Abstract
Background: Low-grade chronic inflammation, which can be modulated by diet, has been suggested as an important risk factor for depression, but few studies have investigated the association between the inflammatory potential of the diet and depression.
Objective: We investigated the prospective association between the inflammatory potential of the diet, measured by the Dietary Inflammatory Index (DII), and incident depressive symptoms and tested the potential modulating effect of sex, age, physical activity, and smoking status.
Methods: This study included 3523 participants (aged 35–60 y) from the SU.VI.MAX (Supplémentation en Vitamines et Minéraux Antioxydants) cohort, who were initially free of depressive symptoms. Baseline DII (1994–1996) was computed by using repeated 24-h dietary records. Incident depressive symptoms were defined by a Center for Epidemiologic Studies–Depression Scale score ≥17 for men and ≥23 for women in 2007–2009. We used multivariable logistic regression models to estimate ORs and 95% CIs, and modeled the DII as a continuous variable and as sex-specific quartiles.
Results: A total of 172 cases of incident depressive symptoms were identified over a mean follow-up of 12.6 y. The DII was not associated with incident depressive symptoms in the full sample. In sex-specific models, men with a higher DII had a higher risk of incident depressive symptoms (quartile 4 compared with quartile 1—OR: 2.32; 95% CI: 1.01, 5.35), but the association was only marginally significant (P-trend = 0.06). When analyses were performed across smoking status, current and former smokers with a higher DII had a higher risk of incident depressive symptoms (quartile 4 compared with quartile 1—OR: 2.21; 95% CI: 1.08, 4.52). A positive association was also observed among less physically active participants (quartile 4 compared with quartile 1—OR: 2.07; 95% CI: 1.05, 4.07).
Conclusion: The promotion of a healthy diet with anti-inflammatory properties may help to prevent depressive symptoms, particularly among men, smokers, or physically inactive individuals. This trial was registered at clinicaltrials.gov as NCT0027242.
Keywords: mental health, depression, diet, inflammation, Dietary Inflammatory Index, prospective study
Introduction
Depression represents one of the main causes of disability worldwide according to the WHO and accounts for 4.3% of the total global burden of diseases (1). Current evidence suggests that inflammation may play a role in the pathophysiology of depression (2). In addition to the cross-sectional association between C-reactive protein (CRP)12 and proinflammatory cytokines, including IL-6 and TNF-α, and current depression (3, 4), there is some evidence from longitudinal studies that inflammation is a possible mediator of risk factors related to depression (5). Moreover, randomized controlled trials found anti-inflammatory agents to be efficient in the treatment of major depression, suggesting a causal relation (6).
In addition, many studies have investigated the influence of diet on chronic inflammation (7–10). Indeed, diet consists of various bioactive compounds exhibiting pro- or anti-inflammatory properties (11). The Dietary Inflammatory Index (DII) was recently developed to estimate the inflammatory potential of the overall diet on the basis of the pro- and anti-inflammatory effects of various dietary components on several inflammatory biomarkers (11). In previous studies, an elevated DII score (reflecting a proinflammatory diet) was associated with a higher risk of several types of cancer, cardiovascular diseases, and worse cognitive functioning (12–17).
To our knowledge, 3 cohort studies specifically investigated the association between the DII and the risk of depression or depressive symptoms (18–20), but none examined the potential effect modifiers. The Australian Longitudinal Study on Women's Health was restricted to women with a mean age of 52.0 y, and the Whitehall II study investigated the association with recurrent depressive symptoms. Another cohort study conducted in women included in the Nurses' Health Study investigated the prospective relation between an inflammatory dietary pattern and the risk of depression with the use of reduced-rank regression to identify a dietary pattern that was positively correlated with inflammatory biomarkers (21). These 4 studies reported a significant association between the DII or a proinflammatory dietary pattern and a higher depression risk among women (18–21). Importantly, in the Whitehall II study, no significant association was found among men (20). Depressive symptoms are more frequent among women than among men (22), but it remains unclear whether women are more or less vulnerable to inflammation-induced mood and behavior changes (23).
The purpose of this study was to investigate the prospective association between the inflammatory potential of diet (as measured by the DII) and the risk of depressive symptoms in a large cohort of French adults and to test the potential modulating effect of sex, age, physical activity, and smoking status, because these factors are associated with both inflammation and depressive symptoms (2, 24–29).
Methods
Study population.
Our analyses are based on the Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX; 1994–2002) study, which was initially a randomized, double-blind, placebo-controlled trial (NCT00272428). It was designed to test the efficacy of daily supplementation with nutritional doses of antioxidant vitamins and minerals (ascorbic acid, vitamin E, β-carotene, selenium, and zinc) on the incidence of cancer, ischemic heart disease, and all-cause mortality. Details on the cohort are available elsewhere (30, 31). Briefly, after a national recruitment campaign with a call for volunteers living in France (women aged 35–60 y and men aged 45–60 y), 13,017 participants who met all of the eligibility criteria (31) were randomly assigned and included in the final study sample. All of the participants were invited to complete the Center for Epidemiologic Studies–Depression Scale (CES-D) questionnaire (baseline CES-D, 1996–1997), and the response rate was 65%. At the end of the trial phase, those who agreed to participate in the postsupplementation follow-up study were included in the observational SU.VI.MAX 2 study (2007–2009; n = 6850). Only those participants who were included in this additional observational follow-up received the CES-D questionnaire a second time (2007–2009).
The SU.VI.MAX and SU.VI.MAX 2 studies were conducted according to the guidelines in the Declaration of Helsinki and were approved by the ethics committees for studies with human subjects of the Paris-Cochin Hospital [Comités de Consultation pour la Protection des Personnes se prêtant à la Recherche Biomédicale (CCPPRB 706 and 2364, respectively) and the Commission Nationale de l'Informatique et des Libertés (CNIL 334641 and 907094, respectively)]. Written informed consent was obtained from all participants.
