Abstract
2-aminothiazoline-4-carboxylic acid (ATCA) is a minor metabolite of cyanide and is suggested to be a promising biomarker for cyanide exposure due to its specificity to cyanide metabolism and its excellent short- and long-term stability during storage. In this study, magnetic carbon nanotubes, including magnetic multi-walled carbon nanotubes (Mag-MWCNT) and magnetic single-walled carbon nanotubes (Mag-SWCNT) were synthesized as a novel sorbent for dispersive micro solid phase extraction (d-μSPE) to extract ATCA from biological matrices. ATCA spiked deionized water samples with the addition of the isotopic internal standard (ATCA – 13C, 15N) were subjected to Mag-CNT/d-μSPE to confirm extraction efficiency of this new technique. The extracted ATCA was derivatized and quantitated using gas chromatography/mass spectrometry (GC/MS) analysis. The extraction parameters were optimized and a detection limits of 15 and 25 ng/mL were obtained for synthetic urine and bovine blood respectively with a linear dynamic range of 30 – 1000 ng/mL. The optimized Mag-CNT/d-μSPE method facilitated efficient extraction of ATCA using 2 mg of Mag-MWCNT with a 10-minute extraction time. The current assay was also found to be effective for the extraction of ATCA with average recoveries of 97.7 ± 4.0% (n=9) and 96.5 ± 12.1% (n=9) from synthetic urine and bovine blood respectively. The approach of using Mag-CNT to facilitate d-μSPE offered a novel alternative to extract ATCA from complex biological matrices.
Keywords: Nanotechnology, Cyanide biomarker, 2-aminothiazoline-4-carboxylic acid, Magnetic carbon nanotubes facilitated dispersive micro solid phase extraction
1. Introduction
Cyanide is infamous for its swift and deadly effects attributed to its uses as a chemical warfare agent and the association of numerous accidental and intentional deaths [1–7]. In 2016, 198 cyanide exposure cases were reported in the United States, of which, nearly 70% were unintentionally exposed and more than 8% were due to intentional poisoning [8]. Although accidental exposure to cyanide is primarily caused by consumption of the seeds and roots of certain plants [4, 9–11], it is commonly encountered in industrial workers who have access to cyanide salts [3, 12] and in smokers and fire victims due to the inhalation of cyanide-containing smoke [13–15]. Confirmation of cyanide exposure is an important task for clinical diagnosis, workplace safety, environmental monitoring, and forensics.
Two major approaches are currently adopted for the detection of cyanide exposure from biological samples: 1) direct detection of cyanide and 2) detection of its major metabolite, thiocyanate. Direct cyanide detection can be performed both at the field and in the laboratory [1, 6, 12, 16–18]. However, the high volatility and short half-life (t1/2 = 0.34 – 1.28 hours) of cyanide narrow the detection window for successful analysis [19]. Previous studies also revealed that cyanide concentration fluctuates over time and at various temperatures upon storage [20, 21]. These create a challenge for the confirmation of exposure due to the difficulty in interpreting the cyanide concentration. Confirmation of cyanide exposure by the detection of thiocyanate also faces similar problems. The concentration of thiocyanate also fluctuates in various biological matrices upon storage at different temperatures and over time [22–24]. Despite thiocyanate has a much longer half-life (t1/2 = 96 – 129 hours), it is present endogenously at a high concentration [19, 25]. Wood et al. suggested that such a high concentration might be due to the involvement of thiocyanate in other metabolic pathways, which makes its detection nonspecific to cyanide exposure [26].
An alternative method that is proposed for confirming cyanide exposure is by the analysis of its minor metabolite: 2-aminothiazoline-4-carboxylic acid (ATCA). Unlike thiocyanate, ATCA does not metabolize further and is not associated with other endogenous pathways except for cyanide [19]. Moreover, ATCA has excellent short- and long-term stability at various storage conditions in working solutions and biological matrices, including post-mortem and putrid samples [21, 23, 27]. These suggest that ATCA may be a stable and specific biomarker for confirming cyanide intoxication.
Sensitive analytical methods have been developed to extract and detect ATCA from a wide range of biological samples [21, 23, 27–32]. Majority of these established ATCA analytical methods focused on solid-phase extraction (SPE) and liquid-liquid extraction (LLE) [21, 27, 31–34]. Although proven sensitive, extractions using SPE or LLE may not be optimal. Commercially available SPE columns capable of extracting ATCA are expensive and the recovery could be inconsistent due to the interferences in complex matrices, such as whole blood [27]. As for LLE, a higher sample volume is usually required, which may not be always available in forensic casework. In 2003, Anastassiades et al. introduced dispersive SPE (d-SPE) as a new sample preparation technique to remove undesirable matrix interferences and coupled with a LLE for the detection of pesticide residues [35]. The solid sorbent in d-SPE method was directly added to the sample, dispersed by shaking, stirring, vortexing or ultrasonicating, and was separated from the supernatant using centrifugation. The d-SPE method was suggested to be a better technique that could avoid most of the drawbacks regarding conventional SPE and LLE methods, such as unequal flow rate in SPE columns and the uses of potentially toxic organic solvents in LLE procedures [30, 36]. Since then, the method evolved and additional preparation steps were incorporated to facilitate the extraction of different analytes in different matrices [37, 38]. In recent years, dispersive micro solid phase extraction (d-μSPE) was developed as a miniaturized d-SPE method to extract and pre-concentrate the analyte from the samples in contrast to sample clean-up. In general, the amount of solid sorbent, sample volume, and organic solvent used in the d-μSPE method is greatly reduced to the μg and μL range respectively. Some of the developed d-μSPE methods [38–40] further facilitate more efficient and cost-effective extractions by eliminating the solvent wash step after extraction of the analytes when compared to conventional SPE methods. However, the reduction in scale of the extraction requires the selection of a highly efficient sorbent to maintain or even enhance the extraction efficiency in comparison to the conventional methods.
In this study, magnetic carbon nanotubes (Mag-CNT) were introduced as an alternative d-μSPE sorbent for the extraction of ATCA from biological matrices. The high surface area and sorption capacity of carbon nanotubes (CNT) are suggested to be an appropriate sorbent to achieve an efficient d-μSPE. With the surface modification in form of magnetization of CNT, it further provides separation convenience and high adsorption capacity contributed to the high surface area. In fact, Mag-CNT facilitated d-μSPE (Mag-CNT/d-μSPE) has been widely applied to modern analytical protocols, including the extractions of heavy metal ions, pesticides, and chemical warfare agents [41–43]. The purpose of this study is to develop a novel Mag-CNT/d-μSPE technique to extract ATCA from biological samples for forensic applications.
