Abstract
In Drosophila melanogaster, transformer-2 (tra2) plays an essential role in the sex-specific splicing of doublesex (dsx) and fruitless (fru), two key transcription factor genes that program sexual differentiation and regulate sexual behavior. In the present study, the sequences and expression profiles of three tra2 (Aalbtra2) genes in the Asian tiger mosquito, Aedes albopictus (Ae. albopictus) were characterized. Phylogenetic analysis revealed that these paralogs resulted from two duplication events. The first occurred in the common ancestor of Culicidae, giving rise to the tra2-α and tra2-β clades that are found across divergent mosquito genera, including Aedes, Culex, and Anopheles. The second occurred within the tra2-α clade, giving rise to tra2-γ in Ae. albopictus. In addition to the conserved RNA recognition motif (RRM), arginine-rich/serine-rich regions (RS domains) and a linker region, a glycine-rich region located between the RRM and RS2 was observed in Tra2–α and Tra2–γ of Ae. albopictus that has not yet been described in the Tra2 proteins of dipteran insects. Quantitative real-time PCR detected relatively high levels of transcripts from all three tra2 paralogs in 0–2 hr embryos, suggesting maternal deposition of these transcripts. All three Aalbtra2 genes were highly expressed in the ovary, while Aalbtra2-β was also highly expressed in the testis. RNAi–mediated knockdown of any or all Aalbtra2 genes did not result in an obvious switch of the sex-specificity in dsx and fru splicing in the whole-body samples. However, knockdown of transcripts from all three tra2 genes significantly reduced the female isoform of dsx mRNA and increased the male isoform of the dsx mRNA in both the ovary and the fat body in adult females. Furthermore, knockdown of either Aalbtra2-α or Aalbtra2-γ or all three Aalbtra2 led to a decrease in ovariole number and ovary size after a blood meal. Taken together, these results indicate that two of the three tra2 genes affect female ovarian development.
Keywords: Aedes albopictus, gene duplication, sex determination, transformer-2 (tra2), doublesex (dsx), ovarian development
Graphical Abstract
1. Introduction
Sexual differentiation is controlled by an array of regulatory gene networks. These networks determine morphological, physiological, and behavioral dimorphism between the two sexes. In the fruit fly Drosophila melanogaster, initiation of the sex determination cascade is dependent upon X chromosome dosage, which determines the transcriptional activation of Sex-lethal (Sxl) (Erickson and Quintero, 2007). Sxl RNA splicing is then autoregulated to produce a transcript that codes for the full-length Sxl protein only in female embryos. The functional Sxl protein regulates the splicing of transformer (tra) pre-mRNA, creating the functional splicing factor Tra. Tra, together with the RNA-binding protein Transformer-2 (Tra2) and other splicing factors, controls the sex-specific splicing of the pre-mRNA of two transcription factor genes, doublesex (dsx) and fruitless (fru), which gives rise to female- and male-specific isoforms of dsx (dsxF and dsxM, respectively) and fru (fruF and fruM, respectively). The sex–specific isoforms of the Dsx and Fru proteins program sexual differentiation (Bell et al., 1991; Inoue et al., 1990).
Although sex–specific splicing of dsx and fru is highly conserved in insects, including mosquitoes (Biedler et al., 2016), the upstream regulators of the sex determination pathway appear to evolve rapidly. For example, Sxl has not been shown to be involved in sex determination outside Drosophilidae (Zhang et al., 2014), and the tra gene has not been found in a number of insects, including mosquitoes (Geuverink and Beukeboom, 2014; Salvemini et al., 2013). On the other hand, the tra2 gene is well conserved among insects and has been reported in a number of insects, including D. melanogaster (Belote and Baker, 1982), Drosophila virilis (Chandler et al., 1997), the housefly Musca domestica (Burghardt et al., 2005), the tephritids Ceratitis capitata (Gomulski et al., 2008; Salvemini et al., 2009), Anastrepha suspense (Schetelig et al., 2012) and Anastrepha spp. (Sarno et al., 2010), the calliphorid Lucilia cuprina (Concha and Scott, 2009), and the silkworm Bombyx mori (Niu et al., 2005). In all cases, tra2 has been found to be transcribed in both sexes throughout development. The Tra2 protein structure consists of an RNA recognition motif (RRM) flanked by two arginine-rich/serine-rich regions (RS domains) that mediate protein-protein interactions and are required for the female-specific splicing of dsx pre–mRNA (Amrein et al., 1994; Mattox et al., 1996; Sarno et al., 2010; Wu and Maniatis, 1993).
Injection of tra2 dsRNA into C. capitata (Salvemini et al., 2009) and M. domestica (Burghardt et al., 2005) embryos resulted in complete sex reversal of genotypically female individuals into fertile adult males, suggesting an essential role of tra2 in female development. Transient knock-down of either tra or tra-2 using corresponding dsRNA led to highly efficient sex reversion in the transgenic strain of A. suspensa chromosomal females with a Y–linked DsRed marker integration, indicating that tra and tra-2 are both necessary for female development (Schetelig et al., 2012). The sex reversion tends to be associated with the switch of sex-specificity of dsx or fru splicing, as shown in Tribolium castaneum (Shukla and Palli, 2013). In D. melanogaster, tra2 is necessary not only for female sexual differentiation but also for normal spermatogenesis in males (Amrein et al., 1988). Tra2 behaves as a splicing inhibitor for tra2 pre-mRNA by binding the specific intronic splicing silencer (ISS) sites of the M1 intron in the germline of Drosophila males (Mattox and Baker, 1991). In the mosquito Ae. aegypti, nix, a dominant male-determining factor on the M chromosome, acts as a primary signal in the sex determination hierarchy (Hall et al., 2015). Knockout of nix, a distant homolog of tra2 results in a shift to female splice isoforms of both dsx and fru. It has not been determined how Nix alters the sex-specific splicing of dsx and fru. We have previously isolated and characterized two tra2 homologous genes in Ae. aegypti, namely, Aaegtra2-α and Aaegtra2-β (Liu et al., 2013). Knockdown of Aaegtra2-β causes a bias in the sex ratio of the next generation due to segregation distortion acting at the level of gametic function and female-specific zygotic lethality (Hoang et al., 2016). However, it is not clear whether dsx and fru are also under the control of tra2 gene in mosquitoes. Here, we report the cloning, evolutionary analysis, and functional characterization of three tra2 genes in the Asian tiger mosquito Aedes albopictus (Aalbtra2 genes).
2. Materials and methods
2.1. Mosquitoes
The Foshan strain of Ae. albopictus was collected in Foshan, Guangdong Province, P.R. China, and established in the laboratory in 1981. All mosquitoes were maintained in humidified incubators at 25 ± 1°C on a 12 hr:12 hr light:dark cycle. Larvae were reared in pans and fed on finely ground fish food mixed 1:1 with yeast powder. Adult mosquitoes were kept in 30 cm cube cages and allowed access to a cotton wick soaked in 20% sucrose as a carbohydrate source.
2.2. Aalbtra2 cloning strategy
tBLASTn searches were performed using the D. melanogaster Tra2 (Dmeltra2–264) (Swiss-Prot: P19018.1) (Amrein et al., 1988) protein sequence as a query against the Ae. albopictus transcriptome and genome (http://www.vectorbase.org/). Furthermore, to explore the common characteristics of tra2 in Culicidae, we also performed a series of tBLASTn searches against the Anopheles stephensi and Culex quinquefasciatus genomic databases. To determine the molecular organization of Aalbtra2, Astetra2 and Cquitra2, reverse transcription PCR (RT–PCR), 3’-rapid amplification of cDNA ends (RACE) and 5’–RACE analyses were performed. The gene cloning strategies for Ae. aegypti tra2 have been described in our previous work (Liu et al., 2013).
2.3. Expression analysis of Aalbtra2
Total RNA from different developmental stages (embryo, larva, pupa, adult male and adult female) and different tissues (head, midgut, Malpighian tubule, ovary and testis) of Ae. albopictus was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by DNase treatment (Ambion, Inc., Austin, TX, USA). First-strand cDNA was synthesized using Superscript III (Invitrogen, USA) reverse transcriptase with oligo-dT primers. RT–PCR amplification of cDNA was performed using gene-specific primers. After PCR, all amplicons were analyzed by electrophoresis in agarose gels, cloned using a CloneJET PCR Cloning Kit (ThermoFisher Scientific, Waltham, MA USA) or a pGEM–T Easy Vector System (Promega, Madison, WI, USA) following the manufacturer’s protocol, and sequenced. In all cases, the mRNA of the Ae. albopictus ribosomal protein 7 (Aalb-RpS7) gene (GenBank: JN132168) was used as an internal control.
2.4. 5’ and 3’ RACE
5’ RACE and 3’ RACE were performed with gene-specific primers and a Smart RACE cDNA Amplification Kit (Clontech, Palo Alto, CA, USA) or a FirstChoice RLM–RACE Kit (Ambion, Austin, TX, USA) according to the manufacturer’s protocol. The sequences of the primers used in RT–PCR and RACE for amplification of nearly full-length cDNA sequences are shown in Supplementary Table 1. The amplified fragments were gel-eluted, subcloned into the pGEM-T Easy Vector and confirmed by sequencing (Beijing Genomics Institute, Beijing, China).
2.5. Molecular evolutionary analyses
The evolutionary analysis included 22 Tra2 sequences belonging to different insects and vertebrates with the following accession numbers:
Insecta: Diptera: Anastrepha amita (EMBL: FN658617), Anastrepha bistrigata (EMBL: FN658616), Anastrepha fraterculus sp. 1 (EMBL: FN658608), Anastrepha grandis (EMBL: FN658612), Anastrepha obliqua (EMBL: FN658607), Anastrepha serpentina (EMBL: FN658613), Anastrepha sororcula (EMBL: FN658614), Anastrepha striata (EMBL: FN658615), Ceratitis capitata (EMBL: EU999754), Bactrocera oleae (EMBL: AJ547623), Musca domestica (EMBL: AY847518), Lucilia cuprina (EMBL: FJ461620), Drosophila melanogaster (EMBL: M23633), Drosophila virilis (EMBL: XM002049663), and Drosophila pseudoobscura (EMBL: XM001360568); Lepidoptera: Bombyx mori (EMBL: NM001126233); Hymenoptera: Apis mellifera (EMBL: XM001121070); Hemiptera: Acyrthosiphon pisum (EMBL: XM_001944790.2); Coleoptera: Tribolium castaneum (EMBL: EFA10734.1).
Vertebrata: Mammalia: Homo sapiens (EMBL: U53209); Aves: Gallus gallus (EMBL: AADN02024287); and Pisces: Oryzias latipes (EMBL: AB079121).
The different domains of the Tra2 protein were defined as described by (Salvemini et al., 2009) as an RS-rich N-terminal region, an RRM, a linker region, and an RS–rich C–terminal region. The ExPASy ScanProsite tool (Gattiker et al., 2002) (http://prosite.expasy.org/scanprosite/) was used to carry out a search for RRMs. RRM and linker region amino acid sequences of Tra2 homologs were aligned using ClustalX version 1.81 (Thompson et al., 1997) with the default program parameters. A phylogenetic tree based on the amino acid sequences was constructed using the distance-based neighbor-joining (NJ) method in the MEGA 4 program (Tamura et al., 2007) with 1000 bootstrap replicates. The tree was rooted with the Tra2 proteins of the non-dipteran insects B. mori, A. mellifera, and T. castaneum.