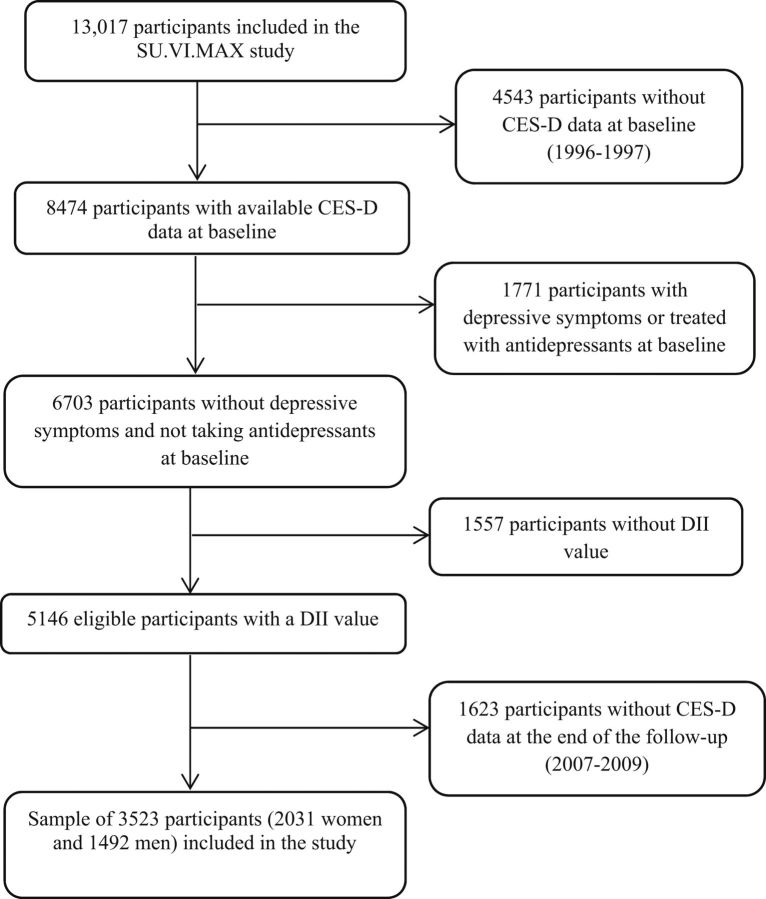
From the sample of 8474 participants with available CES-D data at the first assessment (1996–1997), we excluded participants with baseline depressive symptoms (defined by CES-D ≥17 for men and ≥23 for women; n = 1646) (32) or who were treated with antidepressants at baseline (n = 125), those without a DII value (due to <3 dietary records during the first 2 y of follow-up; n = 1557), and those with missing CES-D follow-up data collected between 2007 and 2009 (n = 1623). The final sample included 3523 participants (Figure 1).
FIGURE 1.
Flow chart of participant selection. CES-D, Center for Epidemiologic Studies–Depression Scale; DII, Dietary Inflammatory Index; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants.
Depressive symptoms.
The French version of the CES-D was used to assess depressive symptoms (33, 34). This questionnaire includes 20 items that evaluate the frequency of symptoms and behavioral characteristics associated with depression during the preceding week with the use of a 4-point scale (0 = <1 d, 1 = 1–2 d, 2 = 3–4 d, and 3 = 5–7 d). The responses were summed to yield a total score between 0 and 60, with higher scores denoting more depressive symptoms. We used the cutoff scores validated for the French population (CES-D ≥17 in men and ≥23 in women) to define the presence of depressive symptoms (32). Incident cases of depressive symptoms were defined as participants who were free of depressive symptoms at the beginning of the study, who did not use antidepressants at baseline, and who exhibited depressive symptoms at the second CES-D data assessment.
Dietary data and the “inflammatory potential” of the diet.
During the trial phase, participants were asked to provide a 24-h dietary record every 2 mo (6 records/y) covering all days of the week and all seasons of the year. Approximately two-thirds of the initial cohort agreed to provide dietary information. To indicate the consumed portion, participants were assisted by an instruction manual with validated photographs of >250 generic food items represented in 3 main, 2 intermediate, and 2 extreme portion sizes (35). A validated French food-composition table was used to estimate nutrient intakes (36). Daily food intake refers to the average consumption as reported on all 24-h dietary records completed during the first 2 y of follow-up, thus accounting for intravariability of intake.
We calculated the DII score to determine the “inflammatory potential” of the diet. The concept and computation of the DII score have been previously described (11). Briefly, the DII score is based on findings from 1943 articles published until 2010, focusing on the effects of a variety of dietary components on inflammation. In the present study, the DII was computed by using data on 36 of the 45 variables, including a number of nutrients, specific food items, and bioactive compounds. In particular, intakes of energy, carbohydrate, protein, total fat, cholesterol, SFAs, vitamin B-12, and iron were computed as proinflammatory factors, whereas dietary intakes of MUFAs, PUFAs (ω-3, ω-6), niacin, thiamin, riboflavin, vitamin B-6, magnesium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavan-3-ol, flavonols, flavonones, flavones, isoflavones, alcohol, fiber, garlic, ginger, pepper, onion, and tea were entered as anti-inflammatory factors. All of these components were linked to 6 major inflammatory biomarkers: IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP. Points were assigned according to whether each variable increased (+1), decreased (−1), or had no effect (0) on inflammation. Dietary intakes were first standardized by using means ± SDs for each variable, with the use of a database that included food-consumption data from 11 populations around the world, then converted to the centered percentile scores. Finally, the centered percentile score for each variable was multiplied by the associated coefficient derived from the literature review; all were then summed to create the overall DII score. A higher DII score represents a more proinflammatory diet and a lower DII score represents a lower inflammatory potential of the diet (11).
Covariates.
At enrollment, self-administered questionnaires provided information on sex, date of birth, educational level (primary, secondary, or university level), marital status (living alone or cohabiting), socio-professional category (unemployed, manual worker, employee/support staff, or white-collar worker), physical activity (irregular or <1 or ≥1 h walking/d), and smoking status (never, former, or current smoker). At the first clinical examination (1995–1996), anthropometric measurements were collected and BMI was calculated as the ratio of weight to squared height (kg/m2). BMI was split into 4 categories: underweight (<18.5), normal weight (≥18.5 and <25), overweight (≥25 and <30), and obese (≥30) (37). Incident cases of cancer and cardiovascular diseases were recorded during the follow-up and validated by an external committee (31). Missing data were handled by multiple imputation (38, 39), with a set of 20 imputations from the available data. The proportion of missing values was <5% for all variables (n = 13 for educational level, n = 75 for socio-professional category, n = 105 for marital status, n = 127 for smoking, and n = 51 for BMI).
Statistical analysis.
We compared participants included in the study with excluded participants by using chi-square tests or t tests, according to the type of variable. Baseline characteristics are presented as means ± SDs or as numbers (percentages) across quartiles of DII scores, and P values were provided by linear contrast or Cochran-Mantel-Haenszel tests. For descriptive purposes, nutrient intakes were energy-adjusted by using the residual method (40).
