Abstract
Objective:
To investigate whether there is a difference in the risk of asthma exacerbations between children with pre-existing asthma who receive live attenuated influenza vaccine (LAIV) compared with inactivated influenza vaccine (IIV).
Material and methods:
We identified IIV and LAIV immunizations occurring between July 1, 2007 and March 31, 2014 among Kaiser Permanente Northern California members aged 2 to <18 years with a history of asthma, and subsequent asthma exacerbations seen in the inpatient or Emergency Department (ED) setting. We calculated the ratio of the odds (OR) of an exacerbation being in the risk interval (1–14 days) versus the comparison interval (29–42 days) following immunization, separately for LAIV and IIV, and then examined whether the OR differed between children receiving LAIV and those receiving IIV (“difference-in-differences”).
Results:
Among 387,633 immunizations, 85% were IIV and 15% were LAIV. Children getting LAIV vs. IIV were less likely to have “current or recent, persistent” asthma (25% vs. 47%), and more likely to have “remote history” of asthma (47% vs. 25%). Among IIV-vaccinated asthmatic children, the OR of an inpatient/ED asthma exacerbation was 0.97 (95% CI: 0.82–1.15). Among LAIV-vaccinated asthmatic children the OR was 0.38 (95% CI: 0.17–0.90). In the difference-in-differences analysis, the odds of asthma exacerbation following LAIV were less than IIV (Ratio of ORs: 0.40, CI: 0.17–0.95, p value: 0.04).
Conclusion:
Among children ≥2 years old with asthma, we found no increased risk of asthma exacerbation following LAIV or IIV, and a decreased risk following LAIV compared to IIV.
Keywords: Vaccines, Influenza, Asthma, Safety
1. Introduction
In 2003, a nasally-administered, live attenuated influenza vaccine (LAIV, FluMist; MedImmune, Gaithersburg, MD) was approved by the US Food and Drug Administration for persons 2 years through 49 years of age [1,2]. From the 2007–2008 influenza season through the 2015–2016 season, the Advisory Committee on Immunization Practices (ACIP) recommended the use of either LAIV or inactivated influenza vaccine (IIV) in healthy children and adolescents ≥2 years of age [3–7], but either recommended, or cautioned, against use of LAIV in children with asthma, depending on the age of the child [4,6,7].
Concerns about use of LAIV in asthmatic children were partly based on a pre-licensure randomized placebo-controlled safety trial that suggested increased wheezing following LAIV [8]. However, asthma was not one of that trial’s pre-specified endpoints, and this finding occurred in the context of >1500 statistical comparisons [8]. Subsequent evidence for increased asthma exacerbations among children older than 2 years of age receiving LAIV is limited. Another randomized trial comparing LAIV with IIV found non-significantly higher rates of hospitalization following LAIV among children 24–47 months old with a history of wheezing illness [9]. An open-label, non-randomized trial [10] found no increase in healthcare utilization attributed to respiratory illnesses in LAIV recipients, and two post-licensure studies (which excluded LAIV-vaccinated children with asthma) also found no increase in respiratory events following LAIV [11,12].
Few studies have examined adverse outcomes following LAIV in children with asthma or related respiratory conditions. An open-label field trial among children with intermittent wheezing, found no increase in acute asthma exacerbations in the first two weeks after LAIV vaccination [13]. Two randomized trials, one among children with asthma [14] and one among children with history of recurrent respiratory tract infections [15], and two post-marketing evaluations among children with asthma or recurrent wheezing [16,17], found no increase in adverse events among children receiving LAIV compared with IIV. A Cochrane review concluded there was no difference in asthma exacerbations between vaccine types in children over 2 years of age, while acknowledging the number of patients on which that conclusion was based was small [18].
In 2016, the ACIP made an interim recommendation that health care providers in the U.S. not use LAIV in the upcoming influenza season, citing poor effectiveness [19]. Nevertheless, some providers may elect to use LAIV [19], it continues to be recommended outside the U.S. [20,21], and may again be recommended for use in the U.S. in future seasons. Therefore, the safety of LAIV use in children with asthma remains a concern.
The goal of this study was to investigate the safety of LAIV administered to children and adolescents with a history of asthma and to evaluate whether its safety profile varied according to asthma severity. We compared LAIV versus IIV with respect to asthma exacerbations and other adverse outcomes in children and adolescents with a history of asthma within Kaiser Permanente Northern California (KPNC).
2. Materials and methods
2.1. Setting
KPNC is a nonprofit, integrated health care delivery system that provides comprehensive health services to 3.5 million members. KPNC databases capture immunizations and inpatient, emergency department (ED), and outpatient diagnoses. Immunizations are provided at no additional cost to members and are almost all received within the system. This study was approved by the Kaiser Permanente Northern California Institutional Review Board.
