Abstract
Background
The effect of alcohol consumption on cognitive decline is not clear. We aimed to study the association between alcohol consumption and cognitive functioning controlling for functional heath status.
Methods
A total of 1610 older adults with a score ≥26 on the Mini-Mental State Examination (MMSE) were followed to assess the change in scores at the 3-year follow-up. Information on alcohol consumption as well as socio-demographic, lifestyle, psychosocial and clinical factors, as well as health service use were assessed at baseline and 3-year follow-up interviews. Linear mixed models with repeated measures were used stratifying by functional status.
Results
Close to 73% reported consuming alcohol in the past 6 months, of which 11% were heavy drinkers (≥11 and ≥16 drinks for women and men). A significant decrease in MMSE scores was observed in low functioning non-drinkers (−1.48; 95% CI: −2.06, −0.89) and light to moderate drinkers (−0.99; 95% CI: −1.54, −0.44) and high functioning non-drinkers (−0.51; 95% CI: −0.91, −0.10).
Conclusions
Alcohol consumption did not contribute to cognitive decline. Cognitive decline was greater in individuals reporting low functional status. Research should focus on the interaction between changing patterns of alcohol consumption and social participation in individuals with low and high functioning status.
Keywords: alcohol consumption, dementia, older people
Introduction
Dementia has become a global public health issue1 affecting close to 50 million people worldwide with societal costs reaching US$ 600 billion.2 Recent meta-analyses focusing on modifiable risk factors of cognitive decline have identified education, physical inactivity3,4 and smoking.4 There is less clear evidence on the effect of alcohol.5,6
Antsey et al.’s7 meta-analysis and Ilomaki et al.’s8 review of systematic reviews showed that, compared to non-drinkers, those reporting light to moderate drinking (up to 28 drinks/week) had up to a 40% decreased risk of dementia, whereas heavy to excessive drinking did not affect the risk.8 In Neafsy and Collins9 systematic review moderate drinking (up to 3 drinks/day) reduced up to 30% the risk of cognitive impairment but not cognitive decline. The results observed may in part be explained by a cohort selection effect where lifetime abstainers are grouped together with past alcohol drinkers more likely to develop disease thus rendering this reference group sicker.7–9 This has been called the ‘sick quitter’ bias.10 Ng Fat et al.11 showed that having limitations due to a chronic illness was associated with the cessation or reduction of alcohol consumption.
More recently, Topiwala et al.12 showed that alcohol consumption was associated with brain atrophy in men, but not in women. The authors also reported that as opposed to abstainers, higher alcohol consumption (>7 drinks a week) was associated with a faster decline on lexical fluency but not on word recall or semantic fluency. There was no association between alcohol consumption and cognitive performance. Differences in baseline cognitive scores among abstainers and alcohol users may have influenced the results.
In Canada, the prevalence of alcohol abstainers among older adults has decreased from 31% in 2001 to 24% in 2014.13 An increasing trend of drinking between 1997 and 2014 among older adults was also recently observed in the USA where the prevalence of heavy episodic drinking increased yearly by 3.7%.14 The fact that more than 80% of those aged ≥65 years are prescribed at least one drug, with an average reaching 5.5 drugs per senior,15 the interaction with alcohol increases the potential for side effects.
The objective of this study was to assess, in a sample of community living older adults recruited in primary care practices, the association between alcohol consumption and cognitive decline over a 3-year period. This study will contribute to the present literature by considering both quantity and frequency of alcohol consumption, as well as assessing the effect of a change in alcohol consumption on cognitive status while controlling for a number of important confounders including self-rated functional status. This variable, not previously controlled for in the literature, will allow to control for the possible effect that non-drinkers stopped drinking due to functional limitations associated with illness and in part control for the ‘sick quitter’ bias.
