Abstract
OBJECTIVE:
To estimate whether a rapid recovery program would reduce length of stay among patients undergoing laparotomy on a gynecologic oncology service.
METHODS:
We conducted a prospective, randomized, controlled trial comparing an enhanced recovery after surgery protocol with routine postoperative care among women undergoing laparotomy on the gynecologic oncology service. Protocol elements included: preoperative counseling, regional anesthesia, intraoperative fluid restriction, and early postoperative ambulation and feeding. A sample size of 50 per group (N=100) was planned to achieve 80% power to detect a two-day difference in our primary outcome, length of hospital stay; secondary outcomes included: total daily narcotics used, time to postoperative milestones, and complications.
RESULTS:
A total of 112 women were enrolled between 2013 and 2015. Nine patients did not undergo laparotomy and were excluded, leaving 52 and 51 patients in the control and intervention groups, respectively. There was no difference in length of stay between the two groups (median 3.0 in both groups;P=.36). Enhanced recovery after surgery patients used less narcotics on day 0 (10.0 compared with 5.5 morphine equivalents in the control and intervention arms, respectively, P=.09) and day 2 (10.0 compared with 7.5 morphine equivalents, respectively; P=.05);however, there was no statistically significant difference between groups in any of the secondary outcomes. Post hoc analysis based on actual anesthesia received also failed to demonstrate a difference in time to discharge.
CONCLUSION:
When compared with usual care, introducing a formal enhanced recovery after surgery protocol did not significantly reduce length of stay.
CLINICAL TRIAL REGISTRATION:
ClinicalTrials.gov, https://clinicaltrials.gov, NCT01705288.
General anesthesia and surgically induced pain affect the sympathetic nervous, endocrine, immunologic, and gastrointestinal systems affecting vascular tone, perfusion, ventilation, and temperature regulation and ultimately end-organ functioning.1,2 These effects linger into the postoperative period manifesting as hypercoagulability and ileus, leading to increased morbidity and prolonged length of hospital stay.3
Enhanced recovery after surgery protocols attempt to minimize the physiologic deviations induced by surgery. Protocol components vary, but most include: preoperative education and expectation setting, regional anesthesia, intraoperative fluid restriction, aggressive management of pain and nausea, early postoperative mobilization, and early resumption of enteral nutrition.4 Our group and others have demonstrated that, among patients undergoing simple, uncomplicated surgeries, enhanced recovery after surgery protocol use was associated with decreased length of stay and reduced postoperative narcotic use.2–7 Furthermore, a recent meta-analysis describes that, compared with historical controls, use of enhanced recovery after surgery protocols resulted in significant improvements in patient satisfaction, cost to patient, and length of stay without increasing morbidity or readmission rates after open hysterectomy.8
Despite these encouraging findings, a Cochrane Database Review in 2015 concluded “We currently have no evidence from high-quality studies to support or refute the use of perioperative enhanced recovery programmes for gynaecological cancer patients. Further well-designed RCTs with standard FT (fast track) programmes are needed.”9 Furthermore, there remains concern regarding application of enhanced recovery after surgery principles to an older, more medically compromised population or to patients undergoing more extensive operations as are typically encountered on the gynecologic oncology service.
We conducted a randomized, controlled trial to test the hypothesis that an organized enhanced recovery after surgery protocol could reduce length of stay among women undergoing laparotomy on a gynecologic oncology service compared with our current practices.
MATERIALS AND METHODS
Approval for this study was obtained by the University of Minnesota institutional review board and the trial was registered with clinicaltrials.gov before the enrollment of any patients (NCT01705288).
We conducted a prospective, randomized, controlled study comparing a standardized enhanced recovery after surgery protocol with our current surgical practices. All patients with a planned laparotomy on the gynecologic oncology service, irrespective of their presumed diagnosis, were considered eligible. Recruitment was at the discretion of the treating physician. Patients undergoing planned laparoscopy, vulvar, or minor procedures were ineligible because the median stay for these patients at our institution is less than 1 day. All patients were counseled about the potential risks and benefits of the enhanced recovery after surgery protocol before being offered enrollment.