Logistic regression models were performed to estimate the association between the DII score (modeled as sex-specific quartiles and as a continuous variable) and the incidence of depressive symptoms over a 13-y follow-up period (first DII quartile as the reference). Linear trend across quartiles of DII was also estimated by modeling quartiles of DII score as an ordinal variable. Model 1 (main model) was adjusted for sex, intervention group, number of 24-h dietary records, mean energy intake without alcohol (kilocalories per day), interval between the 2 CES-D measurements, baseline age, educational level, marital status, socio-professional category, smoking status, physical activity, and BMI. A second model (model 2) was performed to additionally account for incident cancer or major cardiovascular events during follow-up.
In addition, subgroup analyses by sex, baseline age (≤49 compared with >49 y; median value), physical activity (irregular physical activity or <1 h walking/d compared with ≥1 h walking/d), and smoking status (never smoker compared with former or current smoker) were conducted to test for modification effects. Sensitivity analyses were also performed to test the robustness of our findings. First, we used inverse-probability weighting (IPW) to account for a possible selection bias (41). The probability of inclusion in the present study was calculated for each individual with the use of multivariable logistic regression. The inverse of that probability was then multiplied by the sampling proportion (nincluded/neligible) and was used as a weight in logistic regression models. Second, we defined the presence of depressive symptoms in both men and women by using an alternative cutoff value of 16 (34). Subgroup analyses were also conducted to determine whether the association between DII and depressive symptoms was modified by intervention group (active supplementation group compared with placebo group). All of the statistical analyses were conducted by using SAS (version 9.4; SAS Institute), with a significance level of 0.05 for 2-sided tests.
Results
The analyses included 2031 women and 1492 men. Compared with excluded participants from the eligible population (n = 1623), included participants were older, more often men, more often allocated to the active supplementation group during the trial phase, cohabiting, and never smokers at baseline (Supplemental Table 1). The mean ± SD age at baseline was 52.1 ± 4.7 y for men and 47.6 ± 6.4 y for women. The DII score ranged from −5.0 to 5.8, with a mean of 0.5 ± 1.8. The mean ± SD DII score was 0.1 ± 1.7 for men and 0.8 ± 1.8 for women.
Among the 3523 participants included in the study, 172 exhibited incident depressive symptoms at the end of follow-up (mean ± SD follow-up: 12.6 ± 0.5 y). Baseline characteristics of the participants are presented across quartiles of the DII score in Table 1. The highest quartile of DII score (reflecting a proinflammatory diet) included more women than did the lowest quartile (reflecting an anti-inflammatory diet). Participants in the highest quartile were also younger, less educated, less physically active, and more likely to have a BMI value in the normal-weight range. In addition, unadjusted comparisons showed that a higher DII score was significantly associated with lower total daily energy intake and lower intakes of total PUFAs, fiber, and most vitamins and minerals, but a higher intake of SFAs (Table 2).
TABLE 1.
Baseline characteristics of 3523 participants across quartiles of the DII score: the SU.VI.MAX study1
| Q1 (low-inflammatory diet) | Q2 | Q3 | Q4 (high-inflammatory diet) | P-trend2 | |
|---|---|---|---|---|---|
| n | 880 | 881 | 881 | 881 | |
| DII score | −1.8 ± 0.8 | −0.1 ± 0.3 | 1.1 ± 0.4 | 2.9 ± 0.9 | |
| Age, y | 50.9 ± 6.0 | 49.7 ± 6.2 | 49.4 ± 6.1 | 48.1 ± 6.2 | <0.0001 |
| Male, n (%) | 477(54.2) | 427(48.5) | 344(39.1) | 244(27.7) | <0.0001 |
| Intervention group, n (%) | 0.06 | ||||
| Active supplementation group | 483(54.9) | 480(54.5) | 455(51.6) | 451(51.2) | |
| Placebo group | 397(45.1) | 401(45.5) | 426(48.4) | 430(48.8) | |
| Marital status, n (%) | 0.80 | ||||
| Living alone | 121(13.8) | 103(11.7) | 118(13.4) | 119(13.5) | |
| Cohabiting | 734(83.4) | 755(85.7) | 736(83.5) | 732(83.1) | |
| Education, n (%) | <0.0001 | ||||
| Primary | 132(15.0) | 159(18.1) | 168(19.1) | 200(22.7) | |
| Secondary | 329(37.4) | 348(39.5) | 340(38.6) | 364(41.3) | |
| Postsecondary | 416(47.3) | 373(42.3) | 370(42.0) | 311(35.3) | |
| Socio-professional status, n (%) | <0.0001 | ||||
| Unemployed | 57(6.5) | 71(8.1) | 62(7.0) | 98(11.1) | |
| Manual worker | 28(3.2) | 47(5.3) | 40(4.5) | 38(4.3) | |
| Office/support staff | 476(54.1) | 489(55.5) | 507(57.6) | 532(60.4) | |
| White-collar worker | 305(34.7) | 259(29.4) | 250(28.4) | 189(21.5) | |
| Physical activity, n (%) | <0.0001 | ||||
| Irregular | 162(18.4) | 192(21.8) | 206(23.4) | 239(27.1) | |
| <1 h walking/d | 262(29.8) | 286(32.5) | 269(30.5) | 252(28.6) | |
| ≥1 h walking/d | 439(49.9) | 393(44.6) | 390(44.3) | 367(41.7) | |
| Smoking status, n (%) | 0.14 | ||||
| Never smoker | 412(46.8) | 429(48.7) | 427(48.5) | 443(50.3) | |
| Former/current smoker | 435(49.4) | 426(48.4) | 421(47.8) | 403(45.7) | |
| BMI, n (%) | 0.005 | ||||
| Underweight | 9(1.0) | 17(1.9) | 21(2.4) | 26(3.0) | |
| Normal weight | 574(65.2) | 538(61.1) | 577(65.5) | 590(67.0) | |
| Overweight | 234(26.6) | 275(31.2) | 224(25.4) | 205(23.3) | |
| Obese | 52(5.9) | 42(4.8) | 43(4.9) | 45(5.1) | |
| History of CVD/cancer, n (%) | 90(10.2) | 101(11.5) | 90(10.2) | 86(9.8) | 0.57 |
Values are means ± SDs or n (%) unless otherwise indicated. The numbers of participants who were missing data were as follows: 105 for marital status, 13 for education, 75 for socio-professional status, 127 for smoking status, 66 for physical activity, and 51 for BMI. CVD, cardiovascular diseases; DII, Dietary Inflammatory Index; Q, quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants.
P-trend values are based on a linear contrast test or the Cochran-Mantel-Haenszel test.
TABLE 2.