2. Materials and Methods
2.1. Chemicals and Reagents
2-aminothiazoline-4-carboxylic acid (ATCA) was purchased from Chem-Impex International (Wood Dale, IL). The internal standard, 2-aminothiazoline-4-carboxylic acid-13C, 15N (ATCA-13C, 15N), was obtained from Toronto Research Chemical, Inc. (North York, Canada). N-methyl-N-trimethylsilyl-trifluoroacetoamide (MSTFA), single-walled carbon nanotubes (0.7 – 1.3 nm in diameter and 1 μm in length), multi-walled carbon nanotubes (110 – 170 nm in diameter and 5 – 9 μm in length), carbon coated iron nanoparticles (25 nm average particle size), iron (II) chloride hexahydrate (FeCl3 · 6H2O), and iron (III) chloride tetrahydrate (FeCl2 · 4H2O) were purchased from Sigma-Aldrich (St. Louis, MO). Synthetic urine, Surine-, was obtained from Cerilliant (Round Rock, TX). Drug-free human urine was obtained commercially from UTAK Laboratories (Valencia, CA). Bovine blood was obtained from Quad Five (Ryegate, MT). The solvents, except for those used in the synthesis of Mag-CNT, were at least LCMS grade and all of the solvents were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Hampton, NH). Deionized water was obtained from a Merck Millipore water purification system (Burlington, MA).
2.2. Synthesis of Mag-CNT
Mag-CNT were prepared as described by Pardasani et al. with modifications [41]. The synthetic process was applied to both multi-walled (MWCNT) and single-walled carbon nanotubes (SWCNT). Briefly, the CNT were purified by dispersing in a 3:1 ratio of concentrated nitric acid and concentrated sulfuric acid at 60°C with stirring overnight. Purified CNT were washed with copious amount of water, followed by ethanol, and were re-suspended in water and dried at 100°C for 6 hours in a centrifugal evaporation system (Labcono, Kansas City, MO). Subsequently, 50 mg of dried CNT were suspended in 25 mL of deionized water containing 70 mg of iron (II) chloride tetrahydrate and 135 mg of iron (III) chloride hexahydrate. The suspension was sonicated at 50°C for 30 min, followed by a slow addition of 1 mL concentrated ammonium hydroxide (NH4OH). The pH of the suspension was monitored in the range of 10–11 after addition. The temperature of the suspension was raised to 80°C after complete addition of NH4OH and the reaction was held at 80°C for 30 min. The suspension was then cooled to room temperature slowly and the Mag-CNT were separated from the solution with the help of a permanent Neodymium magnet. The Mag-CNT were washed with copious amount of deionized water, followed by ethanol and re-suspended in deionized water. Finally, the cleaned Mag-CNT were dried completely at 100°C in the centrifugal evaporator.
2.3. Calibration and Quantification
A stock solution of ATCA (60 μg/mL) was prepared in methanol with 2% (v/v) formic acid and was used throughout the entire study. Intermediate concentrations (150 and 3000 ng/mL) of the stock solution were prepared by dilution with methanol. Deionized water samples spiked with 1000 ng/mL of ATCA standard solution were used for the development and optimization of the extraction method. Calibration standards were prepared at the concentrations of 5, 10, 25, 30, 50, 100, 250, 500, and 1000 ng/mL to determine the limit of detection (LOD) and limit of quantitation (LOQ). A non-weighted regression was employed for all calibration curves, which were plotted with the response ratio of internal standard and ATCA against the concentration of ATCA in the calibration standards. The low, medium, and high quality control samples (n=3) were prepared at 90, 500, and 800 ng/mL respectively. LOD and LOQ were estimated statistically from the calibration curves by:
and
in which sy is the standard deviation of y-intercept; while Avgm is the average value of the slope. The LOD and LOQ values were then reported by analyzing the calibration standards closest to the corresponding estimated statistical values with the Signal to Noise function on the ChemStation software (Agilent, Santa Clara, CA) with a peak signal to peak noise ratio higher than 3.3 and 10 correspondingly for the standards. Calibration levels of 30, 50, 100, 250, 500, and 1000 ng/mL were used in method development, optimization and method application to synthetic urine and bovine blood samples.
2.4. GC/MS Method
GC/MS analysis was performed on an Agilent GC/MS system, which consists of a 7890A series of gas chromatography, a 5975C series mass spectrometry, and a 7683B series auto-sampler (Agilent, Santa Clara, CA). The column used was a DB-5 bonded phase column (30 m × 0.25 mm × 0.25 μm) with helium as a carrier gas at the flow rate of 1 mL/min. The GC/MS program was adopted from Logue et al. with minor modifications. Briefly, the autosampler was set to inject 1 μL of sample into the injection port, which was held at 290°C and contained a Cyclosplitter liner (Restek, Bellefonte, PA). The total flow was set to be 54 mL/min with the septum purge flow of 3 mL/min and a 10:1 split ratio. Using the described method, the ATCA-(TMS)3 and the ATCA-13C, 15N-(TMS)3 were eluted at approximately 8.76 min, with a total run time of 13 min. The ions selected were as follow: ATCA-(TMS)3 (245, 347, and 362 m/z) and ATCA-13C, 15N-(TMS)3 (248, 350, and 365 m/z) under selected ion monitoring (SIM) mode. The dwell time was set to 50 ms.
2.5. Mag-CNT/d-μSPE Development and Optimization
A simplified flow diagram for the Mag-CNT/d-μSPE development was shown in Figure 1A. Typically, 2 mg of Mag-MWCNT were added to 100 μL water samples spiked with 1000 ng/mL of ATCA in triplicates. The samples were then vortexed and extracted at 50°C for 10 min with sonication. After the d-μSPE process, the samples were then centrifuged and the Mag-MWCNT were isolated with a permanent Neodymium magnet while the supernatants were transferred to separate tubes. The extracted ATCA were desorbed with 150 μL of deionized water with 5% (v/v) ammonium hydroxide. After desorption, the Mag-MWCNT were isolated with the permanent magnet and the desorbed extracts were transferred to separate tubes. After the addition of ATCA – 13C, 15N (833 ng/mL) as the internal standard, each portion (the supernatants, desorbed extracts, and the Mag-CNT after desorption) were dried at 65°C using the centrifugal evaporator. The dried samples were derivatized with 150 μL of 30% (v/v) MSTFA in hexane at 50°C for 10 min with sonication. The Mag-CNT were isolated and the derivatized products were transferred to GC/MS vials fitted with inserts (Microliter, New Jersey, NJ) and subjected to GC/MS analysis for ATCA quantitation.
