Abstract
Breathing is a well-described, vital and surprisingly complex behaviour, with behavioural and physiological outputs that are easy to directly measure. Key neural elements for generating breathing pattern are distinct, compact and form a network amenable to detailed interrogation, promising the imminent discovery of molecular; cellular, synaptic and network mechanisms that give rise to the behaviour. Coupled oscillatory microcircuits make up the rhythmic core of the breathing network. Primary among these is the preBötzinger Complex (preBötC), which is composed of excitatory rhythmogenic interneurons and excitatory and inhibitory pattern-forming interneurons that together produce the essential periodic drive for inspiration. The preBötC coordinates all phases of the breathing cycle, coordinates breathing with orofacial behaviours and strongly influences, and is influenced by, emotion and cognition. Here, we review progress towards cracking the inner workings of this vital core.
We must, and do, breathe without serious interruption from birth to death. In health and at rest, breathing comes naturally, effortlessly and without thought. In the hour or so to read this Review, you will take ~700 normal breaths, plus ~12 sighs. You will probably adjust your breathing to cough, sneeze, yawn (hopefully not induced by this Review), mutter or comment out loud and, if you are eating, coordinate your breathing to chew and swallow, and, for the rodents among you, whisk.
Despite the deceptive simplicity of breathing — the essential elements of which develop in utero — it requires a sophisticated motor program to ventilate the lungs and respond appropriately to physiological challenges and changing environmental conditions (FiG. 1). Like locomotion and other rhythmic behaviours for which the basic movement pattern is generated within the brainstem and/or spinal cord without the need for peripheral or suprapontine input, breathing originates from a central pattern generator (CPG) that is functionally divisible into rhythmogenic components that drive downstream motor pattern-forming components (FiG. 2a).
Fig. 1 |. The anatomy and physiology of respiration.
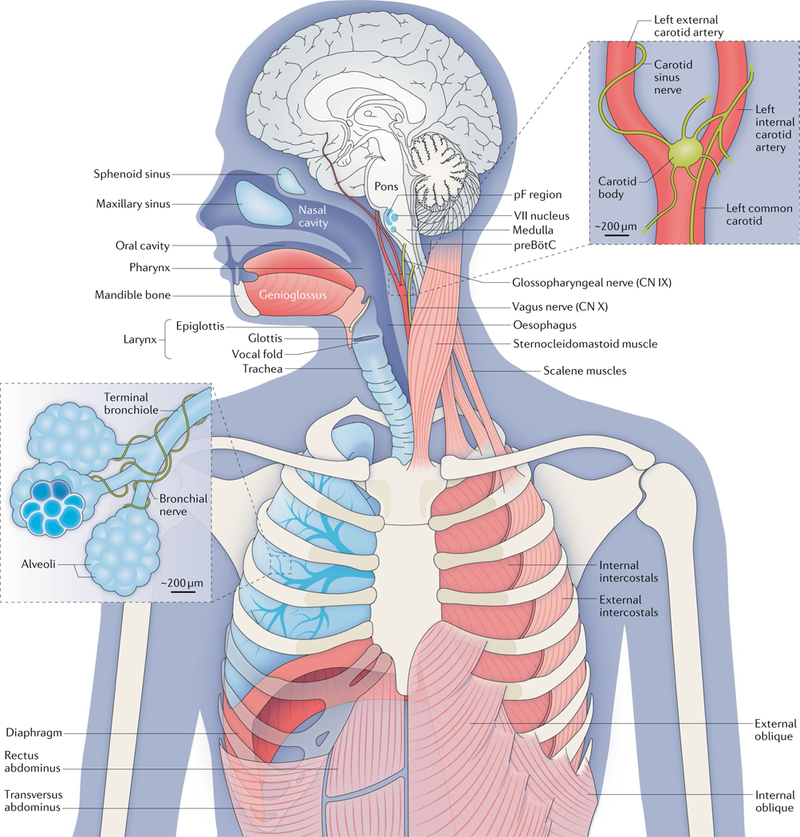
Breathing movements depend on pump, resistance and accessory muscles. Pump muscles include the dome-like, uniquely mammalian diaphragm and the external intercostals. During inspiration, descent of the diaphragm combined with rib elevation by the external intercostals expands the lungs to draw in air (assuming the airways are patent). The oblique abdominals and transversus abdominus and internal intercostals are expiratory pump muscles. Airway resistance muscles, which modulate inspiratory and expiratory airflow, include skeletal muscles of the tongue (including the genioglossus (shown) and the hyoglossus, styloglossus and stylohyoid muscles (not shown)), glottis, larynx and pharynx, as well as smooth muscle of the bronchi. The sternocleidomastoid and scalene muscles are accessory muscles that stabilize the rib cage. The gas-exchanging surface of human lungs, consisting of ~5 × 108 alveoli each measuring 200 μm in diameter, is roughly half the size of a tennis court (~70 m2) but is contained in a volume of <3 litres. At rest, we inhale and exhale ~5 litres of air per minute (~10 × 500 millilitre breaths per minute, containing ~ 1 litre of O2); we extract from the inspired air ~250 millilitres of O2 per minute to support metabolism and add to the expired air ~200 millilitres of CO2 per minute. The regulation of breathing relies on feedback from peripheral and central chemosensors. Carotid bodies, at the branch point of the carotid arteries, monitor the partial pressure of O2 (pO2), the partial pressure of CO2 (pCO2) and pH in arterial blood and signal to the brainstem via the glossopharyngeal nerve (cranial nerve (CN) IX). In the brainstem, chemosensory neurons and glia in the ventral parafacial nucleus (pFV) and other regions detect and respond to fluctuations in CO2 levels and pH in the cerebrospinal fluid. These neurons project paucisynaptically to the preBötzinger Complex (preBötC) and other sites to influence breathing to maintain homeostasis. Breathing in mammals (and other air breathers) under most conditions is extremely sensitive to changes in CO2 levels that directly affect pH. For example, in resting humans, an ~2.5% increase in pCO2 from 40 to 41 mmHg will increase ventilation ~40% from ~5 to ~7 litres per minute. By contrast, breathing is relatively insensitive to changes in O2 levels at rest. The O2–haemog1obin dissociation curve is fairly flat at normal levels of arterial O2 (100 ± 20 mm Hg), meaning that haemoglobin is >96% saturated with O2 even if breathing increases substantially. However, under certain conditions (for example, high altitude or intense exercise), hypoxia provides a powerful stimulus to breathe. Continuous breathing comes at a considerable metabolic cost insofar as the respiratory muscles are the only skeletal muscles that are active during all sleep and wake states. To counter this, mammals evolved a powerful diaphragm that is sufficient to inflate the lungs, and a resting breathing pattern in which inspiration (and usually postinspiration) is active (requiring muscle contraction), whereas expiration is passive. In birds (and most lower vertebrates) that do not have a diaphragm, inspiration and expiration are both active at rest. Central breathing networks are also modulated by mechanosensory feedback regarding the status of the pump muscles and lungs. Stretch- receptor afferents in airway smooth muscle encode volume-related information via the vagus nerve (CN X), which is crucial in maintaining optimal lung volumes for efficient breathing. This feedback also underlies Breuer-Hering reflexes, which are important in controlling the timing and pattern of each breath. Lung stretch-receptor afferents and their central relay interneurons rhythmically inhibit the inspiratory preBötC and excite the expiratory lateral parafacial nucleus (pFL) when lungs are inflated (inspiratory termination reflex) and conversely excite the preBötC and inhibit the pFL when lungs are deflated. VII nucleus, facial motor nucleus. The figure is based on a drawing contributed by J. Milstein.
Fig. 2 |. Elements of the breathing central pattern generator. a.
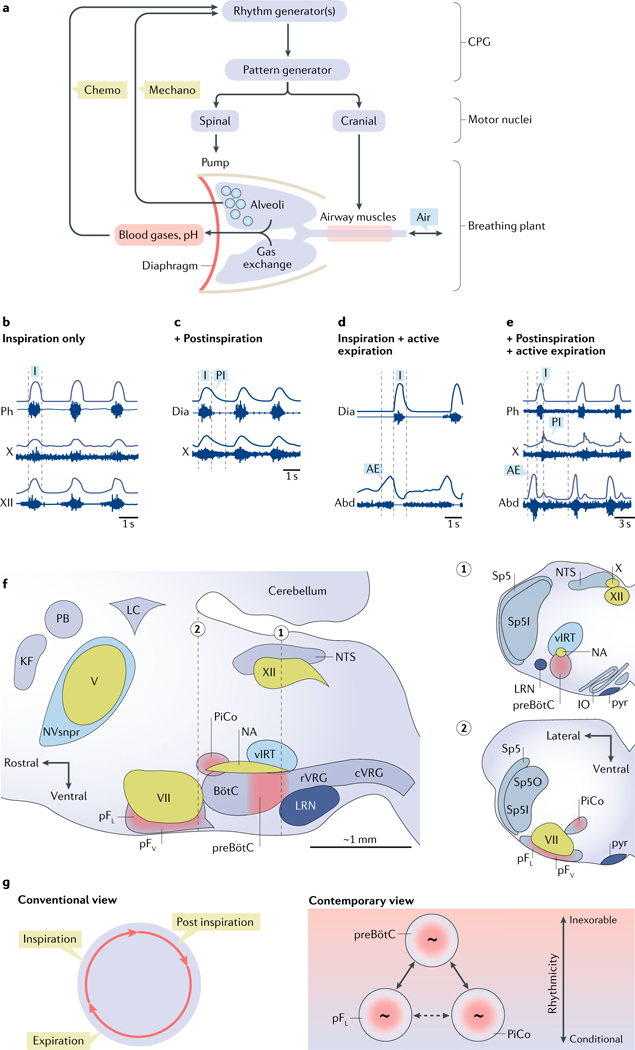
a | Schematic view of breathing neural and physical plant. The image shows a central pattern generator (CPG) composed of rhythm-generating and pattern-generating microcircuits. Spinal and cranial motor nuclei innervate pump and airway muscles, respectively. Airways, lungs, blood gases and sensory (‘chemo’ and ‘mechano’) feedback are indicated. b | Inspiration (I) is the inexorable phase of the breathing cycle. In an anaesthetized rat, the inspiratory cycle is evident in electrical recordings (raw and integrated signals) from the phrenic nerve (Ph), a branch of the vagus nerve (cranial nerve (CN) X) innervating laryngeal adductor muscles, and the hypoglossal nerve (CN XII) innervating the genioglossus muscle (tongue protruder). c | Also in an anesthetized rat, inspiration recorded via diaphragmatic (Dia) electromyography (EMG), shown with postinspiration (PI) recorded from the laryngeal branch of CN X. d | Also in an anesthetized rat, inspiration shown via diaphragmatic EMG (Dia), with active expiration (AE; evoked by disinhibition of the lateral parafacial respiratory group (pFL)) indicated by abdominal EMG (Abd). e | In a reduced in situ rat preparation, inspiration, postinspiration and active expiration measured via electrical recordings from the phrenic (Ph) nerve, CN X and lumbar abdominal (Abd) nerve, respectively. Active expiration was evoked by hypercapnia. f | Parasagittal view of the brainstem containing the breathing CPG. Respiratory rhythmogenic sites are shown in red: the preBötzinger Complex (preBötC; inspiratory), the pFL (expiratory) and the more medial chemosensitive ventral parafacial respiratory group (pFV; rhythmogenic in the perinatal period only), as well as the ‘postinspiratory comp1ex’(PiCo; hypothesized to underlie postinspiration). Other rhythmogenic sites, such as the whisking-related ventral intermediate reticular formation (vIRT) and the masticatory trigeminal principal sensory nucleus (NVsnpr), are shown in blue. Cranial motor nuclei controlling airway resistance muscles, the hypoglossal motor nucleus (XII) and the nucleus ambiguus (NA), as well as facial muscles, the facial motor nucleus (VII) and the trigeminal motor nucleus (V), are shown in green. Brainstem sites associated with breathing motor pattern or sensorimotor integration are shown in grey: the rostral ventral respiratory group (rVRG) containing inspiratory, that is, phrenic and external intercostal, premotor neurons and the caudal ventral respiratory group (rVRG) containing expiratory premotor neurons. Other sites include the pontine Kölliker-Fuse nucleus (KF) and parabrachial nucleus (PB), the nucleus of the solitary tract (NTS) and the expiratory BotC. Also shown is the locus coeruleus (LC) that receives preBötC projections, the cerebellum and the lateral reticular nucleus (LRN). Insets 1 and 2 show transverse sections at the level of the preBötC (dotted line 1) and pF (dotted line 2). Additional structures in the insets include the spinal trigeminal tract (Sp5), the spinal trigeminal sensory nucleus oralis (Sp5O) and the interpolaris (Sp5I), the inferior olive (IO) and the pyramidal tract (pyr). g | In the conventional view of breathing, inspiration, postinspiration and expiration constitute a continuous unitary breathing cycle. According to the contemporary view, inspiration is the inexorable part of the breathing cycle, whereas postinspiration and expiration are conditional, driven by three distinct, coupled oscillators. When all parts of the cycle are manifest, the preBötC coordinates the phases. Part a is adapted with permission from REF227, Elsevier. Part b is adapted with permission from APS, REF228. Part c is republished with permission of Society for Neuroscience, from Role of inhibition in respiratory pattern generation, Janczewski, W. A. et al. 33 (13), 2013 (REF126); permission conveyed through Copyright Clearance Center, Inc. Part d is republished with permission of Society for Neuroscience, from Active expiration induced by excitation of ventral medulla in adult anesthetized rats, Pagliardini, S. et al. 31 (8), 2011 (REF.83); permission conveyed through Copyright Clearance Center, Inc. Part e is adapted with permission from REF.229, Elsevier.