Methods
Data source and study population
The sample consisted of older adults aged ≥65 years (n = 1811) recruited in the ESA-Services study (2011–13) while waiting for medical services in a general practice clinic located in one of the most populated administrative health regions of the province of Quebec representative of metropolitan, urban and rural areas. Participating clinics were recruited via general practitioners practicing in the region full time (>4 days/week). Among eligible physicians, 60% participated. Detailed information on the methodology has been previously published.16
Briefly, patients were contacted within 30 days of the visit to schedule an at home interview. In order to minimize bias associated with cognitive impairment, individuals in the 10th percentile of the Mini-Mental State Examination (MMSE)17 at baseline (MMSE < 26) were excluded from the analyses.18 Among the 1627 eligible individuals, we were able to contact and re-interview at home 1061 of these individuals, 3 years later. Study variables were ascertained at both interviews. Losses to follow-up did not differ with respect to baseline MMSE scores (28.5 versus 28.6, P = 0.27); past or current smoking status (62 versus 61%, P = 0.76); and alcohol consumption group (non-drinkers: 27 versus 31%, light to moderate: 68 versus 66%, heavy drinkers: 4 versus 5%, P = 0.06); low income (97 versus 98%, P = 0.05); the presence of minor (1.9 versus 1.2%, P = 0.20) and major (2.6 versus 1.6%, P = 0.17) depression; reporting 3 or more chronic physical disorders (70 versus 67%, P = 0.18), female gender (62 versus 57%, P = 0.05); completed primary level of education and less (14 versus 11%, P = 0.05). Those lost to follow-up were however younger (65–74 years) (40 versus 47%, P < 0.01), exercised on average fewer times a week (3.2 versus 4.0, P < 0.001) and more likely to have reported an anxiety disorder (12.0 versus 6.4%, P < 0.0001). Reasons given for not participating at follow-up (n = 18) included cognitive impairment. These participants were included in the analyses and given a MMSE median score of 16. Finally, subjects were compensated $15CDN for their participation at each interview. The study was approved by the University of Sherbrooke Institute of Geriatrics ethics committee.
Measures
Cognitive functioning was measured at both baseline (year 1) and follow-up interviews (year 4) with the MMSE with possible scores ranging between 0 and 30.17 The MMSE has been shown to have good psychometric properties in assessing general cognitive functioning, in community living older adults consulting in primary care practices, with sensitivity and specificity estimates reaching 80 and 98%.19,20 In our study sample, the intra-class correlation coefficient was 0.82 (P < 0.001) when examining MMSE scores at baseline by interviewer. Further, we assessed the change in MMSE over an interval that was over 3 years which is recommended when using the MMSE to assess cognitive change.21
Self-reported alcohol consumption was assessed at both interviews with three questions. The first question ‘have you consumed alcohol in the past 6 months’, was categorized as yes/no. Those responding yes were then asked, ‘what has been the usual frequency, with which you have consumed alcohol in the past 6 months. There were six possible answers to this last question: less than once a month; more than once a month but less than once a week;, once a week; two to four times a week; five to seven times a week; and never’. The interviewer then proceeded with the question ‘how many alcoholic drinks (where on glass of white wine or red equals to one beer, equals to a shot of spirit or strong alcohol) do you usually consume on average during a 24-h period’ and ‘were you ever drunk or in an inebriated state, in the past 6 months’.
Alcohol consumption was based on the product between the number of drinks ordinarily consumed in one occasion (24 h period) and the weekly frequency of drinking alcohol10 and categorized according to Canadian guidelines on alcohol consumption11 as follows: non-drinkers (0 drinks), light to moderate drinkers (≤10 drinks/week for women and ≤15 drinks/week for men) and heavy drinkers (>10 drinks/week for women and >15 drinks/week for men). In Canada a standard drink contains 13.6 g of alcohol.11
Smoking status was also measured at both interviews using the following question: ‘Do you smoke cigarettes’ and the possible answers included never smoked, former smoker and current smoker. Physical activity was assessed at baseline and follow-up with the following question: ‘How many times a week do you exercise for more than 20 min (e.g. walking at a rapid pace)’. Similar single self-reported items are just as valid as other questionnaire-based measures of physical activity.22,23
The past 6-month presence of an anxiety disorder (agoraphobia, social or specific phobia, panic disorder, generalized anxiety disorder) and major and minor depression were assessed by trained interviewers using the ESA-Q diagnostic computer-assisted questionnaire based on DSM-5 criteria and similar to the CIDI.24 The questionnaire has reported consistent results25 and was recently included in a large meta-analysis.26
Functional status was also assessed at both interviews and was based on 29 questions relating to the presence of difficulties, ranging from no problem to extreme problem, in activities and instrumental activities of daily living, relating to mobility, self-care and usual activities, pain and the presence of generalised anxiety disorder and psychological distress. As detailed elsewhere,27 responses to these questions were summarised into an index ranging from 0 (worst health) to 1 (perfect health). The calculated functional status index was dichotomized at the lowest quintile (yes/no).
