Abstract
Early childhood trauma can have profound and lifelong effects on adult mental health and psychosocial wellbeing. Nevertheless, responses to trauma are highly variable. Genetic variants may help explain variation in responses to trauma by identifying alleles that associate with changes in mental health measures. Protective factors, such as resilience, likely also play an important role in responses to trauma. The effects of genetic variants, in combination with protective factors, on psychosocial health are not well understood, particularly in non-Western contexts. In this study, we test the relative influence of genetic variants of monoamine oxidase A (MAOA, a gene proposed to influence the impact of childhood trauma on adult violence and antisocial behavior), levels of resilience, and exposure to traumatic events on psychosocial stress and mental health trajectories over time. We use data from a cohort of 12-18-year-old Syrian refugees who were forcibly displaced to neighboring Jordan (n = 399). DNA samples and survey data on trauma exposure, resilience (CYRM-12), and psychosocial stress were collected at three time points: baseline, ~13 weeks, and ~48 weeks. Using multilevel models, we identified an association of MAOA variant, in males only, with symptom scores of psychosocial stress on the Perceived Stress Scale (PSS) over time (p = 8.1 x 10−4). We also found that resilience is strongly associated with PSS (p = 7.9 x 10−9), underscoring the importance of protective factors in influencing levels of psychosocial stress. Furthermore, there was an additive effect wherein the sharpest reductions in perceived psychosocial stress are seen in low-activity MAOA males with low trauma exposure or high resilience levels. Our results highlight the value of studies that integrate genetic and psychosocial factors to better understand complex phenotypes, such as responses to trauma in contexts of high trauma exposure.
Introduction
Childhood traumatic experiences are associated with poor health outcomes that can persist throughout life. In particular, children and adolescents who experience physical and emotional abuse are at increased risk for psychiatric diagnoses, especially mood, anxiety, and substance abuse disorders, as well as suicidality, violent and criminal behavior, poor physical health and increased risk for infectious disease later in life [1–7]. However, there is great variation in how children experience and recover from early trauma [8]. There is a paucity of longitudinal research conducted outside Western contexts on this issue, especially with war-affected children and adolescents. Research on refugee populations is of great importance because refugees experience a high burden of trauma, loss, and stress [9]. Understanding how war-related adversity and trauma impacts health and development is particularly critical in children and adolescents since they account for 52% of refugees worldwide [10]. Many refugees are exposed to substantial trauma during war, as well as ongoing stressors and social marginalization during and after resettlement. Nevertheless, many refugees show notable resilience in psychosocial, developmental, and health trajectories [11, 12]. Now in its seventh year, the Syrian crisis has led more than 5.68 million people to leave Syria, while a further 6.5 million Syrians are internally displaced, two-fifths of whom are under 18 years of age [13, 14]. This is the largest refugee crisis since the Second World War, the consequences of which have led scholars and humanitarian agencies to call for renewed attention to address health and developmental issues for a potentially ‘lost generation’ coping with exposures to ‘toxic stress.’ [9].
The factors that determine how children experience and recover from trauma are not well understood, but likely include both genetic and psychosocial factors [8]. The monoamine oxidase A gene (MAOA) has been widely studied for its role in influencing the impact of childhood trauma on adult aggressive and antisocial behavior. MAOA is located on the X chromosome and the encoded MAOA enzyme catalyzes the degradation of brain neurotransmitters serotonin, dopamine and norepinephrine, thus playing a key role in regulating behavior. A functional repeat polymorphism located approximately 1.2 kb upstream of the MAOA coding sequence was first identified by Sabol et al. [15], who used luciferase reporter gene fusions and transfection experiments to show that alleles with different numbers of repeat sequences were transcribed with different efficiencies in human neuroblastoma and human placental choriocarcinoma cell lines. MAOA deficiency was first studied in humans in five affected males who exhibited borderline mental deficiency as well as impulsive aggression, criminal violence, and hypersexual behavior [16]. In the early 2000’s, Caspi and Moffitt’s [17] landmark Dunedin Longitudinal Cohort study investigated MAOA genetic variants to better understand why some abused children developed antisocial behavior as adults and others did not. They determined that childhood trauma was more likely to lead to antisocial and violent behavior in males who carried a MAOA variant with low transcriptional activity (MAOA-L) [17].
Over the past decade, additional studies have identified associations between MAOA variants and a wide range of aggressive and antisocial behaviors including adolescent antisocial behavior [18], reduced social cooperation [19], physical aggression [20], criminal violence [21], recidivism in male violent offenders [22], and brain morphology in violent offenders with psychopathic traits [23]. Not all associations have been confirmed, however, and the first genome-wide association study on adult antisocial behavior did not find significant associations with any genetic polymorphisms, leading the authors to speculate that the relevant genes may have effect sizes which are so small that they are undetectable with their sample size [24]. In general, MAOA-L is the allele most often associated with increased aggressive or antisocial behaviors and the strongest associations are typically found in males [25, 26].
Interestingly, some studies have found opposite effects in females, i.e. association of the high-activity MAOA (MAOA-H) variant with increased risk of antisocial behavior and aggression [25, 27]. A study of neural activity found that activity in the amygdala and hippocampus increased with childhood stress in MAOA-L males and decreased in MAOA-H males, with the opposite pattern seen in females [27]. Another brain imaging study found increased connectivity between the amygdala and ventromedial prefrontal cortex that indicated dysregulated amygdala activation in MAOA-L males; this effect was not seen in females [28]. Variable levels of testosterone and the specific interaction of MAOA-L with high levels of testosterone [29] likely explain some of the differing effects of MAOA between males and females, and possibly within males as well.
The timing of trauma exposure is critical since brain structures are still developing during childhood and adolescence; disruption of different brain structures will likely impact different psychosocial outcomes [30, 31]. The critical developmental periods, and sensitive windows, for three key regions of the brain (hippocampus, amygdala, prefrontal cortex) all overlap in early and middle childhood [32, 33]. Children across a range of ages have been studied in order to investigate critical windows of trauma exposure. For instance, family adversity experienced in the first three years of life has been found to associate with increased hyperactivity at 4–7 years of age in MAOA-L boys and girls from the U.K. Avon Longitudinal Study of Parents and Children, UK [34]. Fewer studies have focused on adolescents, but Lee [18] found that the effect of deviant peer affiliation on antisocial behavior in participants from the National Longitudinal Study of Adolescent Health study was significantly stronger in MAOA-H carriers than MAOA-L carriers. In sum, different studies identify both MAOA-L and MAOA-H variants as being risk alleles, which may reflect ongoing brain development and exposure to trauma at different ages.
In addition to the effect of MAOA on aggressive and antisocial behaviors, other behaviors have been tested for association with MAOA in order to provide a more comprehensive perspective on MAOA functioning and to better understand the behaviors impacted by MAOA. For instance, MAOA-L variants have been found at slightly higher frequencies in alcoholics (both male and female) with antisocial personality disorder compared to alcoholics with anxiety and depression, suggesting that MAOA variation may contribute to over- vs under-reactive behaviors in the development of alcoholism [26]. MAOA likely also plays a role in the risk of developing stress-related behaviors like obsessive-compulsive disorder [35] as well as addictive behaviors like cigarette smoking [36]. In a study on assessment of risk in a laboratory setting, MAOA-H individuals showed a preference for longshot risks and were less likely to purchase insurance, suggesting that MAOA variation may play a role in real-life decision-making and risk-assessment [37]. Negative associations can also be informative, such as the study that found MAOA was not one of the nine candidate genes associated with response time on an attention task, suggesting that MAOA does not play a role in attentional deficits [38]. Overall, our understanding of the effect of MAOA on complex behaviors is incomplete, but the impact of MAOA is likely to be relatively small, and possibly difficult to detect [39].