The primary objective was to determine whether instituting a formal enhanced recovery after surgery protocol would significantly reduce the length of stay for patients undergoing laparotomy on the gynecologic oncology service. Secondary endpoints included operating time, estimated blood loss, assessment of time to milestones, use of narcotic and or antiemetic medication, and rate of failure to adhere to protocol.
Patients were randomized (1:1) at the time of preoperative evaluation to receive either enhanced recovery after surgery or usual care using blocked randomization with varying block sizes and sequentially numbered sealed opaque envelopes to mitigate selection bias. Patients randomized to receive enhanced recovery after surgery underwent additional, standardized, preoperative counseling, including a one-page scripted discussion of expectations with emphasis on the benefits of decreasing narcotic use as well postoperative expectations with regard to early ambulation, eating, and criteria for discharge. Patients randomized to receive enhanced recovery after surgery were allowed a regular diet until 6 hours before surgery and allowed clear liquids until 2 hours before surgery. Routine mechanical bowel preparation was discouraged but allowed in the setting of planned bowel resection.
The perioperative anesthesia protocol for patients receiving enhanced recovery after surgery included either placement of a spinal block with 16 mg isobaric tetracaine with 0.2 mg epinephrine wash and 0.1 mg preservative-free hydromorphone given at level L3–4 or a T12 epidural using 0.125% bupivacaine at a rate of 8–12 mL per hour. Patients who received spinal or epidural anesthesia also received bilateral transversus abdominis plane infiltration with liposomal bupiva-caine (total dose 266 mg). Patients could undergo intubation and general anesthesia if indicated at the discretion of the attending anesthesiologist.
Intravenous fluid in the enhanced recovery after surgery group was restricted to 1 cc/kg per hour throughout the case, and phenylephrine was used to maintain blood pressure. Patients demonstrating hypotension or hypoperfusion received additional resuscitation at the discretion of the anesthesiologist. Oliguria was tolerated in the absence of other signs of hypoperfusion.
Enhanced recovery after surgery patients were encouraged to ambulate within 2 hours after surgery and offered a regular diet immediately. Pain management included oral acetaminophen and ibuprofen followed by narcotic medications as needed as well as continued epidural use if this was the regional anesthesia used. The Foley catheter was removed when patients were able to ambulate. All enhanced recovery after surgery patients were referred to physical therapy during their hospital stay, whereas patients in the control arm were referred only at the request of the primary surgeon.
Patients randomized to the control arm received perioperative counseling per their primary surgeon. To compare against “current practice,” the control arm was not formally dictated, which permitted the use of any or all enhanced recovery after surgery tenets.
We recorded clinical milestones including onset of flatus, time to ambulation, and time to tolerate a regular diet. Discharge criteria were identical for both groups: hemodynamic stability, euthermia, ability to tolerate oral intake, adequate pain management on oral medications, and sufficient home support. Discharge to transitional care was not considered different than discharge to home. No “target date” for discharge was prescribed for either group.
The primary analysis was by intention to treat. All continuous measures were initially summarized using descriptive statistics and assessed for normality using visual inspection and the Shapiro-Wilk method. Comparisons of demographic and clinical characteristics by randomization group were conducted using t tests and Wilcoxon rank-sum tests for continuous data and χ2 and Fisher exact tests for categorical data as appropriate. The primary outcome of interest, length of hospital stay measured as whole days from the day of surgery until discharge as well as the secondary outcome of total daily narcotic and antiemetic use were not normally distributed and therefore compared using Wilcoxon rank-sum tests. Medians and 95% confidence intervals are presented. Narcotic use was standardized by conversion to morphine equivalents using the methods of Korff et al.10 Secondary outcomes were compared using χ2 and Fisher exact tests as appropriate. P values <.05 were considered statistically significant.