2.6. DsRNA synthesis and adult injection
Aalbtra2-α- and Aalbtra2-β-specific primers containing the T7 promoter sequence at their 5’ ends and cDNA were used to amplify the different Aalbtra2 genes. The purified PCR products were used as templates to synthesize dsRNA using a MEGAscript T7 Kit (Ambion, USA). Green fluorescent protein (GFP) dsRNA was used as a control. The primers used to amplify the Aalbtra2-α, Aalbtra2-β and GFP templates are listed in Supplementary Table 2. A specific dsRNA was also designed to knock down Aalbtra2-γ; however, the preliminary experimental results showed that the designed dsRNA did not significantly reduce Aalbtra2-γ relative expression levels. As a result, a set of double-stranded siRNAs (designated Aalbtra2-γ siRNA) was designed to target the coding sequence of Aalbtra2-γ. A scrambled siRNA (targeting GFP) was used as a negative control. All double-stranded siRNAs were purchased from Convenience Biology (Changzhou, China), and the sequences are shown in Supplementary Table 2.
Approximately 500 nl of dsRNA and siRNA solution (10 nM) was injected laterally into the thorax of 2-day-old adult Ae. albopictus females under a microscope unless stated otherwise. In triple gene knockdown experiments, 600 ng of a 1:1:1 mixture of two kinds of dsRNA and siRNA (injection volume: 500 nl) for each targeted gene was injected into the thorax of 2-day-old adult Ae. albopictus females. The same amount of a gfp dsRNA and siRNA mix was also injected as a negative control. After injection, the adult mosquitoes were immediately transferred to small plastic cups (900 ml, 11 cm top diameter) and provided a 10% sucrose solution.
2.7. DsRNA soaking assay
First instar larvae (n=50 per group) were washed three times with Dulbecco’s phosphate-buffered saline (DPBS, ThermoFisher Scientific, USA) in a petri dish. After a final wash, the DPBS was removed, and the larvae were soaked in 350 μl of premixed dsRNA mixtures diluted with DPBS (600 ng of a 1:1:1 mix of two kinds of dsRNA and siRNA). Larvae soaked in the same amount of gfp dsRNA and siRNA were used as a negative control group. After 24 hr of soaking, the larvae were transferred back to the pans and fed regularly. After 48 hr of regular feeding, the larvae were soaked again. This process was repeated three times. Half of the larvae in each group were collected when they developed into 4th instar larvae, and total RNA and genomic DNA were isolated from each individual using a Quick-DNA/RNA™ Microprep Plus Kit (Zymo Research, Irvine, CA, USA). Sex was determined by PCR amplification of the Ae. albopictus male-determining factor Aalbnix (our unpublished data), the homolog of Ae. aegypti nix (Hall et al., 2015). The RNA of larvae of the same sex were pooled together and then used for further dsx and fru analysis.
2.8. Ovarian measurements and fat body dissection
To explore the effect of tra2 interference on ovarian development and the expression of Ae. albopictus vitellogenin (Aalbvg) mRNA and sex-specific transcripts of dsx and fru, the injected mosquitoes were mated with male mosquitoes and fed defibrinated sheep blood 48 hr post injection (p.i.). At 48 hr post blood meal (p.b.m.), the ovaries were dissected by removing the soft cuticle between the fifth and sixth abdominal sternites with a fine needle and then removing the terminal segments and placing them in a drop of suitable buffered mosquito saline (Hagedorn et al., 1977). The numbers of developed follicles were counted, and the individual follicle size was measured under a stereomicroscope (SMZ1000 with Digital Sight DS–U3, Nikon, Japan).
To explore the effect of tra2 interference on sex-specific transcripts of mosquito dsx in the fat body p.b.m., 24 hr post emergence adult females were fully coupled with males and then injected with dsRNA/siRNA in the thorax, followed by routine rearing. After 36 hr of rearing, the surviving adult females were administered a second injection. The injected mosquitoes were fed with defibrinated sheep blood at 36 hr p.i.. The fat body tissues were dissected from surviving female mosquitoes (72 hr p.i., 36 hr p.b.m.) according to a protocol described previously (Chung et al., 2017). However, only the fat body and ovaries from individuals with obvious ovarian defects (left-right asymmetry, lack of development, etc.) were collected for dsx analysis (Aalbtra2-α interference group: ovarian defects n=16, total n=51; Aalbtra2-γ interference group: ovarian defects n=18, total n=45).
2.9. RT-PCR and qRT-PCR analysis
To analyze the temporal and spatial expression patterns of Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ, total RNA was extracted from different developmental stages and different tissues of Ae. albopictus using TRIzol Reagent (Invitrogen, USA). Any residual DNA was removed using a TURBO DNA-free™ Kit (Ambion, USA). First-strand cDNA was synthesized from the total RNA using oligo-dT primers and a RevertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, USA). The Ae. albopictus rpS7 gene was used as a control (Hall et al., 2015).
To analyze the effects of Aalbtra2 downregulation on the sex-specific mRNA splicing patterns of the dsx and fru genes, the sex-specific transcripts of dsx and fru in RNAi females and males were detected by RT–PCR using specific primers designed to amplify the sex-specific regions. The amplified RT-PCR products from female and male adults were separated on 1.0% or 2.0% agarose gels, depending on the size of the product, and purified using an E.Z.N.A. Gel Extraction Kit (Omega Bio–tek, Doraville, GA, USA). These cDNA fragments were cloned into the plasmid pGEM–T Easy Vector and sequenced.
The inhibition of tra2 and the relative expression levels of the sex-specific transcripts of dsx and fru were quantified by qRT–PCR, using a SYBR Green kit (Roche, Basel, Switzerland) following the manufacturer’s instructions. To analyze the effects of Aalbtra2 downregulation on the mRNA expression of the major yolk protein precursor gene Aalbvg during vitellogenesis, ovaries were dissected from the mosquitoes in the Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ triple interference groups, larvae soaking groups and control groups. At 48 hr p.b.m., total RNA was extracted from batches of 30 females from the Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ interference groups and control groups using TRIzol (Molecular Research Center, Inc., OH, USA) according to the manufacturer’s directions. Three Ae. aegypti vitellogenin orthologs were analyzed. AalbvgA, AalbvgB, and AalbvgC (AALF008766, AALF019930, and AALF008379, respectively [VectorBase]) shared 80.16% nucleotide identity, and primers were designed to match highly conserved regions of the Aalbvg mRNA sequences. To detect the transcript levels of vg in ovary samples from Aalbtra2-α- or Aalbtra2-γ-knockdown adults, only individuals with obvious ovarian defects, as described above, were selected for subsequent quantitative analysis. The transcript levels of Vg mRNA were measured using SYBR dye technology (Applied Biosystems, Foster, CA, USA).
For each treatment described above, three independent biological replicates were analyzed. Ae. albopictus rpS7 was used as an endogenous reference gene for the normalization of the expression data. All quantification was performed using an Applied Biosystems™ 7500 Real-Time PCR System (Applied Biosystems, CA, USA). Gene expression levels were analyzed by the 2−ΔΔCt method (Livak and Schmittgen, 2001). All of the primers used in this study are listed in Supplementary Table 3.
2.10. Statistical Analysis
All experiments were carried out with three biological replicates. Differences in the mean and standard error of the mean (SEM) among the groups in the RNAi study were analyzed by analysis of variance (ANOVA; GraphPad Prism Version 6.01). For one-to-one comparisons, the mean and SEM of the dsx, fru, and vg relative expression levels, and the ovarian ovariole counts were analyzed with GraphPad Prism Version 6.01, and differences between means were analyzed with Student’s t test. The average ovarian follicle size was analyzed by ordinary one-way ANOVA, and the hatching of larvae was compared using Chi–square with Yates’ correction. No statistical methods were used to predetermine sample size. The experiments were not randomized, and the investigators were not blinded to allocation during the experiments and outcome assessment. Statistical significance was assigned when P values were <0.05 using GraphPad Prism Version 6.01 and IBM SPSS Statistics 20.0.
3. Results
3.1. Isolation and molecular characterization of Aalbtra2
A tBLASTn search of the Ae. albopictus genome and transcripts using D. melanogaster Tra2 as a query was performed. Four draft sequences with e-values less than 1e−10, AALF015404, AALF008878-RA, AALF008877-RA and AALF004560-RA, were identified, which were located on scaffolds JXUM01S000397, JXUM01S001946, JXUM01S001946, and JXUM01S001377, respectively.
Molecular cloning of the three Aalbtra2 genes was performed by combining traditional PCR amplification with 3’ and 5’ RACE. The three paralogs of tra2 (Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ corresponding to AALF008877–RA, AALF015404–RA and AALF008878-RA, respectively) with different supercontig locations were cloned and characterized, and their sequences were deposited in GenBank. The sequences included Aalbtra2-α (GenBank: MF682534), Aalbtra2-β (GenBank: MF682535), Aalbtra2-γ.1 (GenBank: MF682536), and Aalbtra2-γ.2 (GenBank: MF682537). The two splice isoforms Aalbtra2-γ.1 and Aalbtra2-γ.2, were cloned from Aalbtra2-γ. However, we failed to clone the full-length cDNA of AALF004560-RA (located on JXUM01S001377) using RT–PCR and RACE.
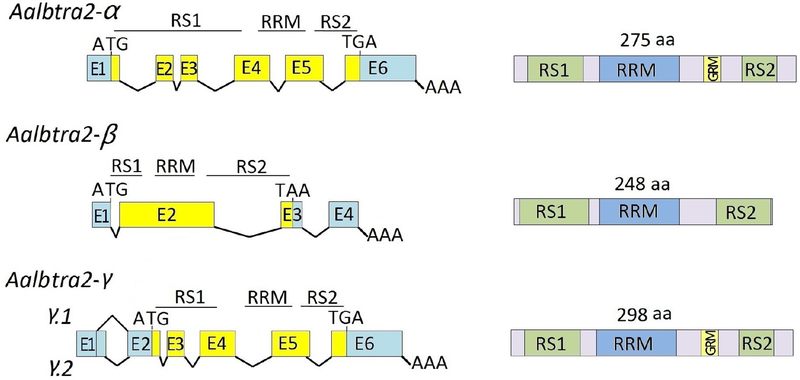
Alignment of the Aalbtra2 cDNA sequences with the corresponding genomic sequence was performed to determine the exon/intron organization and the alternative splicing events (Figure 1). Figure 1 shows the molecular organization of the Aalbtra2 RNA molecules. Aalbtra2-α spans a 2933 bp genomic region located in supercontig JXUM01S001946 with 6 exons and an ORF encoding 275 amino acids. The transcription start site is located at 147 bp upstream of the initiation codon AUG of the ORF. Aalbtra2-β was found in supercontig JXUM01S000397 and consisted of four exons and three introns; the ORF encodes a protein of 248 amino acids (Figure 1). Aalbtra2-γ consists of 6 exons distributed over a 1.2 kb region on supercontig JXUM01S001946 and contains an 897 bp ORF encoding a protein of 298 amino acids. The Aalbtra2-γ.1 isoform differs from Aalbtra2-γ.2 at the splice site used at the 3’ end of noncoding exon 1.