2.2. Study population
We identified all IIV and LAIV immunizations between July 1, 2007 and March 31, 2014 for KPNC members aged 2 through 17 years who were continuous KPNC members for two years prior to immunization (2009 Pandemic monovalent vaccines were not included). We retained immunizations for children with a history of asthma – those who received at least one International Classification of Diseases, 9th Revision, Clinical Modification (ICD9) diagnosis code for asthma (493.xx) any time prior to IIV/LAIV immunization. We included the same child over multiple seasons if they received immunizations in more than one season, and included children who received different vaccine types (IIV or LAIV) in successive seasons.
2.3. Classification by asthma severity
Children were classified into one of three groups at immunization: (1) “current or recent, persistent asthma”; (2) “current or recent, not persistent asthma”; (3) “remote history of asthma only”. Children had “current or recent, persistent asthma” if they met at least one of these criteria in the year prior to immunization:(1) Asthma principal diagnosis from an inpatient hospitalization or ED visit; (2) ≥3 outpatient visits accompanied by an asthma diagnosis; (3) ≥1 prescriptions for an anti-inflammatory medication (i.e., inhaled corticosteroids, oral steroids, methylxanthines, mast cell stabilizers, leukotriene modifiers, and immunomodulators). Children had “current or recent, not persistent asthma” if they met at least one of these criteria during the two years prior to immunization: (1) Asthma principal diagnosis from an inpatient hospitalization or ED visit; (2) ≥1 outpatient visits accompanied by an asthma diagnosis; (3) ≥1 prescriptions for any asthma medication (anti-inflammatory or beta2 agonist); (4) Did not meet criteria for “current or recent, persistent asthma”. Children not meeting the criteria above at the time of immunization had “remote history of asthma only”. A child’s asthma severity could change from one season to the next. We adapted the first two criteria from Wakefield and Cloutier [22] as proxies for asthma severity. Our criteria “current or recent, not persistent asthma” differs from the Wakefield and Cloutier criteria by looking back two-years rather than one, which allowed greater differentiation between it and “current or recent, persistent asthma”.
2.4. Adverse outcomes
The primary outcome was acute asthma exacerbation, defined as: (1) acute inpatient hospitalization or ED visit accompanied by a principal diagnosis of asthma; or (2) a chart-confirmed outpatient asthma visit. We also identified all non-asthma outpatient visits as a “negative control”, since we did not expect such visits to vary between IIV and LAIV recipients. Secondary analyses assessed non-asthma adverse outcomes: afebrile seizure, Bell’s palsy, epistaxis, febrile seizure, fever, gastrointestinal disorders, migraine, otitis media, and sinusitis (Supplemental Table 1).
2.5. Medical record review: Validating outpatient asthma events
We reviewed charts for outpatient asthma visits to validate that the visits were for acute asthma exacerbations rather than routine asthma management or follow-up. We reviewed the medical records for all outpatient asthma visits during risk and comparison intervals following LAIV. These intervals were selected based on the literature or expert opinion. To compare risk for asthma exacerbation following LAIV versus IIV specifically among children with “remote history of asthma only” (who may have the lowest risk of asthma events), we also chart reviewed all post-IIV outpatient asthma visits for those children. Resource limitations precluded reviewing visits for IIV-vaccinated children with “current or recent, persistent” and “current or recent, not persistent” asthma.
2.6. Analyses
We used a case-centered, risk-interval, approach to evaluate the association between immunization and each outcome. The risk-interval aspect of our approach, which compares the odds of an event occurring in the risk interval versus the comparison interval, allows us to include only vaccinated individuals, thus reducing biases that might be introduced by including unvaccinated persons (who might differ from vaccinated persons in unmeasured ways) [27]. The case-centered aspect of our approach is similar to a stratified Cox proportional hazards model, but is much less computationally burdensome [23]. Like a Cox model, the case-centered approach can rigorously adjust for calendar time and reduce biases relating to temporal trends or seasonality. This approach has been described in detail [23] and been used in prior vaccine effectiveness and safety studies [24–27]. For each combination of vaccine (LAIV or IIV) and outcome (e.g., IP and ED asthma exacerbations), a logistic regression model was fit to a dataset consisting of one record for each outcome event that occurred in either the risk or comparison interval. The dependent variable indicated whether or not the outcome occurred during the risk interval. The independent variable was based on the proportion of vaccinees that were in the risk interval on the calendar day of the case’s outcome event, among all vaccinees in the case’s age-sex stratum that were in either the risk or comparison interval on that date (age strata were defined in one-year increments). The logit of this proportion was included as an offset in an intercept-only logistic regression model so that the fitted models yielded estimates of the ratio of the odds of an event being inside the risk interval versus the odds of an event being inside the comparison interval. In the absence of residual confounding, we interpret this difference in odds as attributable to the vaccine. In primary analyses of asthma exacerbation, the risk and comparison intervals were 1–14 days, and 29–42 days, after immunization, respectively.
