Abstract
Background:
Venous thromboembolism (VTE), ischemic stroke, and myocardial infarction in transgender persons may be related to hormone use.
Objective:
To examine the incidence of these events in a cohort of transgender persons.
Design:
Electronic medical record-based cohort study of transgender members of integrated health care systems who had an index date (first evidence of transgender status) from 2006 through 2014. Ten male and 10 female cisgender enrollees were matched to each transgender participant by year of birth, race/ethnicity, study site, and index date enrollment.
Setting:
Kaiser Permanente in Georgia and northern and southern California.
Patients:
2842 transfeminine and 2118 transmasculine members with a mean follow-up of 4.0 and 3.6 years, respectively, matched to 48 686 cisgender men and 48 775 cisgender women.
Measurements:
VTE, ischemic stroke, and myocardial infarction events ascertained from diagnostic codes through the end of 2016 in transgender and reference cohorts.
Results:
Transfeminine participants had a higher incidence of VTE, with 2-and 8-year risk differences of 4.1 (95% CI, 1.6 to 6.7) and 16.7 (CI, 6.4 to 27.5) per 1000 persons relative to cisgender men and 3.4 (CI, 1.1 to 5.6) and 13.7 (CI, 4.1 to 22.7) relative to cisgender women. The overall analyses for ischemic stroke and myocardial infarction demonstrated similar incidence across groups. More pronounced differences for VTE and ischemic stroke were observed among transfeminine participants who initiated hormone therapy during follow-up. The evidence was insufficient to allow conclusions regarding risk among transmasculine participants.
Limitation:
Inability to determine which transgender members received hormones elsewhere.
Conclusion:
The patterns of increases in VTE and ischemic stroke rates among transfeminine persons are not consistent with those observed in cisgender women. These results may indicate the need for long-term vigilance in identifying vascular side effects of cross-sex estrogen.
Transgender persons are a diverse group whose gender identity differs from a male or female sex designation, which usually is assigned at birth (1). Although some transgender persons may not self-identify on the basis of binary definitions (2), a person whose gender identity differs from a male sex designation at birth often is referred to as male-to-female, transfeminine, or trans woman, and a person whose gender identity differs from a female sex designation at birth often is referred to as female-to-male, transmasculine, or trans man (3, 4). Some transgender persons undergo medical treatment to align their physical appearance with their gender identity (5, 6).
A specific area of concern in transgender health is the risk for acute cardiovascular events (ACVEs), including venous thromboembolism (VTE), ischemic stroke, and myocardial infarction, which might plausibly be related to cross-sex hormone therapy (7). As reviewed elsewhere (8 –11), the direct evidence addressing this issue is sparse and inconsistent because of the predominance of small studies with very few reported events.
A direct evaluation of the evidence regarding the incidence of ACVEs requires a longitudinal study that includes large numbers of transfeminine and transmasculine participants, with sufficient follow-up, appropriate control groups, and documented cross-sex hormone use among participants (12). Integrated health care systems with electronic medical records (EMRs) allow efficient identification and follow-up of hard-to-reach population subgroups, such as transgender persons. Our objective was to compare ACVE incidence rates in a cohort of transgender persons enrolled in 3 such health care systems with rates observed in age-, race-, site-, and membership-matched cisgender men and women (reference cohorts).
Methods
Cohort Ascertainment
This study took place at Kaiser Permanente sites in Georgia, northern California, and southern California and was coordinated by Emory University. All activities were reviewed and approved by the institutional review boards of the 4 institutions. The methods of cohort ascertainment were described in detail previously (13, 14). As summarized in the Supplement and Supplement Figure 1 (available at Annals.org), cohort selection involved a 3-step algorithm: an initial EMR search to identify cohort candidates (step 1), validation of transgender status (step 2), and determination of transmasculine or transfeminine status (step 3).
Ten male and 10 female cisgender Kaiser Permanente enrollees were matched to each member of the final validated transgender cohort by race/ethnicity (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic, and other), year of birth (within a 5-year interval), study site, and calendar year of membership based on the index date. Index date was defined as the first recorded evidence of transgender status. We used both male and female cisgender reference groups because hormone serum concentrations among transgender persons may range from normal physiologic male to normal physiologic female levels, depending on receipt and dosage of hormone therapy as well as individual characteristics (15). A 10:1 ratio was used to allow stratified analyses (for example, by hormone therapy type) while ensuring a sufficient number of cisgender referents for each cohort member. Each transgender cohort member was linked to matched referents via a unique cluster identification number (ID) to allow subanalyses.
Data Collection and Analysis
Only persons aged 18 years or older at their index date who were determined to be transmasculine or transfeminine, along with their matched referents, were included. All study participants were characterized with respect to their Kaiser Permanente enrollment history and their cigarette smoking status, body mass index (BMI; kilograms per square meter), blood pressure, and total blood cholesterol level at baseline. Variable categorization is presented in the footnotes to the tables and in the Supplement.
Transgender hormone treatment was determined through EMR linkages to prescription data by using national drug codes. Linkages with the International Classification of Diseases, Ninth Revision and 10th Revision (ICD-9 and ICD-10), and Current Procedural Terminology codes were used to ascertain surgeries and other interventions. Feminizing drugs (such as estradiol and spironolactone) in a participant recorded as male at birth and masculinizing drugs (such as testosterone) in a participant documented as female at birth were considered evidence of hormone therapy.