Figure 1. Molecular organization of the three tra2 genes of Aedes albopictus.
The exons (boxes) and introns (dashed lines) are not drawn to scale. E1–E6 are the exon numbers. The start (ATG) and stop (TGA/TAA) codons and the poly (A) addition sites (AAA) are marked. In the left panel, the 5’– and 3’–untranslated regions (UTRs) are shown in light blue, and the protein-coding regions are shown in yellow. The arginine and serine-rich motifs (RS1 and RS2) and the RNA recognition motif (RRM) are indicated, and the glycine-rich motif (GRM) is also indicated in the schematics on the right showing the three Tra2 protein sequences.
3.2. Comparison of the molecular organization and protein sequences of Culicidae tra2 genes with those of other species
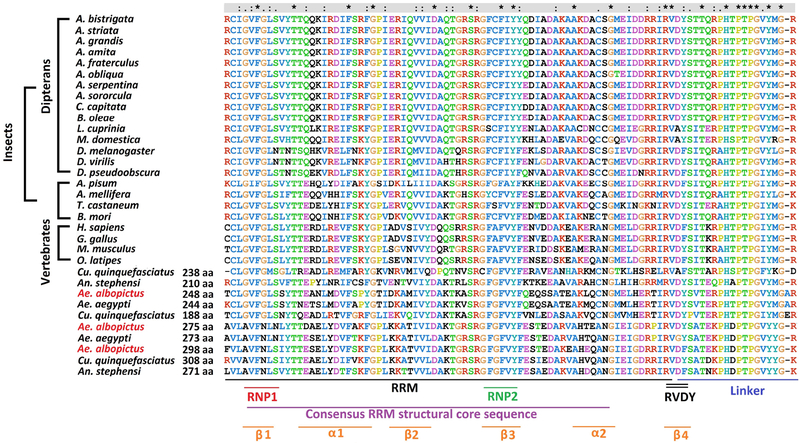
The deduced protein products of all three Aalbtra2 genes are aligned to Tra2 proteins from a few species of mosquitoes (Supplemental Figure 1), other insects and a few representative vertebrate species (Figure 2). The three Ae. albopictus Tra2 proteins contained an RRM flanked by two RS domains (RS1 and RS2), which are characteristics of SR splicing factors. Comparison of these RRMs and linker regions with those of other dipteran insects and vertebrates revealed that the regions are conserved (74 and 19 amino acids, respectively) in all examined species (Figure 2). The highest degree of similarity was observed for the last 7 amino acids of the linker region. In fact, the RRM-linker junction region is not similar to a region in any other known protein and is more likely than other regions to perform conserved functions that are specific to Tra2 (Dauwalder et al., 1996). A conserved RVDY motif was also found downstream of the RRM (Figure 2) (Dauwalder et al., 1996). RVDY (invertebrates) or RVDF (vertebrates) is a conserved motif in nearly all Tra2 sequences that is implicated in protein phosphatase 1 (PP1) binding and is involved in the dephosphorylation of splicing factors (Meiselbach et al., 2006; Novoyatleva et al., 2008; Stamm, 2008). In addition to the motifs previously described in Tra2, a glycine–rich motif (GRM) proposed to be involved in protein-protein interactions (Bocca et al., 2005) was identified in a subgroup (see below) of the mosquito Tra2 proteins. This GRM, which is also found in vertebrate Tra2 (Rodriguez et al., 2016), is absent in the known Tra2 proteins of other dipteran insects.
Figure 2. Amino acid sequence alignment of the RNA recognition motif (RRM) and linker region of the Tra2 homologs.
The asterisks (*) indicate amino acids that are identical in all species. A consensus RRM structural core sequence, putative α-helix or β–sheet regions, and a conserved RVDY motif are all indicated at the bottom of the alignment. Also indicated within the RRM are the positions of two ribonucleoprotein identifier sequences, RNP–1 and RNP–2.
3.3. Phylogeny and molecular evolution of the Tra2 proteins
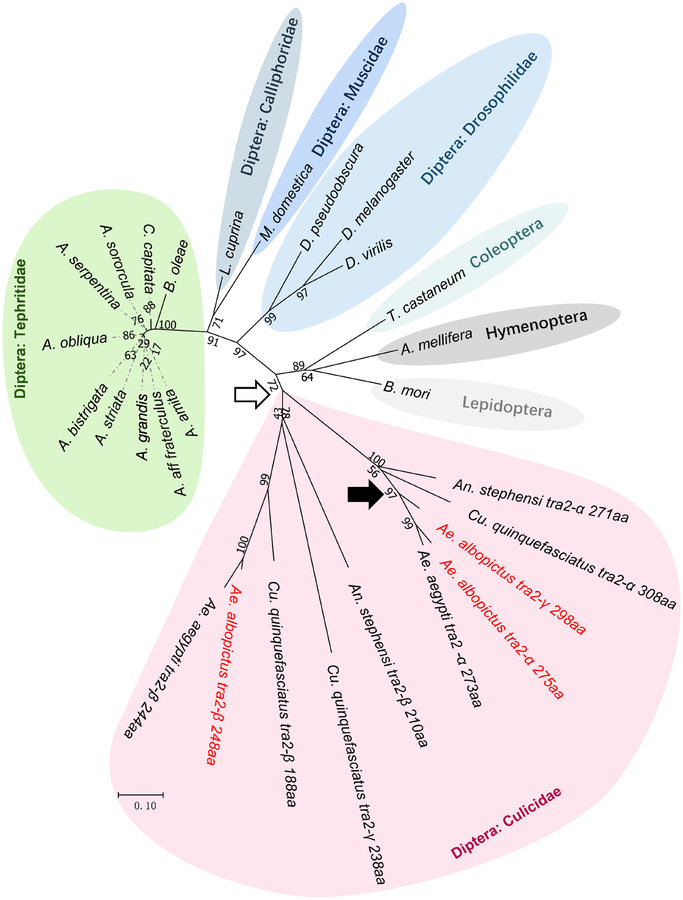
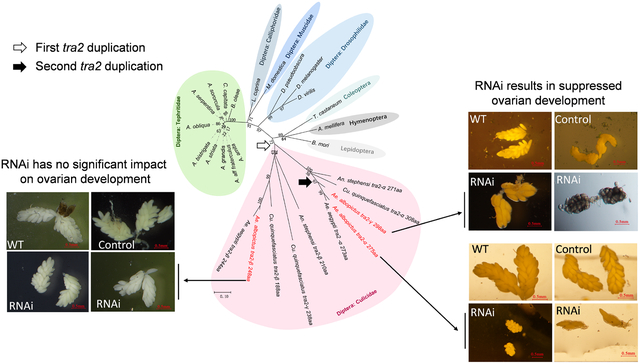
For phylogenetic analysis, the Tra2 proteins encoded by the three Ae. albopictus tra2 genes were aligned with homologous sequences from other species in the Culicidae family, representatives from other dipteran families and a few non-dipteran insects. We used the Tra2 sequences from T. castaneum (Coleoptera), A. mellifera (Hymenoptera) and B. mori (Lepidoptera) as outgroups to root the tree. The topology of the Tra2 protein phylogenetic tree (Figure 3) showed that all mosquito Tra2 sequences originated from the same common ancestor, forming a monophyletic group with 72% bootstrap support. Further groupings suggested that the three Ae. albopictus paralogs resulted from two duplication events. The first occurred in the common ancestor of Culicidae, giving rise to the tra2-α and tra2-β clades that are found across divergent mosquito genera, including Aedes, Culex, and Anopheles. The second duplication occurred within the tra2-α clade, giving rise to tra2-γ in Ae. albopictus. The most parsimonious explanation is that this second duplication occurred prior to the divergence between Ae. albopictus and Ae. aegypti and that tra2-γ was lost in the Ae. aegypti lineage. However, further analysis that includes all Tra2 sequences from additional Aedes species is needed to rule out the alternative hypothesis that the duplication occurred after the divergence between Ae. albopictus and Ae. aegypti and that Aalbtra2-γ underwent rapid evolutionary change after the duplication. A second duplication also occurred in Culex quinquefasciatus, giving rise to Cquitra2-γ. However, Cquitra2-γ and Aalbtra2-γ are not orthologs because Cquitra2-γ is a duplication within the tra2-β clade. Interestingly, the proteins in the mosquito tra2-α clade, including Aalbtra2-γ, contain a GRM that is not found in the tra2-β clade, and the proteins in the tra2-α clade show higher degrees of conservation, as indicated by shorter branch lengths, than the proteins in the tra2-β clade.
Figure 3. Phylogeny of the Transformer-2 (Tra2) protein in mosquitoes and other insects.
Bootstrap values (up to 100%) from 1000 replicate analyses are shown. The scale bar at the bottom indicates 0.10 differences per residue. The taxonomic relationships are indicated right next to the cluster of the tree. The tree was rooted with Tra2 sequences from Tribolium castaneum (Coleoptera), Apis mellifera (Hymenoptera), and Bombyx mori (Lepidoptera). The two duplication events that gave rise to the three Aalbtra2 genes are indicated by an open and a solid arrow, respectively.
3.4. Expression patterns of the three Aalbtra2 genes
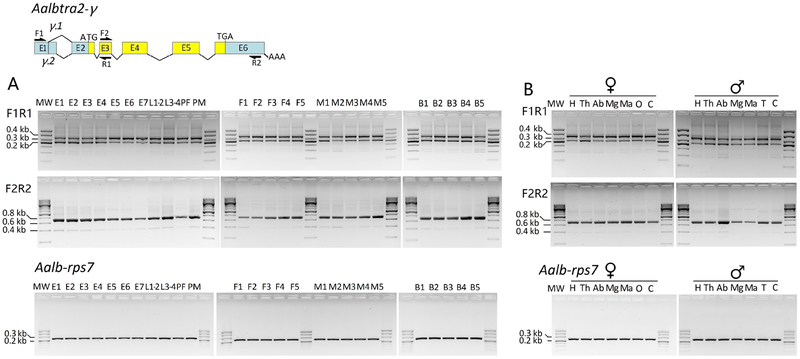
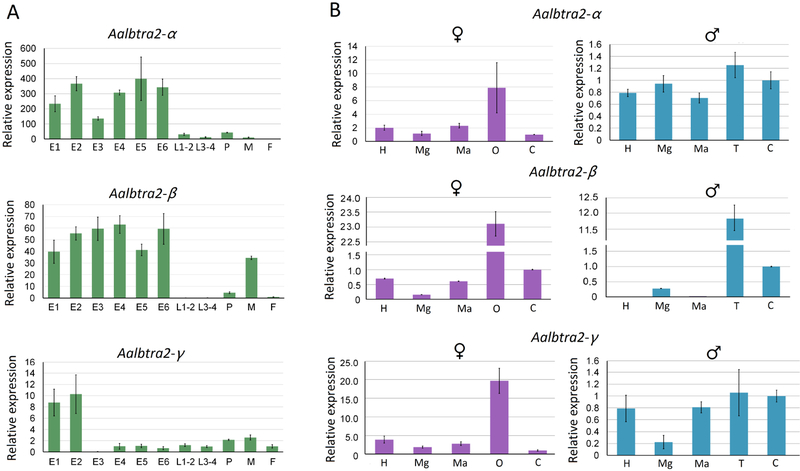
To analyze the temporal and spatial expression patterns of the three Aalbtra2 genes, both RT–PCR and qRT–PCR analyses were performed on total RNA extracted from various stages from embryo to adult using a series of gene-specific primers. Only Aalbtra2-γ produced splice isoforms, Aalbtra2-γ.1 and Aalbtra2-γ.2 (Figure 4A). Both Aalbtra2-α and Aalbtra2-β showed only one transcript (Supplementary Figure 2A). All PCR fragments were cloned and sequenced to confirm the expected sequences. To determine the transcription profiles of the three Aalbtra2 genes, a qPCR assay was used with gene-specific primers. The results showed that the genes Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ were expressed throughout development and during adulthood in both sexes. All three genes showed the highest relative transcript levels at the embryo stage, with Aalbtra2-α and Aalbtra2-β showing high levels throughout embryonic development, whereas Aalbtra2-γ showed a drop in expression after 2–4 hr (Figure 5A). The spatial expression pattern of tra2 in Ae. albopictus was also studied by performing RT–PCR and qPCR on total RNA separately extracted from heads, midguts, Malpighian tubules, ovaries, testes and carcasses. The two Aalbtra2-γ alternative splice products were detected in all tissues (Figure 4B), whereas no additional alternative splicing isoforms were found for either Aalbtra2-α or Aalbtra2-β (Supplementary Figure 2B). Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ were expressed in most tissues in both sexes. Interestingly, Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ all showed enriched expression in the ovary or testis (Figure 5B).