We then examined whether the difference in the odds of an event being in the risk versus comparison interval differed between children vaccinated with LAIV versus IIV – i.e., we examined the “difference-in-differences”. We added to the model an independent variable indicating whether the child received LAIV or IIV. The exponentiated parameter estimate of this binary variable is a ratio of odds ratios and indicates how much more likely it is that the event occurred in the risk versus comparison interval for LAIV versus IIV.
For inpatient/ED asthma exacerbations, we ran the case-centered and case-centered difference-in-differences models for all children in the study, and separately for each asthma severity type. We performed a sensitivity analysis including all eligible children, but using a risk interval of 7–28 days and a comparison interval of 29–50 days.
To assess outpatient asthma exacerbations following LAIV, we used chart confirmed events and case-centered models including LAIV recipients only; for children with remote history of asthma only, we performed case-centered analyses separately for LAIV and IIV, and LAIV versus IIV using the difference-in-differences model. No adjustments were made for multiple comparisons.
3. Results
154,994 children had 387,633 influenza immunizations, of which 330,807 (85%) were IIV and 56,826 (15%) were LAIV (Table 1). Compared with IIV, children who received LAIV were more likely to be female and younger. Children getting LAIV were less likely to have “current or recent, persistent” asthma (25% versus 47% for IIV), and more likely to have “remote history” of asthma only (47% vs. 25%).
Table 1.
Characteristics of influenza immunizations between July 1, 2007 and March 31, 2014 for children 2–18 years of age with a history of asthma prior to the immunization, Kaiser Permanente Northern California.
| Characteristica | Immunization Typec | ||
|---|---|---|---|
| All | IIV | LAIV | |
| Number of unique children | 154,994 | 143,013 | 35,624 |
| Number of immunizations | 387,633 | 330,807 | 56,826 |
| Gender, n (%) | |||
| Female | 168,298 (43) | 142,409 (43) | 25,889 (46) |
| Male | 219,335 (57) | 188,398 (57) | 30,937 (54) |
| Age (years) at immunization, mean (median) | 10.59 (10.77) | 10.63 (10.87) | 10.36 (10.28) |
| Age group (years), n (%) | |||
| 2 | 11,735 (3) | 10,824 (3) | 911 (2) |
| 3–4 | 38,119 (10) | 33,046 (10) | 5073 (9) |
| 5–7 | 71,212 (18) | 59,201 (18) | 12,011 (21) |
| 8–10 | 78,881 (20) | 65,345 (20) | 13,536 (24) |
| 11–14 | 110,105 (28) | 94,061 (28) | 16,044 (28) |
| 15+ | 77,581 (20) | 68,330 (21) | 9251 (16) |
| Asthma type | |||
| Current or recent, persistent | 170,083 (44) | 156,044 (47) | 14,039 (25) |
| Current or recent, not persistent | 108,725 (28) | 92,565 (28) | 16,160 (28) |
| Remote history of asthma only | 108,825 (28) | 82,198 (25) | 26,627 (47) |
| Season of immunizationb | |||
| 2007 | 39,459 (10) | 36,300 (11) | 3159 (6) |
| 2008 | 43,668 (11) | 36,218 (11) | 7450 (13) |
| 2009 | 56,064 (14) | 48,324 (15) | 7740 (14) |
| 2010 | 52,976 (14) | 44,648 (13) | 8328 (15) |
| 2011 | 57,456 (15) | 47,209 (14) | 10,247 (18) |
| 2012 | 65,384 (17) | 54,077 (16) | 11,307 (20) |
| 2013 | 72,626 (19) | 64,031 (19) | 8595 (15) |
For every characteristic, differences between IIV and LAIV children were significant at p ≥ 0.01.
“Season” is defined as running from July 1st of the year listed to June 30th of the following year.
IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine.