Fig. 1.
(A) Workflow for the development of Mag-CNT/d-μSPE, and (B) the optimized workflow for ATCA extraction using Mag-CNT/d-μSPE.
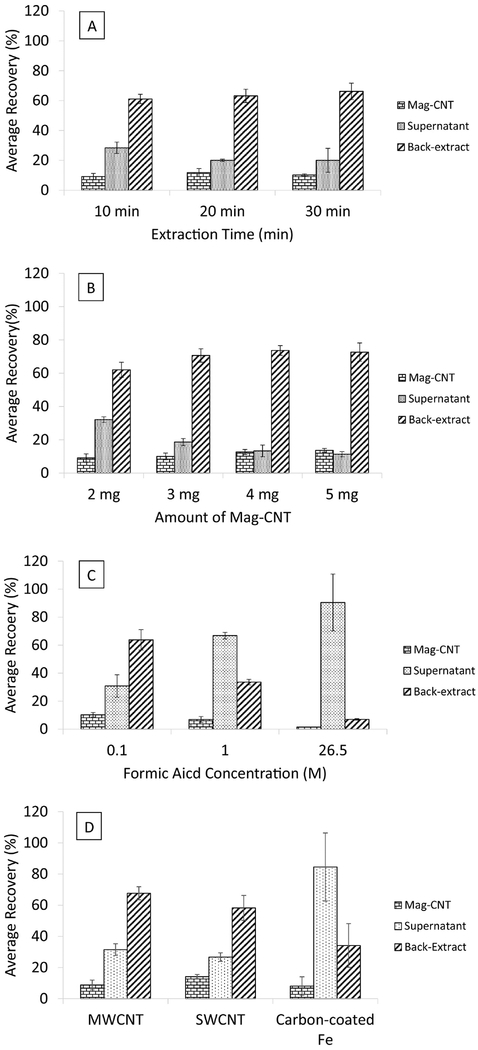
At the initial stage of optimization, deionized water was used as the matrix. Parameters including extraction time, amount of Mag-CNT, types of magnetic nanoparticles, and the concentrations of acid used for pH modification were optimized. Different amount of Mag-MWCNT (2, 3, 4, and 5 mg) and extraction time (10, 20 and 30 min) were evaluated according to the above described Mag-CNT/d-μSPE procedures. Different concentrations of 2% (v/v) formic acid (0.1, 1, and 26.5M) were used to modify the pH value of deionized water prior to the addition of ATCA stock solution and Mag-CNT. The extraction efficiency of Mag-MWCNT, Mag-SWCNT, and the carbon-coated iron nanoparticles was also evaluated with the same experimental procedures.
2.6. Extraction Performance of Mag-CNT/d-μSPE for ATCA in Biological Matrices
The optimized Mag-CNT/d-μSPE conditions were applied for the extraction of ATCA in synthetic urine and bovine blood samples. A simplified flow diagram for the optimized Mag-CNT/d-μSPE method was shown in Figure 1B. Internal standard was added at the beginning of the extraction process. Quality control samples at low, medium, and high concentration levels were spiked with 90, 500, and 800 ng/mL of ATCA respectively and were conducted in triplicates. Protein precipitation was performed on bovine blood samples by adding 200 μL of ice-cold acetonitrile to the spiked samples while vortexing. The samples were then centrifuged and the supernatant was transferred to separate tubes for extraction according to the optimized method described above. Only the desorbed extracts of the synthetic urine and bovine blood samples were derivatized.
2.7. Assay Selectivity
The selectivity of the assay was determined by the recovery of ATCA from synthetic urine and bovine blood as well as the analysis of chromatographic profile of ATCA. To determine the recovery, standard addition of ATCA to pre d-μSPE samples and post d-μSPE final extracts were prepared for each matrix. ATCA was spiked at 90, 500, and 800 ng/mL separately in the synthetic urine and bovine blood samples (n=3 at each level for each matrix). The concentration of ATCA in the final desorbed extract was determined using the linear regression obtained from the calibration curve. Recovery was expressed as the percentage by dividing the ATCA concentration from the pre d-μSPE addition samples by the post d-μSPE addition samples. For chromatographic profile analysis, replicates (n=3) of blank samples of the synthetic urine and bovine blood were subjected to extraction to determine if interference peaks co-eluted at the peak of ATCA – (TMS)3.
2.8. Data analysis
Precision and accuracy of the calibration models and the Mag-CNT/d-μSPE method were calculated from the quality control samples. Inter-assay precision was calculated as %CV by dividing the standard deviation of the calculated concentrations by the mean of the calculated concentrations, and the highest %CV was reported. Accuracy was calculated as a percentage by dividing the absolute error by the expected concentrations. The error involved in analyzing the concentrations of ATCA in the calibration standards and quality controls were reported in the format of mean ± standard deviation. Significant differences between extraction using different extraction time (10, 20, and 30 min), different amount of Mag-MWCNT (3, 4, and 5 mg) were determined by using one-way ANOVA at α = 0.05. Two-tailed t-tests were performed to determine if a significant difference is observed between the 2 and 3 mg of Mag-MWCNT and between SWCNT and MWCNT at 95% confidence level.
3. Results
3.1. Capability of Mag-CNT to extract ATCA
To test the capability of Mag-CNT in extracting ATCA, the distribution of ATCA throughout the extraction process was evaluated. The concentration of ATCA was determined in three main portions of the extraction products: a) the supernatants, which were the ATCA spiked matrices remained after extracting with the Mag-CNT, b) the desorbed extracts, and c) the Mag-CNT isolated after desorption. The internal standard was added before drying under vacuum and the dried samples were derivatized and subjected to GC/MS analysis. The partition of ATCA during Mag-CNT/d-μSPE in the supernatants, desorbed extracts, and the Mag-CNT were found to be 28.4%, 61.1%, and 9.2% respectively.
