast Fourier transform (FFT) was then performed. Power spectral density was calculated by taking the absolute value of the complex FFT terms and then averaging them over the 100 epochs for each frequency value. The frequency resolution at this FFT point size was 1.34 Hz.
Where X(f) is defined as:
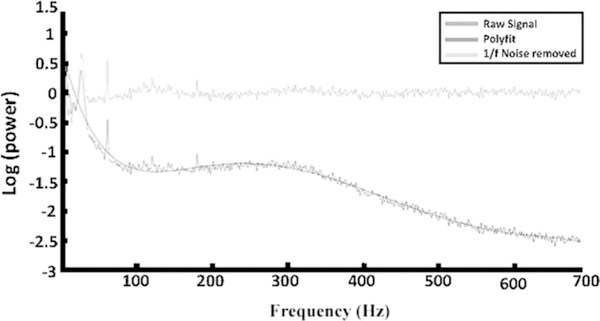
Figure 1 represents the log power spectra from patient 1 during movement.
FIG. 1.
Comparison of raw signal (dark gray line), 7th order polynomial fit (black line), and removal of 1/f noise out to the Nyquist frequency (light gray line). The beta frequencies were easily recognized at around 25 Hz while the 60-Hz noise spike was also seen.
The bispectrum was calculated using the methods described by Shils (26). In short, the bispectrum is a measure of the interaction strength, or coupling, between two different frequencies and is defined as:
Where <> is the average over multiple time epochs, B(ω1,ω2) is the bispectrum, ω=2πf and the value in the <> is the triple product. When frequencies ω1, ω2, and (ω1+ω2) are independent of each other, the bispectrum will be small. Given that the bispectrum is related to the overall power in the signal, the bicoherence is what is used to determine the significance of the bispectral values. There are multiple forms for the bicoherence (see Fig.2 in reference 26 for examples of the bicoherence), yet for the purposes of this work the Kim and Powers (1979) definition was used
which ranges between 0 and 1. Shils demonstrated that for a large number of averages (at least greater than 30) under the null hypothesis of no significant interaction between the 3 different frequencies in the triple product the 95% confidence threshold for Γ(ω1,ω2) can be approximated by
where N is the number of epochs used in the analysis, which for this study was 100 (26,27). The signifigance level for this study is 0.25.
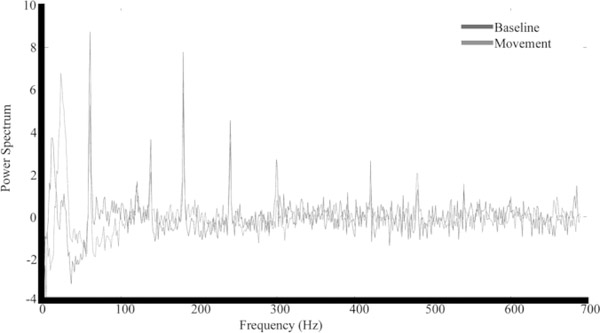
FIG. 2.
Comparison of power spectra between rest (black) and movement (gray). In all hemispheres, the beta peak in the movement data set visually appeared greater than the beta peak in the baseline data set.
The beta band peak power frequencies were defined (beta peak) following the method of Swann et. al. with some modifications (30). The data were normalized using a 7th order polynomial fit to correct for the 1/f noise pattern in the data. (Figure 1). The 7th order polynomial was chosen by running the fit at multiple orders (1–10) and looking at the least square error for every order. The first 6 polynomial fits had errors greater than 20% where the 7th had an error of 15.4% with minimal reductions as the order increased (<1% for each order increase). The least error was defined as the total sum of the squared difference between the fitted polynomial and the original data set.
The polynomial fitted data were then subtracted from the original PSD data set.
Figure 1 shows the overlay of all the above procedures out to the Nyquist frequency. The beta frequencies are easily recognized at around 30 Hz while the 60 Hz noise spike is also seen.
This was done for both the resting and movement data sets (Figure 2). Once the 1/f noise was removed, the data were normalized by subtracting the mean from the data and dividing by the standard deviation.