Figure 4. Developmental (A) and spatial (B) transcription patterns of the Aalbtra2-γ gene.
Overlapping sets of intron-spanning primers were used for RT–PCR to identify the splicing patterns of Aalbtra2-γ mRNA. The arrows denote the locations of the primers used. The F1/R1 primer combination amplified two slightly different cDNA fragments in all developmental samples of Aalbtra2-γ, corresponding to the two alternatively spliced isoforms of exon 1. Aedes albopictus ribosomal protein S7 gene (Aalb-Rps7) was used as an internal control. The analyses were performed on the following samples: E1 = 0–2 hr embryos; E2 = 2–4 hr embryos; E3 = 4–8 hr embryos; E4 = 8–12 hr embryos; E5 = 12–24 hr embryos; E6 = 24–36 hr embryos; E7 = 36–48 hr embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd-4th instar larvae; PF= female pupae; PM= male pupae; F1=0–6 hr emerged female adults; F2=6–12 hr emerged female adults; F3=12–24 hr emerged female adults; F4=24–48 hr emerged female adults; F5=48–72 hr emerged female adults; M1= 0–6 hr emerged male adults; M2= 6–12 hr emerged male adults; M3= 12–24 hr emerged male adults; M4= 24–48 hr emerged male adults; M5= 48–72 hr emerged male adults; B1= 0–6 hr post blood meal (p.b.m.) female adults; B2= 6–12 hr p.b.m. female adults; B3= 12–24 hr p.b.m. female adults; B4= 24–48 hr p.b.m. female adults; B5= 48–72 hr p.b.m. female adults; B4= 24–48 hr p.b.m. female adults; B5= 48–72 hr p.b.m. female adults; H=head; Th = thorax; Ab=abdomen; Mg = midgut; Ma= Malpighian tubule; O=ovary; T=testis; C= carcass; ♀= adult female; ♂=adult male. * The faint band appearing under the main product with the F2/R2 primer sets was a nonspecific amplicon.
Figure 5. Temporal (A) and spatial (B) transcription profiles of the three Aalbtra2 genes as measured by real-time RT-PCR.
(A) Temporal profiles at different developmental stages of Aedes albopictus. E1 = 0–2 hr embryos; E2 = 2–4 hr embryos; E3 = 4–8 hr embryos; E4 = 8–12 hr embryos; E5= 12–24 hr embryos; E6= 24–48 hr embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd–4th instar larvae; P= pupae; M=male; F=female. (B) Spatial transcription profiles in different tissues of Ae. albopictus adult females and males. H=head; Mg = midgut; Ma= Malpighian tubule; O=ovary; T=testis; C= remaining carcass; ♀= adult female; ♂=adult male. The x-axis indicates the sample ID, and the y-axis shows the relative expression value obtained by qPCR. The internal reference gene was Ae. albopictus ribosomal protein 7 (Aalb-RpS7). The error bars represent the SEM.
3.5. Effect of tra2 downregulation on dsx and fru splicing in whole-body samples
In mosquitoes, Aaegdsx (Salvemini et al., 2011; Scali et al., 2005) and Aaegfru transcripts (Salvemini et al., 2013) exhibit sex-specific RNA splicing patterns. In Ae. albopictus, the sex-specific mRNA isoforms of dsx and fru have been identified (our unpublished work), and the full-length coding sequences (CDSs) have been deposited in GenBank under the accession numbers MF682531, MF682532, MF682533, and MG516586, MG516587, and MG516588. The molecular organization of Aalbdsx and Aalbfru is shown in Figure 6A, and the sex-specific splicing patterns are similar to those of orthologous genes in Ae. aegypti (Salvemini et al., 2013; Salvemini et al., 2011).
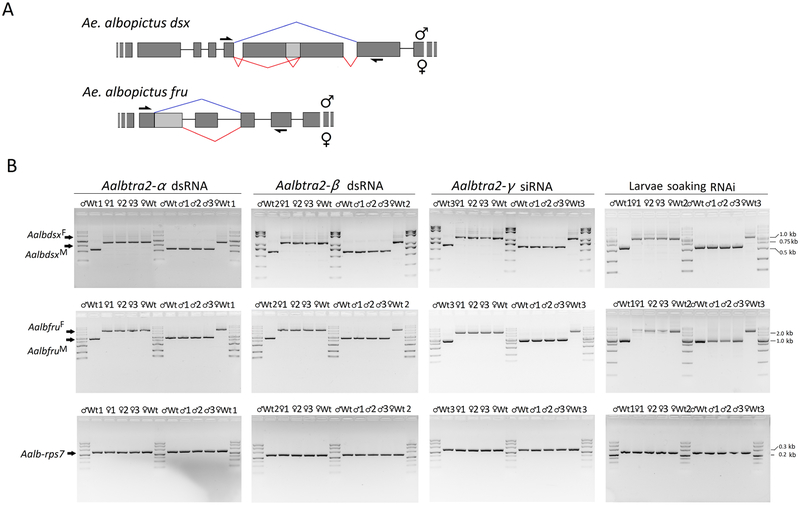
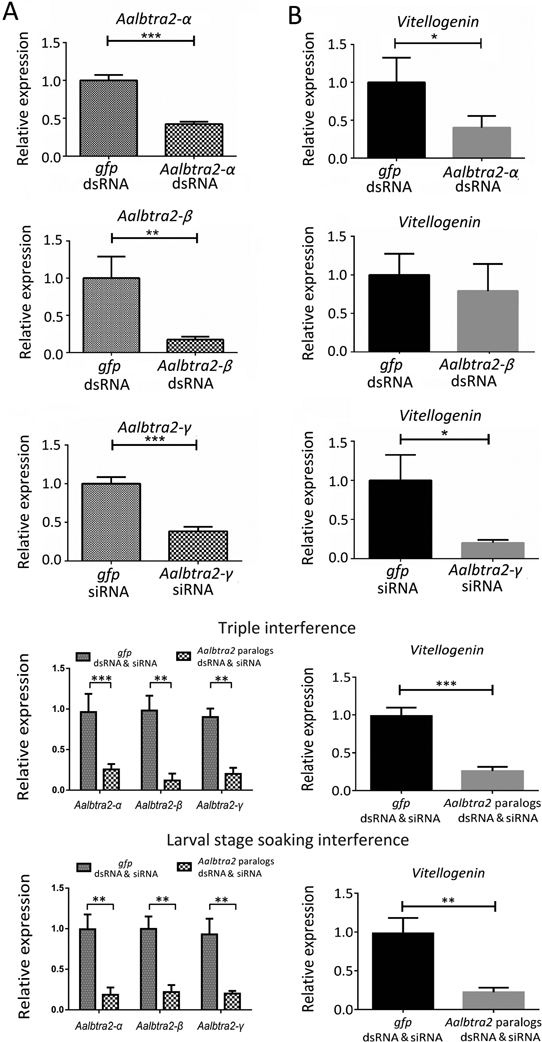
Figure 6. Sex-specific splicing patterns of the dsx and fru transcripts in Aedes albopictus adults after individual or triple RNAi knockdown of Aalbtra2-α, Aalbtra2-β, and Aalbtra2-γ.
(A) Genomic organization and splice variants of dsx and fru in Ae. albopictus. The male-specific and female-specific splicing patterns are marked in blue and red, respectively. The exons and introns are not drawn to scale (see Supplementary Table 3 for further details). The arrows denote the locations of the primers used to amplify the cDNA products of dsx and fru. The primer sets encompassed the alternative splicing junctions and thus produced sex-specific RT–PCR products. (B) RT–PCR products obtained using mRNA from individuals 48 hr after injection of siRNAs/dsRNA targeting one of the three tra2 genes (columns 1–3) or from adult individuals in which all Aalbtra2 genes had been knocked down since the larval stage through soaking with Aalbtra2-α and Aalbtra2-β dsRNA and Aalbtra2-γ siRNA mixtures (column 4).♂1–3 and ♀1–3 refer to the three replicate groups (n=15 adults per replicate) of tra2 RNAi males and females, respectively. Wt♂1–3 and Wt♀1–3 indicate the three replicate groups (n=15 per replicate) of wild-type males and females. Fragments corresponding to the fru female (AalbfruF; 2010 bp) and male (AalbfruM; 987 bp) cDNA sequences and the dsx female (AalbdsxF; 1062 bp) and male (AalbdsxM; 620 bp) cDNA sequences were resolved by electrophoresis on a 1.5% agarose gel that was stained with ethidium bromide and photographed. The numbers with short lines on the right side indicate the molecular weights. Ae. albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control.