Virtually no studies have investigated the influence of MAOA and protective factors on psychosocial and mental health outcomes. We found a single published study of MAOA and a protective factor: high perceived parental care was found to mitigate the effect of childhood stress in females (males were not studied) [40]. Specifically, a three-way interaction between perceived parental care, early life family stressors, and MAOA variant predicted impulsivity/aggression scores. The lack of attention to protective factors is puzzling given the strong effect of protective factors on mental and physical health [41–46] and the opportunity for intervention [47, 48] with potential for intergenerational effects [49, 50]. Furthermore, from an evolutionary perspective, it is unlikely to find common genetic variants whose only effect is to increase the risk of maladaptive behaviors under adverse conditions. Pluess and Belsky have proposed that genetic variants like those at MAOA do not function solely as risk factors, but rather as environmental sensitivity variants whose carriers are more sensitive, or responsive, to both positive and negative exposures [51–53]. In their brain imaging study, Buckholtz et al. [54] proposed that MAOA-L variants may create a less stable developmental framework, e.g. amygdala dysregulation and a compensatory response of increased coupling between the amygdala and the ventromedial prefrontal cortex, that renders MAOA-L males more sensitive to environmental factors relative to MAOA-H males.
In this study, we tested for association of MAOA variants with different measures of psychosocial stress and mental health in a population of Syrian refugee youth who have experienced high levels of war-related trauma. We also investigated the protective aspects of a contextually-specific measure of resilience that measures individual and collective strengths, resources, and capabilities [55]. The goal in studying a range of measures was to identify outcomes other than aggressive and antisocial behavior that associate with MAOA. We predicted there would be an association of MAOA genetic variants, either directly or through an interaction with trauma exposure or resilience, with psychosocial outcomes. Specifically, we hypothesized that MAOA-L individuals would show increased change in the measured outcomes relative to MAOA-H carriers and that this effect would be limited to males, based on findings of dysregulated amygdala activation and proposed increased environmental sensitivity in MAOA-L males [28, 56].
Materials and methods
Study design
Our study population was a cohort of 399 12-18-year-old Syrian refugees in Jordan. Over 671,000 Syrians have taken refuge in Jordan [14]. Participating adolescents were sampled from four sites in northern Jordan (Irbid, Jarash, Mafraq, Zarqa), urban centers which have been heavily affected by the Syrian crisis. Youth in our study were participating in an eight-week stress attunement intervention (Advancing Adolescents; Arabic: Nubader), developed by Mercy Corps for war-affected adolescents in Jordan, Lebanon, Iraq, Syria and Turkey. Buccal samples and data on trauma exposure, resilience, stress, and mental health were collected at three time points: pre-intervention baseline (T1), after ~13 weeks (T2), and after ~48 weeks (T3). Refugees at these four study sites were from disadvantaged families and most were displaced from the cities of Damascus, Daraa, Homs, and surrounding towns in Syria. In order to control for possible population structure created by study sites having different proportions of refugees from different areas of Syria, study site was included as a covariate in all analyses. Participants were sampled in two waves: in the first wave (collected March-June 2015, n = 103), participants were quasi-randomized into treatment groups based on their availability to participate in the intervention immediately, and in the second wave (collected September 2015-February 2016, n = 296), participants were randomly allocated to treatment or wait-list. There were no significant differences in our independent variables (MAOA variant and trauma exposure; data on resilience were collected in the second wave only), enabling us to combine the two waves in order to increase sample size. In this paper, we focus on the 399 adolescents (221 males and 178 females) for whom biological samples were collected. The study received approval from the Prime Minister’s Office of Jordan as well as ethical approval from Yale University (IRB ID 1502015359). Informed consent was obtained in Arabic from all participating adolescents and their parents. No participant who was asked to contribute a buccal sample refused to do so. See previous publications [57–59] for more details on the study method. All data analyzed in this study are presented in S1 and S2 Tables. All data are also available on Mendeley (https://data.mendeley.com/datasets/) doi:10.17632/xhs8tn7nkz.1).
Study population
Individuals with buccal samples and data on trauma exposure, resilience, and the outcome measures were chosen for study (T1 = 399) (Table 1). Some study participants were lost to follow-up at the T2 and T3 time points, resulting in a sample of 263 participants at T2 and 157 participants at T3. Because multilevel models are robust to partial data [60], individuals with missing time points were included in all analyses; thus, each data point contributes to the model.
Table 1. Sample characteristics of Syrian refugee participants.
| Male | Female | |
|---|---|---|
| Sociodemographics | ||
| # Participants at T1 | 221 | 178 |
| # Participants at T2 | 136 | 127 |
| # Participants at T3 | 78 | 79 |
| Age, mean (SD) years | 14.2 (1.81) |
14.5 (1.88) |
| Genetic Data | ||
|
MAOA-H, # Homozygotes or Hemizygotes |
125 | 50 |
|
MAOA-H/L, # Heterozygotes |
NA | 94 |
|
MAOA-L, # Homozygotes or Hemizygotes |
96 | 34 |
| Trauma exposure and Resilience Measures | ||
| Lifetime Exposure to Traumatic Events* (SD) | 6.76 (3.25) |
5.81 (3.21) |
| Resilience, CYRM12 (SD) | 49.8 (6.77) | 49.1 (7.00) |
| Stress and Mental Health Measures | ||
| Perceived Stress Scale, PSS* (SD) | 28.1 (5.37) |
29.4 (6.38) |
| Human Distress Scale, HD (SD) | 39.7 (19.3) |
44.5 (22.7) |
| Human Insecurity Scale, HI* (SD) | 66.3 (20.0) |
70.0 (19.2) |
| Arab Youth Mental Health Scale, AYMH* (SD) | 34.6 (7.72) |
37.2 (9.25) |
| Strengths & Difficulties Questionnaire, SDQ (SD) | 14.9 (5.68) |
16.3 (6.23) |
| Children’s Revised Impact of Events Scale, CRIES-8 (SD) | 19.6 (10.6) |
19.1 (11.7) |
Values are reported as mean with standard deviation in parentheses and are from baseline. An asterisk (*) indicates significant differences between males and females at p < 0.05. For MAOA, repeat lengths 3.5, 4, and 5 were classified as high-activity variants and repeat lengths 2 and 3 were classified as low-activity variants. Since males have only one copy of the X-linked MAOA gene, there were no male heterozygotes. Resilience measures were collected for a subset of 163 males and 127 females.
Psychosocial measures
Lifetime trauma exposure was assessed with the Traumatic Events Checklist, adapted for use with adolescents in conflict regions [61]. Resilience was measured using the Child and Youth Resilience Measure (CYRM) that was specifically developed to assess resilience in youth living in adversity [55]. This measure of resilience is situated within a socioecological framework [55, 62] and assesses protective factors at individual, relational, and contextual levels, rather than treating resilience as an internal trait or using better-than-expected functioning as a proxy for resilience. Since the resilience measure was developed during this study, it was only collected in the second wave of survey with 163 males and 127 females.
We tested six psychosocial and mental health measures. The Perceived Stress Scale (PSS, 14 items) is a generalized measure of psychosocial stress used globally that has been validated in Jordan [63, 64]. Human Distress (HD, 12 items) is a measure of distress and Human Insecurity (HI, 10 items) is a measure of insecurity and captures feelings of fear in conflict-affected areas. Both HD and HI were developed for use in conflict settings in the Middle East and were validated in the West Bank [65]. The Arab Youth Mental Health scale (AYMH, 21 items) is used to screen for depression and anxiety in Arab youth [66]. The Strength and Difficulties Questionnaire (SDQ, 25 items) assesses behavioral and emotional mental health difficulties [67–69]. Finally, the Children’s Revised Impact of Event Scale (CRIES-8, 8 items) measures symptoms of posttraumatic stress [70, 71].