At rest in mammals, a typical breath results from a phase of active contraction of inspiratory pump muscles (the diaphragm and external intercostals), followed by a passive expiratory phase (FiG. 2b). There is often a third phase at the end of inspiration, postinspiration, in which lengthening contraction of the diaphragm and adduction of laryngeal muscles (which affect air-flow resistance) slow expiratory airflow (FiG. 2c). When metabolism increases, expiratory muscles (the abdominals and internal intercostals) are recruited to produce active expiration (FiGS. 1,2d,e).
Therefore, eupnoea always features active inspiration, commonly postinspiration and sometimes active expiration; the phasic composition and pattern of breathing muscle activity are state-dependent. Furthermore, the breathing pattern adapts on a longer timescale to conditions such as growth and maturation, pregnancy, ageing, disease and injury. The essential adaptability of breathing is built into the breathing CPG, which has at its core the preBötzinger Complex (preBötC), a medullary microcircuit that generates the rhythm of inspiration.
Here, we define the preBotC microcircuit and its interactions with additional breathing microcircuits, consider mechanisms of rhythm generation and describe how the inspiratory breathing rhythm provides a signal that coordinates orofacial behaviours and that may affect emotional regulation, even cognitive function.
The inspirational preBötzinger Complex
Inspiratory muscle contraction is the inexorable feature of the mammalian breathing cycle. Its underlying rhythm arises from neural activity in the preBötC, a region identified in fruit bats, moles, goats, cats, rabbits, rats, mice and humans1–7 (FIG. 2f).
The preBötC was initially defined as the region of ventrolateral medulla necessary and sufficient to generate inspiration-related rhythm and motor output in brainstem slices from neonatal rat, albeit with fuzzy borders and indeterminate neuronal constituency1. Most breathing-related preBötC neurons show activity in phase with inspiration. Their axons cross the mid-line to synchronize preBötC neurons bilaterally, and also project to breathing-related medullary premotor regions8–10, some of which in turn project, directly or indirectly, to motor neurons that drive both inspiratory pump and airway resistance muscles (FIGS. 1,2a). There are also numerous direct preBotC projections to other pontomedullary11,12 and suprapontine13 sites. Perturbing preBötC excitability modulates breathing frequency in vitro1,14,15. For example, DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin) activates μ-opioid receptors and substance P activates neuro-kinin 1 receptors (NK1Rs) expressed by subpopulations of preBötC neurons to potently slow or speed up inspiratory rhythm, respectively16–22. Other peptides, including somatostatin (SST), thyrotropin-releasing hormone (TRH), neuromedin B (NMB) and gastrin-releasing peptide (GRP), also act potently on (subsets of) preBötC neurons to modulate inspiratory rhythm and pattern6,23–25 (TABLE 1).
Table 1 |.
Selected markers of interneurons in the preBötzinger Complex
| Marker | (Partially) overlapping markersa |
Relevance to, or effects on, preBötC interneurons | Refs |
|---|---|---|---|
| NK1R | SST and μOR | Peptide receptor that depolarizes (a subset of) rhythmogenic neurons; speeds up inspiratory rhythm | 16,29,230,231 |
| μOR | NK1R | Peptide receptor that hyperpolarizes (a subset of) preBötC neurons; slows down inspiratory rhythm | 16,21 |
| SST | NK1R, VGLUT2 and DBX1 | Marker of pattern-generating output neurons (probably not rhythmogenic) | 1,29,39,40,44,45,230 |
| Reelin | NK1R and SST | Marker for preBötC neurons and bulbospinal premotor neurons | 38 |
| TRH | – | Peptide that depolarizes (a subset of) preBötC neurons; speeds up inspiratory rhythm | 25 |
| VGLUT2 | DBX1and SST | Marker for glutamatergic neurons that are essential for inspiratory rhythmogenesis | 40,44,45,232 |
| DBX1 | VGLUT2, SST, SSTR2A and NK1R | Transcription factor expressed by glutamatergic rhythmgenerating neurons, premotor neurons and midbrain- projecting neurons | 10,12,44,45,169,233 |
| NMBR or GRPR | SST | Peptide receptors that induce sighs | 189 |
| CDH9 | DBX1 | Marker for neurons that project to the (locus coeruleus within the) pons that are potentially involved in arousal | 12 |
μOR, μ-opioid receptor; CDH9, cadherin 9; DBX1, developing brain homeobox protein 1; GRPR, gastrin-releasing peptide receptor; NK1R, neurokinin 1 receptor; NMBR, neuromedin B receptor; preBötC, preBötzinger Complex; SST, somatostatin; SST2AR, somatostatin 2A receptor; TRH, thyrotropin-releasing hormone; VGLUT2, vesicular glutamate transporter 2.
Interneuron populations defined by individual markers overlap to varying degrees with other populations.
Exploiting peptide transmitter and receptor expression has illuminated the key role of the preBötC in breathing. Slowly (over days) killing NK1R-expressing preBötC neurons leads progressively to severe breathing pathology in adult rats26,27 and goats5,28. Rapidly (in minutes) silencing glutamatergic SST-expressing preBötC neurons in awake rats leads to apnoea, requiring rescue by mechanical ventilation29. Alas, peptides and their receptors are imperfect markers, because their expression is not confined to preBötC neurons. For example, expression of NK1R and the glycoprotein reelin extends caudally from the preBötC to the rostral ventral respiratory group that contains phrenic premotor neurons18,30,31, dorsorostrally to densely labelled swallowing-related motor neurons ofthe compact nucleus ambiguus16–18,32–35, and ventrorostrally breathing-related neurons of the parafacial nucleus (pF; see below)36–38 (FIG. 2f). SST is expressed not only by preBötC neurons but also in neighbouring regions4,39,40. Thus, the anatomical boundaries of the preBötC (Fig. 2f) remain fuzzy, which may reflect reality (that is, an actual lack of a sharp anatomical boundary between breathing-related functions ascribed to the preBötC and other functions) or a lack of adequately specific markers.
Nevertheless, delineating preBötC neuronal sub-populations on the basis of features such as anatomy, electrophysiology, transmitter phenotype or peptide and peptide receptor expression is of great value allowing investigation of their roles in generating breathing rhythm or pattern, or in coordinating breathing with other functions, such as whisking or licking41,42.
Genetics, including transcription factors and transmitter-related transcripts, provide another means to classify neuronal subpopulations. One subpopulation of considerable interest consists of glutamatergic preBötC neurons whose precursors express the transcription factor developing brain homeobox protein 1 (DBX1), hereafter referred to as DBX1 neurons, even though the perinatal and adult neurons no longer express DBX1. (In the following, please note that we — and most other groups — have focused on identifying the roles of DBX1 neurons, while not fully considering that Dbx1 -expressing precursors also give rise to preBötC glia43–45, which must factor into interpretations of the effects of Dbx1 knockouts and genetic manipulations of Dbx1-derived cell populations.) Dbx1 -knockout mice do not form a recognizable preBötC and die at birth because they do not breathe; in vitro preparations from these mice, which retain the medulla, do not generate inspiratory rhythm44,45. DBX1 preBötC neurons in vitro express rhythmogenic membrane properties46. Optogenetic photoinhibition in the preBötC of mice expressing archaerhodopsin in DBX1 cells (that is, including neuronal somata, axons and synaptic terminals, as well as glia) gradually slows and ultimately stops inspiratory rhythm in reduced preparations in vitro and breathing in vivo40,47, whereas transient photostimulation of channelrhodopsin-expressing DBX1 cells during expiration can trigger premature inspiration39. Cumulative laser ablation of individual DBX1 neuronal somata in the preBötC slows and then stops rhythmic inspiratory motor output in vitro48. Thus, most of the evidence suggests that DBX1 preBötC neurons are primarily responsible for these effects and that the DBX1 neuronal population includes rhythm-generating neurons, as well as non-rhythmogenic neurons (for example, see REF39), and neurons that transmit preBötC signals throughout the breathing CPG11,12.
Active expiration and dual oscillators
Expiration in mammals is normally passive at rest, presumably because with the appearance of the diaphragm in mammals (FIG. 1), this breathing pattern is more efficient than one with two active phases that is often seen in lower vertebrates (see Supplementary Box 1). With increased demand for O2 (for example, when fleeing a predator), expiration becomes active; this active expiration forces lung volume below its normal resting level, which increases the tidal volume of the subsequent breath (because the next inspiration starts from a smaller lung volume). Assuming no slowing of frequency, the result is an increase in ventilation that delivers more O2 to the lungs.
Expiration becomes active during vigorous exercise when metabolic demand is increased, at altitude where ambient O2 levels are reduced and intermittently in rapid eye movement (REM) sleep49–51. The activation of expiratory muscles in REM sleep is surprising because the activity of most motor neurons is dramatically reduced during REM52. One possible explanation is that the enhancement of breathing resulting from intermittent active expiration counteracts the effects of REM-induced irregular breathing, which is insensitive to chemoreceptor drive49,51,53,54.
Active expiration is also a vital component of various volitional and emotional acts, such as speech, laughing, crying or playing musical instruments. The dual oscillator hypothesis55,56 posits that the normally unceasing preBötC oscillator generates inspiratory rhythm, whereas the normally quiescent, conditional pF oscillator (see below), when turned loose, generates the rhythm underlying active expiration (see, for example, FIG. 2d,e).
The pF abuts the ventral and lateral margins of the facial nucleus; it extends from the dorsal limit of the pyramidal tract medially to the spinal trigeminal tract laterally and contains NKlR-expressing glutamatergic interneurons. Two functionally distinct microcircuits — one laterally located expiratory rhythmogenic site (pFL) and another, ventrally located, chemosensitive site (pFV) — are hypothesized to be crucial subpopulations for central chemoreception of CO2 and pH and for active expiration57,58 (inset 2 in FIG. 2f).
The ventral parafacial nucleus.
Neurons of the pFV — which was originally and is still most often referred to as the retrotrapezoid nucleus (RTN)8,59–61 — detect signals related to CO2 and/or pH levels (possibly with the involvement of glia62,63) and transmit them to the preBötC and other brainstem sites. pFV neurons derive from dorsal progenitor cells that express paired-like homeobox 2B (PHOX2B) and later express atonal homologue 1 (ATOH1)64,65. During late embryonic and early postnatal stages, pFV neurons spontaneously generate late-expiratory bursts66,67 that entrain preBötC rhythm and boost its excitability68,69.
For unknown reasons, postnatal pFV neurons lose their spontaneous, late-expiratory rhythmicity70 while retaining their presumptive role in chemosensation. Neurons of the pFV (that is, the RTN) nonetheless continue to express PHOX2B (for unknown reasons), which has been used as a marker of these neurons in mature rats71–76 to investigate central chemoreflexes59,60,74,77–82.
The lateral parafacial nucleus.
In mature rats, pFL neurons do not express PHOX2B83. In contrast to the extensive characterization of pFV neurons59,60,68,84, the genetic identity of the conditional expiratory pFL neurons remains unresolved.
Neurons of the pFL seem silent at rest but are conditionally rhythmic. Disinhibition or activation of pFL neurons triggers late-expiratory bursts of action potentials in these cells, concurrent with active expiration; this link is hypothesized to be causal56–58,80,83. Consistent with such a role, pFL neurons project to expiratory premotor neurons in the caudal ventral respiratory group (cVRG; FIG. 2f), which in turn project to segmental interneurons that excite lumbar motor neurons that innervate abdominal (expiratory pump) muscles85,86 (FIGS. 1,2a).
