Abstract
Background
The impact of daily or intermittent electronic cigarette (e-cigarette) use on oral health is unknown.
Methods
We performed a cross-sectional analysis using the 2016 Behavioral Risk Factor Surveillance System data. Poor oral health was determined by the number of permanent teeth removed due to non-traumatic causes, and e-cigarette use determined by daily or intermittent use within 30 days prior to survey administration. We performed logistic regression analysis to test associations between e-cigarette use and oral health with adjustment for factors associated with poor oral health, survey clustering, strata and weight.
Results
We included survey responses from 456 343 adults. Over half of respondents (51.5%) reported having at least one permanent tooth removed because of tooth decay or gum disease in their lifetime. Daily e-cigarette use was reported by 4957 (1.1%) of respondents. In multivariable analysis, daily e-cigarette use, was independently associated with a 78% higher odds of poor oral health (adjusted OR = 1.78, 95% CI: 1.39–2.30; P < 0.001).
Conclusions
In a population-based health survey of US adults, self-reported health behavior and outcomes, daily use, but not intermittent use of e-cigarettes was independently associated with poor oral health. Care must be exercised in seeking ‘healthier’ cigarette alternatives.
Keywords: electronic cigarettes, nicotine, oral health
Introduction
Electronic cigarettes (e-cigarettes) are emerging as a popular mode of tobacco consumption.1 These electronic devices consisting of an atomizer with fixed resistance for heat generation, a battery chamber with fixed voltage, and a liquid reservoir intended for loading with products (termed ‘e-juice’ or ‘e-liquid’), include chemicals with the desired volatility, often a mixture of propylene glycol, glycerol and water, along with varied amounts of nicotine, flavoring agents and dyes.2–4 The units are designed to deliver nicotine vapor to users and are marketed for both recreational use and as a smoking cessation aid.5,6 The use of e-cigarettes increased from 1.8% of adults over age 18 years in the USA in 2010 to 13% in 2013.7 The prevalence of e-cigarettes use increased the highest among young adults aged 18–24 years old and among never- and former smokers, raising concerns in the public health community.8
It is widely known that smoking conventional cigarettes leads to poor dental health through impaired innate immunity, local inflammation, and accelerated gingival and periodontal disease.9 However, there are few studies investigating e-cigarettes and oral health. Previous studies have suggested associations between e-cigarette use and tooth fractures in adolescents10 as well as small studies have shown that, as compared to smokers, e-cigarette users and never smokers have less periodontal inflammation and lower self-reported oral symptom scores.11,12
Given these conflicting results and controversy surrounding the use of e-cigarettes in general,13 there is concern about whether e-cigarettes may be associated with impaired oral health. We hypothesized that e-cigarette use is associated with poor oral health. We used data collected from the 2016 Behavioral Risk Factor Surveillance System (BRFSS) to measure associations between daily as well as intermittent e-cigarette use and poor oral health among adults in the USA.
Methods
Data source
To test the hypothesis that e-cigarette use is associated with poor oral health among the US adult population, we performed a cross-sectional analysis using the 2016 BRFSS data. The BRFSS is a population-based health survey conducted by the Center for Disease Control and Prevention (CDC) that contains health-related data collected using telephone surveys that acquire information from residents across the US regarding their health-related risk behaviors, chronic health conditions, and the use of preventive services.14 The 2016 cross section of the BRFSS included 486 303 respondents. The survey was conducted through both landline and cellular telephones using a questionnaire that contained a core set of rotating questions.15 The information obtained was self-reported and provides prevalence estimates for common health conditions. Data from the BRFSS have been comparable to results of self-reported behaviors recorded in other surveys.16,17 For this analysis, we used data from participants aged ≥18 years. The analysis was conducted between September and December 2017.
Poor oral health
Poor oral health was determined by the number of permanent teeth removed due to non-traumatic causes. Respondents were asked ‘How many of your permanent teeth have been removed due to tooth decay or gum disease?’ We defined poor oral health by an affirmative answer to any of the following questions: losing ‘1–5 teeth’, ‘6 or more (but not all) teeth’ or ‘all teeth’. Participants who answered ‘No teeth loss’ were considered to have ‘good oral health’. Participants who responded as ‘not sure’ or who refused to answer the question were classified as missing data and were excluded from the analysis.