Buccal sample collection and DNA extraction
Buccal samples were collected using Transport Swabs (APCO Laboratory Consumable Plastic, Jordan) or DNA Buccal Swabs (Isohelix, United Kingdom). Participants rinsed their mouths with water and then brushed both sides of their mouth with the collection swab for up to 30 seconds. DNA was extracted from the buccal swabs using either the Qiagen DNA Investigator Kit (Qiagen, USA) or Xtreme DNA Isolation Kit (Isohelix, United Kingdom). DNA extractions were performed according to manufacturer’s recommendations with the exception that the AW2 wash was performed twice for swabs extracted using the Qiagen kit.
MAOA assay
Samples were PCR amplified based on a published protocol [34]. All samples used recommended reagent concentrations including 1uL of extracted DNA, Platinum Hot Start PCR Master Mix and Platinum GC Enhancer (Invitrogen, USA), and the following primer sequences: Forward 5’-CCC-AGG-CTG-CTC-CAG-AAA-C-3’ and Reverse 5’-ACT-CAG-AAC-GGA-TGC-TCC-ATT-CG-3’. The PCR profile was as follows: 96°C for 10 min followed by 40 cycles of 94°C for 15 sec, 55°C for 15 sec, and 72°C for 30 sec and a final 3 min step at 72°C. Amplification reactions were electrophoresed on 2% agarose gels using Agarose SFR (VWR Life Science, USA) for 3 hours at 80V. Genotype completion rate was 97%. Genotyping discrepancy was determined by replicating approximately 1/3 of the samples (n = 153); only one sample showed a discrepancy and was typed a third time to resolve the discrepancy.
Statistical analyses
Each MAOA variant was classified according to transcriptional activity: repeat lengths of 3.5, 4, and 5 were classified as high-activity variants while repeat lengths of 2 and 3 were classified as low-activity variants. Since MAOA occurs on the X chromosome, males are hemizygous for MAOA, i.e. they carry only one MAOA variant. In statistical analyses, high-activity male hemizygotes and high-activity female homozygotes were given a score of 1, female heterozygotes with one high-activity and one low-activity variant were given a score of 0.5, and low-activity male hemizygotes and low-activity female homozygotes were given a score of 0. MAOA variant was treated as a categorical variable in males (0 or 1) and was treated as a continuous variable in females or analyses that included both males and females (0–1).
We used multilevel models (time points nested within participants) to test for effects of MAOA genotype, trauma exposure, and resilience on the psychosocial and mental health outcomes over the course of approximately one year. Multilevel models are robust to partially missing data, yielding higher statistical power than linear mixed models and regression analyses, while still taking advantage of a longitudinal design [60, 72]. These features are particularly useful for our data, given that more than half of the participants in this mobile refugee population were lost to follow-up at one year.
All statistical analyses were conducted using R [73]. Multilevel model analysis was conducted using linear mixed effects modeling via the nlme package with measurements at each time point (level one) nested within individuals (level two) [74]. Study site (Irbid, Jarash, Mafraq, Zarqa) and age at T1 were included as fixed effects, and these time-invariant covariates were included in all relevant analyses. Intervention program was treated as a time-varying covariate; all participants were given a score of 0 at T1, while at T2 and T3 those in the control group continued to receive a score of 0 (= has not received the intervention) and those in the treatment group received a score of 1 (= has received the intervention). Measurements of trauma exposure were treated as time-invariant and continuously distributed based on an individual’s score at T1, consistent with previous studies using this sample [57, 75]. Measurements of resilience were treated as time-varying and measurements from all three time points were used. Models that included only Time were used to confirm that sufficient variation existed within the tested outcome to support multilevel modeling. Base models included time and covariates for age, collection site, and intervention. MAOA, trauma exposure, and resilience were added to the base models and were used to test for associations between the outcome measure and MAOA, trauma exposure, or resilience. Models for perceived psychosocial stress (PSS) were run in the following order (see Table 2):
Table 2. Perceived Stress Scale models with male participants.
| Perceived Stress Scale (PSS) | Beta | Std. Error | p value |
|---|---|---|---|
| Model 1. Testing for effects of MAOA on PSS (n = 221) | |||
| Time only | -0.074 | 0.016 | 5.6 x 10−6* |
| PSSMAOA = Base + MAOA + MAOA*Time | |||
| MAOA | 1.5 | 0.67 | 2.4 x 10−2 |
| MAOA*Time | 0.11 | 0.033 | 8.1 x 10−4* |
| Model 2. Testing for effects of MAOA and Trauma exposure on PSS (n = 221) | |||
| Time only | -0.074 | 0.016 | 5.6 x 10−6* |
| PSSMAOA + Trauma = Base + MAOA + MAOA*Time + Trauma + Trauma*Time | |||
| MAOA | 1.5 | 0.67 | 0.025 |
| MAOA*Time | 0.11 | 0.034 | 7.6 x 10−4* |
| Trauma | 0.14 | 0.11 | 0.19 |
| Trauma*Time | 0.00084 | 0.0058 | 0.88 |
| PSSMAOA + Trauma + MAOA*Trauma = Base + MAOA + MAOA*Time + Trauma + Trauma*Time + MAOA*Trauma + MAOA*Trauma*Time | |||
| MAOA*Trauma | 0.011 | 0.21 | 0.95 |
| MAOA*Trauma*Time | 0.0019 | 0.011 | 0.86 |
| Model 3. Testing for effects of MAOA and Resilience on PSS (n = 163) | |||
| Time only | -0.061 | 0.017 | 3.7 x 10−4* |
| PSSMAOA = Base + MAOA + MAOA*Time | |||
| MAOA | 2.2 | 0.78 | 6.1 x 10−3 |
| MAOA*Time | 0.11 | 0.035 | 1.4 x 10−3* |
| PSSMAOA + Resilience = Base + MAOA + MAOA*Time + Resilience | |||
| MAOA | 1.9 | 0.74 | 8.6 x 10−3 |
| MAOA*Time | 0.089 | 0.034 | 8.2 x 10−3 |
| Resilience | -0.026 | 0.053 | 7.9 x 10−9* |
| PSSMAOA + Resilience + MAOA*Resilience = Base + MAOA + MAOA*Time + Resilience + MAOA*Resilience | |||
| MAOA*Resilience | 0.057 | 0.11 | 0.60 |
Time only models do not include covariates. Base models include time and covariates for age, study site, and intervention. Significance was tested individually for MAOA, Trauma exposure or Resilience with respect to association with the intercept of PSS (reported as MAOA or Trauma or Resilience) and for MAOA and Trauma exposure for association with the slope of PSS (reported as Trauma*Time or MAOA*Time). p values with an asterisk (*) remain significant after correction for multiple testing, i.e. p < 1.67 x 10−3.
