Abstract
BACKGROUND:
The risk of febrile seizure is temporarily increased for a few days after the administration of certain vaccines in children aged six to 23 months. Our objective was to determine the febrile seizure risk following vaccination in children aged one to five months, when six different vaccines are typically administered.
METHODS:
We identified emergency department visits and inpatient admissions with International Classification of Diseases, Ninth Revision, febrile seizure codes among children enrolled in nine Vaccine Safety Datalink participating health care organizations from 2006 through 2011. Febrile seizures were confirmed by medical record abstraction. We used the self-controlled risk-interval method to compare the incidence of febrile seizure during postvaccination days 0 to 1 (risk interval) versus days 14 to 20 (control interval).
RESULTS:
We identified 15 febrile seizure cases that occurred after 585,342 vaccination visits. The case patients were aged three to five months. The patients had received a median of four (range two to six) vaccines simultaneously. The incidence rate ratio of febrile seizure after vaccination was 23 (95% confidence interval 5.13 to 100.8), and the attributable risk was 3.92 (95% confidence interval 1.68 to 6.17) febrile seizure cases per 100,000 persons vaccinated.
CONCLUSIONS:
Vaccination in children aged three to five months was associated with a large relative risk of febrile seizure on the day of and the day after vaccination, but the risk was small in absolute terms. Postvaccination febrile seizure should not be a concern for the vast majority of children receiving vaccines, but clinicians might take this risk into consideration when evaluating and treating children susceptible to seizures precipitated by fever.
Keywords: febrile seizure, vaccines, immunization, cohort studies, infant
Introduction
Febrile seizure is the most common type of seizure in childhood, occurring in up to 5% of children.1 Febrile seizure can occur during infections with a variety of pathogens.2,3 Febrile seizure has also been associated with certain types of vaccines. The timing of fever and febrile seizure after vaccination depends on whether the vaccine contains a live attenuated virus, which requires several days to replicate before inducing a febrile response, or contains inactivated components, which may elicit a febrile response within the first 24 hours. The febrile seizure risk with current US live and inactivated vaccines has been studied in children ages six through 23 months, but not in younger children.4,5 The US Advisory Committee on Immunization Practices (ACIP) recommends that all children less than six months old receive six vaccines to prevent eight infectious diseases.6 There are five inactivated vaccines including hepatitis B (HepB) with doses recommended at birth and at one to two months of age, and diphtheria-tetanus-acellular pertussis (DTaP), Haemophilus influenzae type b (Hib), pneumococcal conjugate (PCV), and inactivated poliovirus (IPV) vaccines with doses recommended at two and four months of age. A live attenuated rotavirus (RV) vaccine is also recommended at two and four months of age.
The febrile seizure literature reflects a lack of consensus about whether febrile seizure should be diagnosed in children less than six months of age. There are three frequently cited febrile seizure definitions that list different minimum ages. A National Institutes of Health consensus statement published in 1980 described febrile seizure as usually occurring between ages 3 months and 5 years.7 In 1993, the International League Against Epilepsy defined febrile seizure as occurring in childhood after age one month.8 In 2008, the American Academy of Pediatrics published a simple febrile seizure clinical practice guideline that defined febrile seizure as occurring in children between ages six and 60 months.9 That document did not state the rationale for setting the lower age limit at six months, but a separate systematic review stated that, in practice, the lower age limit is generally considered to be six months because of the concern about serious infections such as meningitis presenting with seizure and fever possibly being mistaken for febrile seizure in children under six months.10
Our objective was to estimate the febrile seizure risk during the time from vaccination through the following day associated with US vaccines administered to children aged one through five months.
Materials and Methods
The Vaccine Safety Datalink (VSD) is a collaboration between the Centers for Disease Control and Prevention and several integrated health care organizations (sites) in the United States that perform vaccine safety research and surveillance.11 Nine sites contributed data to the present study. The VSD population includes over eight million members annually and has been shown to be representative of the general US population in terms of demographic and socioeconomic variations.12
We identified children in the VSD aged one to five months who had an emergency department (ED) visit or inpatient admission from July 1, 2006, through June 30, 2011, associated with an International Classification of Diseases, Ninth Revision (ICD-9) “convulsion” code (780.3, 780.31, 780.32, or 780.39), which include febrile seizure but not epilepsy. We excluded visits that occurred ≤42 days after a previous visit with a convulsion ICD-9 code because those are unlikely to represent a new event. We defined the day of vaccination as “day 0” and only included convulsion visits that occurred on days zero to one or 14 to 20 following the administration of one or more vaccines of any type.