Electronic cigarette use
Our main predictor of interest, e-cigarette use, was determined on a 4-level smoker status within 30 days prior to survey administration (everyday e-cigarette user, someday e-cigarette user, former e-cigarette user and non-e-cigarette user). Respondents were asked to identify themselves with any one category of smoker status. Those who reported ‘everyday e-cigarette user’ were classified as ‘current users’, those who responded as ‘someday e-cigarette user’ was classified as ‘intermittent users’, and those answered as ‘former e-cigarette user’ or ‘non-e-cigarette user’ were classified as ‘non-users’. Participants who responded as ‘not sure’ or who refused to answer the question were classified as missing data and were excluded from the analysis.
Covariates
Based on prior studies that have shown several risk factors associated with poor oral health, we included smoking status, smokeless tobacco (e.g. snuff) use, alcohol use, soda intake, dental visit history, physical health status, depression and diabetes mellitus as potential confounders in our analyses. We categorized cigarette smoking status into current smokers and non/former smokers. Smokeless tobacco use was defined by chewing tobacco, snuff or snus, and was classified into everyday/intermittent users and non-users. Alcohol and soda intake were measured on a continuous scale, as the average number of drinks consumed in the past 30 days prior to survey administration. Finally, we categorized dental visit history based on prior attendance in a dental clinic in the following groups: visit within the past year, visit within the past 2 years, visit within the past 5 years, visit 5 years or more, and never if the participant had not had a previous dental visit. We also included self-reported chronic health conditions such as diabetes mellitus and depression given their known associations with poor oral health. For these, respondents were considered to have the comorbidity if they were ever told by a physician or health professional that they had diabetes or depression. Finally, we evaluated the self-reported physical health status using a three-tier tool which was determined by the number of days of poor physical health, where 0 days was classified as ‘Good’, 1–13 days was classified as ‘Moderate’, and 14–30 days was classified as ‘Poor’. Subjects who were not sure or refused to respond were classified as missing for the purposes of this analysis.
Demographic variables
Sociodemographic variables such as age group (18–24, 25–34, 35–44, 45–54, 55–64, 65 years and above), sex, race (Black, White, Asian and ‘other’ races), education (No education, Some high school education, and College and above), income (<$15 000, $15 000–$35 000, $35 000–$50 000, >$50 000 per annum) were also determined. US region was divided into West, Midwest, South and Northeast regions based on self-reported location.
Statistical analysis
We used descriptive statistics to describe the baseline characteristics stratified by presence or absence of poor oral health. We used chi-square tests to compare the frequencies of nominal variables and independent t-test to compare continuous variables in those with poor and good oral health. We tested the association between the e-cigarette use and covariates of interest with poor oral health using bivariate logistic regression analysis to determine crude odds ratios for each association. Using multivariable logistic regression analysis, we calculated adjusted odds ratios (aOR) and 95% confidence intervals (CI) for the association between e-cigarette use and poor oral health after controlling for covariates significantly different between the two groups in the descriptive analyses. We used sample weights for all our analyses to reduce the variation in respondent’s probability of selection, hence reducing the potential for bias and increasing the representativeness and generalizability of the estimates. We deemed results statistically significant based on a P-value <0.05, and utilized SAS v. 9.4 (Cary, NC) for all analyses.