To calculate sample size, we determined through retrospective assessment of our divisional database that the mean stay for patients who would have qualified for the study using the same inclusion criteria was 5.0±3.4 days. A 2-day reduction in hospitalization in the intervention group as compared with the control group was felt to be clinically meaningful. Given these parameters, a sample size of 50 patients per arm was determined to achieve 80% power to detect a difference of 2 days with a significance level (α) of 0.05 using a two-sided Mann-Whitney test. The study was initially approved for 100 patients, but as a result of enrollment of patients who ultimately did not undergo a qualifying procedure, approval to enroll up to 112 patients was obtained.
RESULTS
A total of 112 patients were enrolled betweenJanuary 1, 2013, and September 1, 2015. Fifty-six patients were randomized to the control group and 56 to the intervention group (Fig. 1). Four patients in the control group and five patients in the intervention group did not undergo eligible surgery (most commonly a change in surgical plan from laparotomy to laparoscopy). Therefore, 52 and 51 patients were included in the control and intervention groups, respectively.
Fig. 1.
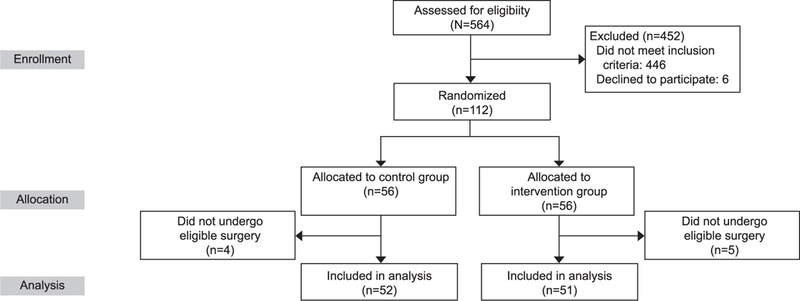
Study enrollment and randomization.
The mean age of participants overall was 55.7 years (95% confidence interval [CI] 53.5–57.9 years) and was similar in both groups. Groups were balanced across for relevant clinical and surgical characteristics, including estimated blood loss, surgery time, and final pathology (Table 1).
Table 1.
Demographic and Surgical Characteristics by Randomized Group (N=103)
| Characteristic | Control | Intervention | P |
|---|---|---|---|
| Demographic | |||
| Age (y) | 52 [56.0 (52.8–59.2)] | 51 [55.4 (52.3–58.5)] | .80* |
| BMI (kg/m2) | 52 [31.3 (29.3–33.3)] | 51 [31.0 (28.5–33.4)] | .84* |
| Smoking status | .96† | ||
| Never | 33 (63.5) | 33 (64.7) | |
| Former | 13 (25.0) | 13 (25.5) | |
| Current | 6 (11.5) | 5 (9.8) | |
| Comorbidities | |||
| Hypertension | 21 (40.4) | 19 (37.3) | .74† |
| Coronary artery disease | 2 (3.9) | 1 (2.0) | 1.00‡ |
| Diabetes | 10 (19.2) | 3 (5.9) | .07‡ |
| Pulmonary disease | 2 (3.9) | 6 (11.8) | .16‡ |
| Other malignancy | 11 (21.2) | 1 (2.0) | .004‡ |
| Surgical characteristics | .15‡ | ||
| Final pathology | |||
| Benign | 15 (28.9) | 20 (39.2) | |
| Borderline | 0 (0.0) | 2 (3.9) | |
| Cancer | 37 (71.2) | 29 (56.9) | |
| EBL (mL) [median (95% CI)] | 51 [200 (150–200)] | 51 [200 (100–300)] | .81§ |
| Bowel surgery | 1 (1.9) | 2 (3.9) | .55 |
| Surgery time (min) [median (95% CI)] | 52 [186 (163–208)] | 50 [171 (147–183)] | .09§ |
Data are n [mean (95% CI)] or n (%) unless otherwise specified.
t test
Chi-squared test.