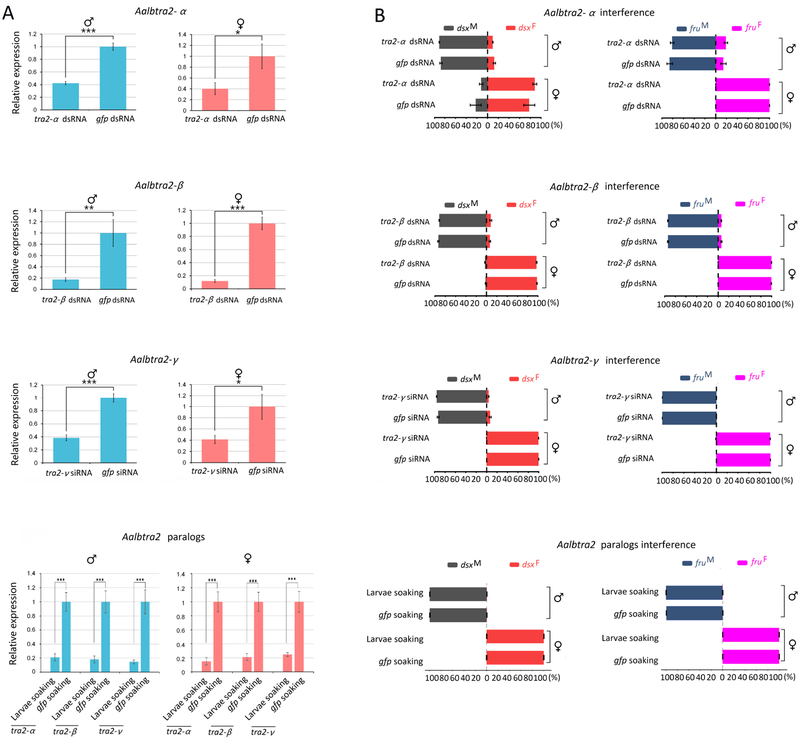
Having established the sex-specific alternative splicing patterns of dsx and fru mRNA, we tested whether silencing of any of the three tra2 genes in adult Ae. albopictus by RNAi would affect the transcription levels or splicing patterns of dsx and fru. RT–PCR was used to display the splicing patterns of the Aalbdsx and Aalbfru genes with the primers dsxF/dsxR and fruF/fruR (Figure 6A), which were designed to produce sex-specific amplicons from the Aalbdsx and Aalbfru transcripts, respectively. In untreated males or females, the expected male- or female-specific dsx and fru cDNA products were detectable. However, changes in the splicing patterns of Aalbdsx and Aalbfru were not observed in either sex after silencing of any of the three tra2 genes in Ae. albopictus (Figure 6B). Successful knockdown of these genes was confirmed by qRT–PCR with GFP RNAi controls (Figure 7A). Real-time PCR was used to detect possible changes in the expression levels of sex-specific isoforms of either dsx or fru using gene-specific primers (Supplementary Table 3) after knockdown of any of the three genes. Moreover, the percentages of AalbdsxF, AalbdsxM, AalbfruF and AalbfruM transcripts among all dsx and fru transcripts after Aalbtra2 RNAi were determined. The results showed that there were no significant changes in the percentages of sex-specific isoforms among total dsx and fru transcripts in either males or females after knockdown of any of the three tra2 genes or all tra2 paralogs simultaneously at the earlier larval stage in development (Figure 7B row 4). Furthermore, no significant changes in the transcription levels of AalbdsxF, AalbdsxM, AalbfruF and AalbfruM were detected (Supplementary Figure 3).
Figure 7. Expression ratios of male- and female-specific transcripts of dsx and fru in Aedes albopictus adults subjected to Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ interference in adulthood or as larvae through soaking.
(A) Analysis of the effects of Aalbtra2 interference in Ae. albopictus adults by qRT–PCR. The bars represent the SEM (n = 3). The x-axis indicates the groups. (B) Expression percentages of male- and female-specific dsx and fru transcripts in groups with Aalbtra2 interference compared to control groups. The y–axis indicates the sample ID, and the x–axis shows the relative percentage of male- or female-specific transcripts among total dsx and fru transcripts based on the relative quantitation (RQ) value obtained by qRT–PCR. The error bars represent the SEM. Ae. albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control (*P < 0.05, **P < 0.01, and ***P < 0.001).
3.6. Knockdown of Aalbtra2-α and Aalbtra2-γ suppresses ovarian development
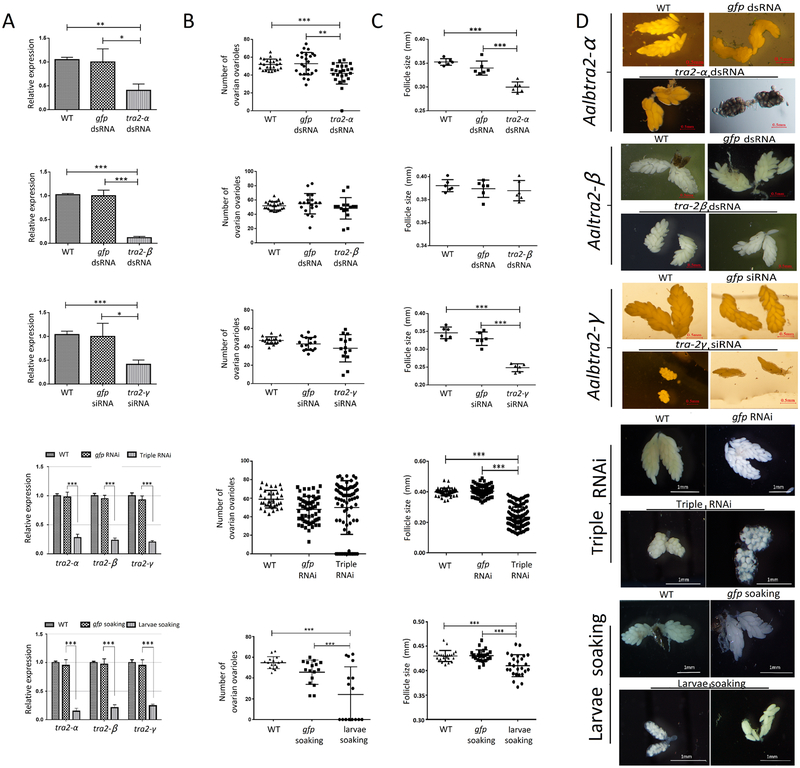
We also investigated the effect of RNAi knockdown of the three tra2 genes on ovarian development. Forty-eight hours after injection of interfering RNAs targeting one of the three tra2 genes, mosquitoes were fed defibrinated sheep blood, and only freshly blood-fed female mosquitoes with obviously engorged, bright red abdomens were selected for subsequent analysis. Mosquitoes in each group were dissected 36 hr p.b.m., and the knockdown efficiency was evaluated by using qRT–PCR with gfp RNAi as the control. The average follicle size (length of the long axis of follicle) was measured, and the ovarioles were also counted for each individual. The results showed that relative levels of Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ mRNA, normalized using the reference gene Aalb-Rps7, were successfully depleted after injection to 40.44 ± 7.741% (P<0.05), 11.90 ± 1.609% (P<0.001) and 41.40 ± 5.205% (P<0.05), respectively, compared to the control groups. Furthermore, Aalbtra2-α downregulation significant decreased (P<0.01) the number of ovarioles in each individual (41.88 ± 2.434, n=24) compared with the control treatment (52.79 ± 2.563, n=24). Although Aalbtra2-γ RNAi did not significantly decrease ovariole numbers, the average follicle size was clearly reduced (P<0.05) (Figure 8A, B, C). Aalbtra2-α and Aalbtra2-γ interference also led to a significant reduction in the average ovarian follicle size compared to the control treatment, and some individuals showed marked left-right asymmetry and growth arrest and even a lack of ovarian development (Figure 8D). There were no statistically significant differences between the control and Aalbtra2-β-downregulated groups with regards to the ovariole number or average follicle size.
Figure 8. Effect of Aalbtra2 knockdown on ovarian development in adult female Aedes albopictus.
(A) Relative expression of Aalbtra2 in dsRNA or siRNA treatment groups (row 1–3), dsRNA and siRNA injection groups (row 4) or soaking knockdown groups (row 5) compared with GFP control groups. The error bars represent the SEM (n = 3). The x–axis indicates the groups; WT indicates wild-type mosquitoes without any treatment. The expression of each Aalbtra2 gene in the GFP control group was set as 1 and used to normalize the Aalbtra2 expression in the RNAi groups (*P < 0.05, **P < 0.01, and ***P < 0.001). (B) Number of ovarian ovarioles per female in the different treatment groups (single dsRNA or siRNA, triple interference and larvae soaking interference groups) compared with the GFP control groups. (C) Average follicle size (length of the long axis) in ovaries isolated from female mosquitoes in the Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ treatment groups (single dsRNA or siRNA, triple interference and larvae soaking interference groups) and control groups. The data represent six biological replicates with 8–10 individuals in each replicate and are shown as the mean ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001). (D) Representative images of ovaries dissected from WT mosquitos and mosquitoes from the Aalbtra2-α, Aalbtra2-β and Aalbtra2-γ treatment groups (single dsRNA or siRNA, triple interference and larvae soaking interference groups) and GFP control groups at 36 hr post blood meal (p.b.m.). The images were taken under a Nikon SMZ1000 stereomicroscope with Digital Sight DS–U3 (scale bar: marked in the picture).
Vitellogenin (Vg) is a large phospholipoglycoprotein that is the precursor of major yolk proteins in oviparous vertebrates and invertebrates, including mosquitoes (Robinson, 2008). Vg is synthesized in the fat body, secreted into the hemolymph, and transported to the ovary. Vitellogenin transcription increases rapidly after a blood meal (Clements and Clements, 1992). Ae. albopictus vg (Aalbvg) displayed the expected expression pattern and reached maximum expression levels at 24 hr p.b.m. (Supplementary Figure 4). To explore whether the repressive effects of Aalbtra2 downregulation on ovarian development may be associated with the downregulation of vg expression, the relative transcript levels of Aalbvg in the Aalbtra2 interference groups were compared to those in the control groups at 36 hr p.b.m., and the silencing efficiency of Aalbtra2 was also analyzed by qRT–PCR. The results showed that Aalbvg mRNA transcript levels were significantly lower in the Aalbtra2-α and Aalbtra2-γ knockdown groups (down to 40.31 ± 8.873% and 20.19 ± 2.203%, respectively; P<0.05) than in the control groups. No significant changes in Aalbvg expression were observed after Aalbtra2-β downregulation (Figure 9A, B row 1, 2, 3).
Figure 9. Effect of Aalbtra2 knockdown on vitellogenin gene transcription in Aedes albopictus post blood meal (p.b.m.).
(A) Relative expression of Aalbtra2 in various treatment groups (dsRNA or siRNA treatment, row 1–3; triple interference, row 4; and larvae soaking interference, row 5) compared with GFP control groups. The x–axis indicates the groups. (B) The relative expression of vitellogenin was quantified in female mosquitos from Aalbtra2-α, Aalbtra2-β, and Aalbtra2-γ treatment groups (dsRNA or siRNA treatment, row 1–3; triple interference, row 4; and larvae soaking interference, row 5) and control groups at 36 hr p.b.m.. The data are presented as the mean ± SEM (n=3; *P < 0.05, **P < 0.01, ***P < 0.001).