Results
Sample characteristics
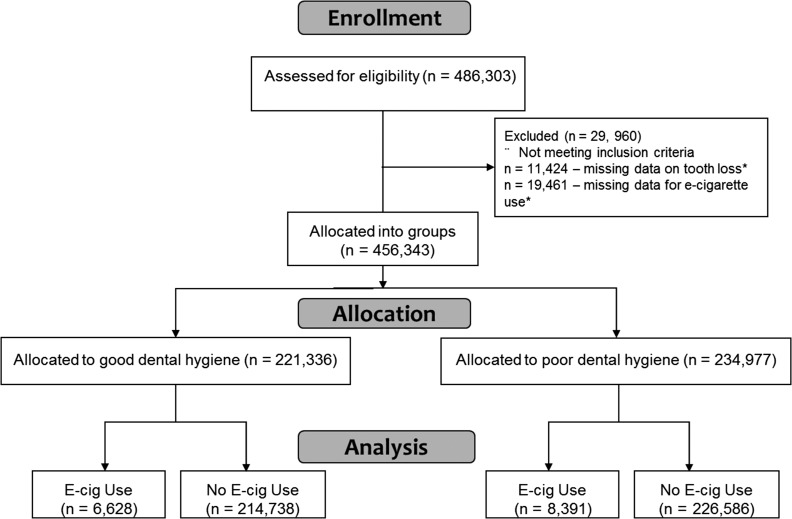
The 2016 BRFSS data included survey responses from 456 343 adults from across the USA, of which 2.3% were excluded for missing tooth loss data and 4.0% were missing e-cigarette use data (Fig. 1). Of those remaining, 34.9% of participants were ≥65 years of age, the majority (83.9%) were White, over half (56.6%) were women, with nearly two-thirds (64.6%) had at least a college education or above, and slightly less than half (47.9%) having an annual household income of >$50 000 (Table 1). The regions of the country were well represented, with 34.4% of respondents from the South, 24.0% from the Midwest, 21.8% from the West and 19.8% from the Northeast. The majority of participants (63.4%) reported their overall health quality as ‘good’, with 13.5% self-reporting diabetes and 17.8% reporting depression. The average [standard deviation (SD)] consumption of soda was 11.7 (43.0) drinks in the past month, and the average (SD) alcoholic beverage consumption was 2.2 (2.5) drinks in the last 30 days.
Fig. 1.
Participant flow diagram. *Some subjects had data missing for both oral health and e-cigarette use.
Table 1.
Baseline characteristics of the 2016 BRFSS participants overall and by oral health status
| Overall | Good oral health | Poor oral health | P value* | |
|---|---|---|---|---|
| (n = 456 343) | (n = 221 366) | (n = 234 977) | ||
| Age, years, n (%) | <0.0001 | |||
| 18–24 | 25 195 (5.5%) | 22 256 (88.3%) | 2939 (11.7%) | |
| 25–34 | 45 895 (10.1%) | 34 141 (74.4%) | 11 754 (25.6%) | |
| 35–44 | 51 979 (11.4%) | 33 720 (64.9%) | 18 259 (35.1%) | |
| 45–54 | 72 906 (16.0%) | 39 860 (54.7%) | 33 046 (45.3%) | |
| 55–64 | 101 206 (22.2%) | 43 126 (42.6%) | 58 080 (57.4%) | |
| 65+ | 159 162 (34.9%) | 48 263 (30.3%) | 110 899 (69.7%) | |
| Race, n (%) | <0.0001 | |||
| White | 374 852 (83.9%) | 185 097 (49.4%) | 189 755 (50.6%) | |
| Black | 39 332 (8.8%) | 15 036 (38.2%) | 24 296 (61.8%) | |
| Asian | 10 580 (2.4%) | 6550 (61.9%) | 4030 (38.1%) | |
| Other | 22 253 (5.0%) | 9935 (44.7%) | 12 318 (55.4%) | |
| Male sex, n (%) | 198 022 (43.4%) | 97 647 (49.3%) | 100 375 (50.7%) | <0.0001 |
| E-cigarette use, n (%) | <0.0001 | |||
| Daily user | 4957 (1.1%) | 2205 (44.5%) | 2752 (55.5%) | |
| Intermittent user | 10 062 (2.2%) | 4423 (44%) | 5639 (56%) | |
| None | 441 324 (96.7%) | 214 738 (48.6%) | 226 586 (51.4%) | |
| Cigarette smoking, n (%) | <0.0001 | |||
| Current smoker | 67 003 (14.8%) | 22 411 (33.4%) | 44 592 (66.6%) | |
| Non-smoker | 387 000 (85.2%) | 197 887 (51.1%) | 189 113 (48.9%) | |
| Smokeless tobacco users, n (%) | 14 948 (3.3%) | 7560 (50.6%) | 7388 (49.4%) | <0.0001 |
| Soda per month, mean (SD) | 11.7 (4.0) | 10.0 (39.0) | 12.0 (46.0) | <0.0001 |
| Alcohol per month, mean (SD) | 2.2 (2.5) | 2.2 (2.3) | 2.2 (2.6) | 0.89 |