Nutritional data of 3523 participants across quartiles of the DII score: the SU.VI.MAX study1
| Q1 | Q2 | Q3 | Q4 | P 2 | |
|---|---|---|---|---|---|
| n | 880 | 881 | 881 | 881 | |
| Number of 24-h dietary records | 10.1 ± 3.1 | 10.5 ± 2.9 | 10.3 ± 2.9 | 10.0 ± 3.2 | 0.30 |
| Alcohol, g/d | 22.1 ± 21.7 | 21.3 ± 21.4 | 17.4 ± 19.5 | 13.7 ± 16.9 | <0.0001 |
| Energy intake, total kcal/d | 2492 ± 641 | 2272 ± 523 | 2042 ± 481 | 1753 ± 439 | <0.0001 |
| Garlic, mg/d | 249 ± 628 | 223 ± 427 | 177 ± 301 | 151 ± 346 | <0.0001 |
| Ginger, mg/d | 5.8 ± 37.1 | 7.8 ± 45.4 | 5.0 ± 31.4 | 7.7 ± 41.7 | 0.61 |
| Pepper, mg/d | 178 ± 150 | 162 ± 128 | 131 ± 104 | 104 ± 94.4 | <0.0001 |
| Onion, g/d | 4.2 ± 4.6 | 3.7 ± 4.0 | 3.2 ± 3.3 | 2.8 ± 2.7 | <0.0001 |
| Tea, g/d | 295 ± 313 | 187 ± 268 | 140 ± 228 | 59.0 ± 122 | <0.0001 |
| Carbohydrate,3 % of energy | 42.6 ± 6.0 | 41.8 ± 5.8 | 41.4 ± 5.9 | 41.3 ± 5.8 | <0.0001 |
| Protein,3 % of energy | 17.5 ± 2.5 | 17.5 ± 2.6 | 17.6 ± 2.7 | 17.8 ± 2.7 | 0.01 |
| Lipids,3 % of energy | 39.9 ± 5.2 | 40.6 ± 4.9 | 40.9 ± 5.0 | 40.8 ± 4.8 | <0.0001 |
| Cholesterol,4 mg/d | 379 ± 114 | 391 ± 100 | 399 ± 93.3 | 403 ± 120 | <0.0001 |
| SFAs,4 g/d | 34.7 ± 6.8 | 36.8 ± 6.0 | 38.2 ± 5.4 | 39.0 ± 4.5 | <0.0001 |
| MUFAs,4 g/d | 34.2 ± 6.2 | 34.6 ± 5.3 | 34.3 ± 4.8 | 33.9 ± 4.1 | 0.18 |
| PUFAs,4 g/d | 14.6 ± 3.7 | 13.9 ± 3.5 | 13.1 ± 3.0 | 12.3 ± 2.3 | <0.0001 |
| 22:6n–3,4 mg/d | 285 ± 237 | 224 ± 175 | 212 ± 162 | 185 ± 132 | <0.0001 |
| 20:4n–6,4 mg/d | 175 ± 65.6 | 174 ± 58.8 | 173 ± 54.1 | 171 ± 49.1 | 0.13 |
| β-Carotene,4 mg/d | 5.4 ± 2.8 | 4.2 ± 2.2 | 3.6 ± 1.8 | 3.0 ± 1.4 | <0.0001 |
| Vitamin A,4 μg/d | 903 ± 949 | 799 ± 746 | 765 ± 610 | 732 ± 452 | <0.0001 |
| Thiamine,4 mg/d | 1.4 ± 0.4 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | <0.0001 |
| Riboflavin,4 mg/d | 1.9 ± 0.4 | 1.8 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.3 | <0.0001 |
| Niacin,4 mg/d | 18.9 ± 4.3 | 17.6 ± 3.7 | 17.2 ± 3.5 | 16.7 ± 3.0 | <0.0001 |
| Pyridoxine,4 mg/d | 1.9 ± 0.4 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.2 | <0.0001 |
| Folic acid,4 μg/d | 371 ± 87.4 | 328 ± 59.1 | 302 ± 49.0 | 270 ± 43.2 | <0.0001 |
| Cobalamin,4 μg/d | 8.2 ± 5.5 | 7.2 ± 4.0 | 6.9 ± 3.6 | 6.5 ± 2.9 | <0.0001 |
| Vitamin C,4 mg/d | 123 ± 45.8 | 101 ± 37.7 | 89.3 ± 34.4 | 73.9 ± 28.6 | <0.0001 |
| Vitamin D,4 μg/d | 3.3 ± 2.4 | 2.8 ± 1.8 | 2.7 ± 1.5 | 2.5 ± 1.3 | <0.0001 |
| Vitamin E,4 mg/d | 14.8 ± 4.3 | 13.5 ± 3.6 | 12.3 ± 3.0 | 11.2 ± 2.6 | <0.0001 |
| Magnesium,4 mg/d | 326 ± 61.1 | 303 ± 46.1 | 290 ± 43.7 | 279 ± 35.7 | <0.0001 |
| Anthocyanidins,4 mg/d | 99.4 ± 58.2 | 88.4 ± 60.1 | 73.6 ± 51.5 | 59.3 ± 44.4 | <0.0001 |
| Flavan-3-ol,4 mg/d | 174 ± 140 | 117 ± 124 | 87.6 ± 102 | 40.9 ± 54.7 | <0.0001 |
| Flavonols,4 mg/d | 71.1 ± 33.8 | 55.2 ± 26.8 | 45.7 ± 22.8 | 32.8 ± 16.5 | <0.0001 |
| Flavanones,4 mg/d | 36.9 ± 37.0 | 28.1 ± 30.0 | 23.0 ± 23.6 | 17.4 ± 20.2 | <0.0001 |
| Flavones,4 mg/d | 36.8 ± 20.1 | 31.9 ± 12.7 | 31.2 ± 12.5 | 29.7 ± 8.6 | <0.0001 |
| Isoflavones,4 μg/d | 30.6 ± 578 | 6.1 ± 19.4 | 15.7 ± 263 | 9.6 ± 15.7 | 0.26 |
| Iron,4 mg/d | 13.9 ± 3.1 | 13.1 ± 2.6 | 12.5 ± 2.4 | 11.9 ± 2.1 | <0.0001 |
| Fiber,4 g/d | 22.9 ± 5.7 | 20.0 ± 4.3 | 18.3 ± 3.4 | 16.9 ± 2.8 | <0.0001 |
Values are means ± SDs unless otherwise indicated. DII, Dietary Inflammatory Index; Q, quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants.
P values are based on a linear contrast test.
Values are percentages of total daily energy intake without alcohol.
Values were energy-adjusted by using the residual method.