Fisher exact test.
Wilcoxon rank-sum test.
CI, confidence interval; BMI, body mass index; EBL, estimated blood loss.
Protocol compliance in the enhanced recovery after surgery arm was generally high, but seven patients (13.7%) randomized to the intervention group received no regional anesthesia in violation of the protocol. The most common protocol deviations were attributed to “anesthesiologist preference.” Whether these deviations reflect oversight, discomfort with the broader application of the technique, or an unrecognized safety or technical concern is unclear. Interestingly, however, we did not identify increased morbidity in the treatment group, and in general compliance improved over time. Similarly, physical therapy was ordered on all patients in the treatment arm but is typically not delivered on weekend days at our institution; as a result, 78.5% of patients in the enhanced recovery after surgery arm actually received this component before discharge. Regional anesthesia was used in 18 (34.6%) patients randomized to the control group and was considered protocol-compliant.
The median length of hospital stay was not significantly different between the groups at 3.0 days (95% CI 2.0–3.0) for the control group compared with 3.0 days (95% CI 2.0–3.0) for the enhanced recovery after surgery group (P=.36; Table 2). There was no significant difference between the amount of narcotic used during postoperative days 0 and 1; less morphine equivalents were used in the intervention group on day 2 (10.0 morphine equivalents [95% CI 2.0–3.0] in the control group compared with 7.5 morphine equivalents [95% CI 3.3–10.0] in the intervention arm; P=.05). There was no statistical difference in time to postoperative ambulation between groups with the majority for both groups ambulating on day 1. There was also no difference in time to passed flatus or the prevalence of emesis. Finally, there were no differences between complication or readmission rates between the randomized groups.
Table 2.
Outcomes by Randomized Group
| Outcome Variable | Control (n=52) | Intervention (n=51) | P |
|---|---|---|---|
| Primary outcome | |||
| Length of hospital stay (d) | 52 [3.0 (2.0–3.0)] | 51 [3.0 (2.0–3.0)] | .36* |
| Secondary outcomes | |||
| Day 0 narcotics† | 51 [10.0 (6.0–14.0)] | 49 [5.5 (2.0–11.0)] | .09* |
| Day 1 narcotics† | 48 [12.6 (10.0–18.5)] | 51 [10.0 (5.0–13.3)] | .16* |
| Day 2 narcotics† | 51 [10.0 (8.3–12.3)] | 48 [7.5 (3.3–10.0)] | .05* |
| When ambulating | .81‡ | ||
| Day 0 | 8 (16.0) | 8 (16.3) | |
| Day 1 | 38 (76.0) | 39 (79.6) | |
| Day 2 | 4 (8.0) | 2 (4.1) | |
| Missing | 2 | ||
| When flatus | .08‡ | ||
| Day 1 | 5 (11.4) | 0 (0.0) | |
| Day 2 | 9 (20.5) | 16 (43.2) | |
| Day 3 | 16 (36.4) | 12 (32.4) | |
| Day 4 or more (in hospital) | 5 (11.4) | 4 (10.8) | |
| None before discharge | 9 (20.5) | 5 (13.5) | |
| Missing | 8 | 14 | |
| Emesis | .86§ | ||
| No | 39 (75.0) | 39 (76.5) | |
| Yes | 13 (25.0) | 12 (23.5) | |
| Physical therapy used | <.001§ | ||
| No | 32 (62.8) | 11 (21.6) | |
| Yes | 19 (37.3) | 30 (78.5) | |
| Missing | 1 | 0 | .36‡ |
| Complications | |||
| No | 48 (92.3) | 44 (86.3) | |
| Yes | 4 (7.7) | 7 (13.7) | |
| ICU admission | 1 (1.9) | 3 (5.9) | |
| Hemorrhage | 0 | 1 (2.0) | |
Data are n [median (95% confidence interval)] or n (%) unless otherwise specified.