We abstracted medical records to determine whether each patient actually had an incident seizure and whether it was accompanied by a fever or not. We defined a “probable” seizure as a clinical diagnosis of seizure by the treating physician. We defined a “possible” seizure as a paroxysmal event for which the treating physician did not make a definitive diagnosis but for which seizure was considered the more likely possibility in the differential diagnosis. We defined fever as a temperature of ≥38°C. We defined a febrile seizure as a seizure in a child who had a fever within the prior 24 hours, excluding patients with central nervous system (CNS) infection, criteria for other symptomatic seizures (e.g., brain structural abnormalities and metabolic disturbance), or a history of afebrile seizure.8 Seizures with fever that did not meet the febrile seizure definition were classified as “seizure with fever other than febrile seizure.”
Statistical methods
We used the self-controlled risk interval (SCRI) method to compare the incidence of febrile seizure during the risk interval (zero to one days post vaccination) to the control interval (14 to 20 days post vaccination).13 The risk interval represents the biologically plausible time period during which inactivated vaccines may induce fever. The control interval represents a time period during which neither inactivated nor live attenuated vaccines should affect the risk of fever or febrile seizure. Comparing two different time intervals for the same individual inherently controls for factors that do not change over time. Choosing a control interval that is relatively short and close in time to the risk interval implicitly controls for factors that change over time, such as age and season. The incidence during each interval was calculated by dividing the number of cases by the number of person-days. The incidence rate ratio (IRR) with 95% confidence interval (CI) was calculated in SAS 9.4 using conditional Poisson regression with an offset term to account for the different interval lengths.14,15 The attributable risk (AR), which is a measure of the excess number of cases that occurred due to exposure to vaccination, was calculated as the difference between the risk interval incidence rate and the control interval incidence rate multiplied by two person-days (i.e., the duration of the risk interval), and the 95% CI was calculated in OpenEpi using the Byar method.16 The primary analysis included only the probable febrile seizure cases.
Institutional review boards at the Centers for Disease Control and Prevention, and each site approved the present study and determined that informed consent was not required.
Results
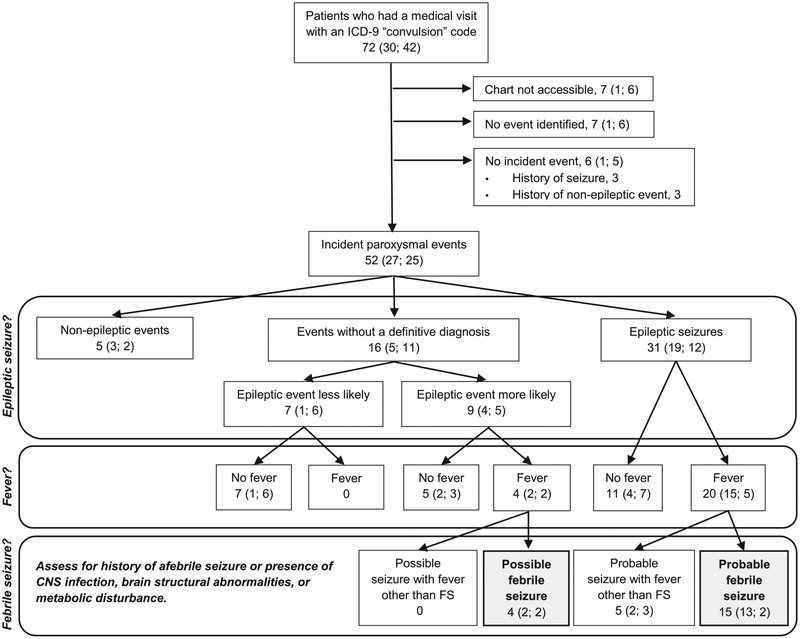
Our study population had 585,342 vaccination visits. Following these, 72 patients had an ED or an inpatient visit associated with a convulsion ICD-9 code (30 during the risk interval and 42 during the control interval). Medical records were available for 65 of these patients (Fig). There were 31 patients with probable seizures, including 11 seizures without fever, 15 febrile seizures, and five seizures with fever other than febrile seizure. Among the seizures with fever other than febrile seizure, three had a history of prior afebrile seizure and two were originally diagnosed as febrile seizure but were later diagnosed with epilepsy following subsequent afebrile seizures. There were nine patients with possible seizures, among which were five seizures without fever and four febrile seizures.