The self-reported presence of a chronic disorder, as diagnosed by a physician, was assessed with a list of 20 disorders (WHO, 2004) and included arthritis, heart and cardiovascular diseases, diabetes and endocrine system disorders, digestive system, and kidney, liver and eye disorders was also measured and categorized as 0–2 versus 3 and over conditions.
The number of ambulatory visits (i.e. emergency department, outpatient clinic) in the year prior to baseline interview was also retained from the ‘Régie de l’assurance maladie du Québec’ (RAMQ), Quebec’s public health insurance plan, where residents are covered for practically all medical consultations with physicians.
Other study variables considered in the analyses as possible confounding factors included age, gender, education (primary and less versus some high school and above) and income (<$55 000 versus ≥$55 000). Social and material deprivation of area of residence was also characterized by the validated Pampalon indices, which are summarized into one index on a scale of 1–5, representing the most advantaged to the most disadvantaged quintiles (highest socioeconomic status, medium high, medium, medium-low, low).28,29 This variable was dichotomized at quintiles 4 and 5, to identify area of residence with high social or material deprivation, versus quintiles 1–3, for low to medium deprivation (quintiles 1–3).
Social support was assessed during the interviews with the three following questions: (i) In your environment, is there someone you can confide in or talk to freely about your problems? (ii) Is there someone in your family or circle of friends who could assist you in time of need? (iii) Is there someone you feel close to, a family member or friend, who shows affection towards you? Social support was then categorized as the presence of all three sources (yes/no).
The number of daily hassles was also assessed and measured using the French version of Daily Hassle scale.30 This French version produced a Cronbach alpha of 0.90 and test–retest reliability coefficients of 0.79 for the frequency of hassles.31 The 30-item questionnaire included seven domains relevant to older people (family, health, money, security and transportation) and experienced during the last month. Possible scores ranged from 0 to 30, with higher scores indicating a higher number of daily hassles.
Statistical analyses
Linear mixed models with SAS PROC MIXED for repeated measures were used.32,33 This allowed for people with missing follow-up data to be included in the analyses as well as to consider the change in study variables at both data collection interviews. The change in MMSE scores was predicted by fitting six dummy time × alcohol groups (non-drinkers, light to moderate and heavy drinkers) controlling for study variables. Linear mixed model analyses with a maximum likelihood method and unstructured correlation structure, with random intercept, were used to examine the correlates of cognitive functioning, as measured with MMSE scores, accounting for within-subject correlation over repeated measures. We also accounted for individuals nested within type of primary care practice recruited in (family medicine group, local community health centres, private practice with ≤3 physicians, and private practice with >3 physicians). We also stratified the analyses by functional status at the lowest quintile. Analyses were carried out with SAS version 9.4.
Results
The characteristics of the study sample with respect to alcohol consumption groups are presented in Table 1. As seen, close to 27% of participants reported no alcohol consumption in the past 6 months whereas 68% were light to moderate and 5% heavy drinkers. There was a significant association between cognitive functioning (MMSE scores) and alcohol consumption at baseline (P < 0.04).
Table 1.