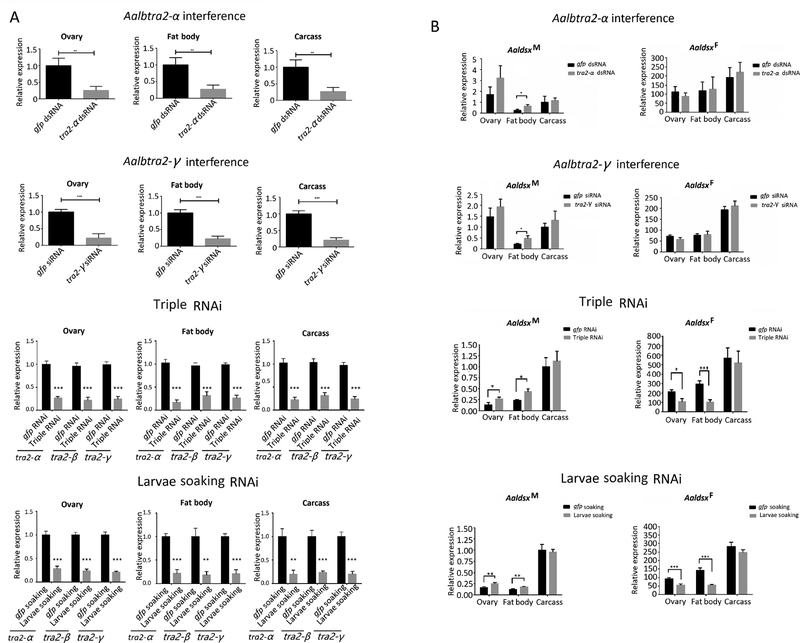
3.7. Effects of Aalbtra2 interference on dsx transcription and splicing patterns in the fat body and ovary
Transcription of the vg gene is under the control of the Dsx protein in T. castaneum (Shukla and Palli, 2012), and the yolk protein genes are also directly regulated by the Dsx protein in D. melanogaster (Burtis et al., 1991; Coschigano and Wensink, 1993). Although we did not detect any changes in the sex-specific splicing patterns or transcript levels of dsx in whole-body RT–PCR and qRT–PCR analyses (Figures 6 and 7 and Supplementary Figure 3), it is possible that slight changes in dsx splicing or transcript levels may have occurred in the fat body due to tra2 knockdown. Changes in tra2 in the fat body, which could be masked in whole-body RT–PCR or qRT–PCR assays, could have a profound effect on ovarian development, as the fat body is the source of Vg synthesis. Therefore, we analyzed the sex-specific splicing patterns of dsx mRNA in adults in which Aalbtra2-α or Aalbtra2-γ was silenced by RNAi using fat body and ovary samples dissected from females 72 hr p.b.m.. Only individuals with obvious ovarian defects upon dissection (for Aalbtra2-α: n=16, total =51; for Aalbtra2-γ: n=18, total=45) were selected for subsequent quantitative analysis. The Aalbtra2-α and Aalbtra2-γ mRNA levels in the ovary, fat body and carcass were lower in the RNAi groups than in the control group (Figure 10A row 1, 2). For all three tissue types, no significant differences were observed in the relative transcript levels of AalbdsxF in either the Aalbtra2-α or Aalbtra2-γ knockdown groups compared with the control group (Figure 10B row 1, 2). However, AalbdsxM transcript levels in the fat body were significantly higher in both knockdown groups than in the control group (Figure 10B, row 1, 2). Notably, the transcript levels of AalbdsxM were two orders of magnitude lower than those of AalbdsxF in the fat body of adult females. Therefore, it is unclear whether the statistically significant change in AalbdsxM expression in the fat body could induce changes in ovarian development. However, knockdown of transcripts of all three tra2 genes either by adult injections or larvae soaking significantly reduced the female isoform of dsx mRNA and increased the male isoform of the dsx mRNA in both the ovary and the fat body in adult females (Figure 10B, row 3 and row 4).
Figure 10. Effect of Aalbtra2-α or Aalbtra2-γ knockdown, triple interference and larvae soaking interference on the levels of sex-specific dsx transcripts in the ovary, fat body, and carcass.
For analysis of the sex-specific splicing patterns of dsx mRNA in adults subjected to Aalbtra2-α or Aalbtra2-γ interference or triple interference by RNAi, fat body and ovary samples were dissected from females at 72 hr post injection (p.i.) and 36 hr post blood meal (p.b.m.). For analysis of the sex-specific splicing patterns of dsx mRNA in adults that had been subjected to larvae soaking interference, fat body and ovary samples were dissected from females 36 hr p.b.m.. (A) Relative expression of Aalbtra2 in dsRNA or siRNA treatment groups (row 1–2), triple interference groups (row 3) and larvae soaking interference groups (row 4) (gray) compared with GFP control groups(black). The error bars represent the SEM (n = 3). The x–axis in row 1–2 indicates the injected dsRNA or siRNA in the treatment and control groups, and the x-axis in row 3–4 indicates the gene. The expression of each Aalbtra2 gene in the GFP control group was set as 1 and used to normalize Aalbtra2 gene expression in the RNAi groups. (B) AalbdsxM and AalbdsxF levels in the ovary, fat body, and carcass were quantified with gene-specific primers in the Aalbtra2-α or Aalbtra2-γ interference groups, triple interference groups and larvae soaking interference groups compared with the control group, respectively. Ae. albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal reference. The data are presented as the mean ± SEM (n = 3; *P < 0.05, **P < 0.01, and ***P < 0.001).
4. Discussion
In this study, we isolated and characterized the three tra2 genes of Ae. albopictus. Although the Tra2 proteins were overall conserved between mosquitoes and other dipteran insects, including D. melanogaster, the mosquito tra2 genes showed some unique features. First, for all mosquito species tested, the tra2 gene has exhibited multiple paralogs, while in other dipterans tra2 has been detected as a single-copy gene. Extensive phylogenetic analysis of Tra2 proteins from divergent species in the Culicidae family and a number of other insects showed that a gene duplication event in the common ancestor of Culicidae gave rise to two tra2 clades, tra2-α and tra2-β, that were found across divergent mosquito genera including Aedes, Culex, and Anopheles. A later duplication gave rise to a third tra2 gene in some mosquitoes, including Ae. albopictus. Only a single tra2 mRNA has been isolated from these non-mosquito dipteran insects, with the exception of D. melanogaster, which produces three tra2 isoforms through alternative splicing and with multiple promoters (Amrein et al., 1990; Mattox et al., 1990). In Drosophila, the tra2 gene also behaves as a splicing inhibitor of the M1 intron in tra2 pre-mRNA to form the male-specific tra2 transcript in the germline of Drosophila males. However, no sex-specific tra2 transcripts were identified in Ae. albopictus.
Like other Tra2 proteins, mosquito Tra2 contains an RRM and a conserved linker region adjacent to the RRM (Figure 1 and 2). There are also two RS domains flanking the RRM. Interestingly a GRM that has been found in human Tra2α (hTra2α; GenBank: AAC50658) (Sievert et al., 1997) was identified between the RRM and RS2 in the mosquito Tra2 proteins in the Tra2–α clade but was missing in the mosquito Tra2 proteins in the Tra2–β clade. The GRM has not been found in any non-mosquito dipteran Tra2. It is thought that GRM is required for multiple mechanisms of splicing regulation (Wang and Burge, 2008), which may suggest that the mosquito Tra2 proteins in the two clades have acquired different functions. In this regard, it is interesting to note that the proteins in the mosquito tra2-α clade, including Aalbtra2-α and Aalbtra2-γ, showed higher degrees of conservation than the protein sequences in the tra2-β clade. This difference may indicate a different level of evolutionary constraint or even neofunctionalization after gene duplication.
The three Aalbtra2 genes showed different levels of transcription at different developmental stages (Figure 5). All three genes were transcribed in both sexes, with relatively high levels in the embryonic stages and in the reproductive organs of adults. The results from a previous study demonstrated that knockdown of tra2 in embryos of B. mori (Suzuki et al., 2012) or A. mellifera (Nissen et al., 2012) can cause abnormal testis formation in some larvae, even leading to embryonic lethality, which suggests that tra2 has vital functions during early embryonic stages.
As a component of the Tra/Tra2 complex, the Tra2 protein has been shown to play an important role in the sex-specific splicing of dsx in several species in Diptera (Concha and Scott, 2009; Peng et al., 2015; Schetelig et al., 2012). In this study, RNAi-mediated knockdown of any of the three Aalbtra2 genes in adult mosquitoes failed to produce any detectable sex-specific splicing changes in dsx and fru in whole body assays. Similar results were observed even in mosquitoes in which all Aalbtra2 genes had been knocked down since the larval stage (Figure 7 row 4). Notably, knockdown of the tra2 gene in B. mori also has no effect on the sex-specific splicing patterns of dsx pre-mRNA (Suzuki et al., 2012). However, knockdown of transcripts of all three tra2 genes significantly reduced the level of the dsxF mRNA and increased the level of the dsxM mRNA in both ovary and fat body in adult females. In these cases, the reduction of the dsxF, which is hundreds of fold more abundant than dsxM, did not result in an equivalent increase in dsxM. Therefore, the relationship between tra2 and the sex-specific splicing of dsx is likely different between Aedes and Drosophila. In this regard, it is worth noting that the Aedes dsx has more than one female-specific introns, which is different from Drosophila (Figure 6, (Biedler and Tu, 2016)).
RNAi–mediated knockdown of Aalbtra2-α or Aalbtra2-γ significantly suppressed ovarian development in females, and the transcription of the major yolk protein gene vitellogenin was significantly reduced. Knockdown of tra2 has also been previously shown to affect the development of the male reproductive system or germline (Amrein et al., 1990; Mattox and Baker, 1991) (Liu et al., 2015; Suzuki et al., 2012) and to affect female fecundity or gonads in several insects (Burghardt et al., 2005; Pomerantz and Hoy, 2015; Schetelig et al., 2012; Shukla and Palli, 2013). It is likely that these effects result from shifts in the alternative splicing of dsx because the Dsx protein has been shown to be involved in the modulation of signaling pathways in both the genital disc (Ahmad and Baker, 2002; Gorfinkiel et al., 2003; Keisman et al., 2001) and gonad (DeFalco et al., 2008; Oliver et al., 1993; Wawersik et al., 2005) and even to directly target yolk protein gene expression in T. castaneum (Shukla and Palli, 2012) and D. melanogaster (Clough et al., 2014).
It is not yet clear how the two tra2 genes affect ovarian development and Vg synthesis in Ae. albopictus. In the current study, when either Aalbtra2-α or Aalbtra2-γ was silenced, a significant increase of dsxM in the adult female fat body was observed, although the relative amount of dsxM is hundreds of fold lower than dsxF. We cannot rule out the possibility that the subtle but significant change in the level of dsxM in the fact body may have an impact on ovarian development and/or Vg synthesis. Vitellogenin is involved in a complex genetic regulatory network (Chen et al., 2004; Martin et al., 2001; Zhu et al., 2007) and is directly or indirectly influenced by multiple factors in mosquitoes, including 20-hydroxyecdysone (20E) (Provost-Javier and Rasgon, 2014), juvenile hormone (JH) (Wang et al., 2017), members of the amino acid/target of rapamycin (AA/TOR) and insulin pathways (Attardo et al., 2006; Boudko et al., 2015; Carpenter et al., 2012; Hansen et al., 2011) and even miRNAs (Bryant et al., 2010). Thus, we cannot rule out the possibility that Tra2 indirectly affects vg via these factors.
In B. mori, Tra2 is apparently not involved in the regulation of the sex-specific splicing of dsx pre-mRNA, but it is required for normal ovary and testis development. The fact that male fertility was not affected by dsRNA soaking or injection may be due to some kind of barrier in the testis (Fairchild et al., 2016; Miranda and Cavicchia, 1986; Toshimori et al., 1979). Further studies are needed to determine the functions and mechanisms of each tra2 gene across different developmental stages in mosquitoes, including the Asian tiger mosquito, which is an important invasive vector of arboviruses.