FIGURE.
Case classification based on medical record abstraction for patients ages 1 to 5 months who had a medical visit with an International Classification of Diseases, Ninth Revision “convulsion” code during specified time intervals following vaccination. The total number of patients is shown first followed in parentheses by the number of patients whose events occurred during the risk interval and the control interval, respectively. The risk interval was postvaccination days 0 to 1 and the control interval was postvaccination days 14 to 20. CNS, central nervous system; FS, febrile seizure.
The characteristics of the patients with febrile seizure are shown in Table 1. The probable febrile seizures all occurred in children aged three to five months, whereas the possible febrile seizures occurred from one to four months of age. The reported seizure duration ranged from five seconds to five minutes. The seizure was witnessed by a heath care provider in only two instances. The provider notes used the term “febrile seizure” to label the diagnosis in 12 of the patients. Six case patients had a non-CNS infection documented at the time the seizure was evaluated (three with upper respiratory tract infection, one with upper respiratory tract infection and acute otitis media, one with sinusitis, and one with pyelonephritis).
TABLE 1.
Characteristics of Patients With a Probable or Possible Febrile Seizure Following Vaccination as Determined by Medical Record Abstraction
| Characteristics | Probable Febrile Seizure n = 15 | Possible Febrile Seizure n = 4 | Total N = 19 |
|---|---|---|---|
| Age (months) | |||
| 1 | 0 | 1 | 1 (5%) |
| 2 | 0 | 1 | 1 (5%) |
| 3 | 4 | 1 | 5 (26%) |
| 4 | 9 | 1 | 10 (53%) |
| 5 | 2 | 0 | 2 (11%) |
| Sex | |||
| Male | 8 | 3 | 11 (58%) |
| Female | 7 | 1 | 8 (42%) |
| Medical history before seizure | |||
| Premature birth | 0 | 1 | 1 (5%) |
| Family history of febrile seizure | 2 | 1 | 3 (16%) |
| Temperature >38°C at the time of vaccination | 1 | 0 | 1 (5%) |
| Vaccines received on day 0 | |||
| Number, median (range) | 4 (2 to 6) | 4 (2 to 4) | 4 (2 to 6) |
| HepB | 1 | 0 | 1 (5%) |
| RV5 | 12 | 4 | 16 (84%) |
| DTaP-HepB-IPV combination vaccine | 11 | 3 | 14 (74%) |
| DTaP-IPV/Hib combination vaccine | 2 | 1 | 3 (16%) |
| Hib | 13 | 3 | 16(84%) |
| PCV 7-valent | 14 | 3 | 17(89%) |
| PCV 13-valent | 1 | 0 | 1 (5%) |
| IIV3 | 1 | 0 | 1 (5%) |
| MIV | 1 | 0 | 1 (5%) |
| Seizure characteristics | |||
| Duration not reported | 6 | 1 | 7 (37%) |
| Duration reported in minutes, median (range) | 1 (0.3 to 5.0) | 0.17 (0.08 to 0.25) | 0.6 (0.08 to 5.0) |
| Complex febrile seizure | 4 | 0 | 4 (21%) |
| Medical visit for febrile seizure | |||
| Non-CNS infection documented* | 5 | 1 | 6 (32%) |
| Seizure witnessed by a health care provider | 2 | 0 | 2(11%) |
| Provider notes used the term “febrile seizure” to label the diagnosis | 9 | 3 | 12(63%) |
| Patient admitted to the hospital | 6 | 3 | 9 (47%) |
Abbreviations:
CNS = Central nervous system
DTaP = Diphtheria-tetanus-acellular pertussis
HepB = Hepatitis B
Hib = Haemophilus influenzae type b
IIV3 = Inactivated influenza vaccine trivalent
IPV = Inactivated poliovirus
MIV = Monovalent pandemic 2009 H1N1 inactivated influenza vaccine
PCV = Pneumococcal conjugate
RV5 = Rotavirus vaccine pentavalent
The five probable cases with infection all occurred during the risk interval, and the one possible case with infection occurred during the control interval.
The 19 probable or possible febrile seizure case patients received a median of four vaccines (range two to six) on day 0 before the febrile seizure (Table 1). Eighteen patients (95%) received PCV, and 17 patients (90%) received a “DTaP-containing” combination vaccine (i.e., DTaP-HepB-IPV or “DTaP-IPV/Hib”). Twelve case patients (63%) received all six recommended vaccine types (Table 2).