3.2. Method sensitivity
The LOD and LOQ of the GC/MS method were estimated statistically from the results obtained from the calibration models and the signal to noise ratio of the closest calibration standard was confirmed using the ChemStation software. Calibration standards with the concentrations of 5, 10, 25, 30, 50, 100, 250, 500, and 1000 ng/mL were used to construct the calibration curves over three separate days. The data were best fitted with a non-weighted linear model with a dynamic range of 30 – 1000 ng/mL. The average R2 values for synthetic urine and bovine blood were 0.9985 and 0.9919 respectively. The statistical estimations of LOD and LOQ were 13.14 and 30.69 ng/mL for synthetic urine and 24.07 and 33.59 ng/mL for bovine blood respectively. After the calculation and analysis of the Signal to Noise ratio from the ChemStation software, the LOD and LOQ for synthetic urine and bovine blood were reported as 15 and 30 ng/mL, and 25 and 30 ng/mL respectively.
3.3. Assay selectivity
Assay selectivity was demonstrated by the calculated recovery values at three different concentration levels in synthetic urine and bovine blood samples. The recovery values at each level and the average value for each matrix were shown in Table 1. An absence of interfering peaks in the extracted ion chromatograms (EIC) was observed at the ATCA peak from the blank matrices. The overlaid EIC of blank synthetic urine, synthetic urine spiked with 1000 ng/mL ATCA, and blank human urine were shown in Figure 2A and that of a blank bovine blood sample and a spiked bovine blood sample (1000 ng/mL ATCA) were illustrated in Figure 2B. As shown in Figure 2A, the peak of ATCA – (TMS)3 eluted at around 8.76 min in synthetic urine and no interference peak was observed in either the blank synthetic urine or the blank human urine samples. No other interference peak was observed to elute at the same retention time as the ATCA – (TMS)3 peak in both the synthetic urine and human urine samples. In previous studies, endogenous ATCA was found in human urine from non-smokers. The human urine that was tested in this study was purchased from a commercial vendor and no ATCA peak was observed using the optimized GC/MS method. Similarly in the blank bovine blood samples, no peak was observed at the retention time of the ATCA – (TMS)3 peak (Figure 2B). Therefore, the selectivity of the method was adequate for both urine and bovine blood. Each matrix was analyzed in triplicates and no interference peaks were observed in all samples.
Table 1:
Average recoveries, bias, and precision of ATCA in the back-extract of low, medium, and high quality control samples in synthetic urine (n=9) and bovine blood (n=9). Error was expressed in percent relative uncertainty of the calculated recoveries for recovery data.
| Synthetic Urine | Bovine Blood | |||||
|---|---|---|---|---|---|---|
| QC Samples | Recovery | Bias | Precision | Recovery | Bias | Precision |
| Low (90 ng/mL) | 96.5 ± 10.4% | −0.72% | 8.00% | 84.9 ± 2.8% | −12.01% | 1.23% |
| Medium (500 ng/mL) | 97.9 ± 4.4% | 2.08% | 2.78% | 97.0 ± 7.0% | −2.24% | 6.93% |
| High (800 ng/mL) | 98.6 ± 4.5% | −0.40% | 3.74% | 107.7 ± 8.5% | −5.37% | 7.98% |
| Average | 97.7 ± 4.0% | 0.32% | 4.84% | 96.5 ± 12.1% | −6.54% | 5.38% |
Fig. 2.
Extraction ion chromatograms (EIC) of ATCA-(TMS)3 at 245, 347, and 362 m/z in (A) a blank synthetic urine, synthetic urine spiked with 1000 ng/mL ATCA, and a human urine sample, and (B) a blank bovine blood and bovine blood sample spiked with 1000 ng/mL ATCA. No interference peak was observed in blank human, synthetic urine, and bovine blood at the retention time of ATCA-(TMS)3.
3.4. Optimized Mag-CNT/d-μSPE method
Different extraction parameters, such as extraction time, amount of Mag-CNT, types of magnetic nanoparticles, and different concentrations of acid used for sample preparation, were optimized for the proposed Mag-CNT/d-μSPE for ATCA extraction. The optimal parameter was chosen for the maximum recovery of ATCA in the desorbed extract and the most efficient for the extraction process.
3.4.1. Extraction Time
Different extraction times (10, 20, and 30 min) were tested to determine whether the duration of extraction will affect the efficiency of Mag-CNT in extracting ATCA. The average recovery in the partition of ATCA in Mag-CNT, supernatants, and desorbed extracts for each extraction time was shown in Figure 3A. No significant difference was found in the average recovery of ATCA in Mag-CNT, supernatant, and desorbed extract among the extraction time of 10, 20, and 30 min at α = 0.05 (p = 0.427). Since no significant difference was found in recovery of ATCA in the desorbed extracts among the three different extraction time settings, 10 minutes was chosen to facilitate a more efficient extraction process.
Fig. 3.
Average recoveries of ATCA in Mag-CNT, supernatant, and in back-ex-tract phases. (A) optimizing extraction time of 10, 20, and 30 mins (n = 3); (B) 2, 3, 4, and 5 mg of Mag-CNT (n = 3); (C) different concentration of formic acid (0.1 M, 1 M, and 26.5 M) were used to acidify the sample before extraction (n=3); (D) multi-walled magnetic carbon nanotubes (MWCNT), single-walled magnetic carbon nanotubes (SWCNT), and commercially available carboncoated iron nanoparticles. Error bars were expressed in ± 1 standard deviation.