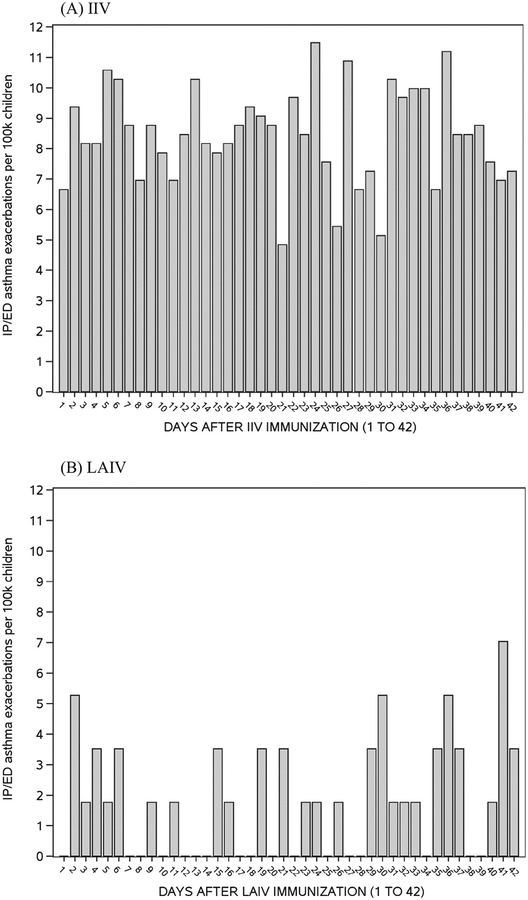
There was no clear temporal pattern of inpatient/ED asthma exacerbations in the 42 days following IIV or LAIV immunization (Fig. 1). Inpatient/ED events following LAIV were infrequent. The rates of inpatient/ED asthma exacerbations during the risk interval were substantially lower in children who received LAIV (19/100,0000 children) compared with those who received IIV (119/100,000 children), an observation seen in all subgroups except those with a remote history of asthma only (Table 2). Regardless of vaccine type, rates of asthma events were highest in children with current or recent, persistent asthma, and lowest in children with remote history of asthma only.
Fig. 1.
Rate of inpatient (IP) and Emergency Department (ED) asthma exacerbations by day following inactivated influenza vaccination (IIV) and live-attenuated influenza vaccination (LAIV).
Table 2.
Adverse event rates in risk and comparison intervals, influenza immunizations between July 1, 2007 and March 31, 2014 for children 2–18 years of age with a history of asthma prior to the immunization, Kaiser Permanente Northern California.
| Event | Group or subgroup analyzed | Interval (days)a | Number of events by type of immunization child receivedb | Events per 100 K children immunized | ||
|---|---|---|---|---|---|---|
| IIV | LAIV | IIV | LAIV | |||
| Inpatient and ER asthma exacerbation visits | All | Risk | 395 | 11 | 119 | 19 |
| Comparison | 389 | 22 | 118 | 39 | ||
| Current or recent, persistent | Risk | 343 | 6 | 220 | 43 | |
| Comparison | 340 | 9 | 218 | 64 | ||
| Current or recent, not persistent | Risk | 46 | 2 | 50 | 12 | |
| Comparison | 44 | 8 | 48 | 50 | ||
| Remote history of asthma only | Risk | 6 | 3 | 7 | 11 | |
| Comparison | 5 | 5 | 6 | 19 | ||
| Outpatient asthma exacerbation visits, after chart review confirmation | All | Risk | c | 242 | c | 426 |
| Comparison | c | 349 | c | 614 | ||
| Current or recent, persistent | Risk | c | 129 | c | 919 | |
| Comparison | c | 187 | c | 1332 | ||
| Current or recent, not persistent | Risk | c | 83 | c | 514 | |
| Comparison | c | 112 | c | 693 | ||
| Remote history of asthma only | Risk | 211 | 30 | 257 | 113 | |
| Comparison | 192 | 50 | 234 | 188 | ||
| All non-asthma related visits | Risk | 51,228 | 8270 | 15,486 | 14,553 | |
| Comparison | 47,633 | 7643 | 14,399 | 13,450 | ||
The risk interval was 1–14 days after immunization. The Comparison interval was 29–42 days after immunization. All events included here occurred in either the risk or comparison intervals in relation to immunization.
IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine.
Not available because chart review abstraction and confirmation was only performed for events following LAIV immunizations and for the subset of events following IIV immunization among children with remote history of asthma only.
Among children with remote history of asthma only, chart review confirmed outpatient asthma exacerbations for 403/572 (70%) events after IIV and 591/846 (70%) after LAIV (Supplemental Table 2). Confirmed outpatient asthma exacerbations rates were higher during the risk interval after IIV (257/100,000 children) than after LAIV (113/100,0000 children; Table 2).
In contrast, IIV and LAIV recipients had similar rates of non-asthma related visits in the risk (15,486/100,000 [IIV] and 14,553/100,000 [LAIV]) and comparison intervals (14,399/100,000 [IIV] and 13,450/100,000[LAIV]).