Active expiration and inspiration are tightly coordinated (see REF58 for a comprehensive description of pre-sumptive preBötC-pFL interactions). In adult rodents, the preBötC provides obligatory excitatory drive that facilitates pFL function; if the preBötC is suppressed, active expiration seems unable to occur58. This drive is putatively tonic and is perhaps provided by tonically firing preBötC neurons rather than those with rhythmic inspiratory activity. Nevertheless, in younger rodents, expiratory rhythm can be sustained without preBötC activity, revealing another form of preBötC-pFL coupling56,87.
The preBötC also transiently inhibits the pFL during inspiration to prevent co-activation of inspiratory and expiratory muscles, which is undesirable during normal breathing but useful during Valsalva manoeuvres (see below). When preBötC activity is suppressed in neonatal or juvenile rats by opioids or excessive lung inflation, the rhythmic activity of late-expiratory pFL neurons, as well as of expiratory muscles and nerves, which are all ordinarily inhibited during inspiration, continues uninterrupted55,56,87,88.
Thus, tonic excitatory projections from the preBötC to the pFL are of most importance in adult mammals for maintaining pFL functionality, whereas phasic inhibitory projections from the preBötC modulate pFL activity on a cycle-to-cycle basis throughout postnatal life to prevent unwanted inspiratory-expiratory co-activation.
Postinspiration
In addition to the continually rhythmic preBötC inspiratory oscillator and the conditionally rhythmic pFL active expiratory oscillator, the motor pattern of breathing in mammals most often incorporates a third phase, postinspiration, which occurs immediately following inspiration (FIG. 2c,e). During postinspiration, shortening contraction of laryngeal adductor muscles (which increases airway resistance) and lengthening contraction of the diaphragm act to retard lung deflation. Postinspiration benefits alveolar gas exchange, by increasing the amount of time that air stays in the lungs89,90, and promotes laminar expiratory airflow that diminishes the likelihood of airway collapse. Modulation of airway resistance during postinspiration also contributes to expulsive reflexes, such as cough91–93, and non-respiratory behaviours, such as swallowing91,94–97 and vocalization98.
Postinspiration is conventionally viewed as an inextricable part of the breathing cycle (FIG. 2g), with a class of models ascribing an essential rhythmogenic role in (normal) tidal breathing to the postinspiratory activity of inhibitory interneurons in the BötC (which is rostral to and distinct from the preBötC)99–101 (FIG. 2f). However, because postinspiratory muscle activity stops intermittently during sleep and under anaesthesia, it is not essential for tidal breathing90 or breathing rhythmogenesis. (Whether chloride-mediated inhibition originating in the BötC is obligatory for rhythmogenesis is discussed below in Synaptic inhibition models).
The neural mechanisms underlying postinspiration are attributed to a distributed, non-rhythmogenic network involving lung mechanoreceptors102 and pontine circuits90,103–105. Recently, postinspiratory activity was proposed to be generated by a noradrenaline-sensitive, conditional oscillator medial to the pF and the VII nucleus, dubbed the ‘postinspiratory complex’(PiCo)106 (inset 2 in FIG. 2f). PiCo interneurons are both glutamatergic and cholinergic, and are modulated by SST and opioids, unequivocally differentiating them from their (active) expiration-related neighbours in the pFL55,56,85,87 and BötC107–110. PiCo is postulated to be an independent breathing-related oscillator for the following reasons: in neonatal rodent slices, peptides independently modulate PiCo and preBötC rhythms; PiCo retains its rhythmicity when isolated; and stimulation of PiCo interneurons in vitro evokes postinspiratory, but never inspiratory, bursts106.
Regardless of where postinspiratory activity originates, it must be coordinated with inspiratory preBötC and expiratory pFL activities (FIG. 2g). Although PiCo-pFL interactions remain to be characterized, the dominant PiCo-preBötC relationship seems to be mutually inhibitory. In vivo, optogenetic activation of PiCo delays the onset of the next inspiration106. In vitro, photoactivation of the preBötC inhibits PiCo interneurons106; in normal breathing, this mutual inhibition would ensure that PiCo activity is not concurrent with inspiration.
Rhythm generation: a rogues’ gallery
More than a quarter of a century since the discovery of the preBötC and the development of rhythmically active in vitro preparations, the mechanisms of rhythm generation remain unresolved. We assert that this is due to the persistence of canonical models of rhythmogenesis in which phase-sequencing synaptic inhibition, or cellular pacemakers (that is, specialized autorhythmic neurons), are essential, despite increasingly strong evidence against them. Further complicating matters, conventional aspects of CPG rhythms typically considered to be crucial, in particular neuronal bursts of action potentials111, may not be so. Here, we discuss several models of rhythmogenesis.
Synaptic inhibition models.
Since the 1970s, the paradigmatic model for breathing was built on the longstanding, familiar ‘half-centre’ concept112,113 that depends on synaptic inhibition. According to this model, regenerative inspiratory activity, produced through recurrent excitation, exceeds a threshold, triggering a powerful inhibitory network that forces a system-wide reset, followed by a refractory period (expiratory phase) before the next inspiratory phase114,115. This model is intuitive and appealing because, with the right parameters, it can readily generate an output pattern resembling phrenic nerve discharge in vivo. However, experiments in reduced preparations in vitro that spontaneously generate rhythmic breathing-related motor output do not support this model. Blocking GABA type A receptor (GABAAR)-mediated and glycine-mediated inhibition in rhythmic slices does not stop or even substantially change the frequency of breathing-related rhythm116, nor do blockers of GABA type B receptor (GABABR)-mediated K+ currents117,118, indicating that conventional postsynaptic inhibition is not obligatory for preBötC rhythmogenesis, at least in vitro.
A revision of the half-centre-like paradigm features a ring ofthree interconnected inhibitory neuronal populations: one in the preBötC and two in the adjacent BötC (that need not be present in rhythmic slices)1,119. The computational inhibitory ring model can be parameterized to produce a breathing pattern with sequential and discrete inspiratory, postinspiratory and late-expiratory phases100,101,120,121 (BOX 1).
Box 1 |. Mathematical models of breathing.
Mathematical modelling complements experimental neuroscience by providing proof of principle and non-trivial, experimentally testable predictions. Before recounting the utility of models, we discuss some provisos of such approaches.
Compared with single-neuron models, network models tend to be more problematic because their real-life connectivity and synaptic properties are highly relevant and yet unknown, and (in many cases) currently unknowable. Therefore, network models of the breathing central pattern generator (CPG) cannot avoid using myriad unmeasured parameters. Given that such parameters can be continually recalibrated or adjusted to fit new data, the risk is that such models represent a sliding proof of principle rather than a substantive verification of the underlying conceptual model.
In contrast to detailed network models, highly abstracted CPG models feature lumped populations or state variables that track average firing rates or plateau-like states rather than actual neuronal variables. Such abstracted models can be useful in understanding how populations interact, if validated by spiking-based models206. However, abstract model components that have no biological analogue risk failing to recapitulate spike-timing-dependent mechanisms that are crucial for many neural functions, including emergent network rhythms (for example, see FIG. 3c).
Models with the potential to advance our understanding of the neural bases of breathing should meet several criteria:
Their equations and parameters should be publicly available (for example, in open-access databases such as Model DB and BioModels Database (see Related Links)) for independent replication
They should adhere to the generally accepted industrial standard of ‘verification, validation, and uncertainty quantification’ (VVUQ)207
They should track membrane potentials and spikes rather than abstracted variables, such as spike rate
They should comprise hundreds to thousands of individual neurons with realistic intrinsic properties and connectivity. For example, the preBötzinger Complex (preBotC) includes ~1,000 core interneurons16,29,48,147,170,173,189,208, among which constituent rhythmogenic neurons connect to one another via excitatory chemical synapses with a one-way probability of ~13% and excitatory postsynaptic potentials measuring ~3 mV (REF.171)
preBötC models should generate burstlets and should not require inhibition, pacemaker neurons, postinspiratory activity or bursts as essential for normal rhythm generation
Stimulation of four to nine constituent neurons in a constructed preBotC model must result in network-wide bursts and motor output148 (if motor circuits are included170)
They must be probed for sensitivity of all parameters that are not tightly constrained by data209 and fit data that were not used to constrain them
They must reproduce the phase-response relationships measured in vivo39,127, 210
Several biophysically realistic models have helped elucidate the breathing CPG. Experimental tests of a preBotC model composed of conditional pacemaker neurons and excitatory synapses133,134 renewed emphasis on synaptic interactions and network (rather than pacemaker) properties in rhythmogenesis129,135,211–213. A group-pacemaker-based network oscillator132 dependent on synaptically triggered conductances for bursting154 generated non-intuitive predictions — some right, some wrong. Although this model’s dependence on bursting conductances probably does not accurately reflect rhythmogenesis111,151, together with cell-photoablation experiments in slices, the model predicted the existence of premotor neurons in the preBotC core170,214. This prediction was later validated by further photoablation studies in slices169 and photostimulation experiments in adult mice39.
Two contemporary models are noteworthy for entirely emphasizing emergent network properties. Schwab et al.215 and Guerrier et al.172 built networks that demonstrated rich dynamics using neurons without bursting conductances and with rudimentary connectivity and synaptic dynamics.
Burstlet theory furthers this progression from cellular to network properties by providing experimental evidence for independent processes that underlie network bursts and rhythm111,151. We propose that breathing rhythmogenesis relies on the dynamics of excitatory synapses and network connectivity rather than pacemakers, inhibition, bursts or postsynaptic conductances.
How does the inhibitory ring model of rhythmogenesis stand up to experimental tests? In preparations in situ (that is, retaining the rostral body and neuraxis of a (usually) juvenile, decerebrate rodent retrogradely perfused through the aorta, allowing recordings of motor activity from multiple spinal and cranial nerves), blocking glycine-mediated inhibition causes the normally distinct inspiratory and postinspiratory phases in phrenic and vagus nerve activity to merge, but the rhythm nevertheless persists122. Along similar lines, blocking all ionotropic receptor-mediated inhibition in situ, either completely by removing chloride from the perfusate101 or by co-application of GABAAR blockers and glycine receptor blockers123, alters the pattern and frequency of nerve activity from rhythmic to tonic.
These in vitro and in situ observations are interpreted to represent validation of the inhibitory ring model101,121–123 because they show perturbation of rhythmicity during diminished or absent inhibition. However, such criteria are insufficient for validation. Rather, the experiments in vitro and in situ show that GABAAR-mediated and glycine-receptor-mediated inhibition influences preBötC excitability and can thus affect the rhythm or pattern of inspiratory motor output and the phasic relationship between inspiration and postinspiration124,125. However, these findings do not show that inhibition is necessary for inspiratory rhythmogenesis.
Of course, the real arbiter of the physiological relevance of inhibition in rhythmogenesis is what happens in (more or less) intact mammals when inhibition is perturbed locally. In adult rodents with intact afferent feedback, blocking inhibition pharmacologically within the preBötC (and the rostrally adjacent BötC) does not stop breathing126. Rather, this manipulation within the preBötC blocks the powerful Breuer-Hering reflex mediated by pulmonary stretch-receptor affer-ents of the vagus nerve, leading to lower-frequency and greater-amplitude inspiration. If this reflex is eliminated by cutting the vagus nerve, which itself lowers the frequency and increases the peak amplitude of inspiration, then blocking inhibition in the preBötC (and/or BötC) has little or no further effect126. Even vagotomized adult rodents lacking peripheral chemoreceptor drive from carotid bodies maintain breathing rhythms after focal blockade of inhibition in the preBötC123. Consistent with these results, transient photoinhibition of preBötC glycinergic neurons during inspiration increases its amplitude but not its duration127. We conclude that synaptic inhibition is not essential for inspiratory rhythmogenesis in intact mammals.
Pacemakers.
The initial identification of the preBötC included recordings from voltage-dependent, rhythmically bursting, putative ‘pacemaker’ neurons1. preBötC neurons with these pacemaker-like properties, subsequently shown to depend on the persistent Na+ current (INaP) or the Ca2+-activated nonspecific cationic current (ICAN) for their autorhythmicity128–131, were hypothesized to underlie the preBötC rhythm1,132. Although mathematical models of preBötC rhythmicity that consist of simulated excitatory pacemaker neurons support the feasibility of this hypothesis133–135, it does not stand up to experimental tests.