3.4.2. Amount of Mag-CNT
Extraction capacity of Mag-CNT was evaluated by determining the recovery from the extraction of 1000 ng/mL of ATCA with different amount of Mag-MWCNT (2, 3, 4, and 5 mg). The average recovery of ATCA in each extraction portion with different amount of Mag-CNT was shown in Figure 3B. By visual examination of the data, the average recovery of ATCA in the desorbed extracts with 2 mg of Mag-CNT was slightly lower than the other groups. Upon statistical analysis using one-way ANOVA (α = 0.05), no significant difference was found in the average recovery in the desorbed extracts, in which 3, 4, and 5 mg of Mag-CNT were used (p = 0.698). Since no significant difference was observed among the average recovery in 3, 4, and 5 mg of Mag-CNT, the data obtained from 3 mg Mag-CNT was used to compare to that of 2 mg of Mag-CNT to determine whether a significant difference was present. The result from two-tailed t-test showed that the average recovery of ATCA in the desorbed extracts in the 2 mg of Mag-CNT extraction was not significantly different than that when using 3 mg of Mag-CNT at a 95% confidence interval. A possible reason for the slightly lower recovery when extracting with 2 mg of Mag-MWCNT might be due to human errors in consistently weighing a small amount of Mag-CNT. During optimization process, caution was taken to accurately and precisly measure Mag-CNT. Based on the bias and precision data obtained from the quality control samples, The method demonstarted good accuracy and precision within 20% of the expected value with the use of 2 mg of Mag-CNT. As a result, 2 mg of Mag-CNT were adopted for the Mag-CNT/d-μSPE method for a cost-effective reason.
3.4.3. Concentrations of Acid for Sample Preparation
The performance of different concentrations of formic acid was evaluated for the acidification of sample before extraction. To facilitate an efficient extraction based on the Henderson-Hasselbalch equation, the pH value of the sample is usually modified to two pH values below the pKa of an acidic analyte, or two pH values above the pKa value of a basic analyte. The pKa values of the moieties on ATCA were shown in Figure 4. Different concentrations of formic acid were tested to determine the optimal condition for extracting ATCA. Over 69% and 90% of the ATCA was measured in the supernatant when 2% (v/v) of the 1 M and 26.5 M of the acid was used to adjust the pH of the samples respectively (Figure 3C). A much higher recovery (64%) in the desorbed extracts was observed when 2% (v/v) of 0.1 M formic acid was added to the samples, and thus was used in subsequent extractions.
Fig. 4.
pKa values of ATCA.
3.4.4. Comparison among Mag-MWCNT, Mag-SWCNT, and Carbon-coated Iron Nanoparticles
The performances of Mag-MWCNT, Mag-SWCNT, and the commercial product (carbon-coated iron nanoparticles) were compared to determine which product would yield higher recovery of ATCA in the desorbed extracts. As shown in Figure 3D, less than 35% of ATCA was extracted by the carbon coated nanoparticles and approximately 85% of the ATCA was retained in the supernatant. As a result, carbon-coated iron nanoparticle was eliminated as a choice of the d-μSPE sorbent for ATCA extraction. When Mag-SWCNT and Mag-MWCNT were considered, no significant difference was observed in the average recovery of ATCA in the desorbed extracts using a two-tailed t-test at a 95% confidence interval. Mag-MWCNT were chosen to be the sorbent for extraction due to a lower variation in the average recovery in the desorbed extracts and lower cost.
3.4.5. Optimized Extraction Procedure
The optimized extraction procedures are summarized as the following. For urine analysis, internal standard and 2% (v/v) of 0.1 M formic acid was first added to 100 μL of samples, followed by adding 2 mg of Mag-MWCNT for d-μSPE. The samples were then vortexed and extracted at 50°C for 10 min with sonication. After the d-μSPE process, the samples were centrifuged first. The supernatants were decanted and discarded while the Mag-CNT was retained inside the vial by a permanent magnet. ATCA was then desorbed from the Mag-CNT with 150 μL of 5% (v/v) ammonium hydroxide in water at 50°C for 10 min with sonication. The Mag-CNT were separated by centrifugation and the permanent magnet. The desorbed extracts were transferred to separate tubes and dried at 65°C in the centrifugal evaporator. The dried samples were derivatized with 150 μL 30% (v/v) MSTFA in hexane at 50°C for 10 min and were then transferred to GC/MS vials fitted with glass inserts for analysis. For blood analysis, internal standard and 2% (v/v) of 0.1 M formic acid was first added to100 μL of sample and vortexed. Ice cold acetonitrile (200 μL) was added to the samples while vortexing. The supernatants were transferred to separate tubes after centrifugation and 2 mg of Mag-CNT were added for d-μSPE. The rest of the extraction process was identical to that of urine samples as described above.
3.5. Application of the optimized Mag-CNT/d-μSPE in synthetic urine and bovine blood
The optimized Mag-CNT/d-μSPE method was applied in synthetic urine and bovine blood samples at three different concentration levels. Internal standard was added at the beginning of the extraction and only the desorbed extracts were derivatized and analyzed. The concentrations of ATCA in the desorbed extracts using the optimized method in both matrices were determined. The recovery, bias, and precision of the experimental results were shown in Table 1. It should be noted that the recovery of ATCA in human urine and whole blood might be different when Mag-CNT/d-μSPE is applied since surrogates of synthetic urine and bovine blood was used in this study. The average recoveries can only be approximated to that of human urine and whole blood in extracting ATCA.
4. Discussion
ATCA is an amphoteric molecule, which contains both an acidic carboxylic acid moiety and a basic amine moiety (Figure 4). Based on the pKa information and the nature of the amine and carboxylic groups, at least one of the moieties, or both, is charged in all pH values in aqueous solutions. Earlier studies in ATCA showed that its ring structure will be opened under heat and strongly basic condition [31, 44]; as a result, the Mag-CNT/d-μSPE method was designed to be at an acidic range to avoid the potential degradation of ATCA. The addition of concentrated sulfuric acid during purification of CNT has been demonstrated to provide stronger oxidizing power [45], in which more carbon atom on the surface of the CNT can be oxidized into hydroxyl and/or carboxyl groups. Under acidic conditions, both the carboxylic acid moiety on ATCA and the hydroxyl and carboxyl groups on the Mag-CNT are protonated, which facilitate the interaction between ATCA and the Mag-CNT through hydrogen bonding (Figure 5). The acidic extraction condition not only prevented the degradation of ATCA, but more importantly, it also promoted a higher affinity between Mag-CNT and ATCA.
Fig. 5.
Proposed molecular interactions between ATCA and Mag-CNT under acidic condition.