TABLE 2.
Simultaneous Vaccination Combinations Received on Day 0 by Case Patients With a Probable or Possible Febrile Seizure Following Vaccination
| Cases, n | Simultaneous Vaccination Combinations | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 | RV5 | DTaP-HepB-IPV | Hib | PCV | ||||
| 2 | DTaP-HepB-IPV | Hib | PCV | |||||
| 1 | RV5 | DTaP-IPV/Hib | PCV | HepB | IIV3 | MIV | ||
| 1 | RV5 | DTaP-IPV/Hib | PCV | |||||
| 1 | RV5 | DTaP-IPV/Hib | ||||||
| 1 | RV5 | Hib | PCV | |||||
| 1 | Hib | PCV | ||||||
Abbreviations:
DTaP = Diphtheria-tetanus-acellular pertussis
HepB = Hepatitis B
Hib = Haemophilus influenzae type b
IIV3 = Inactivated influenza vaccine trivalent
IPV = Inactivated poliovirus
MIV = Monovalent pandemic 2009 H1N1 inactivated influenza vaccine
PCV = Pneumococcal conjugate
RV5 = Rotavirus vaccine pentavalent
Table 3 shows the incidence of probable febrile seizure following vaccination by age in months. The IRR of probable febrile seizure was 23 (95% CI 5.13 to 100.8), and the AR was 3.92 (95% CI 1.68 to 6.17) febrile seizure cases per 100,000 children vaccinated at ages 3 to 5 months. When both probable and possible febrile seizure cases were combined, the IRR was 13 (95% CI 4.36 to 39.6), and the AR was 2.37 (95% CI 1.06 to 3.68) febrile seizure cases per 100,000 children vaccinated at ages one to five months.
TABLE 3.
Number of Probable Febrile Seizure Cases and Incidence Rate Following Vaccination by Age in Months
| Age (Months) | Vaccination Visits | Patients With “Convulsion” ICD-9 Code | Probable Febrile Seizure Cases | |
|---|---|---|---|---|
| Risk Interval, n (Rate*) | Control Interval, n (Rate*) | |||
| 1 | 67,477 | 5 | 0 | 0 |
| 2 | 200,998 | 15 | 0 | 0 |
| 3 | 61,369 | 14 | 4(3.26) | 0 |
| 4 | 202,299 | 34 | 7 (1.73) | 2 (0.14) |
| 5 | 53,199 | 4 | 2 (1.88) | 0 |
| TOTAL | 585,342 | 72 | 13 (1.11) | 2 (0.05) |
Abbreviations:
ICD-9 = International Classification of Diseases, Ninth Revision
Incidence rate is per 100,000 person-days.
Table 4 shows results of additional SCRI analyses. When case patients with a non-CNS infection at the time of the seizure were excluded, the febrile seizure risk after vaccination was still significantly increased. The IRR was also significantly increased when all seizures with fever were included in the analysis. However, in an analysis of only seizure without fever, the IRR was not significantly increased at 2.0 (95% CI 0.59 to 6.83).
TABLE 4.
Incidence Rate Ratio of Seizure Following Vaccination in Children Ages 1 to 5 Months Using the Self-Controlled Risk Interval Method*
| Analysis | total, n | Risk Interval, n | Control Interval, n | IRR (95% CI) |
|---|---|---|---|---|
| Febrile seizure | ||||
| Probable cases | 15 | 13 | 2 | 23 (5.13 to 100.8) |
| Probable and possible cases | 19 | 15 | 4 | 13 (4.36 to 39.6) |
| Febrile seizure cases who received RV5, DTaP-HepB-IPV, Hib, and PCV simultaneously on day 0† | ||||
| Probable cases | 9 | 8 | 1 | 28 (3.50 to 223.9) |
| Probable and possible cases | 12 | 9 | 3 | 11 (2.84 to 38.8) |
| Febrile seizure cases excluding those who had a non-CNS infection at the time of seizure | ||||
| Probable cases | 10 | 8 | 2 | 14 (2.97 to 65.9) |
| Probable and possible cases | 13 | 10 | 3 | 12(3.21 to 42.4) |
| All seizures with fever (including febrile seizure) | ||||
| Probable cases | 20 | 15 | 5 | 10 (3.82 to 28.9) |
| Probable and possible cases | 24 | 17 | 7 | 8.5 (3.52 to 20.5) |
| Seizures without fever | ||||
| Probable cases | 11 | 4 | 7 | 2.0 (0.59 to 6.83) |
| Probable and possible cases | 16 | 6 | 10 | 2.1 (0.76 to 5.78) |
Abbreviations:
CNS = Central nervous system
DTaP = Diphtheria-tetanus-acellular pertussis
HepB = Hepatitis B
Hib = Haemophilus influenzae type b
IPV = Inactivated poliovirus
IRR = Incidence rate ratio
PCV = Pneumococcal conjugate
RV5 = Rotavirus vaccine pentavalent
The risk interval was postvaccination days 0 to 1, and the control interval was postvaccination days 14 to 20.