In both the transgender and the reference cohorts, ACVEs were ascertained on the basis of ICD-9 or ICD-10 codes. The lists of codes and numbers of cases ascertained by each code are specified in Supplement Table 3 (available at Annals.org). Only ACVEs with a diagnosis date during follow-up were used in the analyses. History of ACVEs was defined as having an event with a diagnosis date before the start of follow-up.
Statistical Analysis
All transgender cohort members were characterized as transfeminine or transmasculine and grouped further according to their history of cross-sex hormone use. Follow-up in the overall analysis extended from the index date until the first occurrence of the event of interest, disenrollment from the plan for more than 90 days, death, or the end of the study period (30 November 2016). For participants who began hormone therapy at Kaiser Permanente after the index date (hormone initiation cohort), additional analyses were conducted. In these analyses, follow-up started on the date of the first filled prescription for estrogen or testosterone for transfeminine or transmasculine participants, respectively. Matched referents were assigned the same start date for follow-up.
Missing covariate values for BMI, blood pressure, and total cholesterol level were assigned by using multiple imputation methods (n = 5 imputations). Incidence rates were calculated as the number of cases per 1000 person-years, and the corresponding 95% CIs were calculated by using the Poisson distribution. Both unadjusted Kaplan-Meier curves and weighted cumulative incidence curves adjusted for covariates at the population means were constructed to compare the incidence of each ACVE type in the transmasculine and transfeminine participants with those in the corresponding matched reference cohorts. Risk differences at 2, 4, 6, and 8 years were calculated directly from the adjusted cumulative incidence curves. The 95% CI for each risk difference estimate was calculated via a bootstrapping procedure using 1000 random samples with replacement.
In the primary analysis, we used multivariable Cox proportional hazards models to compare ACVE rates in the overall transfeminine and transmasculine cohorts and among members of the hormone initiation cohorts with those in the matched cisgender reference groups, after controlling for history of any ACVE, smoking status, BMI, blood pressure, and blood cholesterol level ascertained near the index date. Each model was stratified by cluster ID to account for matching. Proportional hazards assumptions were tested by examining log-minus-log plots for each variable in the model and by performing a goodness-of-fit test using Schoenfeld residuals (16). The results of the Cox models were expressed as adjusted hazard ratios with corresponding 95% CIs. Because the weighted cumulative incidence curves could not account for matching, hazard ratios from models that were not stratified by cluster ID were also calculated and are included in Supplement Tables 4 and 5 (available at Annals.org). When evidence (such as log-minus-log survival plots) suggested that the proportional hazards assumption was violated, stratified Cox models were used to control for covariates, and extended Cox models with time-dependent hazard ratio estimates were used for the main independent variables of interest (16).
Although the cohort size precluded detailed analyses by specific hormone therapy regimens, some examination of treatment subcategories was possible. These secondary exploratory analyses focused on transfeminine cohort subgroups defined on the basis of administration route (oral or other) and estrogen type (estradiol or other). In addition, the highest daily hormone dosages were summarized for participants who had an event of interest and in those who received hormone therapy but remained ACVE-free.
We examined the effect of different case and exposure definitions, risk factors, and analytic approaches by conducting a series of sensitivity analyses (Supplement). To investigate the effects of unaccounted confounding, we calculated a range of E-values for the main results and the lower limits of their 95% CIs observed in Cox regression models (17). The data analyses were performed with SAS, version 9.4 (SAS Institute). E-values were obtained by using an online calculator for hazard ratios with an outcome prevalence of less than 15%.
Results
A total of 6456 transgender cohort members were identified in the EMR. After persons younger than 18 years at their index date (n = 1347), those with unknown sex designation at birth (n = 75), and those with no follow-up data (n = 74) were excluded, the study group included 4960 transgender participants and matched reference cohorts of 48 686 cisgender men and 48 775 cisgender women.
The transgender cohort comprised 2842 (57%) transfeminine and 2118 (43%) transmasculine persons (Table 1). More than 50% of participants in both groups were non-Hispanic whites; Hispanics represented 18% of transfeminine and 14% of transmasculine participants, whereas the remainder of the study population was distributed approximately equally among non-Hispanic blacks, Asians/Pacific Islanders, and persons whose race/ethnicity was marked as other or unknown. The proportion of newly identified cohort members increased over time, with approximately 40% of participants identified in the 3 most recent years. About 38% of the transgender cohort had a normal BMI around the index date, which was greater than the proportion of reference men but less than that of reference women. Fewer than 20% of participants were current smokers at or around the index date, with the highest proportion observed in the transmasculine group (Table 1).
Table 1.