Model 1. Testing for effects of MAOA variant on PSS:
Time only
Base (the base model includes time and the covariates: age, site, intervention)
PSSMAOA = Base + MAOA + MAOA*Time
Model 2. Testing for effects of MAOA variant and Trauma exposure on PSS:
Time only
Base (the base model includes time and the covariates: age, site, intervention)
PSSMAOA + Trauma = Base + MAOA + MAOA*Time + Trauma + Trauma*Time
PSSMAOA + Trauma + MAOA*Trauma = Base + MAOA + MAOA*Time + Trauma + Trauma*Time + MAOA*Trauma + MAOA*Trauma*Time
Model 3. Testing for effects of MAOA variant and Resilience on PSS:
Time only
Base (the base model includes time and the covariates: age, site, intervention)
PSSMAOA + Resilience = Base + MAOA + MAOA*Time + Resilience
PSSMAOA + Resilience + MAOA*Resilience = Base + MAOA + MAOA*Time + Resilience + MAOA*Resilience
Significance tests for all variables (as reported in Table 2) were conducted using ANOVAs that compared models with and without the variable being tested, i.e. in Model 1 outlined above, the significance of MAOA*Time was tested by comparing models with and without MAOA*Time. Significance after correction for multiple testing was calculated as p < 0.05 / (5 psychosocial outcomes with sufficient variation for multilevel modeling x 2 sexes x 3 models) = 1.67 x 10−3.
In order to visualize the effects that we report, partial effect plots were constructed using the lme4, remef, and ggplot packages [76–78]. All plots were corrected for the same covariates (age, site, and intervention) that were included in the significance testing. Trauma exposure and resilience scores were treated as continuous variables in the multilevel models and when testing for significance, but were dichotomized when constructing the partial effect plots (Figs 1 and 2) to more clearly illustrate the effects (trauma exposure was dichotomized at <4 vs ≥4 events as this cutoff was previously found to be relevant for this sample [75] and resilience was dichotomized at the median based on averages of all resilience measures for each individual, i.e. median = 51).
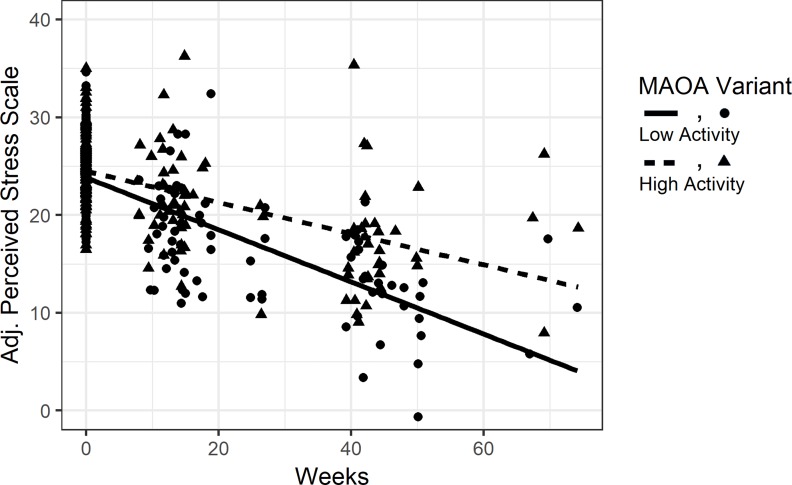
Fig 1. Partial effect plot of perceived psychosocial stress over time by MAOA variant in males.
MAOA-L males had sharper reductions in levels of perceived stress (PSS) over time relative to MAOA-H males. Perceived stress symptom scores were adjusted for the effects of all covariates in the model and plotted (Y axis) for each participant at all time points (X axis).
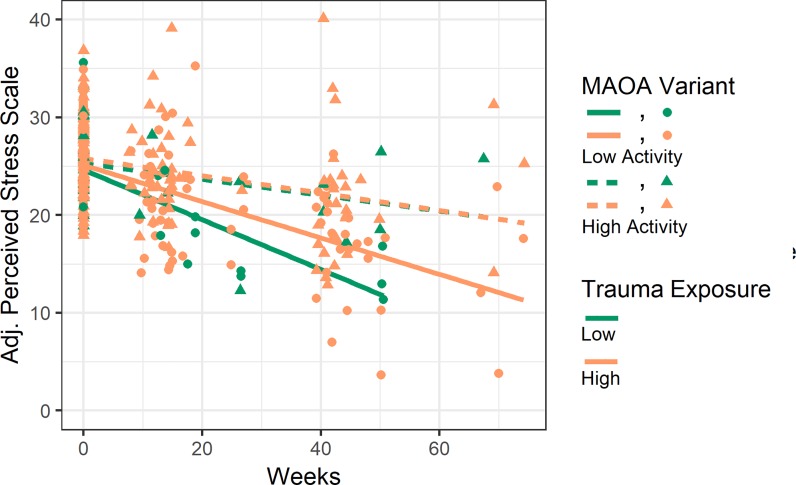
Fig 2. Partial effect plot of perceived psychosocial stress over time by MAOA variant and trauma exposure in males.
MAOA-L males had sharper reductions in levels of perceived stress (PSS) over time relative to MAOA-H males. Perceived stress symptom scores were adjusted for the effects of all covariates in the model and plotted (Y axis) for each participant at all time points (X axis). Partial effect plot lines were fitted for the four categories of MAOA variant (Low-activity/High-activity) and trauma exposure (Low/High). Trauma exposure was dichotomized around <4 vs ≥4 events in the figure for visualization purposes, but was treated as a continuous measure in multilevel models.
Results
Sample characteristics
Sample characteristics for the Syrian refugee cohort are shown in Table 1. Genetic data were collected for 221 males and 178 females at T1, with retention of 66% and 39% of participants at T2 and T3, respectively. Exposure to traumatic events was higher for males relative to female participants, but levels of resilience were comparable by gender. No differences in trauma exposure or resilience levels were seen between MAOA-L and MAOA-H carriers. In terms of outcomes, psychosocial stress (PSS), insecurity (HI), and anxiety and depression (AYMH) symptoms were significantly higher in females.
Initial analyses by gender
We investigated six measures of psychosocial stress and mental health. All but one outcome (HI) had sufficient variation for multilevel modeling. With the cohort of males and females combined (n = 399), we found no significant associations between MAOA and the five tested outcomes (PSS, HD, AYMH, SDQ, or CRIES-8). We then divided the sample to examine males and females separately. In females (n = 178), no significant associations were found between MAOA and the five outcomes. In males (n = 221), associations were only significant for symptoms of psychosocial stress as measured on the Perceived Stress Scale (PSS); no associations were found between MAOA and either AYMH or SDQ, whilst there was insufficient variation for HD and CRIES-8 to test in multilevel models. Thus, all subsequent analyses focus on PSS in males.
MAOA
We found that MAOA was significantly associated with PSS over time, even after correction for multiple testing (MAOA*Time p = 8.1 x 10−4; Table 2, Model 1). Plots of MAOA and PSS show that MAOA variants exhibited different changes in PSS over time (Fig 1). Specifically, MAOA-L males had sharper reductions in PSS levels over time as compared to high MAOA-H males.
MAOA and trauma exposure
We next tested to see if the association identified above between MAOA and PSS was influenced by trauma exposure. Although previous studies have found interactive effects between MAOA and trauma, we found no interactive effect of MAOA and trauma exposure on PSS (MAOA*Trauma p = 0.95 and MAOA*Trauma*Time p = 0.86; Table 2, Model 2). As expected, MAOA was still directly associated with PSS over time, even with trauma exposure included in the model (MAOA*Time p = 7.6 x 10−4; Table 2, Model 2). Plots of MAOA and trauma exposure show that MAOA-L males had the sharpest reduction in PSS (Fig 2). Furthermore, there appeared to be an additive effect wherein MAOA-L males with low trauma exposure had the sharpest reductions in PSS over time, as compared to all MAOA-H males and MAOA-L males with high trauma exposure. In contrast to the difference in PSS shown by MAOA-L males with respect to trauma exposure, MAOA-H males with high and low trauma exposure showed the same PSS trajectory.