Sample characteristics at baseline interview as a function of alcohol consumption defined by Canadian guidelines
| Non-drinkers | Light to moderate drinkers (≤10 for women and ≤15 drinks/week for men) | Heavy drinkers (≥11 for women and ≥16 drinks/week for men) | Chi-square df = 2 | |
|---|---|---|---|---|
| N = 435 (27%) | N = 1111 (68%) | N = 82 (5%) | P-value | |
| Age | ||||
| 65–74 years | 252 (58%) | 745 (67%) | 56 (68%) | 0.003 |
| ≥75 years | 183 (42%) | 366 (33%) | 26 (32%) | |
| Gender | ||||
| Men | 139 (32%) | 505 (45%) | 38 (46%) | <0.001 |
| Women | 296 (68%) | 606 (55%) | 44 (54%) | |
| Education | ||||
| Primary | 61 (15%) | 100 (9%) | 4 (9%) | 0.002 |
| High school/College/University | 350 (85%) | 1052 (91) | 43 (91%) | |
| Income | ||||
| <$55 000 | 422 (97%) | 1091 (98%) | 81 (99%) | 0.29 |
| ≥$55 000 | 13 (3%) | 20 (2%) | 1 (1%) | |
| Married | ||||
| Yes | 249 (57%) | 734 (66%) | 56 (68%) | 0.004 |
| No | 186 (43%) | 377 (34%) | 26 (32%) | |
| Area of residence | ||||
| High deprivation | 261 (60%) | 642 (58%) | 43 (52%) | 0.41 |
| Low/Medium deprivation | 174 (40%) | 469 (42%) | 39 (48%) | |
| Social support | ||||
| 0–2 | 84 (19%) | 992 (89%) | 15 (18%) | <0.0001 |
| 3 | 351 (81%) | 119 (11%) | 67 (82%) | |
| Daily hassles | ||||
| 0–2 | 246 (57%) | 603 (54%) | 48 (59%) | 0.59 |
| 3+ | 189 (43%) | 508 (46%) | 34 (41%) | |
| # of chronic physical disorders | 109 (25%) | 381 (35%) | 30 (37%) | 0.001 |
| 0–2 | 326 (75%) | 730 (65%) | 52 (63%) | |
| ≥3 | ||||
| Functional status index | ||||
| Lowest quintile | 125 (29%) | 195 (18%) | 14 (17%) | <0.0001 |
| Higher quintiles | 310 (71%) | 916 (82%) | 68 (83%) | |
| Depression | ||||
| Yes | 33 (8%) | 49 (4%) | 3 (4%) | 0.03 |
| No | 402 (92%) | 1062 (96%) | 79 (96%) | |
| Anxiety | ||||
| Yes | 67 (15%) | 130 (12%) | 7 (9%) | 0.08 |
| No | 3468(85%) | 981 (88%) | 75 (91%) | |
| Psychotropic drug use | ||||
| Yes | 123 (28%) | 183 (16%) | 18 (22%) | <0.0001 |
| No | 312 (72%) | 928 (84%) | 64 (78%) | |
| Smoking status | ||||
| Never smoker | 199 (46%) | 411 (37%) | 19 (23%) | <0.0001 |
| Past or current smoker | 236 (54%) | 700 (63%) | 63 (77%) | |
| Mean (95% CI) | Pr > F | |||
| Cognitive functioning (MMSE) | 28.5 (28.4, 28.6) | 28.7 (28.6, 28.8) | 28.7 (28.4, 29.0) | 0.04 |
| Exercise (# of time per week) | 3.5 (3.1, 3.9) | 3.6 (3.4, 3.9) | 3.7 (3.0, 4.4) | 0.78 |
| # of ambulatory visits in past year | 9.7 (8.6, 10.8) | 7.9 (7.4, 8.4) | 7.9 (6.3, 9.6) | 0.004 |