The overall and sex-specific analyses are presented in Table 3. No significant association was observed between the DII score and incidence of depressive symptoms in the full sample.
TABLE 3.
Prospective associations between quartiles of DII score and incident depressive symptoms in 3523 participants: the SU.VI.MAX study1
| Q1 | Q2 | Q3 | Q4 | P-trend2 | Continuous DII | P 3 | |
|---|---|---|---|---|---|---|---|
| All participants | |||||||
| DII, range | −4.99, −0.76 | −0.76, 0.49 | 0.49, 1.77 | 1.77, 5.82 | |||
| n | 880 | 881 | 881 | 881 | 3523 | ||
| Number of cases | 45 | 35 | 41 | 51 | 172 | ||
| Model 14 | 1 (ref) | 0.75 (0.47, 1.19) | 0.88 (0.55, 1.39) | 1.07 (0.66, 0.72) | 0.63 | 1.03 (0.93, 1.14) | 0.56 |
| Model 25 | 1 (ref) | 0.74 (0.47, 1.18) | 0.87 (0.55, 1.39) | 1.06 (0.66, 1.71) | 0.63 | 1.03 (0.93, 1.14) | 0.57 |
| Men | |||||||
| DII, range | −4.99, −1.10 | −1.10, 0.003 | 0.01, 1.18 | 1.18, 4.93 | |||
| n | 373 | 373 | 373 | 373 | 1492 | ||
| Number of cases | 15 | 16 | 17 | 21 | 69 | ||
| Model 14 | 1 (ref) | 1.42 (0.66, 3.04) | 1.49 (0.68, 3.25) | 2.32 (1.01, 5.35) | 0.06 | 1.17 (0.99, 1.40) | 0.07 |
| Model 25 | 1 (ref) | 1.40 (0.65, 3.00) | 1.47 (0.67, 3.20) | 2.27 (0.98, 5.23) | 0.06 | 1.17 (0.98, 1.39) | 0.08 |
| Women | |||||||
| DII, range | −4.22, −0.45 | −0.45, 0.83 | 0.84, 2.13 | 2.13, 5.82 | |||
| n | 507 | 508 | 508 | 508 | 2031 | ||
| Number of cases | 27 | 19 | 31 | 26 | 103 | ||
| Model 14 | 1 (ref) | 0.61 (0.33, 1.13) | 0.98 (0.56, 1.70) | 0.72 (0.39, 1.33) | 0.59 | 0.96 (0.85, 1.08) | 0.52 |
| Model 25 | 1 (ref) | 0.62 (0.33, 1.13) | 0.98 (0.56, 1.70) | 0.73 (0.39, 1.34) | 0.63 | 0.96 (0.86, 1.09) | 0.56 |
Values are ORs (95% CIs) unless otherwise indicated. The P value for the sex interaction term is 0.48. CES-D, Center for Epidemiologic Studies–Depression Scale; DII, Dietary Inflammatory Index; Q, quartile; ref, reference; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants.
Estimated by modeling quartiles of DII score as an ordinal variable.
P for linear relation (DII score as a continuous variable).
Adjusted for age, sex, intervention group during the trial phase, educational level, marital status, socio-professional status, energy intake without alcohol, number of 24-h dietary records, interval between the 2 CES-D measurements, smoking status, physical activity, and BMI (all covariates were collected at baseline).
Adjusted for all variables in model 1 plus cancer or cardiovascular events during follow-up.
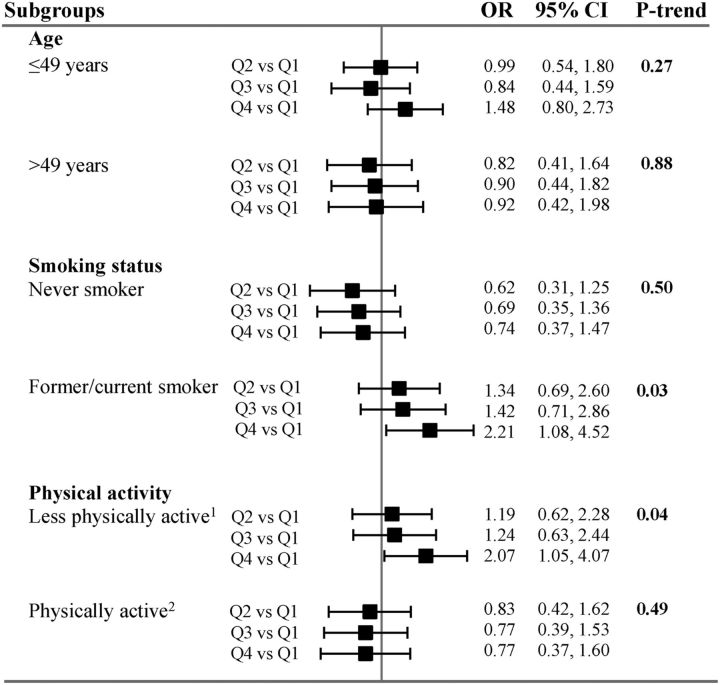
P values for the interaction terms included in the multivariable models were as follows: 0.26 for age, 0.56 for physical activity, 0.88 for smoking status (Figure 2), and 0.48 for sex (Table 3). Because the choice of these potentially modulating factors was hypothesis-driven, and because formal tests for interaction are not robust and extremely conservative (42), we chose to perform subgroup analyses to investigate the modulating effect of all of the above-cited variables. In sex-specific models, we observed a borderline positive association between the highest quartile of DII and the risk of incident depressive symptoms in men (quartile 4 compared with quartile 1—OR: 2.32; 95% CI: 1.01, 5.35; P-trend = 0.06), after taking into consideration a large range of confounding factors (sociodemographic, lifestyle, and BMI; model 1). Of note, the association found among men was not linear and was attenuated after additional adjustment for cancer and cardiovascular events during follow-up (quartile 4 compared with quartile 1—OR: 2.27; 95% CI: 0.98, 5.23; P-trend = 0.06). No significant association was observed among women in any of the models.
FIGURE 2.
Association between quartiles of DII score and incident depressive symptoms in population subgroups. P values for the interaction terms were as follows: 0.26 for age, 0.56 for physical activity, and 0.88 for smoking status. Values are ORs and 95% CIs across quartiles of the DII. Model adjusted for age, sex, intervention group during the trial phase, educational level, marital status, socio-professional status, energy intake without alcohol, number of 24-h dietary records, interval between the 2 CES-D measurements, smoking status, physical activity, and BMI (all covariates were collected at baseline). 1Irregular physical activity or <1 h walking/d. 2Physical activity of ≥1 h walking/d. CES-D, Center for Epidemiologic Studies–Depression Scale; DII, Dietary Inflammatory Index; Q, quartile.