MAOA and resilience
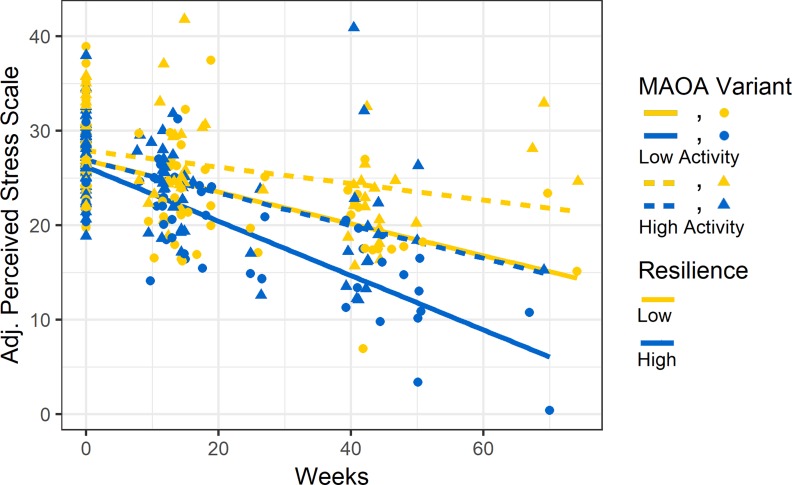
We next tested to see if the association between MAOA and PSS was influenced by participants’ levels of resilience. We first confirmed that the direct effect of MAOA on PSS over time reported above was also present in the smaller sample with resilience data (MAOA*Time p = 1.4 x 10−3; Table 2, Model 3). When levels of resilience were added to the model, we found that MAOA was still associated with PSS, but no longer survived our conservative correction for multiple testing (MAOA*Time p = 8.2 x 10−3; Table 2, Model 3). We also found that resilience was strongly associated with PSS (Resilience p = 7.9 x 10−9; Table 2, Model 3). We found no significant interactive effects between MAOA variants and resilience on PSS (MAOA x Resilience p = 0.28; Table 2, Model 3). Plots show that higher levels of resilience were associated with lower PSS at baseline and with a sharper reduction in PSS over time (Fig 3). Furthermore, there appeared to be an additive effect with resilience wherein MAOA-L males with high resilience had the sharpest reductions in PSS over time compared to all MAOA-H males and MAOA-L males with low resilience (Fig 3).
Fig 3. Partial effect plot of perceived psychosocial stress over time by MAOA variant and resilience levels in males.
MAOA-L males with high resilience had the sharpest reduction in levels of perceived stress (PSS) over time. Perceived stress symptom scores were adjusted for the effects of all covariates in the model and plotted (Y axis) for each participant at all time points (X axis). Partial effect plot lines were fitted for the four categories of MAOA variant (Low-activity/High-activity) and Resilience (Low/High). Resilience was dichotomized around the median (median = 51) in the figure purely for visualization purposes but was treated as a continuous measure in multilevel models.
Discussion
This is the first study to test the effect of early life trauma exposure, MAOA genetic variants, and a culturally-specific measure of resilience on psychosocial outcomes in a population of Syrian refugee youth over time. Specifically, we tested for direct and interactive effects of MAOA variants, trauma exposure, and resilience levels on psychosocial outcomes at baseline and over approximately one year. We tested a range of psychosocial and mental health outcomes in order to better understand the range of behaviors that may be influenced by trauma, MAOA, and resilience.
We found a significant association between MAOA and perceived psychosocial stress (PSS) over time, in males only, which remained significant after strict Bonferroni correction for multiple testing (MAOA*Time p = 8.1 x 10−4; Table 2, Model 1). We found direct effects of MAOA on PSS, but no interactive effects between MAOA and trauma. We also found no associations between MAOA and the tested outcomes in the overall cohort, or in females only. These results suggest that MAOA plays a role in influencing levels of psychosocial stress in the males in our study population independent of their trauma exposure. Furthermore, MAOA was only significantly associated with changes in PSS over time (MAOA*Time p = 8.1 x 10−4 vs MAOA p = 2.4 x 10−2; Table 2). Specifically, MAOA-L males showed sharper reductions in PSS scores over time (Fig 1). The fact that the association of MAOA and PSS only emerges over time highlights the importance of conducting longitudinal research in order to understand the long-term effect of MAOA on psychosocial stress and possibly other measures of mental health.
We report no significant association between trauma and PSS in the males in our study population. However, we have found a significant association between trauma and PSS in the larger dataset of all Syrian refugees including those with no biological samples (n = 446) (unpublished data), so it may be that our smaller sample size of males only (n = 221) is not sufficient to detect an association between trauma and PSS. Interestingly, if we treat trauma as time-varying in the multilevel model, we do detect an association between trauma and PSS in males, although the p value (p = 0.014) is not significant after correction for multiple testing. This result suggests there is a trend of trauma exposure impacting PSS although the effect is not straightforward.
We also found that a culturally-relevant measure of resilience (CYRM-12) was strongly associated with psychosocial stress (p = 7.9 x 10−9; Table 2, Model 3). When resilience was added to the model, MAOA was no longer associated with PSS (after correction for multiple testing). Furthermore, we saw an additive effect with both trauma exposure and resilience levels wherein MAOA-L males with either low trauma exposure or high resilience showed the sharpest reductions in psychosocial stress (Figs 2 and 3).
Very few studies of complex phenotypes, such as response to trauma, include both genetic and psychosocial factors even though both genetic and environmental factors are involved in all complex phenotypes. By including both types of factors, we identified additive effects between MAOA and trauma exposure and between MAOA and resilience on psychosocial stress. Additive effects act independently, and not as gene x environment interaction effects wherein the environmental factor exerts different effects on an individual based on his or her genotype. Furthermore, by comparing the effects of MAOA and resilience, we conclude that MAOA variation impacts levels of psychosocial stress in males, but that resilience appears to be a more influential factor.
Our study is the second to examine the effect of MAOA and resilience on mental health outcomes. We find an additive effect wherein MAOA-L males with high resilience showed the sharpest reductions in perceived psychosocial stress (Fig 3). The only other study to investigate the impact of protective factors and MAOA variants on mental health found a similar result with MAOA-L carriers. Specifically, Kinnally et al. [40] found that high perceived parental care mitigated the effect of a childhood stressor on impulsivity scores in MAOA-L females (males were not studied); level of perceived parental care had no effect on impulsivity in homozygous MAOA-H females.
Our results are consistent with Pluess and Belsky’s model of environmental sensitivity that posits carriers of sensitivity variants are more sensitive to both positive and negative environmental exposures [52, 53, 79]. Pluess [51] proposed a theoretical framework in which environmental sensitivity is a function of specific genetic variants, and genetic factors predict individual differences in environmental sensitivity to both positive and adverse conditions. Belsky and Pluess and colleagues [80, 81] specifically proposed that due to genetic differences, some children would benefit more from intervention efforts and that evaluations of intervention efficacy should account for such genetic variation. In the case of MAOA, our results suggest that MAOA-L variants may function as sensitivity variants and carriers may be more responsive to the effect of both negative (trauma exposure) and positive (levels of resilience) factors. Similarly, other studies have found that the MAOA-L variant is most sensitive to a positive factor (high perceived parental care; [40]) and to negative factors (aggressive and antisocial behavior in the presence of childhood trauma [17, 25, 82, 83]). Furthermore, in a brain imaging study, Buckholtz et al. [28] proposed that MAOA-L individuals may be more susceptible to environmental factors than MAOA-H individuals due to their amygdala dysregulation and compensatory response between the amygdala and the ventromedial prefrontal cortex. It is important to note that Pluess [51] posited that sensitivity variants act through a gene x environment mechanism with no proposed difference between genetic variants in the absence of adverse or positive conditions. We found a direct effect of MAOA on PSS, rather than gene x environment effects with trauma exposure or resilience, which may suggest that sensitivity variants can also act through an additive mechanism.