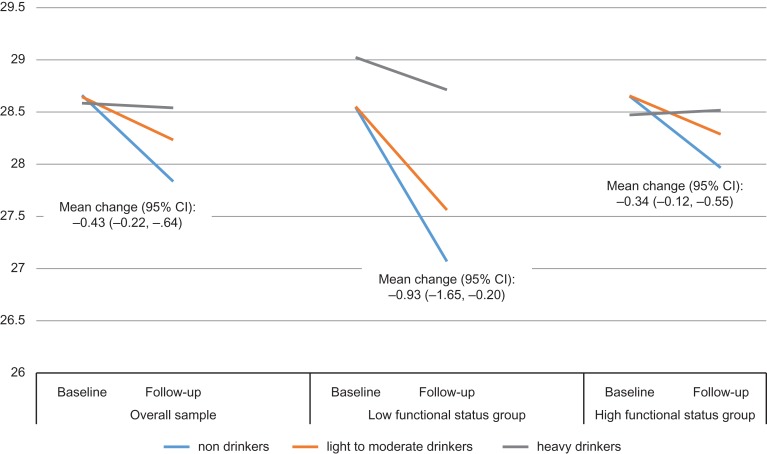
The results also showed significant decline in MMSE scores in the overall sample (mean change: −0.43; 95% CI: −0.22, −0.64) as well as in the sample restricted to those with low functioning (mean change: −0.93; 95% CI: −1.65, −0.20) and high functioning status (mean change: −0.34; 95% CI: −0.12, −0.55) (Fig. 1). The multivariate analysis (Table 2) showed a significant time × alcohol consumption interaction (Χ2(2) = 6.32, P < 0.002) in predicting change in MMSE scores between baseline and follow-up.
Fig. 1.
Cognitive functioning (MMSE scores) at baseline and 3-year follow-up by alcohol consumption group.
Table 2.
Multivariate model assessing change in MMSE scores as a function of study variables in repeated analysis from baseline to follow-up
| Estimate (95% CI) | |
|---|---|
| Time × alcohol consumption | |
| Baseline—non-drinkers | 0.12 (−0.38, 0.62) |
| Baseline—light to moderate drinker | 0.10 (−0.38, 0.59) |
| Baseline—heavy drinker | 0.05 (−0.52, 0.61) |
| Follow-up—non-drinkers | −0.71 (−1.21, −0.20) |
| Follow-up—light to moderate drinker | −0.31 (−0.80, 0.18) |
| Follow-up—heavy drinker | Reference |
| Age (≥75 years versus 65–74 years) | −0.58 (−0.72, −0.45) |
| Gender (women versus men) | 0.33 (0.19, 0.48) |
| Education (primary versus high school/college/university) | −0.74 (−0.94, −0.54) |
| Income (≥$55 000 versus < $55 000) | −1.41 (−1.81, −1.01) |
| Married (yes versus no) | 0.06 (−0.08, 0.20) |
| Area of residence (high versus low/medium deprivation) | −0.15 (−0.28, −0.02) |
| Social support (3 versus 0–2) | 0.49 (0.31, 0.67) |
| Daily hassles (3+ versus 0–2) | 0.03 (0.02, 0.05) |
| # of chronic physical disorders (≥3 versus 0–2) | 0.001 (−0.14, 0.14) |
| Functional status index (lowest quintile versus higher quintiles) | −0.24 (−0.41, −0.08) |
| Depression (yes versus no) | −0.17 (−0.47, 0.13) |
| Anxiety (yes versus no) | −0.26 (−0.47, −0.10) |
| Psychotropic drug use (yes versus no) | −0.11 (−0.31, 0.09) |
| Smoking status (never smoker versus past or current smoker) | −0.06 (−0.19, 0.08) |
| Exercise (# of time per week) | 0.01 (−0.003, 0.03) |
| # of ambulatory visits in past year | −0.004 (−0.01, 0.004) |
| Nested for type of primary care practice individuals were recruited in | |
The results showed cognitive decline in non-drinkers and light to moderate drinkers in the overall sample and in each functional status group (Table 3).
Table 3.