The results of the remaining stratified analyses are presented in Figure 2. No significant associations were observed within the different age subgroups. In analyses stratified by smoking status, we found no significant association between DII score and the risk of incident depressive symptoms among nonsmokers. In the subgroup of current and former smokers, DII score was positively associated with the risk of incident depressive symptoms (quartile 4 compared with quartile 1—OR: 2.21; 95% CI: 1.08, 4.52; P-trend = 0.03).
Finally, no significant association between DII score and incidence of depressive symptoms was observed in the subgroup of physically active participants (physical activity ≥1 h walking/d), whereas among less physically active participants (irregular physical activity or <1 h walking/d), a positive association between DII score and the risk of incident depressive symptoms was observed (quartile 4 compared with quartile 1—OR: 2.07; 95% CI: 1.05, 4.07; P-trend = 0.04).
In sensitivity analyses, when using IPW to account for selection bias, the association observed in men was strengthened (quartile 4 compared with quartile 1—OR: 2.49; 95% CI: 1.07, 5.78; P-trend = 0.03; model 1; Supplemental Table 2). Applying a cutoff value of 16 to define depressive symptoms led to nonsignificant findings (Supplemental Table 3). No significant association was observed in the supplemented and placebo groups (data not shown).
Discussion
In this large prospective study, a proinflammatory diet (as reflected by a higher DII) at midlife was marginally associated with a higher risk of incident depressive symptoms over a 13-y follow-up period among men, but the association was no longer significant after additional adjustment for health events. In sensitivity analyses that used IPW to correct for potential selection bias, the associations remained significant even after adjustment for health events among men. A higher DII was also associated with a higher risk of incident depressive symptoms in specific subgroups of the population, namely current and former smokers and less physically active individuals. No significant associations were observed within any specific age subgroup, among women, nonsmokers, or physically active individuals in both intervention groups or in the full sample.
Our findings of associations of a proinflammatory diet with a higher risk of incident depressive symptoms in men are consistent with those reported by the SUN (Seguimiento Universidad de Navarra) project, including 15,093 men and women, which showed that the risk of depression was 47% (95% CI: 17%, 85%) higher in the highest quintile of DII compared with the first quintile (18). In our study, the associations in men were attenuated after accounting for cancer and cardiovascular events that occurred during follow-up. Because we had previously observed that DII was positively associated with cancer and cardiovascular risk (12, 14), these diseases may partly mediate the association between DII and subsequent depressive symptoms. Next, in contrast to the results reported in the other studies, we did not observe a significant association between DII score and the risk of incident depressive symptoms among women. The Australian Longitudinal Study on Women's Health in 6438 women with a mean age of 52 y at baseline reported an ∼20% lower risk of developing depressive symptoms among women with a more anti-inflammatory diet (quartile 1 compared with quartile 4—RR: 0.81; 95% CI: 0.69, 0.96) (19). In the Nurses' Health Study, the reduced-rank regression approach was used to classify women aged 50–77 y according to an inflammatory dietary pattern related to plasma concentrations of inflammatory markers such as CRP, IL-6, and TNF-α receptor 2. The highest quintile of this dietary pattern was associated with a 41% (95% CI: 22%, 63%) increase in the risk of depression compared with the lowest quintile (with depression defined as diagnosis of depression and use of antidepressants) (21). In addition, in the Whitehall II study, a proinflammatory diet was significantly associated with an increased risk of recurrent depressive symptoms among women (OR for the highest compared with the lowest tertile: 2.83; 95% CI: 1.48, 5.42), whereas a nonsignificant association was observed in men (20). Our findings may also be interpreted in light of previous research that focused on the link between dietary patterns in general and depressive symptoms. In our study, the DII was correlated with a less healthy nutritional profile. Thus, our findings observed among men are consistent with previous work documenting lower depressive symptoms among individuals with a generally healthier diet (43, 44), such as the Mediterranean diet (45, 46), which has been hypothesized to have anti-inflammatory properties and beneficial vascular effects.
With regard to the absence of an association among women, several hypotheses can be advanced. The first is related to potential false-positive cases among women. Indeed, women generally report more depressive symptoms than do men (47). We used French sex-specific cutoffs, which are higher for women than for men (32), and we cannot rule out the possibility of classification bias related to depressive symptoms. Second, diet may be a less prominent factor in the development of depressive symptomology among women than among men. During the period between puberty and menopause, women are more vulnerable to depression than men, which could, among others, be explained by the hormonal fluctuations that accompany the reproductive cycle (48, 49). Indeed, some women present episodes of depression associated with reproductive events, such as the premenstrual period, pregnancy, the postpartum period, and the menopausal transition (48, 50, 51). This is generally due to hormonal imbalances related to estrogen deficiency (48). Another hypothesis is the actual absence of a relation between diet and depressive symptoms in women.
In this longitudinal analysis of the SU.VI.MAX cohort, a proinflammatory diet was associated with a higher risk of incident depressive symptoms among the subgroup of current and former smokers but not in nonsmokers. Tobacco use, an inflammatory agent, is a major risk factor for the development of several chronic diseases (52). Cigarette smoke contains several toxins, including free radicals, metals, and other substances with immunomodulatory effects and that are able to induce chronic inflammation (24, 52). Studies examining the associations between smoking status and inflammation are scant, but some data suggest lower systemic concentrations of immune markers and higher concentrations of acute-phase proteins (including CRP and other proinflammatory cytokines) in smokers than in nonsmokers (53–55).
Moreover, we found that a proinflammatory diet was associated with a higher risk of incident depressive symptoms among less physically active participants but not in physically active participants. Some benefits of physical activity are attributed to its anti-inflammatory properties, mainly through its role on adipose tissue (reduction in visceral fat mass and inflammatory environment), the immune system, skeletal muscle (increasing the production of anti-inflammatory cytokines or reducing proinflammatory cytokines), and blood circulation (56). Overall, the data suggest that regular physical activity reduces concentrations of inflammatory markers (including CRP, IL-6, and TNF-α) and increases concentrations of anti-inflammatory markers such as IL-10, IL-12, and IL-4 (57, 58).
Thus, a possible explanation for the association between DII score and incident depressive symptoms observed in smokers and in less-active participants is the cumulative inflammatory potential of lifestyle, which includes dietary habits. Alternatively, an anti-inflammatory diet may buffer the inflammatory potential of an unhealthy lifestyle (i.e., smoking or low physical activity) so that its protective effect only emerges in individuals displaying these behaviors. Indeed, smoking has been associated with inflammation (25, 59) and depression (26, 60, 61) in various studies. In addition, several epidemiologic studies suggested a reduction in the risk of depressive symptoms among individuals who regularly practice ≥1 physical activities (27, 62, 63).