As noted above, we only identified associations of MAOA and resilience with PSS in males. This result is consistent with other studies that have found the effect of MAOA on measures of psychosocial health and behavior to be weak or non-existent in females [28, 39, 83, 84]. Since MAOA occurs on the X chromosome, males and females differ in copy number of MAOA and random X chromosome inactivation in females means that females likely have variable MAOA levels throughout the body relative to males. Studies of brain morphology and brain activity have found different effects of childhood trauma on male and female brains [23, 27] so MAOA may have different biological effects in males and females.
There are three main limitations to note in our study. First, we focused on a single gene. MAOA is a well-supported candidate gene to investigate the impact of childhood trauma, but additional genes should be studied in order to fully understand the impact of childhood trauma and resilience on adult behaviors, particularly in refugee populations with exposure to high levels of trauma. Second, our focus on Syrian refugees, who have relatively high levels of trauma exposure, may have restricted the level of population variation in some of the psychosocial health outcomes. Third, our sample size is small, particularly since the association we identified was only found in males, which reduced our sample size. A recent meta-analysis found no support for 18 previously identified candidate genes for depression, suggesting that previous studies had been underpowered [85]. Research on high trauma populations can help us understand the impact of trauma on mental health, but collection of a large number of longitudinal samples from a highly mobile refugee population will always be challenging [86]. The fact that, even after correction for multiple testing, we detected significant associations with psychosocial stress in our study population is encouraging. Nevertheless, work with a larger, more heterogeneous sample is needed to more fully evaluate the effects of MAOA, trauma exposure, and resilience on psychosocial stress and mental health measures. In order to fully understand the impact of trauma on mental health, more work with vulnerable populations is necessary, particularly in non-Western contexts with high levels of trauma exposure.
Conclusions
We report significant effects of MAOA genetic variants and resilience levels on changes in perceived psychosocial stress over time, in a population of Syrian refugee male youth. We also find an additive effect wherein males with low-activity MAOA variants who reported low trauma exposure or high resilience had the sharpest reductions in perceived psychosocial stress over time. Our results highlight the potential scientific value of careful and ethical collection of data and biological samples in populations with high levels of trauma exposure. In contexts of war, violence, and forced migration, addressing the root causes of human suffering and the health consequences of toxic stress and trauma exposure is of vital importance. It is also prudent to reach for an in-depth understanding of the interplay between genetic and psychosocial factors that are able to influence the expression of interpersonal and social behaviors over the life course.
Supporting information
See S2 Table for codebook for variable names presented here.
(CSV)
(XLSX)
Acknowledgments
We gratefully acknowledge the Syrian families who participated in this project. We thank Jane Macphail, Director of the Advancing Adolescents programme implemented in Jordan as part of the No Lost Generation initiative in the wider region; Noura Shaed, Natasha Shawarib and Jon Kurtz at Mercy Corps; and the field team affiliated to the organization Taghyeer, especially Dima Hamadmad, Ghufran Abydayyeh, Rahmeh Hyari and Sana’a Bakeer.
Data Availability
All relevant data are reported in the manuscript and its Supporting Information files. All data are also available on Mendeley (https://data.mendeley.com/datasets/) doi:10.17632/xhs8tn7nkz.1.
Funding Statement
Laboratory research was funded by the University of Florida College of Liberal Arts and Sciences (CJM). Field research was funded by Elrha’s Research for Health in Humanitarian Crises (R2HC) Programme (http://www.elrha.org/r2hc/home), which aims to improve health outcomes by strengthening the evidence base for public health interventions in humanitarian crises (CPB). The R2HC programme is funded equally by the Wellcome Trust and the UK Government. Additional funding was received from NSF grant DGE-1315138. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry. 2018;160(8):1453–60. [DOI] [PubMed] [Google Scholar]
- 2.Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, et al. Childhood maltreatment and the structure of common psychiatric disorders. British Journal of Psychiatry. 2012;200(2):107–15. Epub 2018/01/02. 10.1192/bjp.bp.111.093062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott KM, McLaughlin KA, Smith DAR, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: Comparison of prospective and retrospective findings. British Journal of Psychiatry. 2012;200(6):469–75. 10.1192/bjp.bp.111.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens V, Huber-Lang M, Plener PL, Brähler E, Brown RC, Fegert JM. Association of child maltreatment subtypes and long-term physical health in a German representative sample. European Journal of Psychotraumatology. 2018;9(1):1510278 10.1080/20008198.2018.1510278 PMC6136347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MP, Kingree JB, Lamis D. Associations of adverse childhood experiences and suicidal behaviors in adulthood in a U.S. nationally representative sample. Child: Care, Health and Development. 2018;2018(0):1–8. 10.1111/cch.12617 [DOI] [PubMed] [Google Scholar]
- 6.Stepanikova I, Baker E, Oates G, Acharya S, Uddin J, Thon V, et al. Perinatal Maternal Stress and Susceptibility to Infectious Diseases in Later Childhood: An Early Life Programming Perspective. J Psychol. 2018;in press. 10.1080/00223980.2018.1483311 [DOI] [PubMed] [Google Scholar]
- 7.McDade TW, Ryan C, Jones MJ, MacIsaac JL, Morin AM, Meyer JM, et al. Social and physical environments early in development predict DNA methylation of inflammatory genes in young adulthood. Proceedings of the National Academy of Sciences. 2017;114(29):7611–6. 10.1073/pnas.1620661114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obradović J, Boyce WT. Individual Differences in Behavioral, Physiological, and Genetic Sensitivities to Contexts: Implications for Development and Adaptation. Developmental Neuroscience. 2009;31(4):300–8. 10.1159/000216541 [DOI] [PubMed] [Google Scholar]
- 9.SavetheChildren. Invisible wounds: The impact of six years of war on the mental health of Syria’s children. 2018. Available from: https://www.savethechildren.org/content/dam/global/reports/emergency-humanitarian-response/invisible-wounds.pdf. Accessed 13.07.2018. [Google Scholar]
- 10.UNHCR. Syrian regional refugee response in Jordan 2017. Available from: https://data.unhcr.org/syrianrefugees/country.php?id=107. [Google Scholar]
- 11.Masten AS. Global Perspectives on Resilience in Children and Youth. Child Development. 2014;85(1):6–20. 10.1111/cdev.12205 [DOI] [PubMed] [Google Scholar]
- 12.Reed RV, Fazel M, Jones L, Panter-Brick C, Stein A. Mental health of displaced and refugee children resettled in low-income and middle-income countries: risk and protective factors. The Lancet. 2012;379(9812):250–65. 10.1016/S0140-6736(11)60050-0. [DOI] [PubMed] [Google Scholar]
- 13.UNHCR. Global trends: Forced displacement in 2015: Geneva, Switzerland: UNHCR; 2016. [Google Scholar]
- 14.UNHCR. Syria Regional Refugee Response 2018. Available from: https://www.savethechildren.org/content/dam/global/reports/emergency-humanitarian-response/invisible-wounds.pdf. Accessed 13.07.2018. [Google Scholar]
- 15.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–9. [DOI] [PubMed] [Google Scholar]
- 16.Brunner H, Nelen M, Breakefield X, Ropers H, van Oost B. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–80. 10.1126/science.8211186 [DOI] [PubMed] [Google Scholar]