Next, the beta peaks were located in both of the data sets by visual examination of all data within the beta band (13Hz – 30Hz). Once the beta peaks were located, the data set was truncated to +/− 4 Hz surrounding the peak frequency. The amplitude at the peak beta value was compared in the rest condition versus the movement condition using the Wilcoxon rank sum test. In order to ensure that removal of the 1/f noise did not change the characteristics of the signal, data for two patients was analyzed without using the 1/f noise correction method.
Post-operative programming and selection of optimal stimulation contact(s) were performed using clinical criteria. Programming neurologists (G.P. and L.V.M.) were blinded to the LFP data analysis and results.
Results
Local field potentials were recorded in seven patients (11 total hemispheres) undergoing STN DBS surgery (4 bilateral, 3 unilateral, Table 1). The average age of the patients (±SD) was 59.4±9 years. Forty-three percent of subjects were male. The average duration of PD (±SD) was 8.86±3.8 years. Six patients were implanted with the Medtronic Model 3389 lead (Medtronic, Minneapolis, MN) and 1 patient was implanted with the St. Jude Medical Infinity™ directional lead (Abbott, Chicago, IL).
Table 1.
Results. Shaded boxes indicate significant elevations in beta power with movement
| Patient | Age/Gender | Disease Duration (years) | Cardinal Symptom | UPDRS III ON/OFF Medication | Hemisphere | Beta Peak (Hz) | Movement | P value | Bicoherence Rest | Bicoherence Movement |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51F | 6 | Tremor | 12/30 | L | 22.8 | Increase | 0.008 | N | Y |
| R | 25.5 | Increase | 0.03 | Y | Y | |||||
| 2 | 59M | 10 | Bradykinesia | 20/44 | L | 16.1 | Increase | 0.69 | N | Y |
| R | 22.8 | Increase | 0.15 | N | Y | |||||
| 3 | 64F | 11 | Tremor | 12/36 | L | 22.8 | Increase | 0.02 | Y | Y |
| R | 25.5 | Increase | 0.15 | Y | Y | |||||
| 4 | 55M | 16 | Bradykinesia | 13/66 | L | * | * | * | * | * |
| R | 20.14 | Increase | 0.008 | N | N | |||||
| 5 | 48F | 7 | Bradykinesia | 19/49 | R | 21.5 | Increase | 0.008 | N | N |
| 6 | 74F | 5 | Tremor | na/55 | L | 18.8 | Increase | 0.008 | Y | Y |
| 7 | 65M | 7 | Bradykinesia | 24/53 | R | 25.5 | Increase | 0.31 | Y | Y |
na= patient unable to tolerate levodopa
= inadequate LFP signal for analysis, Movement = change in beta power during continuous repetitive hand grasp movement, M=male, F=female, L=left, R=right, Y=yes, N=no, Wilcoxon rank sum test used for statistical analysis with significance set at P< 0.05. UDPRS (Unified Parkinson’s disease dating scale)
Adequate LFP signal was obtained in ten of eleven hemispheres. There were no differences in beta peak characteristics and subsequent statistical comparisons when analyzing either the raw signal or the signal with the 1/f noise pattern removed in two patients thus the 1/f data was analyzed. The bipolar contacts which provided the most robust LFP signal were used for subsequent analysis and are shown in Table 2. The optimal contacts chosen for therapeutic stimulation after programming from two experienced movement disorders specialists (LVM and GP) are also shown in Table 2. The contacts that provided the most robust LFP signal corresponded to the same contacts chosen for therapeutic stimulation in nine of the ten hemispheres.
Table 2.