A range of factors such as stress, chronic anxiety, a poor diet, physical inactivity, obesity, smoking, and sleep disorders have been suggested to increase the risk of depression via an increase in the systemic inflammatory state. Systemic inflammation is the result of the release of proinflammatory cytokines from immune cells and chronic activation of the innate immune system (24). Indeed, several studies have shown high concentrations of proinflammatory cytokines in the serum of patients with major depressive episodes (3, 64). Proinflammatory cytokines may contribute to the development of depression through pathologic activation of the immune response, including the acute-phase reaction as well as tissue damage that can occur at the onset of the inflammatory response. This reaction is evidenced by a large increase in the production of many proteins, including acute-phase proteins (65).
Systemic inflammation affects the brain through several pathways, including leaky regions in the blood-brain barrier, active transport of cytokines through the brain endothelium, and activation of vagal afferent fibers (66). Cytokines and other proinflammatory mediators influence a large number of pathways involved in mood regulation, such as neurotransmitter metabolism, neuroendocrine function, basal ganglia function, and synaptic plasticity (67). In particular, it has been suggested that proinflammatory cytokines affect the neural systems involved in mood regulation by an imbalance in the production and transmission of neurotransmitters such as serotonin, dopamine, noradrenaline, and glutamate, involving, in particular, the activation of the enzyme indoleamine 2,3-dioxygenase, which degrades tryptophan metabolism by depriving the precursor serotonin of melatonin and kynurenine (68).
Some limitations of this study should be mentioned. First, despite the prospective design of our investigation, reverse causality cannot be entirely excluded because our study was observational. We also acknowledge the possibility of residual confounding, even after taking into account a wide range of confounders. Indeed, other factors, such as family history of depressive disorders, stressful life events, and sleep disorders, are associated with depression (2, 24). Other limitations are related to the high exclusion rate of participants and the generalizability of our findings. Among participants of the SU.VI.MAX cohort who were free of depression at the beginning of the study and who had available DII data at baseline (eligible population, n = 5146), we included only participants who had CES-D data at the end of the follow-up (n = 3523). This high rate of exclusion could include a potential bias. However, the use of the IPW to account for selection bias in sex-specific models provides similar results in terms of trend, but the association observed in men was strengthened. In addition, participants of the SU.VI.MAX cohort were aged 35–60 y (women) or 45–60 y (men) at baseline. Any generalization of our findings should be done with caution. Finally, only 36 of the 45 dietary variables were included in the computing of the DII score. Nevertheless, our estimation can be considered acceptable because, according to available data, the average number of variables used for calculating the DII score in previous studies was 27.
In addition, the present study exhibits a number of important strengths, including its prospective design, the large sample size, the availability of repeated CES-D data, and the quality of the dietary data based on repeated 24-h dietary records, reflecting the participants' food habits. Another strength was the use of a DII score specifically designed to measure the inflammatory potential of the overall diet.
In summary, results from this large prospective study suggest that a proinflammatory diet at midlife, as measured by a higher DII score, was associated with a higher risk of incident depressive symptoms, in particular among men and in subgroups with unhealthy lifestyles. These findings indicate that the promotion of a healthy diet exhibiting anti-inflammatory properties, as well as lifestyle overall, could be an important element of primary prevention strategies with respect to depressive symptoms in the course of aging. However, to confirm the role of a proinflammatory diet in depression, and to better target at-risk subgroups, further prospective epidemiologic investigations are needed.
Supplementary Material
Acknowledgments
We thank Younes Esseddik, Paul Flanzy, Yasmina Chelghoum, and Than Duong Van (computer scientists); Rachida Mehroug (logistics assistant); and Nathalie Arnault, Véronique Gourlet, Fabien Szabo, Laurent Bourhis, and Stephen Besseau (statisticians) for their technical contributions to the SU.VI.MAX study. MT, SH, PG, and EK-G were responsible for developing the concept, design, and protocol of the study and for coordinating data collection; MA performed the statistical analysis and wrote the manuscript; EK-G supervised the study and provided methodologic guidance; NS, JRH, and MDW designed and computed the DII score; and MA, VAA, CL, MT, NS, JRH, MDW, SH, PG, CJ, KEA, and EK-G were involved in interpreting the results and editing the manuscript for important intellectual content. All authors read and approved the final manuscript.
Abbreviations
- CES-D
Center for Epidemiologic Studies–Depression Scale
- CRP
C-reactive protein
- DII
Dietary Inflammatory Index
- IPW
inverse-probability weighting
- SU.VI.MAX
Supplémentation en Vitamines et Minéraux Antioxydants
Footnotes
Supported by the French National Research Agency (ANR-05-PNRA-010), a 2013 research grant from the Société Française d'Hypertension Artérielle (R13024KK RAK13204KKA), the French Ministry of Health, the US National Institute for Diabetes, Digestive, and Kidney Diseases (grant R44DK103377), and Paris 13 University.