INaP and ICAN are not limited to excitatory neurons with pacemaker properties; rather, they are widely expressed in all excitatory46,136 and many inhibitory137 preBotC neurons. Thus, any perturbation of these currents will affect most (if not all) preBötC neurons and cannot be ascribed to effects on only pacemaker or pacemaker-like neurons. Moreover, drugs that attenuate INaP and ICAN, such as riluzole and flufenamic acid (which, in experiments in vitro, inconsistently perturb or stop preBötC and motor nerve rhythms), nonspecifically affect other neuronal properties, such as membrane potential and synaptic transmission138,139. In addition, in some studies, these drugs are applied at excessively high concentrations — that is, doses several-fold higher than their half-maximal effective concentration — and without stringent experimental controls that would account for effects outside the preBötC. Indeed, when the off-target effects of drugs such as riluzole or flufenamic acid on excitability are explicitly offset or limited to the preBötC, rhythm and motor output continue unabated129,140. Although gasping-like rhythms in reduced in vitro and in situ preparations may involve INaP-dependent pacemaker properties131,141, more than 20 years of in vitro experiments have failed to produce strong evidence for the rhythmogenic role of pacemaker-like properties in normal eupnoea-like breathing and indeed have yielded considerable data that refute this hypothesis. Nonetheless, some researchers continue to interpret data obtained under these experimental conditions as confirmation of the pacemaker hypothesis131,142–144.
As discussed above, in vivo data are the ultimate arbiter of physiological relevance. Pacemaker or pacemaker-like neurons in the preBötC in vivo are conspicuous by their absence; if they are there and essential, they should be easy to identify. The pacemaker hypothesis may not be dead, but is, at best, gasping.
Recurrent excitation: bursts and burstlets.
A network oscillator in which constituent interneurons, if provided with excitatory drive, interact to produce a rhythm is an alternative to the unlikely half-centre and pacemaker-driven breathing CPG models145,146. The premise of the model is that, starting with a mostly silent network after inspiration, spontaneous activity in a few preBötC neurons induces postsynaptic activity in other preBötC neurons, with connectivity and excitability tuned such that activity percolates among them, building to a crescendo during inspiration147–152. According to the group-pacemaker hypothesis of rhythmogenesis132,153,154, such recurrent excitation evokes inward currents such as ICAN in preBötC neurons, leading to a pronounced network-wide burst. This burst is followed by a transient refractory period proposed to be due to intrinsic activity-dependent outward currents and synaptic depression155–158 but not, for reasons explained above, requiring postsynaptic inhibition. The cycle restarts with the abatement of refractoriness and the spontaneous resumption of activity in a few neurons, which reignites the percolation process.
We posit that a small fraction of preBötC neurons initiates each cycle of recurrent excitation to generate the collective burst148, and that the initiating neurons can differ for each cycle. This notion is based on our observation that concurrently exciting as few as four preBötC inspiratory neurons in vitro (by photolytic glutamate uncaging) reliably triggers, after a 100–400 ms delay, a preBötC network burst and motor output148. Note that breathing-related rhythms in vitro are typically ~0.2 Hz, whereas breathing frequency in intact rodents typically exceeds 2 Hz. At a higher temperature in vivo (~37 °C, versus ~27 °C in vitro), far more preBötC neurons are spontaneously active, providing multiple seed points for recurrent excitation and accelerating the entire process, consistent with frequencies of 2–5 Hz in mice.
A requisite feature of breathing movements is rhythmic bursts of action potentials generated by preBötC neurons that propagate via pattern-related premotor neurons to motor neurons that drive inspiratory muscle contraction. This requirement underlies the long-standing paradigm that rhythmic bursts in the core of the breathing CPG (that is, the preBötC) are an essential rhythmogenic mechanism115,159. We suggest not. Why?
In field potential recordings of the preBötC in vitro, low-amplitude population activity during pre-inspiration precedes high-amplitude inspiratory bursts111 that lead inexorably to motor output. A critical component of the excitatory drive underlying pre-inspiratory and inspiratory activity in the preBötC is a steady Na+ leak current (via the Na+ leak channel NALCN) that tonically depolarizes rhythmogenic preBötC neurons160,161 (and, given its pharmacokinetics, NALCN probably mediates (some of) the effects of peptide modulators on preBötC function162,163). However, pre-inspiratory and inspiratory activity are not inexorably linked; imposing mild reductions in neural excitability in vitro (by reducing extracellular K+ levels) causes intermittent failure of the inspiratory burst, such that only the low-amplitude component (known as the ‘burstlet’) remains. Further reductions in excitability increase the failure rate of these bursts, yet the burstlet rhythm continues, with its frequency similar to the original burst-only rhythm111 (see panels depicting 9 mM to 3 mM K+ in FIG. 3a). preBötC neurons discharge fewer spikes and at lower rates during burstlets than during bursts111. In light of these findings, the burstlet hypothesis111,151 posits that preBötC burstlets are low-amplitude rhythmogenic pre-inspiratory components needed for (but do not always lead to) bursts and that bursts themselves are dispensable for rhythmogenesis.
Fig. 3 |. Emergent network rhythms and burstlet theory.
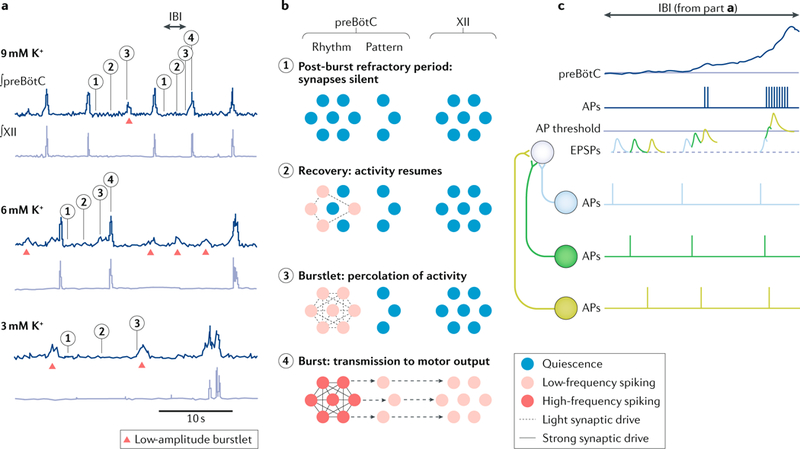
a | In vitro recordings of preBötzinger Complex (preBötC) field potentials and hypoglossal nerve (cranial nerve XII) activity showing rhythmic inspiratory-related output at several levels of excitability determined by changes in the concentration of K+ in the bathing solution. Bursts are associated with full- amplitude activity synchronized in the preBötC and XII nerve root. Lower-amplitude burstlets (without XII output) are marked with red triangles. b | Schematic of network activity underlying burstlets, bursts and XII output. Numerals indicate different stages of the trajectories in part a: 1, refractory state following inspiration; 2, spontaneous spiking resumes in some neuronal constituents; 3, active neurons are mutually reinforcing owing to recurrent synaptic interconnections, resulting in low-amplitude pre-inspiratory activity that percolates through the rhythmogenic population (that is, a burstlet); and 4, the burstlet triggers a full burst, which propagates to pattern-related preBötC neurons, premotor neurons and motor neurons, generating XII motor output. Colour scale: blue reflects quiescence, and pink and red map to progressively higher rates of spiking. Lines connecting the cells represent strength of synaptic drive: the absence of a line reflects synaptic depression; dotted lines reflect light synaptic drive; and solid lines represent strong drive. c | The interburst interval (IBI) in part a (shown at 9 mM K+) is reproduced here to illustrate the role of spike synchronization, schematized by three neurons projecting to a fourth neuron. Left: when action potentials (APs) are unsynchronized, excitatory postsynaptic potentials (EPSPs) do not summate. Middle and right: as the constituent neurons fire APs in progressively greater synchrony, the summation of EPSPs progressively increases, generating more spikes in postsynaptic partners. We suggest that greater synchrony among preBötC neurons ensues during the IBI. Part a is republished with permission of Society of Neuroscience, from Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex, Kam, K. et al. 33 (22), 2013 (REF111); permission conveyed through Copyright Clearance Center, Inc. Part c is adapted from REF152, by permission of Oxford University Press.
If bursts are not essential for rhythmogenesis, then two major assumptions need re-evaluation. The first assumption to be reconsidered is that rhythmogenic mechanisms rely on burst-associated, high-voltage- activated ionic conductances and marked increases in intracellular Ca2+. Instead, voltage-dependent mechanisms that operate at subthreshold membrane potentials associated with burstlets, previously ignored, must now be considered. The second assumption to be reconsidered is that inspiratory motor nerve discharge is determined solely by pattern-generating premotor neuron circuits downstream of the preBötC9,164–168 (FIG. 2f). If bursts are indeed non-rhythmogenic yet essential for motor output, then pattern-generating circuits must now also incorporate preBötC neurons (FIG. 3b). Indeed, some DBX1 preBötC neurons function as inspiration-related hypoglossal premotor neurons48,169,170, and glutamatergic SST-expressing preBotC output neurons11 form part of the pattern generator but not the core oscillator39.
We propose that a burstlet-based rhythmogenic mechanism involves preBötC interneurons transitioning from unsynchronized low-frequency spiking (<5 Hz) to more coincident, higher-frequency spiking (>5 Hz) as the burstlet grows. In this scheme, network assembly corresponds to neuron synchronization, which facilitates the summation of excitatory postsynaptic potential (EPSPs) above a threshold (FIG. 3c). preBötC inspiratory neurons in vitro discharge between 5 Hz and 40 Hz across the breathing cycle, with each action potential producing ~3–5 mV unitary EPSPs171. As individual preBötC neurons do not spike at high enough frequencies to alone produce postsynaptic action potentials, (nearly) convergent input from three to five concurrently spiking neurons would be required to depolarize a postsynaptic neuron to spike threshold (typically from –60 mV to –45 mV). This back-of-the- envelope calculation nicely matches the aforementioned in vitro observation that concurrent activation of four or more preBötC neurons can evoke preBötC and hypoglossal (XII) motor output bursts at delays consistent with the induction of burstlets148. Note that between four and nine preBötC neurons are required to trigger bursts and motor output; that variability may pertain to neuron subtype or may reflect when the neurons are stimulated during the interburst interval, because presumably more neurons would need to be stimulated to evoke a burst during the early part of the interburst interval when constituent preBötC neurons are recovering from refractoriness156,172.
Direct measures from paired neuronal recordings suggest that each excitatory inspiratory preBötC neuron projects to ~13% of the other inspiratory neurons in the preBotC171. (We note that indirect methods of measuring connectivity infer tenfold lower connectivity147 — a discordant result that we speculate is due to decreased sensitivity of these assays.) Extrapolating and assuming a rhythmogenic population of ~1,000 neurons48,152,173, 130 excitatory presynaptic partners project to each inspiratory preBötC neuron. In that context, ~3–5% of its partners would need to spike in tight temporal sequence to drive this neuron to threshold and to depolarize its own postsynaptic partners in turn. This estimation is a testable prediction of burstlet theory (FIG. 3c).
Burstlet theory posits that burst-associated conductances are important for robust output157,174,175 but are not themselves rhythmogenic. Is such a mechanism in which bursts are not themselves rhythmogenic feasible? One computational model (BOX 1) assembled a network of neurons lacking bursting conductances — that is, INaP, ICAN and a high-threshold Ca2+ current — endowed with only spiking conductances and excitatory synapses that are subject to short-term facilitation and depression. This model is rhythmic and can replicate benchmark experimental results172, including the effects of photostimulation or laser ablation of preBötC neurons48,148. That model, together with experimental recordings of burstlets and bursts triggered via small subsets of preBötC neurons111,148, emphasizes the importance of delineating the topology and dynamics of excitatory interconnections. We see the time-consuming and tedious quantification of synaptic connectivity among candidate rhythmogenic preBötC neurons as a worthwhile and unavoidable step in cracking rhythmogenesis.
The role of glia.
Once viewed principally as nutritive and supportive elements, glia are now understood to be functionally much more diverse, and their role in preBötC function is currently being re-evaluated. Although astrocytes lack electrical excitability, they can modulate extracellular ion concentrations, and respond to and release various transmitters, profoundly affecting neighbouring neurons and their constituent circuits and/or microcircuits. For example, masticatory rhythmogenesis is hypothesized to result from oral afferents triggering astrocytic release of an endogenous Ca2+-binding protein that reduces the extracellular concentration of free Ca2+; this modulates neuronal excitability and induces oscillatory bursting in trigeminal interneurons that putatively drive chewing movements176.
In the breathing CPG, astrocytes seem to, at a minimum, metabolically support preBötC neurons. For example, in vitro, inspiratory rhythms are perturbed by glial toxins (such as fluoroacetate or methionine sul-foximine) but are restored when key astrocyte-derived substrates, primarily glutamine, are replaced177,178. A major challenge is to design experiments that distinguish between the various metabolic, ion-homeostatic and transmitter-releasing or transmitter-responding functions of glia. For the breathing CPG, a small subpopulation of putative preBötC astrocytes shows weak and inconsistent increases in intracellular Ca2+ in phase with preBötC inspiratory rhythm in vitro179, but the functional importance of this remains unclear. Gliotransmission probably has an important role in the pFV where astrocytes can detect CO2 and pH62,63, and in the preBotC, where astrocytes can detect hypoxia180,181; astrocytes are likely to facilitate the regulation of these critical physiological variables through effects on breathing rhythm and pattern.