Optimal recorded LFP contacts correlated with nine of the ten contacts independently chosen for postoperative programming. Correlation between the two were based on the contact chosen as the active contact or cathode (− sign immediately following the numbered contact) and its coincidence with one of the recorded LFP contacts. Shaded boxes indicate mismatched contacts.
| Patient | Surgery Type | Left | Right | ||
|---|---|---|---|---|---|
| LFP | Active | LFP | Active | ||
| 1 | Bilateral | 0/1 | 0–1+2+ | 1/2 | 2-c+ |
| 2 | Bilateral | 0/1 | 1-c+, 3-c+ | 0/1 | 1-c+, 3-c+ |
| 3 | Bilateral | 1/2 | 1–3+ | 2/3 | 1–3+ |
| 4 | Bilateral | * | 1-c+ | 0/1 | 1-c+ |
| 5 | Right | - | - | 2/3 | 1-c+, 2-c+ |
| 6 | Left | 0/1 | 1-c+ | - | - |
| 7 | Right | - | - | 0/1 | 1–0+ |
LFP = recorded LFP contact pair, Active = contact(s) chosen for programming, “-“ indicates cathode contact, “+” indicates anode contact
= inadequate LFP signal
Spectral Analysis
In all ten analyzed hemispheres, an increase in beta power was observed during movement (red line) in comparison to rest (blue line) (Figure 2). This increase in beta power achieved statistical significance (p < 0.05) in six of the ten hemispheres when compared to resting beta activity (Table 1). There was no difference in the location of the beta peak between the raw power spectral density data and the data with the 1/f power removed. In all cases the beta peak in the movement data set visually appeared greater than the beta peak in the resting data set, yet in four hemispheres, the beta peak amplitudes were not significantly different. In three of those hemispheres whereby the beta power was not significantly different between movement and rest, the patients were predominantly bradykinetic.
Bicoherence
Changes in bicoherence between rest and movement can be seen in Figure 3. There was significant coupling within the beta frequency band and its first harmonic seen at rest (Figure 4a) and with movement (Figure 4b) in five of the ten hemispheres. For each bicoherence figure all data above 0.25 is significant to the 95% confidence interval (26). In each plot the colored surfaces represent an area of bicoherence values with the x-axis representing f1 and the y-axis representing f2. In some cases, due to spectral spreading, as a result of FFT resolution and filtering artifact, there is spread of the bicoherence from a significant value in the middle to insignificant values. Additionally, there are some areas outside of the alpha and beta bands that look like there is some coupling (see blue surfaces at f1(f2)=26Hz and f2(f1)=9z). In reviewing the raw data these areas are artifact due to mathematical rounding errors (eg. 1e−9/1e−15). For each plot the data is symmetric around the diagonal thus only half the plot is analyzed.
FIG. 3.
Analysis of bicoherence showed no significant (P . 0.05) beta coupling at rest in five out of 10 hemispheres (A). Significant beta coupling during movement was seen in three of the five hemispheres (B), and no beta coupling was seen in the remaining two hemispheres (C). The 95% confidence interval is 0.25. The x- axis represents frequency f1 in Hz, and the y-axis represents frequency f2 in Hz. The colors in the plot represent the bicoherence values at f1, f2, and f112. Each central point is surrounded by an area of nonsignificant bicoherence because of spectral spreading related to the spectral estimate of the FFT. FFT, fast Fourier transform.
FIG. 4.
Significant (P . 0.05) beta coupling was seen at rest in five of the 10 hemispheres (A). This beta coupling persisted during active movement in all five hemispheres (B). The 95% confidence interval is 0.25 The x-axis represents frequency f1 in Hz, and the y- axis represents frequency f2 in Hz. The colors in the plot represent the bicoherence values at f1, f2, and f112. Each central point is surrounded by an area of nonsignificant bicoherence because of spectral spreading related to the spectral estimate of the FFT. FFT, fast Fourier transform.
There was no coupling seen at rest in the remaining five hemispheres (Figure 3a). With movement, coupling was demonstrated in three of these five hemispheres (Figure 3b). There was no coupling seen during movement in the remaining two hemispheres (Figure 3c).
Discussion
We report a persistence of net beta band activity during continuous movement that further increases in power with respect to rest in the PD ‘off’ state when summed over a two-minute epoch. This increase in beta activity reached significance in six of the ten recorded hemispheres. Interestingly, significance was achieved in four of the five tremor dominant hemispheres, but only in two of six bradykinesia dominant hemispheres. Although our sample size is small, this correlation implies that significant changes in beta power may be more specific in patients with tremor predominance. Further analysis of these findings is warranted.