Different concentration of formic acid was tested to determine the effects on the interaction of ATCA and Mag-CNT sorbent in the biological matrix. A decreased recovery was noticed in the desorbed extracts when a high concentration of formic acid (2% (v/v) of 26.5M) was used during the d-μSPE process. Such decreased recovery of ATCA might be due to the disturbance of hydrophobic interaction. Under high acid concentration, both the amine and carboxyl moieties on ATCA were protonated and its hydrophilicity increased. This hindered the hydrophobic interaction between ATCA and Mag-CNT and thus resulted in a low recovery. Another possibility was the competition between formic acid and ATCA to the Mag-CNT. Due to the high concentration of formic acid, ATCA might have less chance to interact with Mag-CNT. A lower concentration of formic acid was found mediating the pH and enhancing the interaction between ATCA and the Mag-CNT. Other choices of acidic pH modifiers could be investigated in future work.
During method development, the capability of Mag-CNT to extract ATCA was determined by the addition of the internal standard at the end of the d-μSPE process. With this approach, we could evaluate the interaction between ATCA and the Mag-CNT sorbent. During the optimization process, the internal standard was added before d-μSPE and only the desorbed extracts were analyzed by GC/MS. The concentration of ATCA was determined from internal standard calibration model, which was plotted with the response ratio between internal standard and ATCA against the ATCA concentration of the calibration standards. The addition of internal standard improved the precision of the method and the possible variations during the d-μSPE process.
To couple with GC/MS analysis, derivatization is needed because ATCA is non-volatile. The derivatization agent, MSTFA, reacts with the active hydrogens on both of the carboxylic and amine moiety on ATCA with replacements of TMS groups. Due to the high reactivity of MSTFA towards active hydrogen on hydroxyl, carboxyl and amino groups, the solvent used for the dilution of MSTFA has to be relatively non-polar and does not contain any active hydrogens. As a result, a basic desorption using deionized water with 5% (v/v) ammonium hydroxide was applied to elute ATCA from the Mag-CNT, and the desorbed extracts were then dried and derivatized using hexane as the solvent.
To date, the application of d-μSPE in ATCA extraction is limited. ATCA-specific molecularly imprinted polymer (MIP) has been developed to extract ATCA by Jackson et al. and Lulinski et al. [30, 36]. Jackson et al. further integrated the MIP and coated them on stir-bars to selectively extract ATCA from urine; however, the limited binding capacity led to low recoveries and limited reproducibility [30]. The optimized d-μSPE method by Lulinski et al. extracted ATCA by using MIP alone and showed better recovery of 81 – 89% of ATCA from post-mortem whole blood samples [36]. However, the synthesis process of the MIP involved lengthy and complicated manufacturing steps, as well as numerous chemicals to achieve the high selectively of the d-μSPE sorbent [30, 36]. In our study, different magnetic nano-particles were studied to determine the most cost-effective and efficient d-μSPE sorbent for the extraction of ATCA. Currently, Mag-CNT are not commercially available. A close product, carbon coated iron nano-particles were studied to determine the potential for their application in the extraction process to avoid the need for in-house synthesis of magnetic nano-particles. Unfortunately, the recovery of ATCA using the commercial magnetic nano-particles was not satisfactory. Once hydroxyl or carboxyl functional group derived CNTs are commercially available, their applications for ATCA extraction after magnetization can be further compared. Furthermore, the commercially available products might offer a more efficient and cost-effective sorbent synthesis process. The use of Mag-CNT for d-μSPE involved fewer chemicals in the sorbent synthesis and offered a new extraction process that simplified the extraction procedures.
d-μSPE methods are frequently used to pre-concentrate the analytes to facilitate the detection of trace level components [38–40]. In this work, we focused on the determination of the capability of Mag-CNT to extract ATCA, therefore pre-concentration was not yet tested. Note that the Mag-CNT/d-μSPE method was optimized using the highest concentration (1000 ng/mL) of the calibration model. The use of the lowest concentration for the optimization could be tested in future to confirm that the signals variation is due to the effect of the variable itself instead of an error in the quantitation.”
The Mag-CNT/d-μSPE method coupled with GC/MS analysis provided a quantitative analysis of ATCA from synthetic urine and bovine blood samples with good accuracy, precision, and sensitivity. An average recovery of 97.7 ± 4.0% and 96.5 ± 12.1% of ATCA was obtained from synthetic urine and bovine blood samples respectively using the optimized Mag-CNT/d-μSPE method. A slightly lower recovery was obtained for bovine blood at the low concentration quality control samples, which suggested a higher interference from the complex blood matrix at the lower concentration. Although direct comparison to the methods from Lulinski et al. and Jackson et al. is not possible due to the difference in matrices, the optimized Mag-CNT/d-μSPE/GC/MS method offered a relatively good recovery of ATCA from biological samples. Moreover, previous studies showed that the average endogenous ATCA level in human urine from non-smokers and that in post-mortem blood was 87 ± 12 ng/mL and 134 ng/mL ± 59 %CV respectively [21, 28]. The Mag-CNT/d-μSPE/GC/MS method in this study offered a LOD and a proper linear dynamic range for further implementation to quantify ATCA from biological samples.
5. Conclusions
Mag-CNT were synthesized as a novel sorbent for d-μSPE to extract ATCA from biological matrices. Compared to the commercial carbon-coated iron nano-particles, carbon nanotubes offered a better recovery of ATCA from aqueous solutions. The optimized Mag-CNT/d-μSPE process showed that Mag-CNT were capable of extracting ATCA from synthetic urine and bovine blood. The application of the Mag-CNT/d-μSPE process coupled with GC/MS analysis for the detection of ATCA demonstrated good accuracy and precision and provided the sensitivity to detect endogenous level of ATCA in urine and blood. The application of Mag-CNT/d-μSPE is promising for the extraction of ATCA from human biological fluid to assist in the confirmation of cyanide exposure. Future study may focus on the improvement of the method, such as the choice of desorption solvents, optimal pH for ATCA extraction in biological samples, and sample volumes. Further development of direct derivatization of adsorbed ATCA on Mag-CNT will potentially assist its complete desorption in one-step. By doing so, better recoverywith avoidance of desorption step can be an additional advantage of the method. To streamline the quantitative analysis of ATCA, Mag-CNT/d-μSPE can be coupled with liquid chromatography/tandem mass spectrometry (LC-MS/MS) to eliminate the use of derivatization.
Highlights.