Among IIV-vaccinated children, odds of an inpatient/ED asthma event occurring during the risk interval was not significantly different from that of the comparison interval (OR 0.97, 95% Confidence Interval [CI]: 0.82–1.15), a finding which was consistent for each asthma severity subset (Table 3). Among LAIV-vaccinated children, odds of an inpatient/ED event occurring during the risk interval was lower than during the comparison interval (OR 0.39, 95% CI: 0.17–0.90). Difference-in-differences analyses found that LAIV was associated with lower odds of asthma exacerbation compared to IIV (Ratio of ORs 0.40, 95% CI: 0.17–0.95, p value: 0.04) among children with all asthma types and among those with current or recent, not persistent asthma (Ratio of OR 0.05, 95% CI: 0.01–0.58). Among children with all asthma types, changing the risk and comparison intervals to 7–28, and 29–50 days, respectively, minimally changed the OR for IIV (0.94, 95% CI: 0.82–1.07), LAIV (OR, 0.36, 95% CI: 0.17–0.78) and LAIV versus IIV (0.38, 95% CI 0.17–0.84).
Table 3.
Relationship between influenza immunization and adverse events among children 2–18 years of age with a history of asthma. Kaiser Permanente Northern California, July 1, 2007 to March 31, 2014.
| Event | Group or subgroup analyzed | Risk Intervalb | Comparison intervalb | Odds Ratio (95% Confidence Interval) of adverse event in the risk interval compared to comparison intervala | Difference-in-Differences | |
|---|---|---|---|---|---|---|
| IIV | LAIV | |||||
| IP and ED asthma exacerbations | All asthma types | 1–14days | 29–42 days | 0.97 (0.82,1.15) | 0.39 (0.17,0.90)* | 0.40 (0.17,0.95)* |
| Current or recent, persistent | 1–14 days | 29–42 days | 0.99 (0.83,1.19) | 0.67 (0.21,2.17) | 0.67 (0.20,2.22) | |
| Current or recent, not persistent | 1–14 days | 29–42 days | 1.03 (0.62,1.71) | 0.06 (0.01,0.56)* | 0.05 (0.01,0.58)* | |
| Remote history of asthma only | 1–14 days | 29–42 days | 0.63 (0.14,2.73) | 0.72 (0.17,3.04) | 1.14 (0.15,8.96) | |
| All | 7–28 days | 29–50 days | 0.94 (0.82,1.07) | 0.36 (0.17,0.78)* | 0.38 (0.17,0.84)* | |
| Outpatient asthma exacerbations | LAIV only, all asthma types | 1–14 days | 29–42 days | c | 0.75 (0.62,0.92)* | c |
| LAIV, current or recent, persistent | 1–14 days | 29–42 days | c | 0.79 (0.61,1.03) | c | |
| LAIV, current or recent, not persistent | 1–14 days | 29–42 days | c | 0.84 (0.62,1.13) | c | |
| Remote history of asthma only | 1–14 days | 29–42 days | 1.06 (0.83,1.35) | 0.67 (0.39,1.16) | 0.63 (0.35,1.15) | |
| All non-asthma-related visits | All | 1–14 days | 29–42 days | 1.06 (1.03,1.08)* | 1.05 (1.00,1.10) | 0.99 (0.94,1.04) |
The analytic dataset had one record per outcome. The dependent variable in the intercept-only logistic regression was “1” if the adverse event occurred during the risk window rather than the comparison window. Only one event per time window was counted. An offset term was used and was determined as follows: On the day of the event, determine the number of children at risk for the event, and who were the same age (in 1-year increments) and sex as the child with the event, and who had received the same type of influenza vaccine. Calculate the proportion of these children who were in the risk window on that day (as opposed to being in the comparison window.) This proportion divided by one minus the proportion is the odds. The logit of the odds is the offset term. For each immunization type, the odds ratio (OR) is the ratio of the odds of an event occurring in the risk interval and the odds of an event occurring in the comparison interval. The “difference-in-differences” is the ratio of the IIV odds ratio and the LAIV odds ratio. For the IIV and LAIV columns, values greater than (less than) one indicate increased (decreased) risk of the event following receipt of that vaccine. For the difference-in-differences column, values greater than (less than) one indicate that the risk of an event following LAIV is higher (lower) than the risk following IIV. IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine.
Intervals represent days after immunization. All events included here occurred in either the risk or comparison intervals.
Not available because chart review abstraction and confirmation was only performed for events following LAIV immunizations and for the subset of events following IIV immunization among children with remote history of asthma only.
Significant at p ≤ 0.05.