| Depression, n (%) | 80 999 (17.8%) | 32 809 (40.5%) | 48 190 (59.5%) | <0.0001 |
| Diabetes mellitus, n (%) | 61 553 (13.5%) | 17 505 (28.4%) | 44 048 (71.6%) | <0.0001 |
| Physical health status, n (%) | <0.0001 | |||
| Good | 283 685 (63.4%) | 150 799 (53.2%) | 132 886 (46.8%) | |
| Moderate | 102 442 (22.9%) | 49 500 (48.3%) | 52 942 (51.7%) | |
| Poor | 61 445 (13.7%) | 18 144 (29.5%) | 43 301 (70.5%) | |
| Visit to dentist, n (%) | <0.0001 | |||
| Within past year | 310 166 (68.6%) | 164 623 (53.1%) | 145 543 (46.9%) | |
| Within past 2 years | 47 761 (10.6%) | 21 051 (44.1%) | 26 710 (55.9%) | |
| Within past 5 years | 40 080 (8.9%) | 15 962 (39.8%) | 24 118 (60.2%) | |
| More than 5 years ago | 50 920 (11.3%) | 16 222 (31.9%) | 34 698 (68.1%) | |
| Never | 3515 (0.8%) | 1937 (55.1%) | 1578 (44.9%) | |
| Education, n (%) | <0.0001 | |||
| College and above | 293 897 (64.6%) | 164 589 (56%) | 129 308 (44%) | |
| Some high school | 160 618 (35.3%) | 55 956 (34.8%) | 104 662 (65.2%) | |
| None | 647 (0.1%) | 227 (35.1%) | 420 (64.9%) | |
| Yearly income, n (%) | <0.0001 | |||
| >$50 K | 185 889 (47.9%) | 114 395 (61.5%) | 71 494 (38.5%) | |
| $35–50 K | 55 907 (14.4%) | 25 134 (45%) | 30 773 (55%) | |
| $15–35 K | 106 815 (27.5%) | 37 992 (35.6%) | 68 823 (64.4%) | |
| <$15 K | 39 212 (10.1%) | 11 517 (29.4%) | 27 695 (70.6%) | |
| Region, n (%) | <0.0001 | |||
| Midwest | 109 694 (24%) | 56 004 (51.1%) | 53 690 (48.9%) | |
| Northeast | 90 433 (19.8%) | 42 889 (47.4%) | 47 544 (52,6%) | |
| South | 156 776 (34.4%) | 69 183 (44.1%) | 87 593 (55.9%) | |
| West | 99 440 (21.8%) | 53 290 (53.6%) | 46 150 (46.4%) |
∗ P value for comparisons between participants with good and poor oral health.
E-cigarette use
While 67 003 (14.8%) of the sample reported current smoking within the previous 30 days, only 4957 (1.1%) reported current daily use of e-cigarettes, 10 062 (2.2%) reported intermittent use of e-cigarettes, and 14 948 (3.3%) reported current use of smokeless tobacco.
Factors associated with oral health in the cohort
The majority of respondents (51.5%) reported having at least one permanent tooth removed because of tooth decay or gum disease in their lifetime. Nearly all respondents (99.2%) had previously seen a dentist, with most (79.1%) having had a visit within the previous 2 years.
Using univariate logistic regression analyses, we found significant association (P < 0.05) between poor oral health and the following variables: age, race, sex, cigarette smoking, use of other smokeless tobacco products, average soda consumption in the past 30 days, education level, income level, physical health status, last visit to a dentist, depression, diabetes mellitus and region. There was no association between average alcohol consumption in the past month in the past 30 days with poor oral health.