Problem of scale.
Noteworthy is that current views of the neural basis of breathing rhythm generation are based almost exclusively on analysis of small mammals. Rodents breathe 2–5 times per second, but large mammals such as elephants breathe 4–12 times per minute and whales (including the bowhead, humpback and grey whales) all breathe about once per minute when not diving. Thus, mice breathe 100–300-fold faster than do whales. Whether similar neural mechanisms operate over disparate timescales is an intriguing question. Upon ultimate identification of the relevant mechanisms in rodents, an interesting challenge will be to determine the transformed mechanisms that generate (much) slower frequencies.
Breathing and orofacial behaviours
With vertebrate evolution, the oropharyngeal cavity became very busy, critical for such diverse behaviours as breathing, vocalization, biting, chewing, swallowing, coughing and sniffing. Humans — through evolution of speech — added to this morass a complex and highly compliant airway, which is, unfortunately, prone to collapse during sleep. Conflicts between breathing and other behaviours, such as swallowing, exist because the pharynx is a common path for delivery of nutrients to the stomach via the oesophagus and air to the lungs via the trachea, but such conflicts must be avoided. Consequently, breathing is normally exquisitely coordinated with a multitude of orofacial behaviours.
The nature of the conflicts and need for coordination of orofacial behaviours and breathing have changed through evolution. The emergence of a spinal motor neuron-driven and axial muscle-driven aspiration pump in reptiles, birds and mammals relieved the oropharynx of its role as a breathing pump, reducing one source of conflict. However, the recruitment of chest wall muscles for breathing introduced new respiratory–locomotor constraints, leading to an elaboration of mechanisms that coordinate breathing with locomotion (Box 2). The emergence of the preBötC as the primary oscillator for breathing in mammals, which was presumably tightly associated with the appearance of the diaphragm as well as the consequent transition to being primarily inspiratory breathers, further reduced oropharyngeal conflicts. For example, the preBötC prevents concurrent inspiration and swallow by inhibiting the swallowing CPG. Thus, swallows induced by afferent signals that arrive during inspiration are delayed such that they occur primarily during expiration91,94,95, which diminishes the probability that food or liquid enters the trachea91,95,96.
Box 2 |. Locomotion entrains respiratory rhythm.
Respiratory and locomotor central pattern generators (CPGs) can operate independently, but locomotion has influenced the evolution of respiratory pumps and their neural control. Breathing is costly for water-breathing vertebrates, owing to the high density and low o2 content of water; diverse strategies evolved in fish to address this challenge. Some cichlid fish synchronize their pectoral fin power stroke with opening of the mouth and opercula216. Other fish, such as the salmonids (salmon and trout), use mechanoreceptive feedback relating to water velocity to trigger so-called ram ventilation, in which breathing movements stop, the mouth and opercula open and forward movement forces water over the gills. Most extreme, some species of pelagic fish, which are rapid swimmers with streamlined bodies (tuna, shark, swordfish and mackerel), are obligate ram ventilators; that is, they never breathe per se — their locomotor muscles act as the respiratory pump217, and they must swim continuously to avoid suffocation. Whether the brainstem in these species retains the ability to generate breathing rhythm is unknown.
The transition to a terrestrial environment and the evolution of aspiration breathing powered by axial muscles introduced the potential for interference between breathing and locomotion. In reptiles, breathing movements require bilateral synchronization of axial chest muscles (for inspiration) and abdominal muscles (for expiration), whereas locomotion requires left-right alternation of chest and abdominal muscles. That intrinsic incompatibility prevents reptiles from increasing ventilation outside of a modest range of running speeds218.
By contrast, multiple neural mechanisms have evolved in birds and mammals that reduce interference, by synchronizing (in a 1:1 ratio) or coordinating breathing with diverse forms of locomotion. Synchronization is common in galloping quadrupeds, hopping kangaroos and flying birds and bats219. However, other relationships are common and often adaptable, including in humans and birds. Breathing rhythm can be synchronized to locomotor rhythm via efferent signals from the spinal locomotor CPG to the brainstem breathing CPG37,220,221. Afferent feedback from moving limbs and pulmonary mechanoreceptors can alter this central synchronization to produce multiple coupling ratios and also independently coordinate breathing with locomotion220,222–224.
Locomotor–respiratory coordination, which is pervasive among birds and terrestrial mammals, may confer a selective advantage by minimizing interference or reducing the cost of breathing and may be exploited in the evolution of new behaviours. For example, descending motor commands in insect-eating bats coordinate wingbeat with breathing (active expiration) and vocalization, exploiting the force generated by wing muscles to minimize the high cost of generating echolocation pulses225,226.
The preBötC also has a central, yet incompletely defined, role in reflexes related to breathing, such as in cough, a powerful protective expulsive reflex produced by brainstem microcircuits, including the breathing CPG92. Cough has several phases. Sensory airway signals or volitional commands trigger the inspiratory phase, which is followed by a postinspiratory phase that involves closing the glottis. Expiratory muscle contraction against a closed glottis generates high positive airway pressure that, upon sudden glottal opening, produces high-velocity expiratory airflow, followed by a prolonged passive expiratory phase to accommodate swallows91. Disrupted coordination of coughing and swallowing — for example, resulting from incomplete glottal closure during swallow, which is unfortunately common in neurodegenerative diseases —increases the risk of aspiration pneumonia93,97.
Inspiratory and expiratory muscle activities are typically sequential and consequently out of phase, but in the Valsalva manoeuvre, powerful co-contraction of abdominal (expiratory) muscles and the crural diaphragm against a closed glottis generates high intra-abdominal pressure that is necessary for defecation and for stabilizing the abdominal and thoracic cavities for heavy lifting.
The preBötC generates the high-frequency, shallow breathing that underlies sniffing, and, in rodents, synchronizes sniffing (>5 Hz) with exploratory oro-facial behaviours such as licking and whisking182,183. Sniffing and whisking can occur independently, but when co-active they are synchronized via cycle-to-cycle resetting of the whisking rhythm by preBötC-driven sniffing41,42,184.
As described above, the preBötC coordinates inspiration with active expiration and postinspiration during the breathing cycle56,106. Thus, the preBötC inspiratory rhythm broadcasts a ‘master clock’ signal that coordinates inspiration with episodic, non-respiratory orofacial behaviours, and possibly provides a timing signal to temporally bind convergent feeding-related sensory inputs concerning smell, taste and touch184,185. Breathing-coupled rhythms in the hippocampus186–188 may even help to form associations between spatial maps (acquired through whisking) and smells and tastes (via sniffing and licking), which we speculate may assist in foraging.
Sighing, arousal and emotion
Mammalian breathing is punctuated periodically (every ~5 minutes in humans, every ~2 minutes in rodents) by sighs — large inspiratory efforts that are roughly double the size of normal breaths and are crucial for re- inflating collapsed alveoli and preventing atelectasis. As alveoli are fluid-lined, collapsed alveoli need very high inspiratory pressure to overcome the surface tension, which binds collapsed alveoli walls together, in order to reinflate. In mammals, the breathing CPG routinely and periodically induces much larger breaths (that is, sighs) for this purpose. Thus, sighing every few minutes is essential to maintain proper lung function. Sigh frequency is especially high in young infants and during hypoxia, when high breathing frequencies may predispose alveoli to atelectasis. On top of this background of rhythmic physiological sighing, we also generate sighs that are associated with emotions such as relief, grief, exasperation, exhaustion, yearning and happiness, among others.
A microcircuit consisting of four neural subpopulations — two in the preBötC and two in the pF — seems to be necessary for physiological sighing189 and might have a role in emotional sighs (FIG. 4 a). A subset of glu-tamatergic pF neurons that expresses PHOX2B71,84,190,191 and the peptides NMB or GRP project to partially overlapping subpopulations of preBötC neurons that express NMB receptors (NMBRs) and/or GRP receptors (GRPRs) (~200 preBötC neurons in total). In adult rats, injection of NMB or GRP into the preBötC increases sigh frequency (FiC. 4b,c). Injection of antagonists of either peptide receptor into the preBötC decreases sigh frequency, and co-injection of both antagonists transiently abolishes sighing. Genetic deletion of either peptide receptor slows endogenous sigh frequency by ~40%189.
Fig. 4 |. A circuit that generates and modulates sighs.
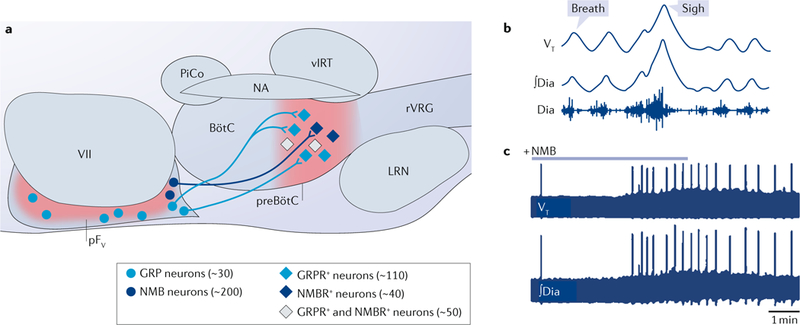
a | Gastrin-releasing peptide (GRP) and neuromedin B (NMB)-expressing neurons of the parafacial nucleus (pF) project to preBötzinger Complex (preBötC) neurons that express GRP receptors (GRPRs), NMB receptors (NMBRs) or both. b | Breathing patterns in an anaesthetized adult rat showing tidal volume (VT; maximal excursion 5 ml), annotated to differentiate an eupnoeic breath from a sigh, and diaphragmatic electromyography (Dia trace shows raw recording; ∫Dia trace shows integrated activity, in arbitrary units). c | Time- compressed recordings of VT and ∫ Dia. Thick baseline reflects continuous eupnoea. Large periodic spikes are sighs, which increase in frequency following infusion of NMB. LRN, lateral reticular nucleus; NA, nucleus ambiguus; pFV, ventral parafacial nucleus; PiCo, postinspiratory complex; rVRG, rostral ventral respiratory group; VII, facial motor nucleus; vIRT, ventral intermediate reticular formation. Part a and c are adapted from ref. 189, Macmillan Publishers Limited. Part b is adapted with permission from ref. 192, Elsevier.
How this microcircuit of pF and preBötC neurons produces a sigh rhythm approximately tenfold slower than the normal breathing rhythm is unknown. One proposed mechanism is that periodic bursts of NMB- expressing and GRP-expressing pF neurons directly evoke preBotC sigh bursts189,192, which — similar to sighs themselves — are larger in magnitude than normal breathing bursts193,194. However, no sigh-associated rhythmicity has been reported in the pF. Indeed, sigh bursts seem to be generated within the preBötC, as demonstrated by their production in medullary slices (which lack the pF) and by the fact that NMB or GRP injection into the preBötC of intact rats progressively increases sigh rate189,193,194. Our favoured hypothesis is that peptidergic drive to the NMBR-expressing and GRPR-expressing preBötC cells (including neurons and perhaps glia) incrementally activates an as-yet unidentified intraneuronal signalling pathway that takes tens of normal breathing cycles to pass a threshold for sigh generation and then resets189.
Another subpopulation of glutamatergic, DBX1 preBötC neurons may also affect arousal and perhaps emotional regulation. Approximately 350 preBötC neurons (175 per side) express the cell adhesion molecule cadherin 9 (CDH9). These DBX1 CDH9+ neurons project to and modulate the activity of locus coeruleus neurons12, which in turn project throughout the brain to influence attention, general arousal, sleep, gastrointestinal function, fear and even panic. Lesioning DBX1 CDH9+ preBötC neurons produces languid mice with a lower basal breathing rate and a shift from theta frequency activity (6–10 Hz) to delta frequency activity (0.5–4 Hz) measured using electrocorticography, suggesting an altered state of preternatural calm12 or, perhaps, lethargy.
Breathing pattern certainly correlates with arousal level. ‘Fight or flight’ responses ensure that maximum exertion, attention and stress are coupled to rapid breathing. Conversely, mental calmness is associated with slow breathing, and low stress levels are linked with slower, deeper breathing195. Taking one or more deep breaths is widely recognized to promote mindfulness and dispel distress. Breathing exercises performed during meditation or yoga can help to ameliorate negative emotional states such as depression, anxiety and stress, and can improve mood and cognitive processing196–203. The underlying mechanisms are likely tied to the breathing rhythms in many suprapontine areas, such as the hippocampus186, which can modulate cognitive function and fear188.