For children receiving LAIV, odds of an outpatient asthma exacerbation during the risk interval was lower than during the comparison interval (OR 0.75, 95% CI: 0.62–0.92; Table 3). For children with remote history of asthma, the difference-in-differences model indicated no significant differences in asthma exacerbations between IIV and LAIV.
There was no significant difference in the odds of non-asthma related visits between LAIV and IIV recipients (Ratio of ORs 0.99, 95% CI: 0.94–1.04; Table 3). For other non-asthma-related outcomes, the number of adverse events were low; incidence rates following IIV tended to be higher than those following LAIV (Table 4). Among these outcomes, only epistaxis had a significant difference, with LAIV versus IIV associated with decreased risk (Ratio of ORs 0.18, 95% CI: 0.04–0.82) – although the number of outcomes in the LAIV group was small (5 in the risk window, 16 in the comparison window).
Table 4.
Non-asthma related event rates in risk and comparison windows, and odds of adverse events, influenza immunizations between July 1, 2007 and March 31, 2014 for children 2–18 years of age with a history of asthma prior to the immunization, Kaiser Permanente Northern California.
| Event | Days after immunization | Number of events | Events per 100 K exposure daysa | Odds Ratio (95% Confidence Interval) of adverse event in the exposure window compared to comparison window after vaccinationb | ||||
|---|---|---|---|---|---|---|---|---|
| IIV | LAIV | IIV | LAIV | IIV | LAIV | Difference-in-Differences | ||
| Afebrile seizures | 1–7 | 13 | 3 | 0.56 | 0.75 | 1.36 (0.58,3.19) | 0.79 (0.14,4.30) | 0.58 (0.09,3.86) |
| 29–42 | 22 | 6 | 0.48 | 0.75 | ||||
| Bell’s palsy | 1–14 | 1 | 1 | 0.02 | 0.13 | c | c | c |
| 29–42 | 3 | 0 | 0.06 | 0.00 | ||||
| Epistaxis | 1–7 | 56 | 5 | 2.42 | 1.26 | 1.04 (0.69,1.57) | 0.19 (0.04,0.80)* | 0.18 (0.04,0.82)* |
| 29–42 | 109 | 16 | 2.35 | 2.01 | ||||
| Fever | 1–2 | 41 | 4 | 6.20 | 3.52 | 0.83 (0.56,1.23) | 0.35 (0.10,1.26) | 0.42 (0.11,1.61) |
| 29–42 | 345 | 55 | 7.45 | 6.91 | ||||
| Febrile seizure | 0–2 | 4 | 0 | 0.40 | 0.00 | c | c | c |
| 29–42 | 13 | 3 | 0.28 | 0,38 | ||||
| Gastrointestinal disorders | 1–7 | 318 | 31 | 13.73 | 7.79 | 0.90 (0.77,1.06) | 0.57 (0.35,0.93)* | 0.63 (0.38,1.06) |
| 29–42 | 688 | 111 | 14.86 | 13.95 | ||||
| Migraine | 1–7 | 105 | 9 | 4.53 | 2.26 | 0.98 (0.73,1.32) | 0.89 (0.32,2.44) | 0.91 (0.32,2.59) |
| 29–42 | 199 | 17 | 4.30 | 2.14 | ||||
| Otitis | 1–7 | 110 | 13 | 4.75 | 3.27 | 0.77 (0.58,1.00) | 0.58 (0.27,1.24) | 0.75 (0.33,1.70) |
| 29–42 | 298 | 39 | 6.43 | 4.90 | ||||
| Sinusitis | 1–7 | 13 | 1 | 0.56 | 0.25 | c | c | c |
| 29–42 | 49 | 1 | 1.06 | 0.13 | ||||
Because the risk and comparison time intervals are not equal for these adverse events, event rates are reported per 100k exposure days, rather than per 100k immunized children. “Exposure days” equals the number of children immunized times the number of days in the time interval.
For each immunization type, the odds ratio (OR) is the ratio of the odds of an event occurring in the risk interval and the odds of an event occurring in the comparison interval. The “difference-in-differences” is the ratio of the IIV odds ratio and the LAIV odds ratio. For the IIV and LAIV columns, values greater than (less than) one indicate increased (decreased) risk of the event following receipt of that vaccine. For the difference-in-differences column, values greater than (less than) one indicate that the risk of an event following LAIV is higher (lower) than the risk following IIV. IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine.
Events were too rare to allow for modeling.
Significant at p ≤ 0.05.
4. Discussion
Evaluating a large population of children and adolescents with history of asthma, we found no evidence that LAIV was associated with increased risk of subsequent asthma exacerbations. On the contrary, our case-centered difference-in-differences analyses suggested that LAIV was associated with lower risk of inpatient/ED visits for asthma exacerbations.