This combination of vaccines was most common in this study population and includes all six vaccine types recommended for children in this age range.
Discussion
Seizures with fever that met the definition of probable febrile seizure occurred after vaccination in children aged three to five months (and possible febrile seizures also occurred in children ages one to two months). The majority of these seizures were labeled as febrile seizure in routine clinical practice, even after the publication of a US clinical practice guideline that stated the lower age limit for febrile seizure to be six months.9 The relative risk of febrile seizure occurring on the day of vaccination or on the day after vaccination was high in this age group, but in absolute terms, the number of febrile seizures attributable to vaccination was 3.92 cases per 100,000 infants vaccinated. This AR is similar to previous estimates for certain vaccines given at ages six or 12 months.4
In this study, all vaccines received by the case patients were age appropriate, except for one child who received influenza vaccine at age five months, which is less than the minimum recommended age of six months. The most common combination of vaccines received simultaneously by patients in the present study was RV5, DTaP-HepB-IPV, Hib, and PCV. This combination includes all six of the vaccine types recommended in this age range, which the ACIP recommendations state may all be given together. This combination of vaccines was associated with an increased risk of febrile seizure in a subgroup analysis. We could not evaluate the risk associated with other specific vaccine combinations due to the small number of individuals who received them, nor could we evaluate the febrile seizure risk associated with individual vaccines, because all but one of the febrile seizure case patients had received PCV. PCV was independently associated with an increased risk of febrile seizure among children ages six to 23 months in a recent VSD study.4 In the current study, we cannot determine if the risk observed is due to PCV alone or if other individual vaccines or interactions between vaccines contributed to the observed risk because we did not have enough children who did not receive PCV for comparison.
Several previous studies that sought to examine febrile seizure risk after individual vaccines also included children less than six months old. “Diphtheria-tetanus-whole-cell” pertussis vaccines, which are no longer used in the United States, were associated with an increased febrile seizure risk in several studies. A VSD study of diphtheria–tetanus-whole-cell pertussis during the period from 1991 through 1993 found an AR of six to nine febrile seizures per 100,000 children on the day of vaccination.17 That study included all children under age seven years but did not report age specific results. Two studies of whole-cell pertussis vaccines in the United Kingdom also found an increased febrile seizure risk, but the first found no increased risk in children less than six months old, whereas the second did not report results specific to this age group.14,18 Whole-cell pertussis vaccines were replaced with acellular pertussis vaccines in the United States in the 1990s. A VSD study of DTaP vaccines (given with or without simultaneous vaccines) during the period from 1997 to 2006 found no increase in seizure risk during the zero to three days postvaccination in children ages six weeks to 23 months, including dose-specific results applicable to children less than six months old.19 The reason why our current study, in which 90% of case patients received a DTaP-containing vaccine, observed an increased febrile seizure risk whereas the DTaP study did not could be due to study design differences or differences in the vaccines included in the studies. The previous study relied on ICD-9 codes without medical record abstraction and did not analyze febrile seizure separately from other types of seizure. The vaccines available and recommended during the previous study’s period were different from the current immunization schedule; PCV and DTaP-HepB-IPV were not yet licensed during the earlier part of that study period. In clinical trials, DTaP-HepB-IPV had higher rates of fever compared with its separately administered component vaccines.20 So the large percentage of case patients in the current study who received PCV or DTaP-HepB-IPV might account for the increased risk we observed. A different DTaP combination vaccine was associated with increased febrile seizure risk specifically in children less than six months of age in another study. A DTaP-IPV-Hib combination vaccine manufactured by the Statens Serum Institut, which is not licensed in the United States, was studied in Denmark from 2003 to 2008, and there was an increased febrile seizure risk on the day of vaccination at ages 3 and 5 months.21 The absolute risk was less than 4 per 100,000 persons. PCV was not used during most of that study period and did not account for that finding.