Characteristics of the Transgender and Matched Reference Cohorts*
| Characteristic | Transfeminine Cohort, n (%) |
Transmasculine Cohort, n (%) |
||||
|---|---|---|---|---|---|---|
| Transfeminine Cohort (n = 2842) |
Reference Men (n = 27 906) |
Reference Women (n = 27 968) |
Transmasculine Cohort (n = 2118) |
Reference Men (n = 20 780) |
Reference Women (n = 20 807) |
|
| Membership site | ||||||
| KPNC | 1585 (56) | 15 620 (56) | 15 650(56) | 1382 (65) | 13 572 (65) | 13 599 (65) |
| KPSC | 1180 (42) | 11 528(41) | 11 553(41) | 678 (32) | 6631 (32) | 6630 (32) |
| KPGA | 77(2.7) | 758 (2.7) | 765 (2.7) | 58 (2.7) | 577 (2.8) | 578 (2.8) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1544 (54) | 15 149 (54) | 15 198(54) | 1281 (60) | 12 532 (60) | 12 557 (60) |
| Non-Hispanic black | 185 (6.5) | 1810 (6.5) | 1825(6.5) | 192 (9.1) | 1885(9.1) | 1890 (9.1) |
| Asian/Pacific Islander | 263 (9.3) | 2600 (9.3) | 2596 (9.3) | 143 (6.8) | 1415 (6.8) | 1421 (6.8) |
| Hispanic | 523 (18) | 5147 (18) | 5157 (18) | 294 (14) | 2900 (14) | 2893 (14) |
| Other/unknown | 327 (12) | 3200 (11) | 3192(11) | 208(10) | 2048(10) | 2046 (10) |
| Age at index date | ||||||
| 18–25 y | 642 (23) | 6246 (22) | 6293 (23) | 737 (35) | 7189(35) | 7210 (35) |
| 26–35 y | 584 (21) | 5715 (20) | 5726 (20) | 701 (33) | 6872 (33) | 6880 (33) |
| 36–45 y | 572 (20) | 5630 (20) | 5624 (20) | 344 (16) | 3392 (16) | 3389 (16) |
| 46–55 y | 547 (19) | 5401 (19) | 5401 (19) | 215(10) | 2125(10) | 2123 (10) |
| >55 y | 497 (17) | 4914(18) | 4924(18) | 121 (5.7) | 1202 (5.8) | 1205 (5.8) |
| Smoking status | ||||||
| Current smoker | 434(15) | 4006 (14) | 2339 (8.4) | 382 (18) | 3167 (15) | 1757 (8.4) |
| Not current smoker | 2408 (85) | 23 900 (86) | 25 629 (92) | 1736(82) | 17 613(85) | 19 050 (92) |
| BMI | ||||||
| Normal weight (<25.0 kg/m2) | 1100 (39) | 6699 (24) | 10 397 (37) | 767 (36) | 6051 (29) | 8717 (42) |
| Overweight (25.0–29.9 kg/m2) | 821 (29) | 9269 (33) | 6846 (24) | 548 (26) | 6762 (33) | 4799 (23) |
| Obese (≥30.0 kg/m2) | 659 (23) | 7814(28) | 7453 (27) | 657 (31) | 5414(26) | 5355 (26) |
| Unknown | 262 (9.2) | 4124 (15) | 3272 (12) | 146 (6.9) | 2553 (12) | 1936 (9.3) |
| Total blood cholesterol level | ||||||
| Normal (<5.2 mmol/L [<200 mg/dL]) | 1589 (56) | 9937 (36) | 10 353 (37) | 1233 (58) | 6298 (30) | 7149 (34) |
| Borderline (5.2–6.2 mmol/L [200–239 mg/dL]) | 528 (19) | 4201 (15) | 4707 (17) | 345 (16) | 2266(11) | 2253 (11) |
| High (>6.2 mmol/L [≥240 mg/dL]) | 170 (6.0) | 1671 (6.0) | 1845(6.6) | 109(5.2) | 892 (4.3) | 784 (3.8) |
| Not done (missing, age <40 y) | 374 (13) | 8696 (31) | 8114(29) | 369 (17) | 9888 (48) | 9280 (45) |
| Unknown (missing, age ≥40 y) | 181 (6.4) | 3401 (12) | 2949(11) | 62 (2.9) | 1436(6.9) | 1341 (6.4) |
| Blood pressure | ||||||
| Normal (systolic: ≤120 mm Hg; diastolic: ≤80 mm Hg) | 1110(39) | 8083 (29) | 13 810(49) | 1070(51) | 6268 (30) | 11 408 (55) |
| Borderline (systolic: 121–139 mm Hg; diastolic: 81–89 mm Hg) | 1187 (42) | 11 607 (42) | 8976 (32) | 787 (37) | 8859 (43) | 6262 (30) |
| Elevated (systolic: ≥140 mm Hg; diastolic: ≥90 mm Hg) | 461 (16) | 4476 (16) | 3131 (11) | 196(9.3) | 2828(14) | 1658 (8.0) |
| Unknown | 84 (3.0) | 3740 (13) | 2051 (7.3) | 65(3.1) | 2825(14) | 1479 (7.1) |
| History of any ACVE before index date | ||||||
| Yes | 54(1.9) | 791 (2.8) | 581 (2.1) | 35(1.7) | 243(1.2) | 246 (1.2) |
| No | 2788 (98) | 27 115 (97) | 27 387 (98) | 2083 (98) | 20 537 (99) | 20 561 (99) |
ACVE = acute cardiovascular event; BMI = body mass index; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California.
We refer to referents as cisgender for expediency but were unable to verify that each of these members was not transgender. Percentages may not sum to 100 due to rounding.