Although the neural substrates that enable breathing to influence cognition and emotion remain speculative, sniffing (rapid breathing), via rhythmic activation of the olfactory bulb, coordinates with hippocampal rhythmicity, which may aid in binding (associating), for example, odours and emotional memory, such as predator odour and fear186,188. preBötC DBX1 CDH9+ neuronal projections to the locus coeruleus12 are a possible source of emotional influence, but there are many additional pathways through which the breathing CPG could influence higher brain centres to affect emotion. Such pathways include the following: preBötC projections to suprapontine nuclei via direct or indirect pathways through the parabrachial nuclei or locus coeruleus or via olfactory nuclei, which receive respiratory-modulated input; vagal afferent input activated by large inspiratory efforts (note that vagal stimulation is effective in treating depression)204; afferent pathways activated by changes in blood gases; and collaterals of descending inputs that drive volitional or emotional breathing. The bottom line is that there are numerous connections between the brainstem breathing CPG and more rostral brain structures186–188 through which physiological, volitional and emotional stimuli that affect breathing in turn affect arousal, emotion and cognition195,205.
Perspectives
The neural mechanisms of breathing are tractable. Often overlooked and underappreciated, the behaviour itself is exceptionally well characterized, and every aspect — for example, blood gases and pH, muscle electromyography and ventilatory parameters — can be reliably and easily measured, mitigating the need for behavioural surrogates. Brainstem breathing circuits are robust, and their constituent neuronal activity and rhythmic outputs can be recorded and manipulated in vivo and in reduced preparations, even in slices.
Breathing in intact mammals depends on coupled neural oscillators: the preBötC drives the inexorable inspiratory rhythm that coordinates presumptive conditional oscillators for other phases of the breathing cycle as well as for sighing and orofacial behaviours in general. Furthermore, the preBötC influences arousal, cognitive function and emotion. Given its highly favourable experimental accessibility and its well-understood roles, we are optimistic that soon the neural underpinnings of breathing will be deeply understood, its underlying microcircuits well defined, its cellular constituents identified and physiologically characterized and its neural mechanisms and dynamics demonstrated at the biophysical level. Ultimately, such understanding would illuminate a fundamentally important and complex mammalian behaviour that is directly relevant to human health.
Supplementary Material
Central pattern generator (CPG).
A network that, generates the rhythm and, basic motor pattern for, behaviours such as locomotion, swimming, chewing and, breathing in vertebrate and, invertebrate animals.
Eupnoea
Breathing typical at rest and in, normal air (~21% O2 and trace amounts of CO2).
Breuer-Hering reflexes
Any of several reflexes mediated by mechanical sensory feedback from the lungs that control inflation and deflation of the lungs.
Phrenic premotor neurons
Neurons that project directly to the diaphragmatic motor neurons of the phrenic cervical motor nuclei, some of which receive input from the preBötzinger Complex.
Bursts
Suprathreshold neuronal depolarizations that drive high-frequency (20–120 Hz) spiking.
Valsalva manoeuvres
Co-contractions of expiratory and inspiratory muscles with a closed glottis, which elevates intra- abdominal pressure
Laminar
Smooth, non-turbulent.
Tidal breathing
Periodic inhalation and exhalation of gas in and out of the gas-exchange structure (lungs) along a common pathway (the trachea).
VII nucleus
The facial motor nucleus, the constituent motor neurons of which innervate facial muscles via the seventh cranial nerve.
Phase-sequencing synaptic Inhibition
Transitions between phases of a network rhythm that are governed by synaptic inhibition.
Vagotomized
Cutting the vagus nerve (cranial nerve X), which removes pulmonary sensory feedback (primarily mechanoreceptive) from the breathing CPG.
Network oscillator
A group of interconnected neurons from which rhythms emerge as a result of synaptic interactions.
Photolytic glutamate uncaging
A technique whereby molecules that chelate glutamate can be cleaved by light at a focal point to locally release the neuromessenger.
Cichlid fish
A large diverse group of ovoid, laterally compressed fish.
Opercula
Plural of operculum; the hard flap covering the gill slits in fishes.
Hypoxia
O2 deficiency.
Aspiration pneumonia
A lung infection that results from the ‘inhalation’ into the lungs of material from the stomach or mouth.
Crural diaphragm
The portion of the diaphragm (the main inspiratory muscle) that surrounds the oesophagus and that, when contracted, prevents gastro-oesophageal reflux.
Atelectasis
Complete or partial collapse of a region of the lung that develops when alveoli (the tiny air sacs within the lung) become deflated.
Acknowledgements
The authors thank W. K. Milsom, T. G. Pitts and numerous colleagues for helpful comments in review and J. Milstein for anatomical drawings that were the basis of Figure 1.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41583-018-0003-6.
RELATED LINKS
Model DB: https://senselab.med.yale.edu/modeldb/
BioModels Database: https://www.ebi.ac.uk/biomodels-main/
References
- 1.Smith JC, Ellenberger HH, Ballanyi K, Richter DW & Feldman JL Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (1991). This seminal work announced the preBötC, which was identified and named in reference 15 cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarzacher SW, Smith JC & Richter DW Pre-Bötzinger complex in the cat. J. Neurophysiol. 73, 1452–1461 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Schwarzacher SW, Rüb U & Deller T Neuroanatomical characteristics of the human pre-Bötzinger Complex and its involvement in neurodegenerative brainstem diseases. Brain J. Neurol. 134, 24–35 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Tupal S et al. Testing the role of preBötzinger complex somatostatin neurons in respiratory and vocal behaviors. Eur. J. Neurosci. 40, 3067–3077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenninger JM et al. Large lesions in the pre- Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J. Appl. Physiol. 97, 1629–1636 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Pantaleo T, Mutolo D, Cinelli E & Bongianni F Respiratory responses to somatostatin microinjections into the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Neurosci. Lett. 498, 26–30 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Bongianni F, Mutolo D, Cinelli E & Pantaleo T Respiratory responses induced by blockades of GABA and glycine receptors within the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Brain Res. 1344, 134–147 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Smith JC, Morrison DE, Ellenberger HH, Otto MR & Feldman JL Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J. Comp. Neurol. 281, 69–96 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Dobbins EG & Feldman JL Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 347, 64–86 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Wu J et al. A V0 core neuronal circuit for inspiration. Nat. Commun. 8, 544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan W, Pagliardini S, Yang P, Janczewski WA & Feldman JL Projections of preBötzinger Complex neurons in adult rats. J. Comp. Neurol. 518, 1862–1878 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yackle K et al. Breathing control center neurons that promote arousal in mice. Science 355, 1411–1415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C & Feldman J Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J. Comp. Neurol. 526, 1389–1402 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. .Funk GD, Smith JC & Feldman JL Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J. Neurophysiol. 70, 1497–1515 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Johnson SM, Smith JC & Feldman JL Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J. Appl. Physiol. 80, 2120–2133 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Gray PA, Rekling JC, Bocchiaro CM & Feldman JL Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger Complex. Science 286, 1566–1568 (1999). This report demonstrates that neuropeptide receptor expression characterizes constituent preBötC rhythmogenic neurons and demarcates the borders of the preBötC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stornetta RL et al. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger Complex. J. Comp. Neurol. 455, 499–512 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Stornetta RL, Rosin DL & Guyenet PG Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J. Comp. Neurol. 434, 128–146 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Guyenet PG & Wang H Pre-Bötzinger neurons with preinspiratory discharges ‘in vivo’ express NK1 receptors in the rat. J. Neurophysiol. 86, 438–446 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Hayes JA & Del Negro CA Neurokinin receptor-expressing pre-Bötzinger complex neurons in neonatal mice studied in vitro. J. Neurophysiol. 97, 4215–4224 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Liu Y-Y et al. Substance P and enkephalinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats. Eur. J. Neurosci. 19, 65–75 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Peña F & Ramirez J-M Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci. 24, 7549–7556 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramírez-Jarquín JO et al. Somatostatin modulates generation of inspiratory rhythms and determines asphyxia survival. Peptides 34, 360–372 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Llona I & Eugenín J Central actions of somatostatin in the generation and control of breathing. Biol. Res. 38, 347–352 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Rekling JC, Champagnat J & Denavit-Saubié M Thyrotropin-releasing hormone (TRH) depolarizes a subset of inspiratory neurons in the newborn mouse brain stem in vitro. J. Neurophysiol. 75, 811–819 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Gray PA, Janczewski WA, Mellen N, McCrimmon DR & Feldman JL Normal breathing requires preBötzinger complex neurokinin-1 receptor- expressing neurons. Nat. Neurosci. 4, 927–930 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay LC, Janczewski WA & Feldman JL Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat. Neurosci. 8, 1142–1144 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenninger JM et al. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J. Appl. Physiol. 97, 1620–1628 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Tan W et al. Silencing preBötzinger Complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat. Neurosci. 11, 538–540 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stornetta RL, Sevigny CP & Guyenet PG Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J. Comp. Neurol. 455, 113–124 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Guyenet PG, Sevigny CP, Weston MC & Stornetta RL Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J. Neurosci. 22, 3806–3816 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coveñas R et al. Mapping of neurokinin-like immunoreactivity in the human brainstem. BMC Neurosci. 4, 3 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YY, Ju G & Wong-Riley MT Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J. Appl. Physiol. 91 1387–1395 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Fukuda H, Nakamura E, Koga T, Furukawa N & Shiroshita Y The site of the anti-emetic action of tachykinin NK1 receptor antagonists may exist in the medullary area adjacent to the semicompact part of the nucleus ambiguus. Brain Res. 818, 439–449 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S & Mizuno N Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J. Comp. Neurol. 347, 249–274 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Onimaru H & Homma I Effect of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vitro study. Brain Res. 599, 272–276 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Le Gal J-P, Juvin L, Cardoit L, Thoby-Brisson M & Morin D Remote control of respiratory neural network by spinal locomotor generators. PLOS ONE 9, e89670 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan W et al. Reelin demarcates a subset of pre-Bötzinger complex neurons in adult rat. J. Comp. Neurol. 520, 606–619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y et al. Defining pre-Bötzinger Complex rhythm-and pattern-generating neural microcircuits in vivo. Neuron 91, 602–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koizumi H et al. Voltage-dependent rhythmogenic property of respiratory pre-Bötzinger complex glutamatergic, Dbx1-derived, and somatostatin¬expressing neuron populations revealed by graded optogenetic inhibition. eNeuro 10.1523/eneuro.0081-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore JD et al. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497, 205–210 (2013). This work shows that preBötC-driven inspiratory rhythms act as a master oscillator for orofacial behaviours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JD, Kleinfeld D & Wang F How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci. 37, 370–380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kottick A, Martin CA & Del Negro CA Fate mapping neurons and glia derived from Dbx1-expressing progenitors in mouse preBötzinger complex. Physiol. Rep. 5, e13300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouvier J et al. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat. Neurosci. 13, 1066–1074 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Gray PA et al. Developmental origin of preBötzinger Complex respiratory neurons. J. Neurosci. 30, 14883–14895 (2010). This work, in conjunction with Ref. 44, shows that rhythmogenic preBötC neurons in perinatal mice are derived from Dbx1-expressing precursors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picardo MCD, Weragalaarachchi KTH, Akins VT & Del Negro CA Physiological and morphological properties of Dbx1-derived respiratory neurons in the pre-Bötzinger complex of neonatal mice. J. Physiol. 591, 2687–2703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vann NC, Pham FD, Hayes JA, Kottick A & Del Negro CA Transient suppression of Dbx1 preBötzinger interneurons disrupts breathing in adult mice. PLOS ONE 11, e0162418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X et al. Laser ablation of Dbx1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. eLife 3, e03427 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagliardini S, Greer JJ, Funk GD & Dickson CT State-dependent modulation of breathing in urethane- anesthetized rats. J. Neurosci. 32, 11259–11270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saini JK & Pagliardini S Breathing during sleep in the postnatal period of rats: the contribution of active expiration. Sleep 10.1093/sleep/zsx172 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Andrews CG & Pagliardini S Expiratory activation of abdominal muscle is associated with improved respiratory stability and an increase in minute ventilation in REM epochs of adult rats. J. Appl. Physiol. 119, 968–974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rekling JC, Funk GD, Bayliss DA, Dong XW & Feldman JL Synaptic control of motoneuronal excitability. Physiol. Rev. 80, 767–852 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burke PGR et al. State-dependent control of breathing by the retrotrapezoid nucleus. J. Physiol.593 2909–2926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boutin RCT, Alsahafi Z & Pagliardini S Cholinergic modulation of the parafacial respiratory group. J. Physiol. 