References
- 1. WHO Mental health action plan 2013–2020. Geneva (Switzerland): WHO; 2013. [Google Scholar]
- 2. Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord 2013;148:12–27. [DOI] [PubMed] [Google Scholar]
- 3. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–57. [DOI] [PubMed] [Google Scholar]
- 4. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013;150:736–44. [DOI] [PubMed] [Google Scholar]
- 6. Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014;71:1381–91. [DOI] [PubMed] [Google Scholar]
- 7. Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 2013;39:99–110. [DOI] [PubMed] [Google Scholar]
- 8. Nowlin SY, Hammer MJ, D'Eramo MG. Diet, inflammation, and glycemic control in type 2 diabetes: an integrative review of the literature. J Nutr Metab 2012;2012:542689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Defagó MD, Elorriaga N, Irazola VE, Rubinstein AL. Influence of food patterns on endothelial biomarkers: a systematic review. J Clin Hypertens (Greenwich) 2014;16:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 2013;71:511–27. [DOI] [PubMed] [Google Scholar]
- 11. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neufcourt L, Assmann KE, Fezeu LK, Touvier M, Graffouillere L, Shivappa N, Hebert JR, Wirth MD, Hercberg S, Galan P, et al. . Prospective association between the dietary inflammatory index and cardiovascular diseases in the SUpplementation en VItamines et Mineraux AntioXydants (SU.VI.MAX) cohort. J Am Heart Assoc 2016;5:e002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer Causes Control 2016;27:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graffouillère L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, Wirth MD, Latino-Martel P, Hercberg S, Galan P, et al. . The Dietary Inflammatory Index is associated with prostate cancer risk in French middle-aged adults in a prospective study. J Nutr 2016;146:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, Rossi M. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. Br J Nutr 2016;115:138–46. [DOI] [PubMed] [Google Scholar]
- 16. Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, Hebert JR, Petersen GM. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis 2016;37:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, Hebert JR, Wirth MD, Hercberg S, Galan P, et al. . Long-term association between the Dietary Inflammatory Index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr 2016. Apr 7 (Epub ahead of print; DOI: 10.1007/s00394-016-1211-3). [DOI] [PubMed] [Google Scholar]
- 18. Sánchez-Villegas A, Ruiz-Canela M, Fuente-Arrillaga C, Gea A, Shivappa N, Hebert JR, Martinez-Gonzalez MA. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr 2015;114:1471–9. [DOI] [PubMed] [Google Scholar]
- 19. Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women's Health. Br J Nutr 2016;116:1077–86. [DOI] [PubMed] [Google Scholar]
- 20. Akbaraly TN, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, Hebert JR, Kivimaki M. Dietary inflammatory index and recurrence of depressive symptoms results from the Whitehall II study. Clin Psychol Sci 2016;4:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas M, Chocano-Bedoya P, Schulze MB, Mirzaei F, O'Reilly EJ, Okereke OI, Hu FB, Willett WC, Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun 2014;36:46–53. Erratum in: Brain Behav Immun 2015;46:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013;34:119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt-Glaser JK. Sex differences in depression: does inflammation play a role? Curr Psychiatry Rep 2015;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, et al. . So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013;11:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonçalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 2011;60:409–24. [DOI] [PubMed] [Google Scholar]
- 26. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284:2606–10. [DOI] [PubMed] [Google Scholar]
- 27. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001;322:763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vulser H, Wiernik E, Tartour E, Thomas F, Pannier B, Czernichow S, Hanon O, Simon T, Simon JM, Ducolombier C, et al. . Smoking and the association between depressive symptoms and absolute neutrophil count in the Investigations Preventives et Cliniques Cohort study. Psychosom Med 2015;77:1039–49. [DOI] [PubMed] [Google Scholar]
- 29. Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2009;8:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hercberg S, Preziosi P, Briancon S, Galan P, Triol I, Malvy D, Roussel AM, Favier A. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: the SU.VI.MAX study—design, methods, and participant characteristics. Control Clin Trials 1998;19:336–51. [DOI] [PubMed] [Google Scholar]
- 31. Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335–42. [DOI] [PubMed] [Google Scholar]
- 32. Führer R, Rouillon F. The French version of the Center for Epidemiologic Studies-Depression Scale. Psychiatr Psychobiol 1989;4:163–6. [Google Scholar]
- 33. Morin AJ, Moullec G, Maiano C, Layet L, Just JL, Ninot G. Psychometric properties of the Center for Epidemiologic Studies Depression Scale (CES-D) in French clinical and nonclinical adults. Rev Epidemiol Sante Publique 2011;59:327–40. [DOI] [PubMed] [Google Scholar]
- 34. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 35. Le Moullec N, Deheeger M, Preziosi P, Monteiro P, Valeix P, Rolland-Cachera M-F, Potier de Courcy G, Christides J-P, Cherouvrier F, Galan P, et al. . Validation du manuel photos utilisé pour l'enquête alimentaire de l'étude SU.VI.MAX [Validation of the photo manual used for the dietary assessment of the SU.VI.MAX study]. Cah Nutr Diet 1996;31:158–64 (in French). [Google Scholar]
- 36. Hercberg SC. Table de composition SU.VI.MAX des aliments [Food composition table SU.VI.MAX]. Paris: Les éditions INSERM/Economica; 2005;182p(in French). [Google Scholar]
- 37. WHO Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452. [PubMed] [Google Scholar]
- 38. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. [DOI] [PubMed] [Google Scholar]
- 39. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 41. Shen C, Li X, Li L, Were MC. Sensitivity analysis for causal inference using inverse probability weighting. Biom J 2011;53:822–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marshall SW. Powe r for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahe C, Unrath M, Berger K. Dietary patterns and the risk of depression in adults: a systematic review of observational studies. Eur J Nutr 2014;53:997–1013. [DOI] [PubMed] [Google Scholar]
- 44. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr 2014;99:181–97. [DOI] [PubMed] [Google Scholar]
- 45. Skarupski KA, Tangney CC, Li H, Evans DA, Morris MC. Mediterranean diet and depressive symptoms among older adults over time. J Nutr Health Aging 2013;17:441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sánchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra ML, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry 2009;66:1090–8. [DOI] [PubMed] [Google Scholar]
- 47. Kessler RC. Epidemiology of women and depression. J Affect Disord 2003;74:5–13. [DOI] [PubMed] [Google Scholar]
- 48. Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci 2008;33:331–43. [PMC free article] [PubMed] [Google Scholar]
- 49. Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry 2015;172:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Freeman EW. Associations of depression with the transition to menopause. Menopause 2010;17:823–7. [DOI] [PubMed] [Google Scholar]
- 51. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas 2014;79:142–6. [DOI] [PubMed] [Google Scholar]
- 52. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 2012;91:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, Kitahara CM, Furr M, Li Y, Kemp TJ, et al. . Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014;106:dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest 2007;131:1557–66. [DOI] [PubMed] [Google Scholar]
- 55. Tonstad S, Cowan JL. C-reactive protein as a predictor of disease in smokers and former smokers: a review. Int J Clin Pract 2009;63:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid Med Cell Longev 2016;2016:7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010;411:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005;45:1563–9. [DOI] [PubMed] [Google Scholar]
- 59. Johannsen A, Susin C, Gustafsson A.. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontology 2000;64:111–26. [DOI] [PubMed] [Google Scholar]
- 60. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among U.S. adults. Addict Behav 2009;34:491–7. [DOI] [PubMed] [Google Scholar]
- 62. Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633–8. [DOI] [PubMed] [Google Scholar]
- 63. Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2012;201:180–5. [DOI] [PubMed] [Google Scholar]
- 64. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71:171–86. [DOI] [PubMed] [Google Scholar]
- 65. Lim W, Hong S, Nelesen R, Dimsdale JE. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med 2005;165:910–5. [DOI] [PubMed] [Google Scholar]
- 66. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoyo-Becerra C, Schlaak JF, Hermann DM. Insights from interferon-alpha-related depression for the pathogenesis of depression associated with inflammation. Brain Behav Immun 2014;42:222–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.