The average follow-up was 4.0 (SD, 3.0) years in the transfeminine group and 4.4 (SD, 3.1) years in the matched reference cohort. For transmasculine participants and their matched reference cohort, the mean follow-up was 3.6 (SD, 2.7) and 3.9 (SD, 2.9) years, respectively.
The transfeminine participants had 148 ACVEs since the index date: 61 VTEs, 54 ischemic strokes, and 33 myocardial infarctions. In the transmasculine cohort, 23 VTEs, 16 ischemic strokes, and 9 myocardial infarctions occurred.
The transfeminine cohort had an increase in post-index date incidence of VTE compared with either reference cohort, and the difference seemed more pronounced with increased follow-up, with 2-and 8-year risk differences of 4.1 (95% CI, 1.6 to 6.7) and 16.7 (CI, 6.4 to 27.5) per 1000 persons relative to cisgender men and 3.4 (CI, 1.1 to 5.6) and 13.7 (CI, 4.1 to 22.7) per 1000 persons relative to cisgender women (Table 2 and Supplement Figure 2, A and D, available at Annals.org). The incidence of ischemic stroke was about the same in all 3 cohorts (Table 2 and Supplement Figure 2, B and E, available at Annals.org). The incidence of myocardial infarction in the transfeminine cohort was greater than in reference women but no different from the incidence in reference men (Table 2 and Supplement Figure 2, C and F, available at Annals.org).
Table 2.
Incidence Rates and Adjusted HRs for ACVEs Among Transfeminine Cohort Members Compared With Matched Reference Cohorts From KPNC, KPSC, and KPGA, 2006–2016
| Cohort and Event of Interest | Transfeminine Cohort |
Adjusted HR (95% CI)* |
||
|---|---|---|---|---|
| ACVEs, n |
Incidence Rate (95% CI)† |
Versus Reference Men |
Versus Reference Women |
|
| Transfeminine overall cohort (n = 2842) | ||||
| VTE | 61 | 5.5 (4.3–7.0) | 1.9 (1.4–2.7) | 2.0 (1.4–2.8) |
| Ischemic stroke | 54 | 4.8 (3.7–6.3) | 1.2 (0.9–1.7) | 1.9 (1.3–2.6) |
| Myocardial infarction | 33 | 2.9 (2.1–4.1) | 0.9 (0.6–1.5) | 1.8 (1.1–2.9) |
| Transfeminine estrogen initiation cohort (n = 853) | ||||
| VTE‡ | 17 | 6.6 (4.1–10.6) | 3.2 (1.5–6.5) | 2.5 (1.2–5.0) |
| At 0–2 y of follow-up | 6 | 4.3 (1.9–9.6) | 1.5 (0.5–5.1) | 1.7 (0.5–5.5) |
| At >2 y of follow-up | 11 | 9.3 (5.2–16.8) | 5.1 (2.1–12.6) | 3.2 (1.3–7.6) |
| Ischemic stroke‡ | 17 | 6.6 (4.1–10.6) | 2.3 (1.2–4.3) | 2.9 (1.5–5.5) |
| At 0–6 y of follow-up | 9 | 3.8 (2.0–7.3) | 1.3 (0.6–2.9) | 2.3 (1.0–5.4) |
| At >6 y of follow-up | 8 | 36.2 (18.1–72.4) | 9.9 (3.0–33.1) | 4.1 (1.5–11.4) |
| Myocardial infarction in the cohort overall | 4 | 1.5 (0.6–4.1) | 1.0 (0.3–3.2) | 2.4 (0.6–9.4) |
ACVE = acute cardiovascular event; HR = hazard ratio; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California; VTE = venous thromboembolism.
Stratified by cluster identification number and history of any ACVE; body mass index (normal, overweight, or obese), smoking status (current vs. not current), blood pressure (elevated, borderline, or normal), and total blood cholesterol level (normal, not done [for persons <40 y], borderline, or high) are included in the model as covariates (see the Supplement [available at Annals.org] for details of variable characterization).
Calculated as number of cases per 1000 person-years.
Models were extended because of violation of proportional hazards assumptions.
In the analyses limited to transfeminine persons who initiated estrogen therapy after the index date (estrogen initiation cohort), the differences in VTE and ischemic stroke incidence rates compared with either reference cohort were evident. The adjusted survival curves, and particularly the Kaplan-Meier curves (Supplement Figure 3, available at Annals.org), suggested inflection points around 2 years of follow-up for VTE (Figure 1) and at 6 years of follow-up for ischemic stroke (Figure 2). The corresponding adjusted cumulative incidence curves for myocardial infarction in the estrogen initiation cohort did not show statistically significant differences (Figure 3), but the hazard ratio estimates in Table 2 are imprecise because few events (n = 4) occurred in the exposed group.
Figure 1.