- 17.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of Genotype in the Cycle of Violence in Maltreated Children. Science. 2002;297(5582):851–4. 10.1126/science.1072290 [DOI] [PubMed] [Google Scholar]
- 18.Lee SS. Deviant Peer Affiliation and Antisocial Behavior: Interaction with Monoamine Oxidase A (MAOA) Genotype. Journal of Abnormal Child Psychology. 2011;39(3):321–32. 10.1007/s10802-010-9474-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertins V, Schote AB, Hoffeld W, Griessmair M, Meyer J. Genetic Susceptibility for Individual Cooperation Preferences: The Role of Monoamine Oxidase A Gene (MAOA) in the Voluntary Provision of Public Goods. PLOS ONE. 2011;6(6):e20959 10.1371/journal.pone.0020959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, et al. Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype. PLoS ONE. 2007;2(5):e486 10.1371/journal.pone.0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stetler DA, Davis C, Leavitt K, Schriger I, Benson K, Bhakta S, et al. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. Journal of Psychiatric Research. 2014;58:69–75. 10.1016/j.jpsychires.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, et al. MAOA Alters the Effects of Heavy Drinking and Childhood Physical Abuse on Risk for Severe Impulsive Acts of Violence Among Alcoholic Violent Offenders. Alcoholism: Clinical and Experimental Research. 2010;34(5):853–60. 10.1111/j.1530-0277.2010.01157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolla NJ, Patel R, Meyer JH, Chakravarty MM. Association of monoamine oxidase-A genetic variants and amygdala morphology in violent offenders with antisocial personality disorder and high psychopathic traits. Scientific Reports. 2017;7(1):9607 10.1038/s41598-017-08351-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tielbeek JJ, Medland SE, Benyamin B, Byrne EM, Heath AC, Madden PAF, et al. Unraveling the Genetic Etiology of Adult Antisocial Behavior: A Genome-Wide Association Study. PLOS ONE. 2012;7(10):e45086 10.1371/journal.pone.0045086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrd AL, Manuck SB. MAOA, Childhood Maltreatment, and Antisocial Behavior: Meta-analysis of a Gene-Environment Interaction. Biological Psychiatry. 2014;75(1):9–17. 10.1016/j.biopsych.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt LG, Sander T, Kuhn S, Smolka M, Rommelspacher H, Samochowiec J, et al. Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. Journal of Neural Transmission. 2000;107(6):681–9. 10.1007/s007020070069 [DOI] [PubMed] [Google Scholar]
- 27.Holz N, Boecker R, Buchmann AF, Blomeyer D, Baumeister S, Hohmann S, et al. Evidence for a Sex-Dependent MAOA× Childhood Stress Interaction in the Neural Circuitry of Aggression. Cerebral Cortex. 2016;26(3):904–14. 10.1093/cercor/bhu249 [DOI] [PubMed] [Google Scholar]
- 28.Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008;13:313–24. 10.1038/sj.mp.4002020 https://www.nature.com/articles/4002020#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 29.Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell'Osso L, Virkkunen M, et al. A Non-Additive Interaction of a Functional MAO-A VNTR and Testosterone Predicts Antisocial Behavior. Neuropsychopharmacology. 2007;33:425 10.1038/sj.npp.1301417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary Evidence for Sensitive Periods in the Effect of Childhood Sexual Abuse on Regional Brain Development. The Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20(3):292–301. 10.1176/appi.neuropsych.20.3.292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox SE, Levitt P, Nelson III CA. How the Timing and Quality of Early Experiences Influence the Development of Brain Architecture. Child Development. 2010;81(1):28–40. 10.1111/j.1467-8624.2009.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giedd JN. Structural Magnetic Resonance Imaging of the Adolescent Brain. Annals of the New York Academy of Sciences. 2004;1021(1):77–85. 10.1196/annals.1308.009 [DOI] [PubMed] [Google Scholar]
- 33.Tottenham N, Sheridan M. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3(68). 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enoch M-A, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene‐environment interactions predict behavioral disinhibition in children. Genes, Brain and Behavior. 2010;9(1):65–74. 10.1111/j.1601-183X.2009.00535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGregor NW, Hemmings SMJ, Erdman L, Calmarza-Font I, Stein DJ, Lochner C. Modification of the association between early adversity and obsessive-compulsive disorder by polymorphisms in the MAOA, MAOB and COMT genes. Psychiatry Research. 2016;246:527–32. 10.1016/j.psychres.2016.10.044 [DOI] [PubMed] [Google Scholar]
- 36.Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-Wide and Candidate Gene Association Study of Cigarette Smoking Behaviors. PLOS ONE. 2009;4(2):e4653 10.1371/journal.pone.0004653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong S, Israel S, Xue H, Ebstein RP, Chew SH. Monoamine Oxidase A Gene (MAOA) Associated with Attitude Towards Longshot Risks. PLOS ONE. 2010;4(12):e8516 10.1371/journal.pone.0008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundwall RA, Watkins JK. Genetic Influence on Slope Variability in a Childhood Reflexive Attention Task. PLOS ONE. 2015;10(6):e0130668 10.1371/journal.pone.0130668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman D, Rosser AA. MAOA–Environment Interactions: Results May Vary. Biological Psychiatry. 2014;75(1):2–3. 10.1016/j.biopsych.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinnally EL, Huang Y-y, Haverly R, Burke AK, Galfalvy H, Brent DP, et al. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatric Genetics. 2009;19(3):126–33. 10.1097/YPG.0b013e32832a50a7 00041444-200906000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aro H. Risk and protective factors in depression: a developmental perspective. Acta Psychiatrica Scandinavica. 1994;89(s377):59–64. 10.1111/j.1600-0447.1994.tb05804.x [DOI] [PubMed] [Google Scholar]
- 42.Betancourt TS, Khan KT. The mental health of children affected by armed conflict: Protective processes and pathways to resilience. International Review of Psychiatry. 2008;20(3):317–28. 10.1080/09540260802090363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: Past, present, and future. Development and Psychopathology. 2013;25(4pt2):1359–73. Epub 2013/12/17. 10.1017/S0954579413000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masten AS, Narayan AJ. Child Development in the Context of Disaster, War, and Terrorism: Pathways of Risk and Resilience. Annual Review of Psychology. 2012;63(1):227–57. 10.1146/annurev-psych-120710-100356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masten A, Barnes A. Resilience in Children: Developmental Perspectives. Children. 2018;5(7):98 10.3390/children5070098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan S-SA, Bower JE, Almeida DM, Cole SW, Dahl RE, Irwin MR, et al. Parental support buffers the association of depressive symptoms with cortisol and C-reactive protein during adolescence. Brain, Behavior, and Immunity. 2016;57:134–43. 10.1016/j.bbi.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labella MH, Kalstabbakken A, Johnson J, Leppa J, Robinson N, Masten AS, et al. Promoting Resilience by Improving Children’s Sleep: Feasibility Among Families Living in Supportive Housing. Progress in Community Health Parterships: Research, Education, and Action. 2017;11(3):285–93. 10.1353/cpr.2017.0033 [DOI] [PubMed] [Google Scholar]
- 48.Koni E, Moradi S, Arahanga-Doyle H, Neha T, Hayhurst JG, Boyes M, et al. Promoting resilience in adolescents: A new social identity benefits those who need it most. PLOS ONE. 2019;14(1):e0210521 10.1371/journal.pone.0210521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belsky J, Conger R, Capaldi DM. The intergenerational transmission of parenting: Introduction to the special section. Developmental Psychology. 2009;45(5):1201–4. 10.1037/a0016245 [DOI] [PubMed] [Google Scholar]