Mag-CNT was synthesized as a novel QuEChERS material for ATCA extraction
Mag-CNT/d-SPE/GC/MS was successfully applied for quantitative analysis of ATCA
The optimized method was applied to synthetic urine and bovine blood samples
Mag-CNT/d-SPE offers a great potential in chemical extraction for forensics
7. Acknowledgements
The authors would gratefully acknowledge the support from the College of Criminal Justice at Sam Houston State University and the partial support from the CounterACT Program, The National Institute of Allergy and Infectious Diseases, and the National Institutes of Health as an Interagency Agreement between NIH and USAMRICD (AOD16026-001-0000/A120—B.P2016-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
There is no declaration of interest from the authors. This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
References
- [1].Lee SK, Rhee JS, Yum HS, Cyanide poisoning deaths detected at the national forensic service headquarters in seoul of Korea: a six year survey (2005~2010), Toxicological Research, 28 (2012) 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rotenberg JS, Cyanide as a Weapon of Terror, Pediatric Annals, 32 (2003) 236. [DOI] [PubMed] [Google Scholar]
- [3].Jethava D, Gupta P, Kothari S, Rijhwani P, Kumar A, Acute cyanide Intoxication: A rare case of survival, Indian Journal of Anaesthesia, 58 (2014) 312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].ÜNal Ö, ÖZen Ö, ÇAksen H, Acute Cyanide Intoxication Related to Apricot Seed: The Findings of Cranial Magnetic Resonance Imaging, Journal of Neurological Sciences, 33 (2016) 171–176. [Google Scholar]
- [5].Geller RJ, Barthold C, Saiers JA, Hall AH, Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs, Pediatrics, 118 (2006) 2146–2158. [DOI] [PubMed] [Google Scholar]
- [6].Gill JR, Marker E, Stajic M, Suicide by cyanide: 17 deaths, J. Forensic Sci, 49 (2004) 826–828. [PubMed] [Google Scholar]
- [7].Nnoli MA, Legbosi NL, Nwafor PA, Chukwuonye II, Toxicological Investigation of Acute Cyanide Poisoning of a 29-year-old Man: A Case Report, Iranian Journal of Toxicology, 7 (2016) 831–835. [Google Scholar]
- [8].Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL, 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report, Clinical Toxicology, 53 (2015) 962–1147. [DOI] [PubMed] [Google Scholar]
- [9].Borron SW, Baud FJ, Mégarbane B, Bismuth C, Hydroxocobalamin for severe acute cyanide poisoning by ingestion or inhalation, The American Journal of Emergency Medicine, 25 (2007) 551–558. [DOI] [PubMed] [Google Scholar]
- [10].Bradbury JH, Cliff J, Denton IC, Uptake of wetting method in Africa to reduce cyanide poisoning and konzo from cassava, Food And Chemical Toxicology: An International Journal Published For The British Industrial Biological Research Association, 49 (2011) 539–542. [DOI] [PubMed] [Google Scholar]
- [11].Nadia C, Ines G, Amira D, Fathia K, Anouer N, Wafa M, Ines B, Hayet G, Abderazzek H, Potential Toxic Levels of Cyanide in Almonds (Prunus amygdalus), Apricot Kernels (Prunus armeniaca), and Almond Syrup, ISRN Toxicology, Vol 2013 (2013), DOI 10.1155/2013/610648(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gotardo AT, Hueza IM, Manzano H, Maruo VM, Maiorka PC, Górniak SL, Intoxication by Cyanide in Pregnant Sows: Prenatal and Postnatal Evaluation, Journal of Toxicology, 2015 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Elevated Blood Cyanide Concentrations in Victims of Smoke Inhalation, The New England Journal of Medicine, 325 (1991) 1761–1766. [DOI] [PubMed] [Google Scholar]
- [14].Yoshida M, Adachi J, Watabiki T, Tatsuno Y, Ishida N, A study on house fire victims: Age, carboxyhemoglobin, hydrogen cyanide and hemolysis, Forensic Science International, 52 (1991) 13–20. [DOI] [PubMed] [Google Scholar]
- [15].Jones J, McMullen MJ, Dougherty J, Toxic smoke inhalation: Cyanide poisoning in fire victims, The American Journal of Emergency Medicine, 5 (1987) 317–321. [DOI] [PubMed] [Google Scholar]
- [16].Chen L, Nie H, Zhang G, Gong F, Yang Y, Gong C, Tang Q, Xiao K, Cyanide ion colorimetric chemosensor based on protonated merocyanine in EtOH, Tetrahedron Letters, 55 (2014) 3017–3023. [Google Scholar]
- [17].Juhyen L, Eun Jung C, Inwon K, Minhe L, kumar CS, Changsik S, Tuning Sensory Properties of Triazole-Conjugated Spiropyrans: Metal-Ion Selectivity and Paper-Based Colorimetric Detection of Cyanide, Sensors (14248220), 17 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee D-N, Seo H, Shin I-S, Hong J-I, Paper Strip-based Fluorometric Determination of Cyanide with an Internal Reference, Bulletin of the Korean Chemical Society, 37 (2016) 1320. [Google Scholar]
- [19].Logue BA, Hinkens DM, Baskin SI, Rockwood GA, The Analysis of Cyanide and its Breakdown Products in Biological Samples, Critical Reviews in Analytical Chemistry, 40 (2010) 122–147. [Google Scholar]
- [20].Kała M, Chudzikiewicz E, The influence of post-mortem changes in biological material on interpretation of toxicological analysis results, Problems of Forensic Sciences, 54 (2003) 32–59. [Google Scholar]
- [21].Rużycka M, Giebułtowicz J, Fudalej M, Krajewski P, Wroczyński P, Application of 2-Aminothiazoline-4-carboxylic Acid as a Forensic Marker of Cyanide Exposure, Chemical Research in Toxicology, 30 (2017) 516–523. [DOI] [PubMed] [Google Scholar]
- [22].Ballantyne B, In vitro production of cyanide in normal human blood and the influence of thiocyanate and storage temperature, Clinical Toxicology, 11 (1977) 173–193. [DOI] [PubMed] [Google Scholar]