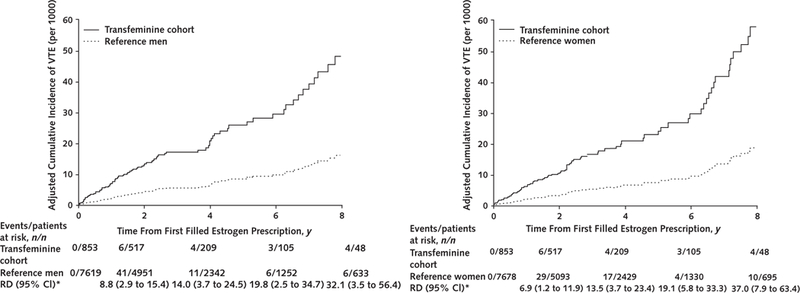
Adjusted cumulative incidence curves comparing rates of VTE among transfeminine cohort members who initiated estrogen therapy after the index date with matched reference men (left) and reference women (right) from KPNC, KPSC, and KPGA, 2006–2016.
Adjustment for covariates was made at the population mean values. KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California; RD = risk difference; VTE = venous thromboembolism.
* Per 1000 persons.
Figure 2.
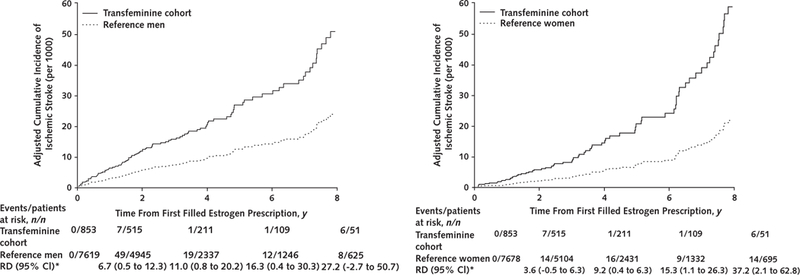
Adjusted cumulative incidence curves comparing rates of ischemic stroke among transfeminine cohort members who initiated estrogen therapy after the index date with matched reference men (left) and reference women (right) from KPNC, KPSC, and KPGA, 2006–2016
Adjustment for covariates was made at the population mean values. KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California; RD = risk difference.
* Per 1000 persons.
Figure 3.
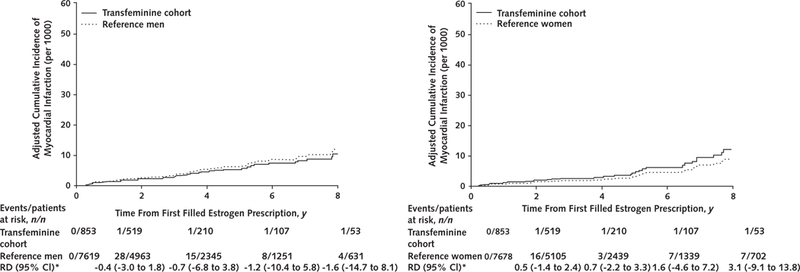
Adjusted cumulative incidence curves comparing rates of myocardial infarction among transfeminine cohort members who initiated estrogen therapy after the index date with matched reference men (left) and reference women (right) from KPNC, KPSC, and KPGA, 2006–2016
Adjustment for covariates was made at the population mean values. KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California; RD = risk difference.
* Per 1000 persons.
Although results of the Schoenfeld goodness-of-fit test were not statistically significant, changes in relative rates overtime and a violation of the proportional hazards assumption were suggested by log-minus-log plots (Supplement Figure 4, available at Annals.org) in the analyses for VTE and ischemic stroke in the estrogen initiation cohort. For this reason, the hazard ratios for VTE in this category of participants are presented separately for follow-up of 2 years or less and for follow-up longer than 2 years, whereas the analyses for ischemic stroke used 6 years as the cutoff. As shown in Table 2, the hazard ratio estimates–after adjustment for cluster ID, BMI, history of ACVE of interest, blood pressure, blood cholesterol level, and smoking–in the 0-to 2-year follow-up for VTE and the 0-to 6-year follow-up for ischemic stroke were closer to the null than the corresponding estimates in the later periods; this was consistent with the inflection points observed on log-minus-log plots.
The cumulative incidence curves for most analyses in the transmasculine cohort closely followed those of the 2 matched reference cohorts (Supplement Figure 5, available at Annals.org). These curves were consistent with the results of the multivariable Cox regression analyses (Table 3). The hazard ratio estimates for VTE in the overall transmasculine cohort that used cisgender men and cisgender women as the reference categories were 1.6 (CI, 0.9 to 2.9) and 1.1 (CI, 0.6 to 2.1), respectively. The corresponding results were 1.1 (CI, 0.6 to 2.0) and 1.3 (CI, 0.7 to 2.5) for ischemic stroke and 0.7 (CI, 0.3 to 1.8) and 1.3 (CI, 0.5 to 3.9) for myocardial infarction. Analyses restricted to the testosterone initiation cohort were limited because of relatively few events (Table 3).
Table 3.