Estimated mean cognitive change (95% CI) from baseline to 3-year follow-up in each alcohol consumption group
| Non-drinkers | Light to moderate drinkers (≤10 for women and ≤15 drinks/week for men) | Heavy drinkers (≥11 for women and ≥16 drinks/week for men) | |
|---|---|---|---|
| Overall sample | −0.83 (−1.05, −0.60) | −0.41 (−0.56, −0.27) | −0.05 (−0.61, 0.52) |
| Low functional status | −1.48 (−2.06, −0.89) | −0.99 (−1.54, −0.44) | −0.31 (−2.26, 1.65) |
| High functional status | −0.69 (−0.93, −0.44) | −0.37 (−0.52, −0.22) | 0.04 (−0.53, 0.62) |
| Comparing heavy drinkers at baseline to all other groups | |||
| Overall sample | |||
| Baseline | ns | ns | Reference |
| Follow-up | −0.75 (−1.14, −0.37) | −0.35 (−0.71, 0.0) | ns |
| Low functional status | |||
| Baseline | ns | ns | Reference |
| Follow-up | −1.96 (−3.06, −0.85) | −1.46 (−2.56, −0.36) | ns |
| High functional status | |||
| Baseline | ns | ns | Reference |
| Follow-up | −0.51 (−0.91, −0.10) | ns | ns |
Estimates are adjusted for age, gender, education, marital status, income, area of residence deprivation, social support, number of daily hassles, number of chronic conditions, the presence of depression and anxiety, psychotropic drug use, ambulatory visits in previous year of interview and smoking status, exercise, and functional status, and type of primary care practice subjects were recruited in.
*NS: not significantly different from reference group, P > 0.05.
Discussion
Main finding of this study
There is no evidence in this study that alcohol contributes to cognitive decline. In this study, the 6-month prevalence of alcohol use was 73%, of which 7% was heavy drinking.13 Within this observation period, the results showed cognitive decline in non-drinkers and light to moderate drinkers in the overall sample as well as in each of the functional status subsamples. We did not observe a change in MMSE scores in heavy drinkers. When looking at individuals in the low functioning group, prior heavy drinkers reporting no alcohol consumption and light to moderate drinking at follow-up had a 2 and 1.5 point decrease in MMSE scores.
What is already known on this topic
In a Spanish cohort study of adults aged ≥55 years there was no association between low to moderate alcohol consumption and cognitive decline with the MMSE over 4.5 years.34 In a US cohort study of older adults participating in the Rancho Bernardo Study,35 moderate and heavy drinkers, compared to non-drinkers, were more likely to survive up to age 85 without cognitive impairment on the MMSE.
What this study adds
The sample consisted of older adults not presenting signs of cognitive impairment at baseline to control for the possibility for reverse causation that is if patients with cognitive problems had already altered their exposure to alcohol in response to their impairment. The repeated measures during the two at home interviews 3 years apart also allowed to account for the change in alcohol consumption in the analyses minimizing the potential misclassification of alcohol status. The protective effect of light to moderate drinking observed by some may be confounded by what has been called the ‘sick quitter’ bias where current non-drinkers, have health disabilities leading them to quit.7–11,34 This was the case in this study sample where non-drinkers reported lower functional status. In order to control for this potential confounding, this study adds to prior research by stratifying the analyses by functional status and showing on average cognitive decline among non-drinkers and light to moderate drinkers in those with and without disabilities. Further to this, we also controlled for lifestyle factors such as smoking status, exercise as well as the presence of physical and psychiatric disorders but also characteristics of the patient’s living environment,21 such as socio-demographic and economic factors, health service use, daily stressors and social support, previously not accounted for systematically in the literature.
Limitations of this study
This study also includes a number of limitations. First, although losses to follow-up did not differ with respect to baseline cognitive scores and alcohol consumption, they were more likely younger. When looking at the characteristics of the sample at baseline, younger participants were more likely at risk alcohol consumers. A more detailed look into the MMSE scores of younger participants, those lost to follow-up did not differ with respect to baseline scores than those who persisted in the study (P = 0.77). Given that there was no difference in the baseline MMSE scores, the effect of losing these younger participants at follow-up, which may have other lifestyle factors, is difficult to predict.
Second, one may argue that excluding from the analyses those with MMSE scores < 26 and potentially eliminating individuals with mild and major neurocognitive disorders due to alcohol consumption, as mentioned in the DSM-5, may have underestimated the damages related to alcohol abuse. However excluding these individuals may limit the selection bias in the study of cognitive decline and alcohol consumption at follow-up. Others have also chosen a similar methodology.36 Additional analysis including these individuals however showed similar results.