E-cigarette use and poor oral health
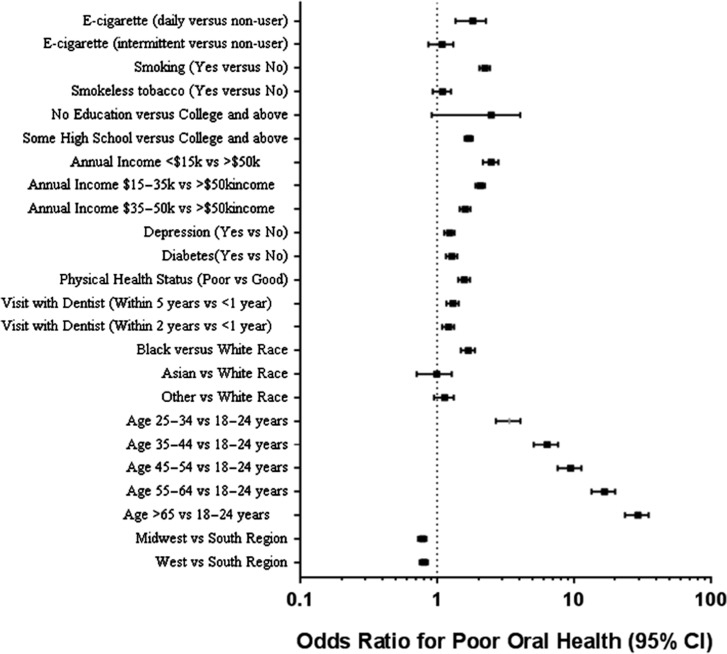
Poor oral health was more prevalent than good oral health among daily e-cigarette users (55.5 versus 44.5%, P < 0.0001) and intermittent e-cigarette users (56 versus 44%, P < 0.0001). Table 2 shows crude and adjusted odds ratios for the association between e-cigarette use and poor oral health after accounting for survey clustering, strata, and weight, and the significant variables listed above. Intermittent e-cigarette users had 18% higher odds of having poor oral health in crude models (OR = 1.18, 95% CI: 1.00–1.39), but after adjustment, the association was attenuated and was no longer significant (OR = 1.08, 95% CI: 0.87–1.32). Daily e-cigarette use was associated with 42% higher odds of having poor oral health in crude models. After adjustment for age, race, cigarette smoking, use of other smokeless tobacco products, education level, income level, physical health status, last visit to a dentist, depression, diabetes mellitus, region, survey clustering, strata and weight, daily e-cigarette use was associated with 78% higher odds of having poor oral health (OR = 1.78, 95% CI: 1.39–2.30; Table 2). The associations between e-cigarette use, demographic factors, and other exposures and poor oral health are shown in Fig. 2.
Table 2.
The association between e-cigarette use and poor oral health
| Variable | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI)a |
|---|---|---|
| e-Cigarette use | ||
| None | Ref. | Ref. |
| Intermittent | 1.18 (1.00–1.39)* | 1.08 (0.87–1.32) |
| Daily | 1.42 (1.12–1.79)** | 1.78 (1.39–2.30)*** |
CI = confidence intervals.
aAdjusted for age, race, sex, cigarette smoking, smokeless tobacco use, number of alcoholic drinks/day, number of soda/day, depression, diabetes mellitus, physical health status, education, income level and visit to a dentist. Data were analyzed after adjustment to account for survey clustering, strata, and weight. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Associations between clinical variables and poor oral health in BRFSS. Daily e-cigarette use, as well as other factors were independently associated with poor oral health in a logistic regression model adjusting for all listed covariates.
Discussion
Main findings of this study
In a population-based health survey of self-reported health behavior and outcomes, we found that daily use, but not intermittent use, of e-cigarettes was independently associated with poor oral health. To our knowledge, this is the first study to assess the relationship between e-cigarette exposure and poor dental health in adults in the USA. This finding is important as it highlights another potential consequence of e-cigarette use on health and disease. To date, most published studies on the health consequences of e-cigarettes have focused on the effects of vaping on the pulmonary or cardiovascular systems given the strong links between conventional cigarette smoking and diseases of these organ systems. Indeed, there have been many conflicting reports on ill-health effects of e-cigarette use, leading to difficulty in understanding the safety and regulatory aspects of e-cigarettes. However, our findings suggest that e-cigarette use is not without consequences for oral health.