Our results indicate that febrile seizure risk may increase on the day of or the day after administration of an inactivated vaccine, but not all febrile seizures occurring during this time interval will necessarily be caused by vaccination. In our study, 32% of patients with a febrile seizure during the risk interval after vaccination had a non-CNS infection documented at the time of their febrile seizure, which may have led to or contributed to the febrile seizure. Some of these seizures may also be due to noninfectious preexisting conditions that have not yet been diagnosed. A study in The Netherlands of children under two years old who experienced seizures following vaccination (including but not limited to febrile seizure) found that 4.5% were diagnosed with epilepsy by age two years.22 In more than half of the children with epilepsy, the first seizure had occurred in temporal association with vaccination. In those whose epilepsy onset had been temporally associated with vaccination, 65% had an identifiable genetic or structural cause. Genetic mutations have been associated with epilepsy syndromes characterized by seizures with fever such as Dravet syndrome (also known as severe myoclonic epilepsy of infancy) and generalized epilepsy with febrile seizures plus.23 These syndromes may not be recognized at the time of the first seizure. In our study, two patients who initially had a seizure with fever went on to later have afebrile seizures and be diagnosed with epilepsy. So perhaps the diagnosis of an apparent febrile seizure in a child under six months who has a seizure with fever should be considered a provisional diagnosis, with the recognition that the diagnosis might change as the child ages and additional syndromic features present. Our secondary analysis that included all seizures with fever also revealed a significantly increased risk, which could suggest an increased risk of epileptic seizure precipitated by fever following vaccination regardless of the underlying mechanism of seizure susceptibility. In as much as certain types of vaccine may elicit fever, and fever in turn may precipitate a seizure in susceptible individuals, this would not be surprising.
This study had several potential limitations. First, we searched only for patients with ED visits or inpatient admissions because a prior VSD study of postvaccination ICD-9 code chart confirmation rates in children ages six weeks to 23 months found that clinic visits with the convulsion codes rarely represent true incident seizures, particularly in children less than one year old.24 So there might have been a few additional febrile seizure case patients seen only in the clinic setting who were not included in our study. Not including such patients would result in a slight underestimation of the incidence rate, but would not affect the IRR estimates derived from the SCRI method, which does not require the capture of all cases in the source population. Second, postvaccination febrile seizure is a rare event, so despite our large study population, our analysis is based on a relatively small number of cases, particularly in the control interval. This led to fairly wide CIs around some of our risk estimates. Third, the present study relied on the treating physician’s clinical diagnosis of a seizure as documented in the medical record. The clinical notes rarely contained detailed descriptions of seizure signs and symptoms needed to utilize the Brighton Collaboration generalized convulsive seizure definition, which is a standardized case definition that was developed for vaccine safety studies.25 Most of the paroxysmal events were not witnessed by a health care provider, and the physician did not make a definitive diagnosis for 31% of the patients. We accounted for this uncertainty by defining a category of possible seizure in our case classification scheme and excluded the possible cases from the primary analysis. Fourth, the present study focused on a risk interval appropriate for febrile seizure risk with inactivated vaccines, which may not be an appropriate risk interval for studying febrile seizure following live-attenuated RV vaccine. Pentavalent (RV5) was the only RV vaccine received by patients in the present study. The rate of fever following RV5 in clinical trials was not higher than placebo, so the likelihood of an acute febrile seizure risk with RV5 is probably low.26
The vaccine products included in the present study are still available and in use today. The ACIP recommendations for routine immunization of children in the one- to five month age range in effect during this study period are the same as the current ACIP recommendations. Therefore, the present study represents the best estimate of the risk of febrile seizure associated with the current US immunization schedule for children ages 1 to 5 months. The AR of febrile seizure following vaccination in this age range is small. Therefore, postvaccination febrile seizure should not be a concern for the vast majority of children receiving vaccines, but clinicians might take this risk into consideration when evaluating and treating children susceptible to seizures precipitated by fever.
Acknowledgments
The authors thank the following organizations for contributing data to this study: Harvard Vanguard Medical Associates and Harvard Pilgrim Health Care, HealthPartners, Kaiser Permanente Colorado, Kaiser Permanente Georgia, Kaiser Permanente Hawaii, Kaiser Permanente Northern California, Kaiser Permanente Northwest, Kaiser Permanente Washington, Marshfield Clinic. The authors also thank all of the Vaccine Safety Datalink project staff at each of the previously mentioned sites whose work made this study possible.