We further found no evidence that LAIV increases the risk of outpatient visits for asthma exacerbations. In particular, we found no relationship between LAIV and outpatient asthma exacerbations for children with “remote history of asthma only”. This result is reassuring since it has not been clear whether LAIV can be safely administered to these children. Despite the ACIP’s caution against it, children with a remote history of asthma represent asthmatics who would be most likely to inadvertently receive LAIV, related to either parents being unaware of cautions against LAIV or providers’ being unaware of asthma history. Together with the results from inpatient/ED setting, this study suggests that concerns about administering LAIV to children with asthma–especially those with current or recent, not persistent asthma or only a remote history of asthma – may not be warranted.
The observation that rates of asthma exacerbations were lower among LAIV recipients than among IIV recipients suggests that LAIV-vaccinated children differed from IIV-vaccinated children in their risk of subsequent exacerbations. Any residual confounding in our case-centered difference-in-differences analysis would be related to timing of events following immunization, and could occur if clinicians (or parents) are able to predict who is going to have an imminent asthma exacerbation 1–14 days following vaccination versus having an exacerbation 29–42 days following vaccination. For example, a clinician might note that a patient with asthma has a cough or an upper respiratory infection (URI) and, being concerned about imminent asthma exacerbation, select IIV for that patient (or postpone LAIV vaccination until a later time); whereas for an identical patient without cough or URI they may have selected LAIV. Observations such as these at the time of vaccination may not be recorded in such a way as to allow for adjustment. While it is possible that residual confounding contributes to our finding that LAIV tends to be associated with decreased risk of asthma exacerbations, our results demonstrate that among children who actually received LAIV, asthma exacerbations were relatively rare and did not increase in the period following immunization. At the very least, LAIV appears safe for the type of children who have been receiving it through 2014.
Strengths of this study include KPNC’s influenza vaccination program which provides encouragement to KPNC’s membership to receive annual influenza vaccines. In the 2013–2014 influenza season, approximately 50% of KPNC child members with prior diagnosis of asthma received an influenza vaccination. Although KPNC follows ACIP guidelines, clinicians can order vaccines outside of ACIP recommendations. Thus, KPNC has a significant population of children with pre-existing asthma diagnoses who received LAIV between 2007 and 2014. Our data also allowed us to re-evaluate asthma severity each year, which could therefore be treated as time-varying. Another strength is that we reviewed the charts of all post-LAIV outpatient asthma visits and post-IIV remote asthma visits, and found the majority of these were for asthma exacerbations rather than routine follow-up or management. It was reassuring to find similar asthma confirmation rates whether children received IIV or LAIV, and whether the event was during a risk or comparison interval.
In addition to potential residual confounding noted above, this study had limitations. We relied on diagnosis codes to identify asthma. Our modified HEDIS classification of asthma type likely included variations in severity within each type. In particular, our findings regarding children with current or recent, persistent asthma, may not apply to the smaller subset of children with very severe asthma. Further, despite the large number of children receiving LAIV, the number of inpatient/ED asthma exacerbations in this group was small. This is a reassuring finding, although the small number of events reduces the precision of results. We also did not attempt to statistically account for persons who had an outcome in more than one season. However, this accounted for only 3.7% of inpatient/ED events, and 1.2% of chart review confirmed outpatient asthma exacerbations; thus, any effect on the results is likely very small. Finally, KPNC providers – like those in some other health systems – have access to the full electronic medical record which likely affected their decisions regarding vaccine type. Our findings may not be as applicable in situations where providers do not have access to the full medical record.
In conclusion, among children and adolescents with a history of asthma, we found no evidence that those who received LAIV were more likely to have an asthma exacerbation than those who received IIV, and we found that incidence of such events following immunization with LAIV was relatively low regardless of asthma severity. Our findings indicate that use of LAIV in children ≥2 years old with asthma did not increase the risk of asthma exacerbations.
Supplementary Material
Acknowledgements
This work was supported by the Centers for Disease Control and Prevention, Contract number: 200-2012-53581, Task Order “Evaluation of safety of live attenuated influenza vaccine versus inactivated influenza vaccine in asthmatic children 2–18 years of age (LAIV)”. We thank John Hansen and Julius Timbol of the Kaiser Permanente Vaccine Study Center for their facilitation of the medical record chart review.