Incidence Rates and Adjusted HRs for ACVEs Among Transmasculine Cohort Members Compared With Matched Reference Cohorts From KPNC, KPSC, and KPGA, 2006–2016
| Cohort and Event of Interest | Transmasculine Cohort |
Adjusted HR (95% CI)* |
||
|---|---|---|---|---|
| ACVEs, n |
Incidence Rate (95% CI)† |
Versus Reference Men |
Versus Reference Women |
|
| Transmasculine overall cohort (n = 2118) | ||||
| VTE | 23 | 3.1 (2.0–4.6) | 1.6 (0.9–2.9) | 1.1 (0.6–2.1) |
| Ischemic stroke | 16 | 2.1 (1.3–3.5) | 1.1 (0.6–2.0) | 1.3 (0.7–2.5) |
| Myocardial infarction | 9 | 1.2 (0.6–2.3) | 0.7 (0.3–1.8) | 1.3 (0.5–3.9) |
| Transmasculine testosterone initiation cohort (n = 585) | ||||
| VTE | 4 | 3.3 (1.3–8.9) | 2.7 (0.6–12.1) | 1.5 (0.4–5.6) |
| Ischemic stroke | 2 | 1.7 (0.4–6.7) | NC‡ | NC‡ |
| Myocardial infarction | 0 | - | - | - |
ACVE = acute cardiovascular event; HR = hazard ratio; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California; KPSC = Kaiser Permanente Southern California; NC = not calculated; VTE = venous thromboembolism.
Stratified by cluster identification number and history of any ACVE; body mass index (normal, overweight, or obese), smoking status (current vs. not current), blood pressure (elevated, borderline, or normal), and total blood cholesterol level (normal, not done [for persons <40 y], borderline, or high) are included in the model as covariates (see the Supplement [available at Annals.org] for details of variable characterization).
Calculated as number of cases per 1000 person-years.
Because of small numbers.
Hormone dosages are described only for members of the estrogen initiation cohort who received oral estradiol alone, the most common formulation-route combination. The average maximum daily dosage of estradiol received during follow-up was 4.1 mg (range, 1 to 10 mg) for transfeminine participants with either VTE or ischemic stroke (n = 11) and 4.2 mg (range, 0.3 to 10 mg) for those with neither event (n = 391). Among transfeminine participants who had either event, the average maximum daily dosage during the first 2 years of follow-up was lower (3.6 mg; range, 1.0 to 7.0 mg) than the corresponding dosage after 2 years of follow-up (5.6 mg; range, 2.0 to 10.0 mg). The mean values for transfeminine participants with no ACVE were similar before and after 2 years of follow-up (4.1 vs. 4.4 mg), and the dose ranges for the 2 intervals were the same (0.3 to 10 mg).
The results of most sensitivity analyses were similar to those of the main analyses (Supplement Tables 6 and 7, available atAnnals.org). However, the precision of some estimates decreased because of fewer events. Supplement Table 8 (available at Annals.org) presents E-value calculations for the main associations observed in this study, with the focus on statistically significant results. The E-values for observed point estimates ranged from 1.7 to 19.3. The corresponding E-values for lower estimates of 95% CIs that excluded 1.0 ranged from 1.7 to 5.5.
Discussion
The results of this EMR-based cohort study of transgender persons indicate that transfeminine participants had higher rates of VTE and, to a lesser extent, ischemic stroke relative to the corresponding rates among cisgender men and women. Myocardial infarction rates were greater among transfeminine participants than in matched cisgender women but were similar to those observed among cisgender men. The evidence was insufficient to draw conclusions about increased risk for any of the ACVEs of interest among transmasculine participants.
Results further indicate that the increases in VTE and ischemic stroke rates were most pronounced among transfeminine participants who initiated estrogen therapy during follow-up and that the patterns of these increases differed substantially from those reported in previous research. For example, in a clinical trial of hormone replacement therapy in postmenopausal women, VTE rates increased relatively rapidly after the intervention began and seemed to decline and then plateau by 5 years of follow-up (18). Likewise, in a case-control study of VTE and estradiol use in Sweden, the risk was elevated only during the first year after the start of therapy (19). In contrast, in our estrogen initiation cohort, VTE rates increased only after 2 years of follow-up and continued to rise for another 5 to 6 years. Likewise, the ischemic stroke rates in the estrogen initiation and 2 reference cohorts did not differ during the first 6 years of follow-up but clearly diverged afterward.
Previous studies of cisgender women demonstrated that the presence and magnitude of the association between estrogen hormone therapy and both VTE and ischemic stroke may differ by medication type, drug combinations, and administration routes (20–26). Although the data on hormone replacement therapy in cisgender women come from high-quality studies, including large randomized placebo-controlled clinical trials, the results of these studies may not apply to transgender persons.
Unlike research of hormone replacement therapy in cisgender populations, placebo-controlled clinical trials of transgender hormones may not be ethically acceptable (12). For this reason, evidence pertaining to the risks and benefits of hormone therapy in transgender persons must be obtained from observational studies (27–31). One of the largest studies to date included a cohort of 816 transfeminine participants who received oral ethinyl estradiol (100 mcg/d) or transdermal estradiol and 293 transmasculine patients who received parenteral testosterone esters or oral testosterone undecanoate (30). The study participants were seen in the outpatient department of Free University Hospital in Amsterdam between 1975 and 1994. Among the transfeminine participants, 45 cases of VTE occurred, only 5 of which arose after surgery. With the general male population of the Netherlands used as a reference, this number of VTE cases was 20-fold higher than expected. Only 1 case of VTE was observed in transmasculine cohort members. A cross-sectional study in Belgium examined morbidity in transgender patients who received transgender care at Ghent University Hospital (31). Transfeminine (n = 214) and transmasculine (n = 138) patients who had at least 3 months of cross-sex hormone therapy between 1986 and 2012 were each age matched to 3 cisgender men and 3 cisgender women. Most transfeminine patients received 2 mg/d of oral estradiol valerate (43%), transdermal estradiol gel (36%), or estradiol patches (14%). Prevalence estimates for history of VTE among transfeminine and transmasculine persons were 6.7% and 1.5%, respectively. No cases of VTE occurred in any of the referent participants.