Supplementary Material
The exons (boxes) and introns (dashed lines) are not drawn to scale. The numbers inside the boxes indicate the exon numbers. The translation start and stop sites and the poly (A) addition sites are marked. In the molecular organization schematics, the 5’– and 3’–UTR regions are indicated in light blue, and the protein-coding exons are indicated in yellow; RS1, RS2, and RRM (underlined) denote the corresponding domains of the putative Tra2 proteins. In the protein schematics, the RS1 and RS2 domains are shown in light green, the RRM is shown in navy, and the GRM is shown in yellow.
Overlapping sets of intron-spanning primers were used in RT–PCR to identify the splicing patterns of Aalbtra2-α and Aalbtra2-β mRNA. The arrows denote the locations of the primers used. The Aedes albopictus ribosomal protein S7 gene (Aalb-Rps7) was used as an internal control. The analyses were performed on the following samples: E1 = 0–2 hr embryos; E2 = 2–4 hr embryos; E3 = 4–8 hr embryos; E4 = 8–12 hr embryos; E5= 12–24 hr embryos; E6= 24–36 hr embryos; E7= 36–48 hr embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd–4th instar larvae; PF= female pupae; PM= male pupae; F1=0–6 hr emerged female adults; F2=6–12 hr emerged female adults; F3=12–24 hr emerged female adults; F4=24–48 hr emerged female adults; F5=48–72 hr emerged female adults; M1= 0–6 hr emerged male adults; M2= 6–12 hr emerged male adults; M3= 12–24 hr emerged male adults; M4= 24–48 hr emerged male adults; M5= 48–72 hr emerged male adults; B1= 0–6 hr PBM female adults; B2= 6–12 hr PBM female adults; B3= 12–24 hr p.b.m. female adults; B4= 24–48 hr p.b.m. female adults; B5= 48–72 hr p.b.m. female adults; H=head; Th = thorax; Ab=abdomen; Mg = midgut; Ma= Malpighian tubule; O=ovary; T=testis; C= remaining carcass.
(A) Schematic diagram of Aalbdsx and Aalbfru gene-specific primer locations. The male-specific and female-specific splicing patterns are marked in blue and red, respectively. The exons and introns are not drawn to scale. The red and blue arrows denote the locations of the primers used to amplify the cDNA products of AalbdsxF/AalbfruF and AalbdsxM/AalbfruM. In the upper panel, the blue arrow with a dotted line indicates one primer spanning an exon–exon boundary; these two exons are located on each side of the female-specific exon. In the lower panel, the mixed two-color arrow indicates the common forward primer. The primer sets encompass the alternative splicing junctions and thus produce sex-specific RT–PCR products. The relative expression levels of (B) AalbdsxF and AalbdsxM and of (C) AalbfruF and AalbfruM were quantified with gene-specific primers in female and male adults subjected to Aalbtra2-α, Aalbtra2-β, and Aalbtra2-γ interference and in controls at 48 hr post injection (p.i.). (D) AalbdsxF, AalbdsxM, AalbfruF and AalbfruM were quantified with gene-specific primers in triple interference groups (upper panel) and larvae soaking interference groups (lower panel) dissected from females 36 hr post blood meal (p.b.m.). ♀= adult female; ♂=adult male. The data are presented as the mean ± SEM (n = 3; *P < 0.05, **P < 0.01, and ***P < 0.001).
qRT–PCR analyses were performed on the indicated samples. E= embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd–4th instar larvae; PE= posteclosion; PBM= post blood meal. Ae. albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control. The data represent the results for three biological replicates with 20 individuals in each replicate and are shown as the mean ± SEM.
Expression ratios were measured by qRT–PCR in ovary, fat body and carcass samples dissected from adult females with RNAi-silenced Aalbtra2-α or Aalbtra2-γ 72 hr post injection (p.i.) and 36 hr post blood meal (p.b.m.) or from adult females at 36 hr p.b.m. that had been subjected to larvae soaking interference. The error bars represent the SEM (n = 3). The x–axis indicates the groups (treatment and control groups). (B) Expression percentages of AaldsxF and AaldsxM in the ovary, fat body, and carcass in interference groups compared to control groups. The y–axis indicates the sample ID, and the x–axis shows the relative percentages of AaldsxF and AaldsxM transcripts among total dsx transcripts based on the relative quantitation (RQ) values obtained by qRT–PCR. The error bars represent the SEM. Aedes albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control (*P < 0.05, **P < 0.01 and ***P < 0.001).
Highlights.
Two duplication events have resulted in three tra2 genes in Ae. albopictus.
A glycine-rich region has been identified in Tra2-α and Tra2-γ.
Knockdown of Aalbtra2 paralogs didn't shift dsxF and dsxM in the ovary and fat body.
Knockdown of Aalbtra2-γ and Aalbtra2-α in adult females reduced post-blood meal ovariole number and ovary size.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81672054); International (Regional) Cooperation and Exchange Program NSFC (81420108024); and the Research Team Program of the Natural Science Foundation of Guangdong (2014A030312016). ZT is supported by NIH grant AI123338. We have no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad SM, Baker BS, 2002. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109, 651–661. [DOI] [PubMed] [Google Scholar]
- Amrein H, Gorman M, Nothiger R, 1988. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell 55, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Amrein H, Hedley ML, Maniatis T, 1994. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer 2. Cell 76, 735–746. [DOI] [PubMed] [Google Scholar]
- Amrein H, Maniatis T, Nothiger R, 1990. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. The EMBO journal 9, 3619–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Shiao SH, Raikhel AS, 2006. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. The Journal of experimental biology 209, 3071–3078. [DOI] [PubMed] [Google Scholar]
- Bell LR, Horabin JI, Schedl P, Cline TW, 1991. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65, 229–239. [DOI] [PubMed] [Google Scholar]
- Belote JM, Baker BS, 1982. Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proceedings of the National Academy of Sciences of the United States of America 79, 1568–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J, Tu Z, 2016. Sex Determination in Mosquitoes.
- Biedler JK, Hall BA, Jiang X, Tu ZJ, 2016. Chapter 10 - Exploring the Sex-Determination Pathway for Control of Mosquito-Borne Infectious Diseases A2 - Adelman, Zach N, Genetic Control of Malaria and Dengue. Academic Press, Boston, pp. 201–225. [Google Scholar]
- Bocca Nora S, Magioli Claudia, Mangeon Amanda, Junqueira Magrani, R., Cardeal Vanessa, Margis Rog¨¦rio, Sachetto-Martins Gilberto, 2005. Survey of glycine-rich proteins (GRPs) in the Eucalyptus expressed sequence tag database (ForEST). Genetics and Molecular Biology. [Google Scholar]
- Boudko DY, Tsujimoto H, Rodriguez SD, Meleshkevitch EA, Price DP, Drake LL, Hansen IA, 2015. Substrate specificity and transport mechanism of amino-acid transceptor Slimfast from Aedes aegypti. Nature communications 6, 8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant B, Macdonald W, Raikhel AS, 2010. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America 107, 22391–22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt G, Hediger M, Siegenthaler C, Moser M, Dubendorfer A, Bopp D, 2005. The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Development genes and evolution 215, 165–176. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC, 1991. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. The EMBO journal 10, 2577–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter VK, Drake LL, Aguirre SE, Price DP, Rodriguez SD, Hansen IA, 2012. SLC7 amino acid transporters of the yellow fever mosquito Aedes aegypti and their role in fat body TOR signaling and reproduction. Journal of insect physiology 58, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D, McGuffin ME, Piskur J, Yao J, Baker BS, Mattox W, 1997. Evolutionary conservation of regulatory strategies for the sex determination factor transformer-2. Molecular and cellular biology 17, 2908–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhu J, Sun G, Raikhel AS, 2004. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. Journal of molecular endocrinology 33, 743–761. [DOI] [PubMed] [Google Scholar]
- Chung HN, Rodriguez SD, Carpenter VK, Vulcan J, Bailey CD, Nageswara-Rao M, Li Y, Attardo GM, Hansen IA, 2017. Fat Body Organ Culture System in Aedes Aegypti, a Vector of Zika Virus. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN, Clements AN, 1992. The biology of mosquitoes. Chapman & Hall, London; New York. [Google Scholar]
- Clough E, Jimenez E, Kim YA, Whitworth C, Neville M, Hempel L, Pavlou H, Chen ZX, Sturgill D, Dale R, 2014. Sex- and Tissue-Specific Functions of Drosophila Doublesex Transcription Factor Target Genes. Developmental cell 31, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha C, Scott MJ, 2009. Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics 182, 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC, 1993. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes & development 7, 42–54. [DOI] [PubMed] [Google Scholar]
- Dauwalder B, Amaya-Manzanares F, Mattox W, 1996. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proceedings of the National Academy of Sciences of the United States of America 93, 9004–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Camara N, Le Bras S, Van Doren M, 2008. Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Developmental cell 14, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JW, Quintero JJ, 2007. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol 5, e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild MJ, Yang L, Goodwin K, Tanentzapf G, 2016. Occluding Junctions Maintain Stem Cell Niche Homeostasis in the Fly Testes. Current biology : CB 26, 2492–2499. [DOI] [PubMed] [Google Scholar]
- Gattiker A, Gasteiger E, Bairoch A, 2002. ScanProsite: a reference implementation of a PROSITE scanning tool. Applied bioinformatics 1, 107–108. [PubMed] [Google Scholar]
- Geuverink E, Beukeboom LW, 2014. Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation 8, 38–49. [DOI] [PubMed] [Google Scholar]
- Gomulski LM, Dimopoulos G, Xi Z, Soares MB, Bonaldo MF, Malacrida AR, Gasperi G, 2008. Gene discovery in an invasive tephritid model pest species, the Mediterranean fruit fly, Ceratitis capitata. BMC genomics 9, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Sanchez L, Guerrero I, 2003. Development of the Drosophila genital disc requires interactions between its segmental primordia. Development (Cambridge, England) 130, 295–305. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Wheelock G, Flanagan TR, 1977. Postemergence growth of the ovarian follicles of Aedes aegypti. Journal of insect physiology 23, 203–206. [DOI] [PubMed] [Google Scholar]
- Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK, Sharakhova MV, Elahi R, Anderson MAE, Chen X-G, Sharakhov IV, Adelman ZN, Tu Z, 2015. A male-determining factor in the mosquito Aedes aegypti. Science (New York, N.Y.) 348, 1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Boudko DY, Shiao SH, Voronov DA, Meleshkevitch EA, Drake LL, Aguirre SE, Fox JM, Attardo GM, Raikhel AS, 2011. AaCAT1 of the yellow fever mosquito, Aedes aegypti: a novel histidine-specific amino acid transporter from the SLC7 family. The Journal of biological chemistry 286, 10803–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang KP, Teo TM, Ho TX, Le VS, 2016. Mechanisms of sex determination and transmission ratio distortion in Aedes aegypti. Parasites & vectors 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Hoshijima K, Sakamoto H, Shimura Y, 1990. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature 344, 461–463. [DOI] [PubMed] [Google Scholar]
- Keisman EL, Christiansen AE, Baker BS, 2001. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Developmental cell 1, 215–225. [DOI] [PubMed] [Google Scholar]