Third, participants having more than three chronic conditions were less likely to consume alcohol. If these participants were more likely to have changed their alcohol consumption habits because of their chronic diseases, it is possible that this group also included past at risk drinkers which may have limited our ability to detect an increased risk of cognitive decline with at risk alcohol consumption, which has been previously hypothesized.37
Fourth, alcohol consumption in this study was measured over a 6-month period to better represent individual drinking patterns over a longer period of time. Alcohol consumption was based on both frequency and quantity. The latter may be subject to misclassification bias as this was not based on a number of standard drinks but on the number of reported drinks where respondents were asked to consider that one glass of wine equals to one beer equals to one shot of spirit or strong alcohol. Measures are also usually larger when drinking at home. Participants may have therefore underestimated the average quantity consumed. This would have a greater effect on those reporting a higher number of drinks and this may have led to underestimating the effect of heavy drinking on cognitive decline.
Finally, the participants were recruited in primary care settings and therefore may exhibit more health problems than those from the general older adult population and also receive more recommendations from physicians on healthier lifestyle habits, which may limit the generalizability of results. Further, the majority of the sample consisted of white French-speaking Canadians in the province of Quebec, limiting extrapolation of results to other populations with different lifestyle factors.
Funding
This study was supported in part by a CIHR operating (Grant # 201403) and by a Fonds de Recherche du Québec - Santé (FRQ-S) operating (Grant # 22251).
References
- 1. World Health Organization Global strategy and action plan on ageing and health. Resource Document World Health Organization. 2015. http://www.who.int/ageing/ageing-global-strategy-draft1-en.pdf?ua=1.
- 2. World Health Organization Dementia: a public health priority. World Health Organization and Alzheimer’s Disease International 2012. http://www.who.int/mental_health/publications/dementia_report_2012/en/.
- 3. Norton S, Matthews FE, Barnes DE et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- 4. Beydoun MA, Beydoun HA, Gamaldo AA et al. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peters R, Peters J, Warner J et al. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 2008;37:505–12. [DOI] [PubMed] [Google Scholar]
- 6. Panza F, Frisardi V, Seripa D et al. Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatry 2012;27:1218–38. [DOI] [PubMed] [Google Scholar]
- 7. Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry 2009;17:542–55. [DOI] [PubMed] [Google Scholar]
- 8. Ilomaki J, Jokanovic N, Tan EC et al. Alcohol consumption, dementia and cognitive decline: an overview of systematic reviews. Curr Clin Pharmacol 2015;10:204–12. [DOI] [PubMed] [Google Scholar]
- 9. Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat 2011;7:465–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 1988;332:1267–73. [DOI] [PubMed] [Google Scholar]
- 11. Ng Fat L, Cable N, Shelton N. Worsening of health and a cessation or reduction in alcohol consumption to special occasion drinking across three decades of the life course. Alcohol Clin Exp Res 2015;39:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topiwala A, Allan CL, Valkanova V et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. Br Med J 2017;357:j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Public Health Agency of Canada The Chief Public Health Officer’s Report on the State of Public Health in Canada. Alcohol Consumption in Canada. 2015. Cat.: HP2-10E-PDF, ISSN: 1924–7087, 76 pp.
- 14. Breslow RA, Castle IP, Chen CM et al. Trends in alcohol consumption among older Americans: National Health Interview Surveys, 1997 to 2014. Alcohol Clin Exp Res 2017;41:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Commission à la santé et au bien-être Québec Prescribed Medications: Current Situation in Quebec (Les médicaments d’ordonnance: état de la situation au Québec). http://www.csbe.gouv.qc.ca/fileadmin/www/2014/Medicaments/CSBE_Medicaments_EtatSituation_2e.pdf (June 2017, date last accessed).