What is already known on this topic
Poor oral health and tooth loss impacts quality of life and is a significant health concern.18,19 Between 1990 and 2015, the number of people with untreated oral conditions rose by 40% and now affects an estimated 3.5 billion people worldwide.20 This increase in disease burden has garnered the attention of policy makers, and oral health is now being recognized as a modifiable target to improve global public health. There are many known risk factors for poor oral health, including age, race, socioeconomic status, depression, diabetes mellitus, lack of recent dental check-ups and cigarette smoking.21,22 We identified associations between these characteristics and poor oral health in our study, increasing the generalizability of our findings.
Little is known about the impact of e-cigarettes on oral health. Nicotine, a main constituent in many e-cigarettes, may have a pathogenic role in tooth loss due to its ability to reduce tooth mineralization through altered genetic signaling,23 and activation of inflammatory pathways.24 There is emerging evidence that suggests other components of e-cigarette promote oral inflammation and senescence of periodontal fibroblasts.25 For example, propylene glycol, a major component of the e-liquid, can also decrease tooth integrity through altering calcium release and tooth mineralization.26 In addition, e-cigarette use can lead to inflammation, oxidative stress, altered cellular senescence, impaired host response and dysregulated repair mechanisms that can lead to periodontal disease and poor oral hygiene.27
What this study adds
We found that daily e-cigarette use, even when adjusted for these traits, was independently associated with poor oral health. This is the first study to investigate this association and to adjust for known confounders. Our observed increase in odds of poor oral hygiene from never-users, to intermittent users, to daily e-cigarette users suggests there may be a dose–reponse relationship between e-cigarette exposure and epithelial and endothelial cells in the oral cavity. Our findings, although cross-sectional in nature, provide the basis needed to further explore these mechanisms related to e-cigarette use and oral health.
Limitations of this study
The main strength of our study is that we included a large sample of US adults across all demographics and geographic locations. Our study also has some limitations. First, this is a cross-sectional study and hence we cannot establish causality between daily e-cigarette use and poor oral health. Second, the exposure and outcome are self-reported and the use of questionnaires is subject to information bias, such that both e-cigarette use and poor oral health may be under-reported. Third, we had data for e-cigarette use for only 30 days prior to survey administration. Fourth, our definition of poor oral health was limited to the loss of any tooth from non-traumatic causes, thus due to the nature of the survey question; alternative definitions could not be explored. Finally, the BRFSS survey is administered only to community-dwelling, non-institutional people with telephone connections, and hence this may limit generalizability. Adults in long-term care such as nursing homes are more likely to have poor oral health but potentially less likely to use e-cigarettes. Adults who were mentally or physically unable to respond to survey questions were excluded. However, the use of sampling weights adjusts for some of these issues of subject selection.
Conclusions
We found that daily e-cigarette use was associated with significantly increased odds of poor oral health in adults in the USA. These results suggest that e-cigarette use may be a risk factor for poor oral health outcomes including periodontal disease and tooth loss.
Acknowledgements
JMW had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis. PH, SPB, NB, NCW and JMW contributed to the conception and design of the study, acquisition of the data, drafting of the article, and contributed to revisions of the manuscript for critically important intellectual content. All the authors approved this version of the article to be published.
Conflict of interest
PH, NB and SPB have no conflicts of interest. NCW has received research contract from Amgen for projects outside the submitted work, and is a consultant for Pfizer. JMW has received grants from NIH and the Cystic Fibrosis Foundation, consulting fees from AstraZeneca, GlaxoSmithKline, Mereo BioPharma, Quintiles and Mylan; and contracts for clinical trial funding from GlaxoSmithKline, AstraZeneca and Gilead for projects outside the submitted work.