While a number of studies have implicated excessive beta activity in the PD ‘off’ state, they have only indirectly demonstrated that an excess of STN beta activity is a reliable biomarker of the ‘off’ state (18,21,23,29). Furthermore, while beta oscillatory activity has been shown to decrease prior to task-oriented movement (event-related desynchronization) in PD patients (5,13,18), our findings of increased beta activity measured during continuous repetitive movements as opposed to rest are reflective of the net summation of beta oscillatory activity over a two-minute recording (in contrast to temporal analyses of beta power before, during, and after a task-oriented movement shown in the prior studies). Our finding of excessive beta activity that escalates during repetitive hand grasp movements implies that it may not only be the presence of beta frequency activity alone but instead relative changes in beta power between rest and movement that are a more specific signature of the Parkinsonian ‘off’ state. Additionally, this finding adds to the work of Johnson et. al. in that it demonstrates that as the PD patient tries to move over a continuous period of the time, the beta energy increases and thus only locating the beta power may not be as beneficial as using changes in the beta power to help modulate closed loop DBS systems (12,20,21).
Marceglia et al. found that the administration of L-dopa reduced cross frequency coupling in the analyzed hemispheres of PD patients with DBS (23). In our study, harmonic coupling within the beta band during rest and movement were less reliable biomarkers amongst our subjects. We found significant bicoherence of LFP rhythms at rest in five of the ten hemispheres while non-significant coupling at rest and during movement was seen in three hemispheres. Given the increases in beta power seen in all hemispheres, one would expect to see a greater number of hemispheres with significant cross frequency coupling within the beta band.
We also found a correlation between optimal LFP signal recorded from contact pairs and those chosen for stimulation postoperatively in nine of the ten hemispheres. This finding is in agreement with several other studies (32,34–36) (Yoshida et al. 2010; Zaidel et al. 2010; Telkes et al. 2016; van Wijk et al. 2017) and supports the notion that real-time LFP analysis may be useful in determining optimal placement of the DBS electrode during surgery. Furthermore, beta band activity in PD has been considered a potential biomarker of the ‘off’ state for modulation by adaptive DBS technology (21). The present study adds to the current body of literature regarding closed-loop paradigms aimed at exploiting changes and patterns of the recorded LFP signal. Presently, some strategies focus particularly on changes in STN beta activity itself, while others focus on exploiting the communication (phase-amplitude coupling) between cortical broadband beta and gamma activity and STN beta activity (9,21,25,28,31). As implantable closed-loop systems move toward clinical implementation, further understanding of the neurophysiological underpinnings of the basal ganglia circuit, in particular the subthalamic nucleus, is critical in the optimization/engineering of these closed-loop devices (20). Additionally, as the number of contacts on the implanted lead increases, more anatomical and physiological data from the active contact(s) will help focus the current space and offset the spatial complexity of the new stimulating patterns generated by these additional leads (10).
The goal of the present study was to compare STN LFP activity between rest and movement in each hemisphere over a predetermined two-minute epoch. The present study has several limitations. In order to make adequate comparison between rest and movement, we analyzed the LFP signal over a two-minute interval and did not assess temporal changes of the signal over the time course of each individual movement. Recordings were also started with the subjects’ hand already moving. This likely explains why our findings of increased beta power during movement differ from those of prior studies where a decrease in beta power was observed just prior to the initiation of movement. Specifically, Oswal et al. observed both a beta band desynchronization and gamma band synchronization just prior to movement which was then followed by an increase in beta power (24). Beta power was also found to be decreased with increased task complexity just prior to movement (24). Imbach et al. demonstrated beta band desynchronization during the execution of two different motor tasks (grip vs press) when assessing the event related potential 250 ms prior to and after the task (11). Furthermore, Fischer et al. recently demonstrated a reduction in beta power during imagined levels of grip strength when assessed over a four second epoch (7). Thus, comparing beta activity over a two-minute interval lacks the temporal resolution required to assess and subsequently interpret the LFP signal in real-time in order to provide the appropriate feedback stimulus in a closed-loop system. However, by choosing a non-complex, stereotyped movement, we still believe that comparisons between rest and movement, although simplified, are of value. Androulidakis et al. have previously demonstrated that STN beta activity is amplitude-modulated over time with repetitive movements in the PD ‘off’ state (2). Importantly, in that study, the LFP signal was analyzed temporally over a 100 sec time span and comparisons of the LFP signal were not made to the resting state (2). In contrast, we recorded LFP data during stereotyped movement as well as rest for direct comparison. Another limitation is that we did not use an accelerometer, goniometer or electromyography (EMG) to correlate with LFP recordings. Rather, the motor task was observed for accuracy and consistency by the examiner, which is a qualitative assessment. All subjects included in the study consistently performed the motor task throughout the recording period. Of note, this study was aimed at analyzing beta frequency as a function of continuous repetitive movement over a predetermined epoch rather than analysis based on movement related to a specific trigger. While EMG may have provided utility in assessing the impact of tremor related movement artifact on the task or at rest, none of the tremor predominant patients exhibited their phenotypic tremor during LFP recordings. Therefore, additional EMG data would unlikely have had a significant impact on our findings.