595, 1377–1392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mellen NM, Janczewski WA, Bocchiaro CM & Feldman JL Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37, 821–826 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janczewski WA & Feldman JL Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. 570, 407–420 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huckstepp RTR, Cardoza KP, Henderson LE & Feldman JL Role of parafacial nuclei in control of breathing in adult rats. J. Neurosci. 35, 1052–1067 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huckstepp RT, Henderson LE, Cardoza KP & Feldman JL Interactions between respiratory oscillators in adult rats. eLife 5, e14203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guyenet PG & Bayliss DA Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961 (2015). This is a comprehensive review of chemosensation in the pFv (that is, the RTN). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guyenet PG et al. Proton detection and breathing regulation by the retrotrapezoid nucleus. J. Physiol. 594 1529–1551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldman JL, Mitchell GS & Nattie EE Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 26, 239–266 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gourine AV et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wenker IC, Kreneisz O, Nishiyama A & Mulkey DK Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J. Neurophysiol. 104, 3042–3052 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose MF et al. Mathl is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron 64, 341–354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubreuil V et al. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J. Neurosci. 29, 14836–14846 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Onimaru H & Homma I A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 23, 1478–1486 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Onimaru H, Ikeda K & Kawakami K CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J. Neurosci. 28, 12845–12850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruffault P-L et al. The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2. eLife 4, e07051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thoby-Brisson M et al. Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat. Neurosci. 12, 1028–1035 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Fortuna MG, West GH, Stornetta RL & Guyenet PG Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J. Neurosci. 28, 2506–2515 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stornetta RL et al. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci. 26, 10305–10314 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang BJ et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J. Comp. Neurol. 503, 627–641 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Abbott SBG et al. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J. Neurosci. 33, 3164–3177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abbott SBG, Stornetta RL, Coates MB & Guyenet PG Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J. Neurosci. 31, 16410–16422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holloway BB, Viar KE, Stornetta RL & Guyenet PG The retrotrapezoid nucleus stimulates breathing by releasing glutamate in adult conscious mice. Eur. J. Neurosci. 42, 2271–2282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikeda K et al. A Phox2b BAC transgenic rat line useful for understanding respiratory rhythm generator neural circuitry. PLOS ONE 10, e0132475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abbott SBG et al. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 29, 5806–5819 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bochorishvili G, Stornetta RL, Coates MB & Guyenet PG Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J. Comp. Neurol. 520, 1047–1061 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosin DL, Chang DA & Guyenet PG Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 499, 64–89 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Abdala APL, Rybak IA, Smith JC & Paton JFR Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J. Physiol. 587, 3539–3559 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moraes DJA, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH & Zoccal DB Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J. Neurophysiol. 108, 882–890 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Marina N et al. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J. Neurosci. 30, 12466–12473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pagliardini S et al. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J. Neurosci. 31, 2895–2905 (2011). This report demonstrates active expiratory functions of the pFL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Y et al. Neuromedin B expression defines the mouse retrotrapezoid nucleus. J. Neurosci. 37, 11744–11757 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janczewski WA, Onimaru H, Homma I & Feldman JL Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J. Physiol. 545, 1017–1026 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silva JN, Tanabe FM, Moreira TS & Takakura AC Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respir. Physiol. Neurobiol. 227 9–22 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Takeda S et al. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95, 740–749 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Sears TA, Berger AJ & Phillipson EA Reciprocal tonic activation of inspiratory and expiratory motoneurones by chemical drives. Nature 299, 728–730 (1982). [DOI] [PubMed] [Google Scholar]
- 89.Tuck SA, Dort JC & Remmers JE Braking of expiratory airflow in obese pigs during wakefulness and sleep. Respir. Physiol. 128, 241–245 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Dutschmann M, Jones SE, Subramanian HH, Stanic D & Bautista TG The physiological significance of postinspiration in respiratory control. Prog. Brain Res. 212, 113–130 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Pitts T et al. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir. Physiol. Neurobiol. 189, 543–551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shannon R et al. Production of reflex cough by brainstem respiratory networks. Pulm. Pharmacol. Ther. 17, 369–376 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Hammond Smith C. A. et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 135, 769–777 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bautista TG, Sun Q-J & Pilowsky PM The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Prog. Brain Res. 212, 253–275 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Wheeler Hegland K, Huber JE, Pitts T, Davenport PW & Sapienza CM Lung volume measured during sequential swallowing in healthy young adults. J. Speech Lang. Hear. Res. 54, 777–786 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Jean A Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 81, 929–969 (2001). [DOI] [PubMed] [Google Scholar]
- 97.Pitts T et al. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 135, 1301–1308 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hernandez-Miranda LR et al. Genetic identification of a hindbrain nucleus essential for innate vocalization. Proc. Natl Acad. Sci. USA 114, 8095–8100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richter DW & Spyer KM Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 24, 464–472 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Smith JC, Abdala APL, Borgmann A,Rybak IA & Paton JFR Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 36, 152–162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith JC, Abdala APL, Koizumi H, Rybak IA & Paton JFR Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 98, 3370–3387 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richter D in Comprehensive Human Physiology: from Cellular Mechanisms to Integration (eds Greger R & Windhorst U) 2079–2095 (Springer, 1996). [Google Scholar]
- 103.Dutschmann M & Herbert H The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 24, 1071–1084 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Dutschmann M & Dick TE Pontine mechanisms of respiratory control. Compr. Physiol. 2, 2443–2469 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poon C-S & Song G Bidirectional plasticity of pontine pneumotaxic postinspiratory drive: implication for a pontomedullary respiratory central pattern generator. Prog. Brain Res 209, 235–254 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Anderson TM et al. A novel excitatory network for the control of breathing. Nature 536, 76–80 (2016). This paper proposes that an autonomous postinspiratory oscillator circuit in the rostral medulla ordinarily couples with the preBotC during breathing to aid in inspiratory-expiratory phase transition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ezure K & Tanaka I GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience 127, 409–417 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Ezure K, Tanaka I & Kondo M Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J. Neurosci. 23, 8941–8948 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian GF, Peever JH & Duffin J Mutual inhibition between Bötzinger-complex bulbospinal expiratory neurons detected with cross-correlation in the decerebrate rat. Exp. Brain Res. 125, 440–446 (1999). [DOI] [PubMed] [Google Scholar]
- 110.Tian GF, Peever JH & Duffin J Bötzinger- complex, bulbospinal expiratory neurones monosynaptically inhibit ventral-group respiratory neurones in the decerebrate rat. Exp. Brain Res. 124, 173–180 (1999). [DOI] [PubMed] [Google Scholar]
- 111.Kam K, Worrell JW, Janczewski WA, Cui Y & Feldman JL Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J. Neurosci. 33, 9235–9245 (2013). This paper presents the idea that burstlets, which are subthreshold for motor output, are nonetheless rhythmogenic in the preBotC.23719793 [Google Scholar]
- 112.Stuart DG & Hultborn H Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res. Rev. 59, 74–95 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Brown TG On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol. 48, 18–46 (1914). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Euler C On the central pattern generator for the basic breathing rhythmicity. J. Appl. Physiol. 55, 1647–1659 (1983). [DOI] [PubMed] [Google Scholar]
- 115.Feldman JL in Handbook of Physiology 463–524 (American Physiology Society, 1986). [Google Scholar]
- 116.Feldman JL & Smith JC Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann. NY Acad. Sci. 563, 114–130 (1989). [DOI] [PubMed] [Google Scholar]
- 117.Zhang W, Barnbrock A, Gajic S, Pfeiffer A & Ritter B Differential ontogeny of GABAB-receptor- mediated pre- and postsynaptic modulation of GABA and glycine transmission in respiratory rhythm-generating network in mouse. J. Physiol. 540, 435–446 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brockhaus J & Ballanyi K Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur. J. Neurosci. 10, 3823–3839 (1998). [DOI] [PubMed] [Google Scholar]
- 119.Funk GD & Greer JJ The rhythmic, transverse medullary slice preparation in respiratory neurobiology: contributions and caveats. Respir. Physiol. Neurobiol. 186, 236–253 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Richter DW Generation and maintenance of the respiratory rhythm. J. Exp. Biol. 100, 93–107 (1982). [DOI] [PubMed] [Google Scholar]
- 121.Richter DW & Smith JC Respiratory rhythm generation in vivo. Physiology 29, 58–71 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dutschmann M & Paton JFR Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat. J. Physiol. 543, 643–653 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marchenko V et al. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within pre-Bötzinger and Bötzinger complexes. eNeuro 10.1523/eneuro.0011-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cregg JM, Chu KA, Dick TE, Landmesser LT & Silver J Phasic inhibition as a mechanism for generation of rapid respiratory rhythms. Proc. Natl Acad. Sci. USA 114, 12815–12820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baertsch NA, Baertsch HC & Ramirez JM The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat. Commun. 843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janczewski WA, Tashima A, Hsu P, Cui Y & Feldman JL Role of inhibition in respiratory pattern generation. J. Neurosci. 33, 5454–5465 (2013). This paper demonstrates that blockade of inhibition in the preBotC and other sites in the medulla does not stop respiratory rhythm and breathing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sherman D, Worrell JW, Cui Y & Feldman JL Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat. Neurosci. 18, 408–414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Del Negro CA, Koshiya N, Butera RJ Jr & Smith JC Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J. Neurophysiol. 88, 2242–2250 (2002). [DOI] [PubMed] [Google Scholar]
- 129.Del Negro CA et al. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J. Neurosci. 25, 446–453 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thoby-Brisson M & Ramirez JM Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J. Neurophysiol. 86, 104–112 (2001). [DOI] [PubMed] [Google Scholar]
- 131.Peña F, Parkis MA, Tryba AK & Ramirez J-M Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43, 105–117 (2004). [DOI] [PubMed] [Google Scholar]
- 132.Rekling JC & Feldman JL PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 60 385–405 (1998). [DOI] [PubMed] [Google Scholar]
- 133.Butera RJ, Rinzel J & Smith JC Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J. Neurophysiol. 82, 382–397 (1999). [DOI] [PubMed] [Google Scholar]
- 134.Butera RJ, Rinzel J & Smith JC Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J. Neurophysiol. 82, 398–415 (1999). [DOI] [PubMed] [Google Scholar]
- 135.Del Negro CA, Johnson SM, Butera RJ & Smith JC Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J. Neurophysiol. 86, 59–74 (2001). [DOI] [PubMed] [Google Scholar]
- 136.Koizumi H et al. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Bötzinger complex respiratory microcircuits. J. Neurosci. 33, 2994–3009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morgado-Valle C, Baca SM & Feldman JL Glycinergic pacemaker neurons in preBötzinger Complex of neonatal mouse. J. Neurosci. 30, 3634–3639 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Doble A The pharmacology and mechanism of action of riluzole. Neurology 47, S233–241 (1996). [DOI] [PubMed] [Google Scholar]
- 139.Guinamard R, Simard C & Del Negro C Flufenamic acid as an ion channel modulator. Pharmacol. Ther. 138, 272–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pace RW, Mackay DD, Feldman JL & Del Negro CA Role of persistent sodium current in mouse preBötzinger Complex neurons and respiratory rhythm generation. J. Physiol. 580, 485–496 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paton JFR, Abdala APL, Koizumi H, Smith JC & St-John WM Respiratory rhythm generation during gasping depends on persistent sodium current. Nat. Neurosci. 9, 311–313 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Koizumi H & Smith JC Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J. Neurosci. 28, 1773–1785 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chevalier M, Toporikova N, Simmers J & Thoby-Brisson M Development of pacemaker properties and rhythmogenic mechanisms in the mouse embryonic respiratory network. eLife 5, e16125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramirez J-M, Tryba AK & Peña F Pacemaker neurons and neuronal networks: an integrative view. Curr. Opin. Neurobiol. 14, 665–674 (2004). [DOI] [PubMed] [Google Scholar]