Wilcoxon rank-sum test.
Morphine equivalents.
Fisher exact test.
Chi-squared test.
ICU, intensive care unit.
To determine whether crossover (defined for this analysis as patients in the enhanced recovery after surgery group who received no regional anesthesia or patients in the control arm who did receive regional anesthesia) was a primary reason for the failure to detect an improvement in time to discharge, an additional post hoc analysis was conducted based on actual anesthesia received, irrespective of randomization. In total, 41 patients received general anesthesia and a patient-controlled analgesia and 62 received primarily a regional anesthesia. Results were similar to the intent-to-treat analysis with the median length of hospital stay 3 days in both groups (3.0 days [95% CI 2.0–4.0] for the control group compared with 3.0 days [95% CI 2.0–3.0]; P=.44). However, there was a significant reduction in narcotic use on postoperative days 0 and 1 between the general and regional anesthesia groups. On postoperative day 0, a median of 10.3 (95% CI 7.0–18.0) morphine equivalents were used in the general anesthesia group compared with 5.5 (95% CI 2.0–12.0) in the regional anesthesia group (P=.03). On postoperative day 1, a median of 13.3 (95% CI 10.0–26.8) morphine equivalents was used in the general anesthesia compared with 8.6 (95% CI 5.0–13.3) in the regional anesthesia group (P=.01). This difference was not significantly different on day 2. Patients who received regional anesthesia also passed flatus earlier that those who received general anesthesia (P=.02).
DISCUSSION
Our data demonstrate that introducing an enhanced recovery after surgery protocol was feasible but did not reduce time to discharge after laparotomy on the gynecologic oncology service compared with our current practices. Notably, length of stay was reduced compared with historical controls; however, this benefit accrued equally to patients in the experimental and control arms, suggesting that the reduction was either unrelated or at best an indirect effect of enhanced recovery after surgery introduction.
There is growing acceptance of enhanced recovery after surgery practices in gynecologic oncology surgery with several case-control studies demonstrating reduced narcotic use and length of stay.11–16 Despite a preponderance of positive studies, however, evaluation of individual protocol tenets has met with mixed results,7,17,18 suggesting either synergy of enhanced recovery after surgery components or bias. As a prospective, randomized, controlled trial, many of the potential biases intrinsic to retrospective or serial cohort studies are mitigated in our analysis. There are multiple potential explanations for a failure to observe a reduction in length of stay comparable with those previously reported. These include: 1) a high rate of use of enhanced recovery after surgery tenets in the control group, which may have blunted the magnitude of any treatment effect; 2) a relatively short prestudy length of stay compared with previous reports; and 3) possible publication bias regarding previous studies.
Multiple enhanced recovery after surgery tenets applied in this study are routine in the practice of our group, including relatively high baseline use of regional anesthesia.19 The authors acknowledge that the high prevalence of a major enhanced recovery after surgery tenet in the control arm would tend to bias the results toward the null hypothesis if the tenet was important; however, restricting regional anesthesia from the control arm was felt to neither reflect nor be superior to our current “best practices.” Similarly, protocol violations involving omission of regional anesthesia among enhanced recovery after surgery patients were present in 13.7% of patients and would also tend to bias the results toward the null hypothesis.
Despite these concerns, our post hoc analysis, based on actual anesthesia received, also failed to demonstrate a difference in length of stay, suggesting that high rates of regional anesthesia in the control arm were not likely the primary reason no effect was observed. Furthermore, the use of regional anesthesia in the control group did not increase from the prestudy baseline; therefore, the reduction in length of stay (from 5 days prestudy to 3 days in the control group) cannot be the result of use of regional anesthesia among control patients.