- 50.Shaffer A, Burt KB, Obradović J, Herbers JE, Masten AS. Intergenerational continuity in parenting quality: The mediating role of social competence. Developmental Psychology. 2009;45(5):1227–40. 10.1037/a0015361 [DOI] [PubMed] [Google Scholar]
- 51.Pluess M. Vantage Sensitivity: Environmental Sensitivity to Positive Experiences as a Function of Genetic Differences. Journal of Personality. 2017;85(1):38–50. 10.1111/jopy.12218 [DOI] [PubMed] [Google Scholar]
- 52.Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. 2013;139(4):901–16. 10.1037/a0030196 [DOI] [PubMed] [Google Scholar]
- 53.Hartman S, Belsky J. Prenatal stress and enhanced developmental plasticity. Journal of Neural Transmission. 2018. 10.1007/s00702-018-1926-9 [DOI] [PubMed] [Google Scholar]
- 54.Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2007;13:313 10.1038/sj.mp.4002020 https://www.nature.com/articles/4002020#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 55.Panter-Brick C, Hadfield K, Dajani R, Eggerman M, Ager A, Ungar M. Resilience in Context: A Brief and Culturally Grounded Measure for Syrian Refugee and Jordanian Host-Community Adolescents. Child Development. 2017:Epub ahead of print. 10.1111/cdev.12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, R. Hariri A, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences. 2006;103(16):6269–74. 10.1073/pnas.0511311103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dajani R, Hadfield K, van Uum S, Greff M, Panter-Brick C. Hair cortisol concentrations in war-affected adolescents: A prospective intervention trial. Psychoneuroendocrinology. 2018;89:138–46. 10.1016/j.psyneuen.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 58.Panter-Brick C, Kurtz J, Dajani R. What strong partnerships achieve: innovations in research and practice. Humanitarian Exchangee. 2018;72:15–9. [Google Scholar]
- 59.Panter-Brick C, Wiley K, Sancilio A, Dajani R, Hadfield K. C-reactive protein, Epstein-Barr virus, and cortisol trajectories in refugee and non-refugee youth: Links with stress, mental health, and cognitive function during a randomized controlled trial. Brain, Behavior, and Immunity. 2019. 10.1016/j.bbi.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. Journal of cognition and development: official journal of the Cognitive Development Society. 2010;11(2):121–36. 10.1080/15248371003699969 PMC3131138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panter-Brick C, Eggerman M, Gonzalez V, Safdar S. Violence, suffering, and mental health in Afghanistan: a school-based survey. Lancet. 2009;374(9692):807–16. Epub 2009/08/25. 10.1016/S0140-6736(09)61080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ungar M, Liebenberg L. Assessing Resilience Across Cultures Using Mixed Methods: Construction of the Child and Youth Resilience Measure. Journal of Mixed Methods Research. 2011;5(2):126–49. 10.1177/1558689811400607 [DOI] [Google Scholar]
- 63.Almadi T, Cathers I, Hamdan Mansour AM, Chow CM. An Arabic version of the Perceived Stress Scale: Translation and validation study. International Journal of Nursing Studies. 2012;49(1):84–9. 10.1016/j.ijnurstu.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 64.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24(4):385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 65.Ziadni M, Hammoudeh W, Rmeileh NMEA, Hogan D, Shannon H, Giacaman R. Sources of Human Insecurity in Post-War Situations: The Case of Gaza. Journal of human security. 2011;7(3):10.3316/JHS0703023. 10.3316/JHS0703023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahfoud Z, Abdulrahim S, Taha MB, Harpham T, El Hajj T, Makhoul J, et al. Validation of the Arab Youth Mental Health scale as a screening tool for depression/anxiety in Lebanese children. Child and Adolescent Psychiatry and Mental Health. 2011;5:9–. 10.1186/1753-2000-5-9 PMC3070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almaqrami MH, Shuwail AY. Validity of the self-report version of the strengths and difficulties questionnaire in Yemen. Saudi Medical Journal. 2004;25(5):592–601. [PubMed] [Google Scholar]
- 68.Alyahri A, Goodman R. Validation of the Arabic Strengths and Difficulties Questionnaire and the Development and Well-Being Assessment. Eastern Mediterranean Health Journal. 2006;12 Suppl 2:S138–S46. [PubMed] [Google Scholar]
- 69.Goodman A, Goodman R. Strengths and Difficulties Questionnaire as a Dimensional Measure of Child Mental Health. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(4):400–3. 10.1097/CHI.0b013e3181985068. [DOI] [PubMed] [Google Scholar]
- 70.Punamäki R-L, Palosaari E, Diab M, Peltonen K, Qouta SR. Trajectories of posttraumatic stress symptoms (PTSS) after major war among Palestinian children: Trauma, family- and child-related predictors. Journal of Affective Disorders. 2015;172:133–40. 10.1016/j.jad.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 71.Veronese G, Pepe A. Psychometric properties of IES-R, short Arabic version in contexts of military violence. Research on Social Work Practice. 2013;23(710–718). [Google Scholar]
- 72.Muthén BO, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2(4):371–402. 10.1037/1082-989X.2.4.371 [DOI] [Google Scholar]
- 73.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 74.Pinherio J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: Linear and nonlinear mixed effects models. 3.1–137 ed; 2016. [Google Scholar]
- 75.Panter-Brick C, Dajani R, Eggerman M, Hermosilla S, Sancilio A, Ager A. Insecurity, distress and mental health: experimental and randomized controlled trials of a psychosocial intervention for youth affected by the Syrian crisis. J Child Psychol Psychiatry. 2018;59(5):523–41. Epub 2017/10/03. 10.1111/jcpp.12832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 77.Hohenstein S, Kliegl R. remef: Remove partial effects. 1.0.6.9000 ed; 2018. [Google Scholar]
- 78.Wickham H. ggplot2:Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 79.Pluess M, Assary E, Lionetti F, Lester KJ, Krapohl E, Aron EN, et al. Environmental sensitivity in children: Development of the Highly Sensitive Child Scale and identification of sensitivity groups. Developmental Psychology. 2018;54(1):51–70. 10.1037/dev0000406 [DOI] [PubMed] [Google Scholar]
- 80.Belsky J, van Ijzendoorn MH. Genetic differential susceptibility to the effects of parenting. Current Opinion in Psychology. 2017;15:125–30. 10.1016/j.copsyc.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 81.Pluess M, Boniwell I. Sensory-Processing Sensitivity predicts treatment response to a school-based depression prevention program: Evidence of Vantage Sensitivity. Personality and Individual Differences. 2015;82:40–5. 10.1016/j.paid.2015.03.011. [DOI] [Google Scholar]
- 82.Ficks CA, Waldman ID. Candidate Genes for Aggression and Antisocial Behavior: A Meta-analysis of Association Studies of the 5HTTLPR and MAOA-uVNTR. Behavior Genetics. 2014;44(5):427–44. 10.1007/s10519-014-9661-y [DOI] [PubMed] [Google Scholar]
- 83.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene–environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903 10.1038/sj.mp.4001851 [DOI] [PubMed] [Google Scholar]
- 84.Guo G, Ou X-M, Roettger M, Shih JC. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. European Journal Of Human Genetics. 2008;16:626 10.1038/sj.ejhg.5201999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, et al. No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. American Journal of Psychiatry. 2019;176(5):376–87. 10.1176/appi.ajp.2018.18070881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moghames P, McEwen F, Pluess M. Mental health research among Syrian refugees in Lebanon: challenges and solutions. Humanitarian Exchangee [Internet]. 2018; 72:[39–42 pp.]. Available from: https://odihpn.org/magazine/mental-health-research-among-syrian-refugees-in-lebanon-challenges-and-solutions/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See S2 Table for codebook for variable names presented here.
(CSV)
(XLSX)
Data Availability Statement
All relevant data are reported in the manuscript and its Supporting Information files. All data are also available on Mendeley (https://data.mendeley.com/datasets/) doi:10.17632/xhs8tn7nkz.1.