- 16. Préville M, Mechakra-Tahiri SD, Vasiliadis HM et al. Family violence among older adult patients consulting in primary care clinics: results from the ESA (Enquête sur la santé des aînés) services study on mental health and aging. Can J Psychiatry 2014;59:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 18. Hudon C, Potvin O, Turcotte MC et al. Normative date for the Mini-Mental State Examination (MMSE) in a sample of community dwelling French speaking residents from Quebec aged 65 and older. Can J Aging 2009;28:347–57. [DOI] [PubMed] [Google Scholar]
- 19. Iliffe SR, Booroff A, Gallivan S et al. Screening for cognitive impairment in the elderly using the Mini-Mental State Examination. Br J Gen Pract 1990;40:277–9. [PMC free article] [PubMed] [Google Scholar]
- 20. MacKenzie DM, Copp P, Shaw RJ et al. Brief cognitive screening of the elderly: a comparison of the Mini-Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychol Med 1996;26:427–30. [DOI] [PubMed] [Google Scholar]
- 21. Clark CM, Sheppard L, Fillenbaum GG et al. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol 1999;56:857–62. [DOI] [PubMed] [Google Scholar]
- 22. Marques A, Peralta M, Martins J et al. Associations between physical activity and self-rated wellbeing in European adults: a population-based, cross-sectional study. Prev Med 2016;91:18–23. [DOI] [PubMed] [Google Scholar]
- 23. Wanner M, Probst-Hensch N, Kriemler S et al. What physical activity surveillance needs: validity of a single-item questionnaire. Br J Sports Med 2014;48:1570–6. [DOI] [PubMed] [Google Scholar]
- 24. Arlington VA. Diagnostic and Statistical Manual of Mental Disorders, 5th edn American Psychiatric Publishing. American Psychiatric Association, 2013. [Google Scholar]
- 25. Preville M, Boyer R, Grenier S et al. The epidemiology of psychiatric disorders in Quebec’s older adult population. Can J Psychiatry 2008;53:822–32. [DOI] [PubMed] [Google Scholar]
- 26. Volkert J, Schulz H, Harter M et al. The prevalence of mental disorders in older people in Western countries—a meta-analysis. Ageing Res Rev 2013;12:339–53. [DOI] [PubMed] [Google Scholar]
- 27. Vasiliadis HM, Bélanger MF. The prospective and concurrent effect of exercise on health related quality of life in older adults over a 3 year period. Health Qual Life Outcomes 2018;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pampalon R, Hamel D, Gamache P et al. An area-based material and social deprivation index for public health in Québec and Canada. Can J Public Health 2012;103:S17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pampalon R, Hamel D, Gamache P et al. Validation of a deprivation index for public health: a complex exercise illustrated by the Quebec index. Chronic Dis Inj Can 2014;34:12–22. [PubMed] [Google Scholar]
- 30. Kanner AD, Coyne JC, Schaefer C et al. Comparison of two modes of stress measurement: daily hassles and uplifts versus major life events. J Behav Med 1981;4:1–39. [DOI] [PubMed] [Google Scholar]
- 31. Vézina J, Giroux L L’Échelle des Embêtements: une étude de validation et d’adaptation du Hassles Scale pour une population adulte âgée. Paper Presented at the Meeting of the Canadian Psychological Association, Montreal 1988.
- 32. Gossett J, Simpson P, Casey P et al. Growing Growth Curves Using PROC MIXED and PROC NLMIXED. 2007. http://www.lexjansen.com/pharmasug/2007/pr/pr03.pdf.
- 33. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 1998;24:323–55. http://gseweb.harvard.edu/~faculty/singer/. [Google Scholar]
- 34. Lobo E, Dufouil C, Marcos G et al. Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol 2010;172:708–16. [DOI] [PubMed] [Google Scholar]
- 35. Richard EL, Kritz-Silverstein D, Laughlin GA et al. Alcohol intake and cognitively healthy longevity in community-dwelling adults: the Rancho Bernardo Study. J Alzheimers Dis 2017;59:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moussa MN, Simpson SL, Mayhugh RE et al. Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults. Front Aging Neurosci 2015;6:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hagger-Johnson G, Sabia S, Brunner EJ et al. Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. Br J Psychol 2013;203:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]