Our prestudy length of stay was notably shorter than in many previous reports, which used almost exclusively a sequential cohort design, comparing a novel enhanced recovery after surgery strategy against historical controls11–16 (Table 3). This strategy is subject to significant observer bias and Hawthorne effect (inadvertent introduction of an effect through the act of studying the intervention). Pressure on both surgeons and hospital systems to reduce length of stay has been increasing for more than two decades. Interventions instituted during this time (and especially ones that accrued patients over multiple years or had no contemporaneous control group) would likely be associated with shorter lengths of stay, from nonintervention-related pressures to earlier discharge, and should therefore be interpreted with caution. In point of fact, had this study been conducted as a sequential cohort (without a control arm), we would have observed the target 2-day reduction in length of stay among enhanced recovery after surgery patients and concluded, albeit erroneously, that enhanced recovery after surgery implementation led to the outcome. Similarly, multiple previous studies included a targeted discharge date in the experimental but not the control arms, which introduces bias in favor of the experimental arms.
Table 3.
Enhanced Recovery Protocols in Gynecologic Oncology Surgery in Which Primary Outcome Included Length of Hospital Stay
| Study |
|||||||
|---|---|---|---|---|---|---|---|
| Design Variable | Marx et al11 | Chase et al12 |
Gerardi et al13 |
Carter14 | Kalogera et al15 | Wijk et al16 | Present Study |
| Prospective Design | No Sequential cohorts | No Cohort | No Case–control | No Cohort | No Sequential cohorts | Yes Sequential cohorts | Yes RCT |
| Sample size | |||||||
| Control | 72 | NA | 45 | NA | 158* | 120 | 52 |
| ERAS | 69 | 880 | 19 | 389 | 165† | 85 | 51 |
| % with gynecologic malignancy | |||||||
| Control | 100 | NR | 100 | NR | 100 | 28 | 71 |
| ERAS | 100 | 48 | 100 | 58 | 100 | 38 | 57 |
| % with bowel surgery | |||||||
| Control | 13 | NR | 100 | NR | 24 | NR | 9.6 |
| ERAS | 7 | NR | 100 | NR | 22 | NR | 11.8 |
| Length of stay (median) | |||||||
| Control | 6 | 10 | 4 staging; 8 cytoreduction | 2 | 3 | ||
| ERAS | 5 | 2 | 7 | 3 | 4 staging; 5 cytoreduction | 2 | 3 |
| % readmitted | |||||||
| Control | 9.7 | 33 | 15 | 4.2 | 5.8 | ||
| ERAS | 2.9 | 5 | 21 | 4 | 20 | 3.5 | 7.8 |
| Protocol directed length of stay (target) | ERAS only (POD 4) | Yes (POD 2) | No | No | NR | Yes (POD 2) | No |
Seventy-seven vaginal cases excluded.
Seventy-six vaginal cases excluded.
RCT, randomized controlled trial; NA, not applicable; ERAS, enhanced recovery after surgery; NR, not reported; POD, postoperative day.
Another possible explanation is that the dissemination and adoption of beneficial surgical practices, combined with increasing pressure to demonstrate cost-efficacy, has pushed surgical recovery closer to the asymptote of “perfect optimization.” Demonstrating significant improvement in length of stay becomes increasingly difficult as the length of stay approaches “optimal.” Even with the implementation of enhanced recovery after surgery programs, length of stay in many reports remained longer than our pretrial length of stay, suggesting that the gains achieved in these settings may accrue from efforts to modernize post-operative management rather than the specific intervention11–16 (Table 3).