- [23].Logue BA, Maserek WK, Rockwood GA, Keebaugh MW, Baskin SI, The analysis of 2-amino-2-thiazoline-4-carboxylic acid in the plasma of smokers and non-smokers, Toxicology Mechanisms And Methods, 19 (2009) 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vinnakota CV, Peetha NS, Perrizo MG, Ferris DG, Oda RP, Rockwood GA, Logue BA, Comparison of cyanide exposure markers in the biofluids of smokers and non-smokers, Biomarkers: Biochemical Indicators Of Exposure, Response, And Susceptibility To Chemicals, 17 (2012) 625–633. [DOI] [PubMed] [Google Scholar]
- [25].Sousa AB, Manzano H, Soto-Blanco B, Górniak SL, Toxicokinetics of cyanide in rats, pigs and goats after oral dosing with potassium cyanide, Archives Of Toxicology, 77 (2003) 330–334. [DOI] [PubMed] [Google Scholar]
- [26].Wood JL, Williams EF Jr., The metabolism of thiocyanate in the rat and its inhibition by propylthiouracil, The Journal Of Biological Chemistry, 177 (1949) 59–67. [PubMed] [Google Scholar]
- [27].Giebułtowicz J, Rużycka M, Fudalej M, Krajewski P, Wroczyński P, LC-MS/MS method development and validation for quantitative analyses of 2-aminothiazoline-4-carboxylic acid – a new cyanide exposure marker in post mortem blood, Talanta, 150 (2016) 586–592. [DOI] [PubMed] [Google Scholar]
- [28].Logue BA, Kirschten NP, Petrikovics I, Moser MA, Rockwood GA, Baskin SI, Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography–mass spectrometry, Journal of Chromatography B, 819 (2005) 237–244. [DOI] [PubMed] [Google Scholar]
- [29].Bhandari RK, Oda RP, Petrikovics I, Thompson DE, Brenner M, Mahon SB, Bebarta VS, Rockwood GA, Logue BA, Cyanide toxicokinetics: the behavior of cyanide, thiocyanate and 2-amino-2-thiazoline-4-carboxylic acid in multiple animal models, Journal Of Analytical Toxicology, 38 (2014) 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jackson R, Petrikovics I, Lai EPC, Yu JCC, Molecularly imprinted polymer stir bar sorption extraction and electrospray ionization tandem mass spectrometry for determination of 2-aminothiazoline-4-carboxylic acid as a marker for cyanide exposure in forensic urine analysis, Analytical Methods, 2 (2010) 552–557. [Google Scholar]
- [31].Lundquist P, Kagedal B, Nilsson L, Rosling H, Analysis of the Cyanide Metabolite 2-Aminothiazoline-4-Carboxylic Acid in Urine by High-Performance Liquid Chromatography, Analytical Biochemistry, 228 (1995) 27–34. [DOI] [PubMed] [Google Scholar]
- [32].Yu JCC, Martin S, Nasr J, Stafford K, Thompson D, Petrikovics I, LC-MS/MS analysis of 2-aminothiazoline-4-carboxylic acid as a forensic biomarker for cyanide poisoning, World Journal of Methodology, 2 (2012) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petrikovics I, Thompson DE, Rockwood GA, Logue BA, Martin S, Jayanna P, Yu JCC, Organ-distribution of the metabolite 2-aminothiazoline-4-carboxylic acid in a rat model following cyanide exposure, Biomarkers: Biochemical Indicators Of Exposure, Response, And Susceptibility To Chemicals, 16 (2011) 686–690. [DOI] [PubMed] [Google Scholar]
- [34].Petrikovics I, Yu JCC, Thompson DE, Jayanna P, Logue BA, Nasr J, Bhandari RK, Baskin SI, Rockwood G, Plasma persistence of 2-aminothiazoline-4-carboxylic acid in rat system determined by liquid chromatography tandem mass spectrometry, Journal Of Chromatography. B, Analytical Technologies In The Biomedical And Life Sciences, 891–892 (2012) 81–84. [DOI] [PubMed] [Google Scholar]
- [35].Anastassiades M, Lehotay JS, Stajnbaher D, Schenck JF, Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce, Journal of AOAC International, 86 (2003) 412–431. [PubMed] [Google Scholar]
- [36].Luliński P, Giebułtowicz J, Wroczyński P, Maciejewska D, A highly selective molecularly imprinted sorbent for extraction of 2-aminothiazoline-4-carboxylic acid – Synthesis, characterization and application in post-mortem whole blood analysis, Journal of Chromatography A, 1420 (2015) 16–25. [DOI] [PubMed] [Google Scholar]
- [37].Schmidt ML, Snow NH, Making the case for QuEChERS-gas chromatography of drugs, TrAC Trends in Analytical Chemistry, 75 (2016) 49–56. [Google Scholar]
- [38].Khezeli T, Daneshfar A, Development of dispersive micro-solid phase extraction based on micro and nano sorbents, TrAC Trends in Analytical Chemistry, 89 (2017) 99–118. [Google Scholar]
- [39].Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J, Miniaturized solid-phase extraction techniques, TrAC Trends in Analytical Chemistry, 73 (2015) 19–38. [Google Scholar]
- [40].Ahmadi M, Elmongy H, Madrakian T, Abdel-Rehim M, Nanomaterials as sorbents for sample preparation in bioanalysis: A review, Analytica Chimica Acta, 958 (2017) 1–21. [DOI] [PubMed] [Google Scholar]
- [41].Pardasani D, Kanaujia PK, Purohit AK, Shrivastava AR, Dubey DK, Magnetic multi-walled carbon nanotubes assisted dispersive solid phase extraction of nerve agents and their markers from muddy water, Talanta, 86 (2011) 248–255. [DOI] [PubMed] [Google Scholar]
- [42].Ruan X-L, Qiu J-J, Wu C, Huang T, Meng R-B, Lai Y-Q, Magnetic single-walled carbon nanotubes–dispersive solid-phase extraction method combined with liquid chromatography–tandem mass spectrometry for the determination of paraquat in urine, Journal of Chromatography B, 965 (2014) 85–90. [DOI] [PubMed] [Google Scholar]
- [43].Ngomsik A-F, Bee A, Talbot D, Cote G, Magnetic solid–liquid extraction of Eu(III), La(III), Ni(II) and Co(II) with maghemite nanoparticles, Separation & Purification Technology, 86 (2012) 1–8. [Google Scholar]
- [44].Bradham LS, Catsimpoolas N, Wood JL, Determination of 2-iminothiazolidine-4-carboxylic acid, Analytical Biochemistry, 11 (1965) 230–237. [DOI] [PubMed] [Google Scholar]
- [45].Haider A, Preparation and characterization of multi walled carbon naotubes/Ag nanoparticles hybrid materials, 2014. [Google Scholar]