A distinguishing feature of our study is that it represents one of the largest cohorts of transgender persons in the United States and, to our knowledge, is the only study of this size that carefully validated transfeminine or transmasculine status in the participants. Although the information on hormone therapy and surgical transgender treatment received within the Kaiser Permanente system is relatively high quality, one of the study’s main limitations was the inability to determine from the data which participants received care elsewhere. This limitation restricted our ability to identify a subcategory of transgender cohort members with no history of transgender treatment of any kind. Our results suggest that some persons with no evidence of hormone therapy at Kaiser Permanente may have received it from other sources. This observation has both research and clinical implications because it illustrates the challenges of reconstructing the full, lifetime history of hormone therapy use.
Even with the relatively large group of patients who initiated hormone therapy at Kaiser Permanente, many useful subanalyses evaluating risks associated with specific types of hormone treatment were not feasible because of sparse drug-and dose-specific strata. In a set of exploratory analyses, we could examine the data on subgroups of patients who received estrogen orally, the most common route of administration. We also could study the estradiol-only estrogen group and the group that received other estrogen formulations. These exploratory analyses do not provide evidence of substantial heterogeneity across subcohorts, but limited conclusions may be drawn because of the paucity of counts in some of the smaller strata. We also found some evidence that dosages increased for participants who had a VTE or an ischemic stroke but not for those who were event-free. No statistical comparisons of the dosage distributions were possible because of the small numbers of events. Of note, the documented dosages in our cohort exceeded those reported in the European studies (30, 31), which also found greater risks for VTE.
Although the current analysis was adjusted for several covariates, some information on possible confounders was not available. For example, we did not have data on statin use or various comorbid conditions. In addition, because transgender persons receive much of their care from a select group of health care providers who do not necessarily practice in the same clinics as providers with primarily cisgender patients, we could not account for differences by clinical site. However, sensitivity analyses indicate that the most important observed associations, such as those reported for VTE, are probably not a result of any potential effect of unmeasured confounders.
It is important to keep in mind that no single study may be considered sufficient for answering all relevant research questions regarding comparative risks and benefits of various treatment options. This is true particularly in observational research, in which the ability to examine specific formulation-route-dosage combinations or rates of rare events depends on the available data. For example, although we did not observe statistically significantly elevated rates of some of the less common events, such as myocardial infarction among transfeminine or any ACVEs among transmasculine patients, interpreting these data as conclusive evidence of no association would be premature. In particular, our analyses suggest that transmasculine persons receiving testosterone may be at higher risk for myocardial infarction, but the number of events was insufficient because of the relatively young age of the transmasculine cohort. A larger cohort and extended follow-up will allow additional analyses of more specific treatment options (such as various combinations of estradiol with antiandrogens and progesterone) and less common events, such as myocardial infarction among trans men or different subtypes of VTE among trans women.
In summary, the present study demonstrated that cross-sex estrogen is a risk factor for VTE and probably ischemic stroke among transfeminine persons. We also observed that patterns of VTE and ischemic stroke incidence among transfeminine persons receiving hormone therapy were different from those reported in cisgender women receiving hormone replacement therapy. If confirmed, these results may indicate the need for increasing vigilance in identifying long-term vascular side effects of estrogen therapy in transgender patients. In the meantime, it is critical to keep in mind that the risk for ACVEs in this population must be weighed against the benefits of treatment.
Supplementary Material
Acknowledgment:
This work would not be possible without continuous support and advice from the Stakeholder Advisory Group, which included representatives of the transgender community and health care providers and researchers who work with transgender persons. The authors thank Dr. Lemuel Arnold, Dr. Nancy Baisch, Dr. John Blosnich, Dr. George Brown, Dr. Robin Dea, Ms. Cheryl Courtney-Evans, Dr. Shawn Giammattei, Ms. Monica Helms, Ms. Cadence Valentine, Mr. Willy Wilkinson, Ms. Savannah Winter, and Mr. Evan Young for their valuable input and willingness to collaborate.
Grant Support: By contract AD-12-11-4532 from PCORI and grant R21HD076387 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Primary Funding Source: Patient-Centered Outcomes Research Institute and Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Role of the Funding Source
This study was funded by the Patient-Centered Outcomes Research Institute (PCORI) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Disclosures: Ms. Nash, Ms. Millman, and Dr. Goodman report a contract from PCORI and grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the conduct of the study. Dr. Becerra-Culqui and Ms. Cromwell report grants from PCORI during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17-2785.
Reproducible Research Statement: Study protocol and statistical code: Available from Dr. Goodman (e-mail, mgoodm2@emory.edu). Data set: Not available.
Current author addresses and author contributions are available at Annals.org.