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- CI
Confidence Interval
- ED
Emergency Department
- HEDIS
Health Plan Employer Data and Information Set
- ICD9
International Classification of Disease, 9th Revision, Clinical Modification
- IIV
inactivated influenza vaccine
- KPNC
Kaiser Permanente of Northern California
- LAIV
live, attenuated influenza vaccine
- OR
odds ratio
- URI
upper respiratory infection
Footnotes
Potential conflicts of interest
G. Thomas Ray has received research support on grants to Kaiser Permanente Division of Research in the past 3 years from Pfizer, Merck & Co, Genentech, and Purdue Pharma. Roger Baxter has received research grants through his institution from MedImmune, GlaxoSmithKline (GSK), Sanofi Pasteur, and Protein Science. Nicola Klein has received research grants through her institution from MedImmune, GSK, Sanofi Pasteur, Novartis (now GSK), Protein Science, Merck & Co, and Pfizer. The remaining authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendix A. Supplementary material Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.03. 082.
References
- [1].Meadows M Nasal flu vaccine approved. FDA Consum 2003;37:27. [PubMed] [Google Scholar]
- [2].MedImmune. Flumist Quadrivalent [Draft package insert]. Gaithersburg, MD: MedImmune; 2015. [Google Scholar]
- [3].Centers for disease control and prevention. Expansion of use of live attenuated influenza vaccine (FluMist) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217–9. [Google Scholar]
- [4].Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008;57:1–60. [PubMed] [Google Scholar]
- [5].Grohskopf LA, Uyeki TM, Bresee JS, Cox N. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- [7].Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep 2015;64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bergen R, Black S, Shinefield H, Lewis E, Ray P, Hansen J, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatr Infect Dis J 2004;23:138–44. [DOI] [PubMed] [Google Scholar]
- [9].Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007;356:685–96. [DOI] [PubMed] [Google Scholar]
- [10].Piedra PA, Gaglani MJ, Riggs M, Herschler G, Fewlass C, Watts M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics 2005;116:e397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Toback SL, Ambrose CS, Eaton A, Hansen J, Aukes L, Lewis N, et al. A postlicensure evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 24–59 months of age. Vaccine. 2013;31:1812–8. [DOI] [PubMed] [Google Scholar]
- [12].Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, et al. A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 5 through 17 years of age. Vaccine 2012;30:2989–98. [DOI] [PubMed] [Google Scholar]
- [13].Gaglani MJ, Piedra PA, Riggs M, Herschler G, Fewlass C, Glezen WP. Safety of the intranasal, trivalent, live attenuated influenza vaccine (LAIV) in children with intermittent wheezing in an open-label field trial. Pediatr Infect Dis J 2008;27:444–52. [DOI] [PubMed] [Google Scholar]
- [14].Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006;25:860–9. [DOI] [PubMed] [Google Scholar]
- [15].Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006;25:870–9. [DOI] [PubMed] [Google Scholar]
- [16].Tennis P, Toback SL, Andrews E, McQuay LJ, Ambrose CS. A postmarketing evaluation of the frequency of use and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5 years. Vaccine 2011;29:4947–52. [DOI] [PubMed] [Google Scholar]
- [17].Tennis P, Toback SL, Andrews EB, McQuay LJ, Ambrose CS. A US postmarketing evaluation of the frequency and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5 years: 2009–2010 season. Vaccine 2012;30:6099–102. [DOI] [PubMed] [Google Scholar]
- [18].Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev 2013(2):CD000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2016;65:1–54. [DOI] [PubMed] [Google Scholar]
- [20].Public Health Agency of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2016–2017; 2016.
- [21].Public Health England. The National Childhood Flu Immunisation Programme 2016/17 Information for healthcare practitioners; 2016.
- [22].Wakefield DB, Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr Pulmonol 2006;41:962–71. [DOI] [PubMed] [Google Scholar]
- [23].Fireman B, Lee J, Lewis N, Bembom O, Van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol 2009;170:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rowhani-Rahbar A, Klein NP, Lewis N, Fireman B, Ray P, Rasgon B, et al. Immunization and Bell’s palsy in children: a case-centered analysis. Am J Epidemiol 2012;175:878–85. [DOI] [PubMed] [Google Scholar]
- [25].Baxter R, Lewis E, Fireman B, DeStefano F, Gee J, Klein NP. Case centered analysis of Optic Neuritis following vaccines. Clin Infect Dis 2016;63:79–81. [DOI] [PubMed] [Google Scholar]
- [26].Baxter R, Lewis N, Bohrer P, Harrington T, Aukes L, Klein NP. Sudden-onset sensorineural hearing loss after immunization: a case-centered analysis. Otolaryngol Head Neck Surg 2016;155:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klein NP, Lewis E, Fireman B, Hambidge SJ, Naleway A, Nelson JC, et al. Safety of measles-containing vaccines in 1-year-old children. Pediatrics 2015;135: e321–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.