- Liu G, Wu Q, Li J, Zhang G, Wan F, 2015. RNAi-Mediated Knock-Down of transformer and transformer 2 to Generate Male-Only Progeny in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). PloS one 10, e0128892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Chen Y, Gu J, Chen X, 2013. [Isolation and expression profiling of transformer 2 gene in Aedes aegypti]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University 33, 1583–1589. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Martin D, Wang SF, Raikhel AS, 2001. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Molecular and cellular endocrinology 173, 75–86. [DOI] [PubMed] [Google Scholar]
- Mattox W, Baker BS, 1991. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes & development 5, 786–796. [DOI] [PubMed] [Google Scholar]
- Mattox W, McGuffin ME, Baker BS, 1996. A negative feedback mechanism revealed by functional analysis of the alternative isoforms of the Drosophila splicing regulator transformer-2. Genetics 143, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattox W, Palmer MJ, Baker BS, 1990. Alternative splicing of the sex determination gene transformer-2 is sex-specific in the germ line but not in the soma. Genes & development 4, 789–805. [DOI] [PubMed] [Google Scholar]
- Meiselbach H, Sticht H, Enz R, 2006. Structural analysis of the protein phosphatase 1 docking motif: molecular description of binding specificities identifies interacting proteins. Chemistry & biology 13, 49–59. [DOI] [PubMed] [Google Scholar]
- Miranda JC, Cavicchia JC, 1986. A permeability barrier in the testis of an insect Triatoma: A freeze-fracture and lanthanum tracer study. Tissue & cell 18, 461–468. [DOI] [PubMed] [Google Scholar]
- Nissen I, Muller M, Beye M, 2012. The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. Genetics 192, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu BL, Meng ZQ, Tao YZ, Lu SL, Weng HB, He LH, Shen WF, 2005. Cloning and alternative splicing analysis of Bombyx mori transformer-2 gene using silkworm EST database. Acta biochimica et biophysica Sinica 37, 728–736. [DOI] [PubMed] [Google Scholar]
- Novoyatleva T, Heinrich B, Tang Y, Benderska N, Butchbach MER, Lorson CL, Lorson MA, Ben-Dov C, Fehlbaum P, Bracco L, Burghes AHM, Bollen M, Stamm S, 2008. Protein phosphatase 1 binds to the RNA recognition motif of several splicing factors and regulates alternative pre-mRNA processing. Human molecular genetics 17, 52–70. [DOI] [PubMed] [Google Scholar]
- Oliver B, Kim YJ, Baker BS, 1993. Sex-lethal, master and slave: a hierarchy of germ-line sex determination in Drosophila. Development (Cambridge, England) 119, 897–908. [DOI] [PubMed] [Google Scholar]
- Peng W, Zheng W, Handler AM, Zhang H, 2015. The role of the transformer gene in sex determination and reproduction in the tephritid fruit fly, Bactrocera dorsalis (Hendel). Genetica 143, 717–727. [DOI] [PubMed] [Google Scholar]
- Pomerantz AF, Hoy MA, 2015. RNAi-mediated knockdown of transformer-2 in the predatory mite Metaseiulus occidentalis via oral delivery of double-stranded RNA. Experimental & applied acarology 65, 17–27. [DOI] [PubMed] [Google Scholar]
- Provost-Javier KN, Rasgon JL, 2014. 20-hydroxyecdysone mediates non-canonical regulation of mosquito vitellogenins through alternative splicing. Insect molecular biology 23, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R, 2008. For mammals, loss of yolk and gain of milk went hand in hand. PLoS Biol 6, e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Y, Gonzalez-Mendez RR, Cadilla CL, 2016. Evolution of the Twist Subfamily Vertebrate Proteins: Discovery of a Signature Motif and Origin of the Twist1 Glycine-Rich Motifs in the Amino-Terminus Disordered Domain. 11, e0161029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini M, D’Amato R, Petrella V, Aceto S, Nimmo D, Neira M, Alphey L, Polito LC, Saccone G, 2013. The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: evolution of genomic organisation and alternative splicing. PloS one 8, e48554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini M, Mauro U, Lombardo F, Milano A, Zazzaro V, Arca B, Polito LC, Saccone G, 2011. Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC evolutionary biology 11, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini M, Robertson M, Aronson B, Atkinson P, Polito LC, Saccone G, 2009. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. The International journal of developmental biology 53, 109–120. [DOI] [PubMed] [Google Scholar]
- Sarno F, Ruiz MF, Eirin-Lopez JM, Perondini AL, Selivon D, Sanchez L, 2010. The gene transformer-2 of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. BMC evolutionary biology 10, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali C, Catteruccia F, Li Q, Crisanti A, 2005. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. The Journal of experimental biology 208, 3701–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetelig MF, Milano A, Saccone G, Handler AM, 2012. Male only progeny in Anastrepha suspensa by RNAi-induced sex reversion of chromosomal females. Insect biochemistry and molecular biology 42, 51–57. [DOI] [PubMed] [Google Scholar]
- Shukla JN, Palli SR, 2012. Doublesex target genes in the red flour beetle, Tribolium castaneum. Scientific reports 2, 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla JN, Palli SR, 2013. Tribolium castaneum Transformer-2 regulates sex determination and development in both males and females. Insect biochemistry and molecular biology 43, 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert V, Kuhn S, Traut W, 1997. Expression of the sex determining cascade genes Sex-lethal and doublesex in the phorid fly Megaselia scalaris. Genome 40, 211–214. [DOI] [PubMed] [Google Scholar]
- Stamm S, 2008. Regulation of alternative splicing by reversible protein phosphorylation. The Journal of biological chemistry 283, 1223–1227. [DOI] [PubMed] [Google Scholar]
- Suzuki MG, Suzuki K, Aoki F, Ajimura M, 2012. Effect of RNAi-mediated knockdown of the Bombyx mori transformer-2 gene on the sex-specific splicing of Bmdsx pre-mRNA. The International journal of developmental biology 56, 693–699. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S, 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular biology and evolution 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshimori K, Iwashita T, Oura C, 1979. Cell junctions in the cyst envelope in the silkworm testis, Bombyx mori Linne. Cell and tissue research 202, 63–73. [DOI] [PubMed] [Google Scholar]
- Wang JL, Saha TT, Zhang Y, Zhang C, Raikhel AS, 2017. Juvenile hormone and its receptor methoprene-tolerant promote ribosomal biogenesis and vitellogenesis in the Aedes aegypti mosquito. The Journal of biological chemistry 292, 10306–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Burge CB, 2008. Splicing regulation: From a parts list of regulatory elements to an integrated splicing code. RNA (New York, N.Y.) 14, 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M, 2005. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Maniatis T, 1993. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Klein J, Nei M, 2014. Evolution of the Sex-lethal Gene in Insects and Origin of the Sex-Determination System in Drosophila. Journal of Molecular Evolution 78, 50–65. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS, 2007. Distinct roles of Broad isoforms in regulation of the 20-hydroxyecdysone effector gene, Vitellogenin, in the mosquito Aedes aegypti. Molecular and cellular endocrinology 267, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The exons (boxes) and introns (dashed lines) are not drawn to scale. The numbers inside the boxes indicate the exon numbers. The translation start and stop sites and the poly (A) addition sites are marked. In the molecular organization schematics, the 5’– and 3’–UTR regions are indicated in light blue, and the protein-coding exons are indicated in yellow; RS1, RS2, and RRM (underlined) denote the corresponding domains of the putative Tra2 proteins. In the protein schematics, the RS1 and RS2 domains are shown in light green, the RRM is shown in navy, and the GRM is shown in yellow.
Overlapping sets of intron-spanning primers were used in RT–PCR to identify the splicing patterns of Aalbtra2-α and Aalbtra2-β mRNA. The arrows denote the locations of the primers used. The Aedes albopictus ribosomal protein S7 gene (Aalb-Rps7) was used as an internal control. The analyses were performed on the following samples: E1 = 0–2 hr embryos; E2 = 2–4 hr embryos; E3 = 4–8 hr embryos; E4 = 8–12 hr embryos; E5= 12–24 hr embryos; E6= 24–36 hr embryos; E7= 36–48 hr embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd–4th instar larvae; PF= female pupae; PM= male pupae; F1=0–6 hr emerged female adults; F2=6–12 hr emerged female adults; F3=12–24 hr emerged female adults; F4=24–48 hr emerged female adults; F5=48–72 hr emerged female adults; M1= 0–6 hr emerged male adults; M2= 6–12 hr emerged male adults; M3= 12–24 hr emerged male adults; M4= 24–48 hr emerged male adults; M5= 48–72 hr emerged male adults; B1= 0–6 hr PBM female adults; B2= 6–12 hr PBM female adults; B3= 12–24 hr p.b.m. female adults; B4= 24–48 hr p.b.m. female adults; B5= 48–72 hr p.b.m. female adults; H=head; Th = thorax; Ab=abdomen; Mg = midgut; Ma= Malpighian tubule; O=ovary; T=testis; C= remaining carcass.
(A) Schematic diagram of Aalbdsx and Aalbfru gene-specific primer locations. The male-specific and female-specific splicing patterns are marked in blue and red, respectively. The exons and introns are not drawn to scale. The red and blue arrows denote the locations of the primers used to amplify the cDNA products of AalbdsxF/AalbfruF and AalbdsxM/AalbfruM. In the upper panel, the blue arrow with a dotted line indicates one primer spanning an exon–exon boundary; these two exons are located on each side of the female-specific exon. In the lower panel, the mixed two-color arrow indicates the common forward primer. The primer sets encompass the alternative splicing junctions and thus produce sex-specific RT–PCR products. The relative expression levels of (B) AalbdsxF and AalbdsxM and of (C) AalbfruF and AalbfruM were quantified with gene-specific primers in female and male adults subjected to Aalbtra2-α, Aalbtra2-β, and Aalbtra2-γ interference and in controls at 48 hr post injection (p.i.). (D) AalbdsxF, AalbdsxM, AalbfruF and AalbfruM were quantified with gene-specific primers in triple interference groups (upper panel) and larvae soaking interference groups (lower panel) dissected from females 36 hr post blood meal (p.b.m.). ♀= adult female; ♂=adult male. The data are presented as the mean ± SEM (n = 3; *P < 0.05, **P < 0.01, and ***P < 0.001).
qRT–PCR analyses were performed on the indicated samples. E= embryos; L1–2= 1st–2nd instar larvae; L3–4= 3rd–4th instar larvae; PE= posteclosion; PBM= post blood meal. Ae. albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control. The data represent the results for three biological replicates with 20 individuals in each replicate and are shown as the mean ± SEM.
Expression ratios were measured by qRT–PCR in ovary, fat body and carcass samples dissected from adult females with RNAi-silenced Aalbtra2-α or Aalbtra2-γ 72 hr post injection (p.i.) and 36 hr post blood meal (p.b.m.) or from adult females at 36 hr p.b.m. that had been subjected to larvae soaking interference. The error bars represent the SEM (n = 3). The x–axis indicates the groups (treatment and control groups). (B) Expression percentages of AaldsxF and AaldsxM in the ovary, fat body, and carcass in interference groups compared to control groups. The y–axis indicates the sample ID, and the x–axis shows the relative percentages of AaldsxF and AaldsxM transcripts among total dsx transcripts based on the relative quantitation (RQ) values obtained by qRT–PCR. The error bars represent the SEM. Aedes albopictus ribosomal protein 7 (rpS7) mRNA was used as an internal control (*P < 0.05, **P < 0.01 and ***P < 0.001).