Although publication bias (the tendency to disseminate positive but not negative results) cannot be excluded as a possible reason for the discrepancy between our observations and those previously reported, we consider this less likely. Both our previous retrospective data in gynecologic surgery as well as multiple properly designed studies in parallel surgical subspecialties suggest optimization of perioperative strategies results in improved surgical outcomes.5
One potential benefit observed in the enhanced recovery after surgery arm of the study was a reduction of postoperative narcotic use. Although multiple studies have suggested a benefit to narcotic reduction with regard to gastrointestinal function, nausea, early enteral feeding, and alterations in the gut microbiome, it is unclear whether the reductions observed in our study were clinically significant, especially as neither time to flatus nor length of stay was obviously affected.1,2,8
Acknowledgments
Supported by the National Institutes of Health (NIH) T-32 training grant 5T32-CA132715 and NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
REFERENCES
- 1.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–41. [DOI] [PubMed] [Google Scholar]
- 2.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466–77. [DOI] [PubMed] [Google Scholar]
- 3.Mayer J, Boldt J, Schellhaass A, Hiller B, Suttner SW. Bispectral index-guided general anesthesia in combination with thoracic epidural analgesia reduces recovery time in fast-track colon surgery. Anesth Analg 2007;104:1145–9 table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011;146:571–7. [DOI] [PubMed] [Google Scholar]
- 5.Dickson E, Argenta PA, Reichert JA. Results of introducing a rapid recovery program for total abdominal hysterectomy. Gynecol Obstet Invest 2012;73:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller C, Kehlet H, Friland SG, Schouenborg LO, Lund C, Ottesen B. Fast track hysterectomy. Eur J Obstet Gynecol Reprod Biol 2001;98:18–22. [DOI] [PubMed] [Google Scholar]
- 7.de Groot JJ, Ament SM,Maessen JM, Dejong CH, Kleijnen JM, Slangen BF. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2016;95:382–95. [DOI] [PubMed] [Google Scholar]
- 8.Nelson G, Kalogera E, Dowdy SC. Enhanced recovery path-ways in gynecologic oncology. Gynecol Oncol 2014;135: 586–94. [DOI] [PubMed] [Google Scholar]
- 9.Lu D, Wang X, Shi G. Perioperative enhanced recovery programmes for gynaecological cancer patients. The Cochrane Database of Systematic Reviews 2015, Issue 3 Art. No.: CD008239. DOI: 10.1002/14651858.CD008239.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx C, Rasmussen T, Jakobsen DH, Ottosen C, Lundvall L, Ottesen B, et al. The effect of accelerated rehabilitation on recovery after surgery for ovarian malignancy. Acta Obstet Gynecol Scand 2006;85:488–92. [DOI] [PubMed] [Google Scholar]
- 12.Chase DM, Lopez S, Nguyen C, Pugmire GA, Monk BJ. A clinical pathway for postoperative management and early patient discharge: does it work in gynecologic oncology? Am J Obstet Gynecol 2008;199:541.e1–7. [DOI] [PubMed] [Google Scholar]
- 13.Gerardi MA, Santillan A, Meisner B, Zahurak ML, Diaz Montes TP, Giuntoli RL II, et al. A clinical pathway for patients undergoing primary cytoreductive surgery with rectosigmoid colectomy for advanced ovarian and primary peritoneal cancers. Gynecol Oncol 2008;108:282–6. [DOI] [PubMed] [Google Scholar]
- 14.Carter J Fast-track surgery in gynaecology and gynaecologic oncology: a review of a rolling clinical audit. ISRN Surg 2012; 2012:368014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalogera E, Bakkum-Gamez JN, Jankowski CJ, Trabuco E, Lovely JK, Dhanorker S, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol 2013;122:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijk L, Franzen K, Ljungqvist O, Nilsson K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand 2014;93:749–56. [DOI] [PubMed] [Google Scholar]
- 17.de Leon-Casasola OA, Karabella D, Lema MJ. Bowel function recovery after radical hysterectomies: thoracic epidural bupivacaine-morphine versus intravenous patient-controlled analgesia with morphine: a pilot study. J Clin Anesth 1996;8: 87–92. [DOI] [PubMed] [Google Scholar]
- 18.Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology 2005;103:1079–88. [DOI] [PubMed] [Google Scholar]
- 19.Rivard C, Dickson EL, Vogel RI, Argenta PA, Teoh D. The effect of anesthesia choice on post-operative outcomes in women undergoing exploratory laparotomy for a suspected gynecologic malignancy. Gynecol Oncol 2014;133:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