In patients with PD, beta band activity that increases with movement may be an exploitable signature of the ‘off’ state. Further investigations should be aimed at refining techniques to identify the beta rhythm and understanding how this signal changes in relationship to other LFP signals.
Footnotes
Conflicts of interest and sources of funding: All authors declare no conflicts of interest
The content of this manuscript has been presented at the following meetings:
World Society of Stereotactic and Functional Neurosurgery Quadrennial Meeting, Berlin, Germany (June 29, 2017)
International Neuromodulation Society 13th World Congress, Edinburgh, Scotland (May 31, 2017)
American Association of Neurological Surgeons Annual Scientific Meeting, Los Angeles, CA (April 24, 2017)
References
- 1.Alegre M, Alonso-Frech F, Rodríguez-Oroz MC, et al. Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur J Neurosci. 2005;22(9):2315–2324. [DOI] [PubMed] [Google Scholar]
- 2.Androulidakis AG, Brücke C, Kempf F, et al. Amplitude modulation of oscillatory activity in the subthalamic nucleus during movement. Eur J Neurosci. 2008;27(5):1277–1284. [DOI] [PubMed] [Google Scholar]
- 3.Brittain J-S, Brown P. Oscillations and the basal ganglia: Motor control and beyond. NeuroImage. 2014;85(Part 2):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2003;18(4):357–363. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy M, Mazzone P, Oliviero A, et al. Movement‐related changes in synchronization in the human basal ganglia. Brain. 2002;125(6):1235–1246. [DOI] [PubMed] [Google Scholar]
- 6.Deletis V, Shils J. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. Academic Press; 2002. [Google Scholar]
- 7.Fischer P, Pogosyan A, Cheeran B, et al. Subthalamic nucleus beta and gamma activity is modulated depending on the level of imagined grip force. Exp Neurol. 2017. July Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foffani G, Priori A, Egidi M, et al. 300-Hz subthalamic oscillations in Parkinson’s disease. Brain J Neurol. 2003;126(Pt 10):2153–2163. [DOI] [PubMed] [Google Scholar]
- 9.de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn A, Neumann W-J, Degen K, Schneider G-H, Kühn AA. Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. 2017. April 8 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imbach LL, Baumann-Vogel H, Baumann CR, Sürücü O, Hermsdörfer J, Sarnthein J. Adaptive grip force is modulated by subthalamic beta activity in Parkinson’s disease patients. Neuroimage Clin. 2015. September 29 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB, Vitek JL. Closed-Loop Deep Brain Stimulation Effects on Parkinsonian Motor Symptoms in a Non-Human Primate - Is Beta Enough? Brain Stimulat. 2016;9(6):892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempf F, Kühn AA, Kupsch A, et al. Premovement activities in the subthalamic area of patients with Parkinson’s disease and their dependence on task. Eur J Neurosci. 2007;25(10):3137–3145. [DOI] [PubMed] [Google Scholar]
- 14.Kochanski RB, Bus S, Pal G, Metman LV, Sani S. Optimization of Microelectrode Recording in DBS Surgery Using Intraoperative CT. World Neurosurg. 2017. April 10 Epub. [DOI] [PubMed] [Google Scholar]
- 15.Kochanski RB, Pal G, Bus S, Metman LV, Sani S. Improving the accuracy of microelectrode recording in deep brain stimulation surgery with intraoperative CT. J Clin Neurosci. 2017. March 2 Epub. [DOI] [PubMed] [Google Scholar]
- 16.Kühn AA, Kempf F, Brücke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci Off J Soc Neurosci. 2008;28(24):6165–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kühn AA, Kupsch A, Schneider G-H, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23(7):1956–1960. [DOI] [PubMed] [Google Scholar]