Funding source: This study was funded by the Centers for Disease Control and Prevention. No external funding was obtained for this study.
Financial disclosure: Dr. Klein reports receiving research support from GlaxoSmithKline, MedImmune, Merck & Co., Pfizer, Protein Science, and Sanofi-Pasteur for unrelated studies. Dr. Naleway reports receiving research funding from MedImmune, Merck & Co., and Pfizer for unrelated studies. The remaining authors have no financial relationships to disclose.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Patel N, Ram D, Swiderska N, Mewasingh LD, Newton RW, Offringa M. Febrile seizures. BMJ. 2015;351:h4240. [DOI] [PubMed] [Google Scholar]
- 2.Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006;35:165–172. [DOI] [PubMed] [Google Scholar]
- 4.Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein NP, Fireman B, Yih WK, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126:e1–e8. [DOI] [PubMed] [Google Scholar]
- 6.Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/vaccines/acip/index.html. Accessed June 29, 2017.
- 7.Statement Consensus. Febrile seizures: long-term management of children with fever-associated seizures. Pediatrics. 1980;66:1009–1012. [PubMed] [Google Scholar]
- 8.Guidelines for Epidemiologic Studies on Epilepsy. Commission on epidemiology and prognosis, international league against epilepsy. Epilepsia. 1993;34:592–596. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121:1281–1286. [DOI] [PubMed] [Google Scholar]
- 10.Mewasingh LD. Febrile seizures. BMJ Clin Evid. 2014;2014. [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32:5390–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukumaran L, McCarthy NL, Li R, et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): a comparison with the United States population. Vaccine. 2015;33:4446–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker MA, Lieu TA, Li L, et al. A vaccine study design selection framework for the postlicensure rapid immunization safety monitoring program. Am J Epidemiol. 2015;181:608–618. [DOI] [PubMed] [Google Scholar]
- 14.Farrington P, Pugh S, Colville A, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet. 1995;345:567–569. [DOI] [PubMed] [Google Scholar]
- 15.Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51:228–235. [PubMed] [Google Scholar]
- 16.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available at: www.OpenEpi.com. Accessed June 29, 2017.
- 17.Barlow WE, Davis RL, Glasser JW, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345:656–661. [DOI] [PubMed] [Google Scholar]
- 18.Andrews N, Stowe J, Wise L, Miller E. Post-licensure comparison of the safety profile of diphtheria/tetanus/whole cell pertussis/haemophilus influenza type b vaccine and a 5-in-1 diphtheria/tetanus/acellular pertussis/haemophilus influenza type b/polio vaccine in the United Kingdom. Vaccine. 2010;28:7215–7220. [DOI] [PubMed] [Google Scholar]
- 19.Huang WT, Gargiullo PM, Broder KR, et al. Lack of association between acellular pertussis vaccine and seizures in early childhood. Pediatrics. 2010;126:263–269. [DOI] [PubMed] [Google Scholar]
- 20.Pediarix Prescribing Information. GSK; 2016. Available at: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm. Accessed June 29, 2017.
- 21.Sun Y, Christensen J, Hviid A, et al. Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type B. JAMA. 2012;307:823–831. [DOI] [PubMed] [Google Scholar]
- 22.Verbeek NE, Jansen FE, Vermeer-de Bondt PE, et al. Etiologies for seizures around the time of vaccination. Pediatrics. 2014;134:658–666. [DOI] [PubMed] [Google Scholar]
- 23.Marini C, Mei D, Temudo T, et al. Idiopathic epilepsies with seizures precipitated by fever and SCN1A abnormalities. Epilepsia. 2007; 48:1678–1685. [DOI] [PubMed] [Google Scholar]
- 24.Shui IM, Shi P, Dutta-Linn MM, et al. Predictive value of seizure ICD-9 codes for vaccine safety research. Vaccine. 2009;27:5307–5312. [DOI] [PubMed] [Google Scholar]
- 25.Bonhoeffer J, Menkes J, Gold MS, et al. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2004;22:557–562. [DOI] [PubMed] [Google Scholar]
- 26.RotaTeq Prescribing Information. Merck; 2017. Available at: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm. Accessed 29 June 2017.