References
- 1.Lombardi E Enhancing transgender health care. Am J Public Health. 2001;91:869–72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bockting W From construction to context: gender through the eyes of the transgendered. SIECUS Rep. 1999;28:3–7. [Google Scholar]
- 3.Giami A, Beaubatie E. Gender identification and sex reassignment surgery in the trans population: a survey study in France. Arch Sex Behav. 2014;43:1491–501. [PMID: ] doi: 10.1007/s10508-014-0382-3 [DOI] [PubMed] [Google Scholar]
- 4.Reisner SL, Gamarel KE, Dunham E, Hopwood R, Hwahng S . Female-to-male transmasculine adult health: a mixed-methods community-based needs assessment. J Am Psychiatr Nurses Assoc. 2013;19:293–303. [PMID: ] doi: 10.1177/1078390313500693 [DOI] [PubMed] [Google Scholar]
- 5.Knudson G, De Cuypere G, Bockting W. Recommendations for revision of the DSM diagnoses of gender identity disorders: consensus statement of the World Professional Association for Transgender Health. Int J Transgend. 2010;12:115–8. [Google Scholar]
- 6.Murad MH, Elamin MB, Garcia MZ, Mullan RJ, Murad A, Erwin PJ, et al. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin Endocrinol (Oxf). 2010;72:214–31. [PMID: ] doi: 10.1111/j.1365-2265.2009.03625.x [DOI] [PubMed] [Google Scholar]
- 7.Shatzel JJ, Connelly KJ, DeLoughery TG. Thrombotic issues in transgender medicine: a review. Am J Hematol. 2017;92:204–8. [PMID: ] doi: 10.1002/ajh.24593 [DOI] [PubMed] [Google Scholar]
- 8.Elamin MB, Garcia MZ, Murad MH, Erwin PJ, Montori VM. Effect of sex steroid use on cardiovascular risk in transsexual individuals: a systematic review and meta-analyses. Clin Endocrinol (Oxf). 2010;72: 1–10. [PMID: ] doi: 10.1111/j.1365-2265.2009.03632.x [DOI] [PubMed] [Google Scholar]
- 9.Weinand JD, Safer JD. Hormone therapy in transgender adults is safe with provider supervision; a review of hormone therapy sequelae for transgender individuals. J Clin Transl Endocrinol. 2015;2: 55–60. [PMID: ] doi: 10.1016/j.jcte.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med. 2017; 167:256–67. [PMID: ] doi: 10.7326/M17-0577 [DOI] [PubMed] [Google Scholar]
- 11.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102:3914–23. [PMID: ] doi: 10.1210/jc.2017-01643 [DOI] [PubMed] [Google Scholar]
- 12.Reisner SL, Deutsch MB, Bhasin S, Bockting W, Brown GR, Feldman J, et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes. 2016;23:198–207. [PMID: ] doi: 10.1097/MED.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roblin D, Barzilay J, Tolsma D, Robinson B, Schild L, Cromwell L, et al. A novel method for estimating transgender status using electronic medical records. Ann Epidemiol. 2016;26:198–203. [PMID: ] doi: 10.1016/j.annepidem.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn VP, Nash R, Hunkeler E, Contreras R, Cromwell L, Becerra-Culqui TA, et al. Cohort profile: Study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open. 2017;7:e018121. [PMID: ] doi: 10.1136/bmjopen-2017-018121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–903. [PMID: ] doi: 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- 16.Kleinbaum D Survival Analysis: A Self-Learning Text. New York: Springer-Verlag; 1995. [Google Scholar]
- 17.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. [PMID: 28693043] doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 18.Rossouw JE, Anderson GL, Prentice RL, AZ LaCroix, C Kooperberg, ML Stefanick, et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288: 321–33. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 19.Høibraaten E, Abdelnoor M, Sandset PM. Hormone replacement therapy with estradiol and risk of venous thromboembolism–a population-based case-control study. Thromb Haemost. 1999;82: 1218–21. [PMID: ] [PubMed] [Google Scholar]
- 20.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Lévesque H, et al. ; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 21.Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THrombo Embolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thrombo-embolism risk. Lancet. 2003;362:428–32. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22.Canonico M, Fournier A, Carcaillon L, Olié V, Plu-Bureau G, Oger E, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30:340–5. [PMID: ] doi: 10.1161/ATVBAHA.109.196022 [DOI] [PubMed] [Google Scholar]
- 23.Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11:124–31. [PMID: ] doi: 10.1111/jth.12060 [DOI] [PubMed] [Google Scholar]
- 24.Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8:979–86. [PMID: ] doi: 10.1111/j.1538-7836.2010.03839.x [DOI] [PubMed] [Google Scholar]
- 25.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. ; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. [PMID: [DOI] [PubMed] [Google Scholar]
- 26.Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–96. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 27.Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. J Clin Endocrinol Metab. 2012;97:4422–8. [PMID: ] doi: 10.1210/jc.2012-2030 [DOI] [PubMed] [Google Scholar]
- 28.Brown GR, Jones KT. Racial health disparities in a cohort of 5,135 transgender veterans. J Racial Ethn Health Disparities. 2014;1: 257–66. [Google Scholar]
- 29.Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016; 3:122–31. [PMID: ] doi: 10.1089/lgbt.2015.0058 [DOI] [PubMed] [Google Scholar]
- 30.van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47:337–42. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169:471–8. [PMID: ] doi: 10.1530/EJE-13-0493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