- 18.Kühn AA, Williams D, Kupsch A, et al. Event‐related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(4):735–746. [DOI] [PubMed] [Google Scholar]
- 19.Lalo E, Gilbertson T, Doyle L, Lazzaro VD, Cioni B, Brown P. Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human. Exp Brain Res. 2007;177(1):137–145. [DOI] [PubMed] [Google Scholar]
- 20.Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(7):717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little S, Pogosyan A, Neal S, et al. Adaptive Deep Brain Stimulation In Advanced Parkinson Disease. Ann Neurol. 2013;74(3):449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Azcárate J, Tainta M, Rodríguez-Oroz MC, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci Off J Soc Neurosci. 2010;30(19):6667–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marceglia S, Foffani G, Bianchi AM, et al. Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease. J Physiol. 2006;571(Pt 3):579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oswal A, Litvak V, Brücke C, et al. Cognitive Factors Modulate Activity within the Human Subthalamic Nucleus during Voluntary Movement in Parkinson’s Disease. J Neurosci. 2013;33(40):15815–15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowland NC, De CH, Swann NC, et al. Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: changes in beta and gamma bands. Front Hum Neurosci. 2015. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shils JL. The bispectrum of the human electroencephalogram. ProQuest Dissertations and Theses. 1995. January 1 Epub. [Google Scholar]
- 27.Shils JL, Litt M, Skolnick BE, Stecker MM. Bispectral analysis of visual interactions in humans. Electroencephalogr Clin Neurophysiol. 1996;98(2):113–125. [DOI] [PubMed] [Google Scholar]
- 28.Steiner LA, Neumann W-J, Staub-Bartelt F, et al. Subthalamic beta dynamics mirror Parkinsonian bradykinesia months after neurostimulator implantation. Mov Disord Off J Mov Disord Soc. 2017;32(8):1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storzer L, Butz M, Hirschmann J, et al. Bicycling suppresses abnormal beta synchrony in the Parkinsonian basal ganglia. Ann Neurol. 2017. September 11 Epub. [DOI] [PubMed] [Google Scholar]
- 30.Swann NC, de Hemptinne C, Miocinovic S, et al. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci. 2016;36(24):6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swann NC, de Hemptinne C, Miocinovic S, et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J Neurosurg. 2017. April 14 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan H, Pogosyan A, Anzak A, et al. Frequency specific activity in subthalamic nucleus correlates with hand bradykinesia in Parkinson’s disease. Exp Neurol. 2013. February Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telkes I, Jimenez-Shahed J, Viswanathan A, Abosch A, Ince NF. Prediction of STN-DBS Electrode Implantation Track in Parkinson’s Disease by Using Local Field Potentials. Front Neurosci. 2016. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trottenberg T, Fogelson N, Kühn AA, et al. Subthalamic gamma activity in patients with Parkinson’s disease. Exp Neurol. 2006;200(1):56–65. [DOI] [PubMed] [Google Scholar]
- 35.van Wijk BCM, Pogosyan A, Hariz MI, et al. Localization of beta and high-frequency oscillations within the subthalamic nucleus region. Neuroimage Clin. 2017. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida F, Martinez-Torres I, Pogosyan A, et al. Value of subthalamic nucleus local field potentials recordings in predicting stimulation parameters for deep brain stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81(8):885–889. [DOI] [PubMed] [Google Scholar]
- 37.Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain J Neurol. 2010;133(Pt 7):2007–2021. [DOI] [PubMed] [Google Scholar]