- 145.Grillner S Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Grillner S The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4 573–586 (2003). [DOI] [PubMed] [Google Scholar]
- 147.Carroll MS & Ramirez J-M Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J. Neurophysiol. 109, 296–305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kam K, Worrell JW, Ventalon C, Emiliani V & Feldman JL Emergence of population bursts from simultaneous activation of small subsets of preBötzinger Complex inspiratory neurons. J. Neurosci. 33, 3332–3338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feldman JL & Del Negro CA Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 7, 232–242 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feldman JL, Del Negro CA & Gray PA Understanding the rhythm of breathing: so near, yet so far. Annu. Rev. Physiol. 75, 423–452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feldman JL & Kam K Facing the challenge of mammalian neural microcircuits: taking a few breaths may help. J. Physiol. 593, 3–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kam K & Feldman JL in Handbook of Brain Microcircuits 2nd edn 624 (Oxford Univ. Press, 2018). [Google Scholar]
- 153.Rekling JC, Champagnat J & Denavit-Saubié M Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J. Neurophysiol. 75 795–810 (1996). [DOI] [PubMed] [Google Scholar]
- 154.Rubin JE, Hayes JA, Mendenhall JL & Del Negro CA Calcium-activated nonspecific cation current and synaptic depression promote network- dependent burst oscillations. Proc. Natl Acad. Sci. USA 106, 2939–2944 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krey RA, Goodreau AM, Arnold TB & Del Negro CA Outward currents contributing to inspiratory burst termination in preBötzinger Complex neurons of neonatal mice studied in vitro. Front. Neural Circuits 4, 124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kottick A & Del Negro CA Synaptic depression influences inspiratory-expiratory phase transition in Dbx1 interneurons of the preBötzinger complex in neonatal mice. J. Neurosci. 35, 11606–11611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mironov SL Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J. Physiol. 586, 2277–2291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Del Negro CA, Kam K, Hayes JA & Feldman JL Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J. Physiol. 587, 1217–1231 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Orlovskiǐ GN Neuronal Control of Locomotion: from Mollusc to Man. (Oxford Univ. Press, 1999). [Google Scholar]
- 160.Lu B et al. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129, 371–383 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Lu B et al. Extracellular calcium controls background current and neuronal excitability via an UNC79- UNC80-NALCN cation channel complex. Neuron 68, 488–499 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu B et al. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457, 741–744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeh S-Y et al. Respiratory network stability and modulatory response to substance P require Nalcn. Neuron 94, 294–303.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ono T, Ishiwata Y, Inaba N, Kuroda T & Nakamura Y Hypoglossal premotor neurons with rhythmical inspiratory-related activity in the cat: localization and projection to the phrenic nucleus. Exp. Brain Res. 98, 1–12 (1994). [DOI] [PubMed] [Google Scholar]
- 165.Dobbins EG & Feldman JL Differential innervation of protruder and retractor muscles of the tongue in rat. J. Comp. Neurol. 357, 376–394 (1995). [DOI] [PubMed] [Google Scholar]
- 166.Ellenberger HH & Feldman JL Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 513, 35–42 (1990). [DOI] [PubMed] [Google Scholar]
- 167.Koizumi H et al. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J. Neurosci. 28 2353–2365 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stanek E 4th, Cheng S, Takatoh J, Han B-X & Wang F Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. eLife 3, e02511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Revill AL et al. Dbx1 precursor cells are a source of inspiratory XII premotoneurons. eLife 4, e12301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song H et al. Functional interactions between mammalian respiratory rhythmogenic and premotor circuitry. J. Neurosci. 36, 7223–7233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rekling JC, Shao XM & Feldman JL Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J. Neurosci. 20, RC113 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guerrier C, Hayes JA, Fortin G & Holcman D Robust network oscillations during mammalian respiratory rhythm generation driven by synaptic dynamics. Proc. Natl Acad. Sci. USA 112, 9728–9733 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hayes JA, Wang X & Del Negro CA Cumulative lesioning of respiratory interneurons disrupts and precludes motor rhythms in vitro. Proc. Natl Acad. Sci. USA 109, 8286–8291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pace RW, Mackay DD, Feldman JL & Del Negro CA Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J. Physiol. 582, 113–125 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crowder EA et al. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J. Physiol. 582, 1047–1058 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morquette P et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat. Neurosci. 18, 844–854 (2015). [DOI] [PubMed] [Google Scholar]
- 177.Hulsmann S, Oku Y, Zhang W & Richter DW Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur. J. Neurosci. 12, 856–862 (2000). [DOI] [PubMed] [Google Scholar]
- 178.Huxtable AG et al. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J. Neurosci. 30, 3947–3958 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Okada Y et al. Preinspiratory calcium rise in putative pre-Bötzinger complex astrocytes. J. Physiol. 590, 4933–4944 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Angelova PR et al. Functional oxygen sensitivity of astrocytes. J. Neurosci. 35, 10460–10473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rajani V et al. Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+ -dependent P2Y, receptor mechanism. J. Physiol. 589, 4583–4600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Travers JB, DiNardo LA & Karimnamazi H Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp. Brain Res. 130, 78–92 (2000). [DOI] [PubMed] [Google Scholar]
- 183.Welzl H & Bures J Lick-synchronized breathing in rats. Physiol. Behav. 18, 751–753 (1977). [DOI] [PubMed] [Google Scholar]
- 184.Kleinfeld D, Deschênes M, Wang F & Moore JD More than a rhythm of life: breathing as a binder of orofacial sensation. Nat. Neurosci. 17, 647–651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kurnikova A, Moore JD, Liao S-M, Deschênes M & Kleinfeld D Coordination of orofacial motor actions into exploratory behavior by rat. Curr. Biol.27 688–696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chi Nguyen V. et al. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J. Neurosci. 36, 162–177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grion N, Akrami A, Zuo Y, Stella F & Diamond ME Coherence between rat sensorimotor system and hippocampus is enhanced during tactile discrimination. PLoS Biol. 14, e1002384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zelano C et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 36, 12448–12467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li P et al. The peptidergic control circuit for sighing. Nature 530, 293–297 (2016). This report demonstrates that pF peptidergic neurons project to the preBotC to influence physiological sighing behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stornetta RL Identification of neurotransmitters and co-localization of transmitters in brainstem respiratory neurons. Respir. Physiol. Neurobiol. 164, 18–27 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dubreuil V et al. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc. Natl Acad. Sci. USA 105, 1067–1072 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li P & Yackle K Sighing. Curr. Biol. 27, R88–R89 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Lieske SP, Thoby-Brisson M, Telgkamp P & Ramirez JM Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci. 3, 600–607 (2000). [DOI] [PubMed] [Google Scholar]
- 194.Ruangkittisakul A et al. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J. Neurosci. 28, 2447–2458 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Boiten FA, Frijda NH & Wientjes CJ Emotions and respiratory patterns: review and critical analysis. Int. J. Psychophysiol. 17, 103–128 (1994). [DOI] [PubMed] [Google Scholar]
- 196.Arch JJ & Craske MG Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behav. Res. Ther. 44, 1849–1858 (2006). [DOI] [PubMed] [Google Scholar]
- 197.Brown RP & Gerbarg PL Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II — clinical applications and guidelines. J. Altern. Complement. Med. 11, 711–717, (2005). [DOI] [PubMed] [Google Scholar]
- 198.Brown RP, Gerbarg PL & Muench F Breathing practices for treatment of psychiatric and stress- related medical conditions. Psychiatr. Clin. North Am. 36, 121–140 (2013). [DOI] [PubMed] [Google Scholar]
- 199.Descilo T et al. Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. ActaPsychiatr. Scand. 121, 289–300 (2010). [DOI] [PubMed] [Google Scholar]
- 200.Jella SA & Shannahoff-Khalsa DS The effects of unilateral forced nostril breathing on cognitive performance. Int. J. Neurosci. 73, 61–68 (1993). [DOI] [PubMed] [Google Scholar]
- 201.Katzman MA et al. A multicomponent yoga-based, breath intervention program as an adjunctive treatment in patients suffering from generalized anxiety disorder with or without comorbidities. Int. J. Yoga 5, 57–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Paul NA, Stanton SJ, Greeson JM, Smoski MJ & Wang L Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Soc. Cogn. Affect. Neurosci. 8, 56–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zeidan F, Johnson SK, Diamond BJ, David Z & Goolkasian P Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 19, 597–605 (2010). [DOI] [PubMed] [Google Scholar]
- 204.Carreno FR & Frazer A Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14 716–727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Masaoka Y, Izumizaki M & Homma I Where is the rhythm generator for emotional breathing? Prog. Brain Res. 209, 367–377 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Dayan P & Abbott LF Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. (Massachusetts Institute of Technology Press, 2001). [Google Scholar]
- 207.Pathmanathan P & Gray RA Verification of computational models of cardiac electro-physiology. Int. J. Num Method. Biomed. Eng. 30, 525–544 (2013). [DOI] [PubMed] [Google Scholar]
- 208.Carroll MS, Viemari J-C & Ramirez J-M Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J. Neurophysiol. 109, 285–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Prinz AA, Bucher D & Marder E Similar network activity from disparate circuit parameters. Nat. Neurosci. 7, 1345–1352 (2004). [DOI] [PubMed] [Google Scholar]
- 210.Alsahafi Z, Dickson CT & Pagliardini S Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo. J. Physiol. 593, 3673–3692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Del Negro CA, Morgado-Valle C & Feldman JL Respiratory rhythm: an emergent network property? Neuron 34, 821–830 (2002). [DOI] [PubMed] [Google Scholar]
- 212.Purvis LK, Smith JC, Koizumi H & Butera RJ Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J. Neurophysiol. 97 1515–1526 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Smith JC et al. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir. Physiol. 122, 131–147 (2000). [DOI] [PubMed] [Google Scholar]
- 214.Song H, Hayes JA, Vann NC, Drew LaMar M & Del Negro CA Mechanisms leading to rhythm cessation in the respiratory preBötzinger complex due to piecewise cumulative neuronal deletions. eNeuro 10.1523/eneuro.0031-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schwab DJ, Bruinsma RF, Feldman JL & Levine AJ Rhythmogenic neuronal networks, emergent leaders, and k-cores. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 82, 051911 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Webb PW Synchrony of locomotion and ventilation in Cymatogaster aggregata. Can. J. Zool. 53, 904–907 (1975). [Google Scholar]
- 217.Wegner NC, Sepulveda CA, Aalbers SA & Graham JB Structural adaptations for ram ventilation: gill fusions in scombrids and billfishes.J. Morphol. 274, 108–120 (2013). [DOI] [PubMed] [Google Scholar]
- 218.Wang T, Carrier DR & Hicks JW Ventilation and gas exchange in lizards during treadmill exercise. J. Exp. Biol. 200, 2629–2639 (1997). [DOI] [PubMed] [Google Scholar]
- 219.Bramble DM & Carrier DR Running and breathing in mammals. Science 219, 251–256 (1983). [DOI] [PubMed] [Google Scholar]
- 220.Funk GD, Steeves JD & Milsom WK Coordination of wingbeat and respiration in birds. II. ‘Fictive’ flight. J. Appl. Physiol. 73, 1025–1033 (1992). [DOI] [PubMed] [Google Scholar]
- 221.Persegol L, Jordan M, Viala D & Fernandez C Evidence for central entrainment of the medullary respiratory pattern by the locomotor pattern in the rabbit. Exp. Brain Res. 71, 153–162 (1988). [DOI] [PubMed] [Google Scholar]
- 222.Funk GD, Milsom WK & Steeves JD Coordination of wingbeat and respiration in the Canada goose. I. Passive wing flapping. J. Appl. Physiol. 73, 1014–1024 (1992). [DOI] [PubMed] [Google Scholar]
- 223.Funk GD, Valenzuela II & Milsom WK Energetic consequences of coordinating wingbeat and respiratory rhythms in birds. J. Exp. Biol. 200, 915–920 (1997). [DOI] [PubMed] [Google Scholar]
- 224.Potts JT, Rybak IA & Paton JFR Respiratory rhythm entrainment by somatic afferent stimulation. J. Neurosci. 25, 1965–1978 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lancaster WC, Henson OW & Keating AW Respiratory muscle activity in relation to vocalization in flying bats. J. Exp. Biol. 198, 175–191 (1995) [DOI] [PubMed] [Google Scholar]
- 226.Speakman JR & Racey PA No cost of echolocation for bats in flight. Nature 350, 421–423 (1991). [DOI] [PubMed] [Google Scholar]
- 227.Feldman JL & McCrimmon DR Fundamental Neuroscience 3rd edn (eds Squire LR et al. ) 855–872 (Academic Press, 2008). [Google Scholar]
- 228.Hayashi F & McCrimmon DR Respiratory motor responses to cranial nerve afferent stimulation in rats. Am. J. Physiol. 271, R1054–R1062 (1996). [DOI] [PubMed] [Google Scholar]
- 229.Jenkin SEM, Milsom WK & Zoccal DB The Kolliker-Fuse nucleus acts as a timekeeper for late-expiratory abdominal activity. Neuroscience 348, 63–72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pagliardini S, Ren J & Greer JJ Ontogeny of the pre-Bötzinger complex in perinatal rats. J. Neurosci. 23 9575–9584 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Thoby-Brisson M, Trinh J-B, Champagnat J & Fortin G Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J. Neurosci. 25 4307–4318 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wallén-Mackenzie A et al. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J. Neurosci. 26, 12294–12307 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gray PA Transcription factors define the neuroanatomical organization of the medullary reticular formation. Front. Neuroanat. 10.3389/fnana.2013.00007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