Funding
Authors have received funding from the National Institutes of Health (NIH) through the National Heart, Lung and Blood Institute (NHLBI) to [K23HL133438 to S.P.B.] and [K08HL123940 to J.M.W.] and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) [K01AR068400 to N.C.W.].
References
- 1. Kasza KA, Ambrose BK, Conway KP et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 2017;376(4):342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinakar C, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med 2016;375(14):1372–81. [DOI] [PubMed] [Google Scholar]
- 3. Jensen RP, Luo W, Pankow JF et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 2015;372(4):392–4. [DOI] [PubMed] [Google Scholar]
- 4. Brown JE, Luo W, Isabelle LM et al. Candy flavorings in tobacco. N Engl J Med 2014;370(23):2250–2. [DOI] [PubMed] [Google Scholar]
- 5. Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med 2014;174(5):812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullen C, Howe C, Laugesen M et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382(9905):1629–37. [DOI] [PubMed] [Google Scholar]
- 7. McMillen RC, Gottlieb MA, Shaefer RM et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):1195–202. [DOI] [PubMed] [Google Scholar]
- 8. Sutfin EL, McCoy TP, Morrell HE et al. Electronic cigarette use by college students. Drug Alcohol Depend 2013;131(3):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 2012;91(2):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho JH. The association between electronic-cigarette use and self-reported oral symptoms including cracked or broken teeth and tongue and/or inside-cheek pain among adolescents: a cross-sectional study. PLoS One 2017;12(7):e0180506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Javed F, Abduljabbar T, Vohra F et al. Comparison of periodontal parameters and self-perceived oral symptoms among cigarette-smokers, individuals vaping electronic-cigarettes and never-smokers: a pilot study. J Periodontol 2017;88(10):1059–65. [DOI] [PubMed] [Google Scholar]
- 12. Tatullo M, Gentile S, Paduano F et al. Crosstalk between oral and general health status in e-smokers. Medicine (Baltimore) 2016;95(49):e5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nutt DJ, Phillips LD, Balfour D et al. E-cigarettes are less harmful than smoking. Lancet 2016;387(10024):1160–2. [DOI] [PubMed] [Google Scholar]
- 14. Center for Disease Control and Prevention Behavioral Risk Factor Surveillance System (BRFSS). 2016.
- 15. Hu SS, Pierannunzi C, Balluz L. Integrating a multimode design into a national random-digit-dialed telephone survey. Prev Chronic Dis 2011;8(6):A145. [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson DE, Holtzman D, Bolen J et al. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soz Praventivmed 2001;46(Suppl 1):S3–42. [PubMed] [Google Scholar]
- 17. Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res Methodol 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerritsen AE, Allen PF, Witter DJ et al. Tooth loss and oral health-related quality of life: a systematic review and meta-analysis. Health Qual Life Outcomes 2010;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kassebaum NJ, Bernabe E, Dahiya M et al. Global burden of severe tooth loss: a systematic review and meta-analysis. J Dent Res 2014;93(7 Suppl):20S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kassebaum NJ, Smith AGC, Bernabe E et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res 2017;96(4):380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kailembo A, Preet R, Stewart Williams J. Common risk factors and edentulism in adults, aged 50 years and over, in China, Ghana, India and South Africa: results from the WHO Study on global AGEing and adult health (SAGE). BMC Oral Health 2016;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kassebaum NJ, Bernabe E, Dahiya M et al. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 2014;93(11):1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanagita M, Kashiwagi Y, Kobayashi R et al. Nicotine inhibits mineralization of human dental pulp cells. J Endod 2008;34(9):1061–5. [DOI] [PubMed] [Google Scholar]
- 24. Manuela R, Mario M, Vincenzo R et al. Nicotine stimulation increases proliferation and matrix metalloproteinases-2 and -28 expression in human dental pulp cells. Life Sci 2015;135:49–54. [DOI] [PubMed] [Google Scholar]
- 25. Sundar IK, Javed F, Romanos GE et al. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016;7(47):77196–77204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Natu VP, Dubey N, Loke GC et al. Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. J Appl Oral Sci 2015;23(4):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Javed F, Kellesarian SV, Sundar IK et al. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis 2017;23(8):1052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]