Abstract
Purpose
To determine whether signs and symptoms of ocular surface disease improve after placement of a self-retained, cryopreserved amniotic membrane (CAM) in patients with Sjögren syndrome (SS).
Methods
The medical records of SS patients who received a self-retained CAM implant (Prokera or Prokera Slim; TissueTech Inc, Doral, FL) for the treatment of ocular surface disease between August 2012 and August 2016 at a single, large academic institution were reviewed retrospectively. Visual acuity, results of slit-lamp examination of the cornea and conjunctiva, and dry eye symptoms, were evaluated before and after CAM insertion.
Results
A total of 6 eyes of 6 patients (all female; mean age, 62.5 ± 13.0 years [range, 49–86 years]) were included. All patients were on topical medications at the time of the study and had signs of ocular surface dryness. There were reductions in corneal and/or conjunctival staining in 5 eyes (83%) after the CAM dissolved. All patients who completed therapy (5/5) experienced a relapse in their signs and symptoms within 1 month of removal of the CAM, with an average time to relapse of 24.6 days. Mean follow-up time was 54.5 days. Foreign body sensation and blurred vision were the most common complaints associated with the CAM implant.
Conclusions
In this small case series, self-retained CAM implantation was found to be beneficial in SS patients with ocular surface disease that is refractory to standard therapies; however, we found that the effects were temporary. Future larger studies are needed to confirm these benefits.
Human amniotic membrane is the innermost layer of the placenta that encases the developing fetus in the amniotic fluid. Cryopreserved amniotic membrane (CAM) has been used as a temporary biologic bandage for the cornea to restore the health of the ocular surface in a wide range of disorders.1 The success of amniotic membrane for the treatment of ocular surface disease has been attributed to its anti-inflammatory, antifibrotic, and antiangiogenic properties in addition to its ability to enhance epithelial healing.1–3
Initially, grafting of amniotic membranes was performed exclusively using sutures, but the emergence of sutureless, self-retaining CAM has facilitated the use of amniotic membrane for the treatment of ocular surface disease in the office setting. Prokera (or Prokera Slim; TissueTech Inc, Doral, FL), approved by the Federal Drug Administration, is clipped to a polycarbonate ring system. This sutureless amniotic membrane is easily applied in a clinical setting—thereby eliminating surgical time and reducing cost—and has the added benefit of eliminating postoperative discomfort, irritation, and suture-related complications.4
Prokera has been used to treat a variety of ocular surface diseases, including Stevens-Johnson syndrome, toxic epidermal necrolysis, neuropathic corneal pain, dry eye disease (DED), neurotrophic keratopathy, and recurrent corneal erosions.1,4–6 A recent study evaluated the effect of CAM in dry eye patients.7 To our knowledge, there have not been any reports regarding its utility in managing patients with Sjögren syndrome (SS), a chronic and potentially life-threatening autoimmune disorder that causes irreversible damage to the lacrimal and salivary glands, resulting in a loss of tear and saliva production. In SS, the tear film becomes insufficient, leading to ocular surface inflammation and DED. The ocular surface symptoms associated with SS can be severe and have been shown to significantly impair quality of life.8 Treatment of DED in SS is aimed at symptomatic relief and interrupting the inflammatory cascade to prevent damage to the ocular surface. Current therapies include artificial tears, punctal occlusion, topical anti-inflammatory eye drops, bandage contact lenses, scleral contact lenses, autologous serum drops, and oral secretagogues, all of which have been shown to help control symptoms and improve the signs of ocular dryness.9–10 Additional therapies include the treatment of concomitant meibomian gland dysfunction with topical or oral antibiotics, warm compresses, or procedures such as intense pulsed light or thermal pulsation.10 However, in a subset of SS patients, dryness of the ocular surface can be refractory to treatment, ultimately resulting in significant complications, including corneal abrasions, infection, ulceration, scarring, and perforation.11 As a result, additional treatments are needed. The purpose of this study was to investigate whether Prokera or Prokera Slim was a well-tolerated and effective treatment in SS patients with ocular surface disease refractory to standard therapy.
Subjects and Methods
The study was approved by the Institutional Review Board at the University of Pennsylvania and adhered to the tenets of the Declaration of Helsinki. The medical records of patients with SS who underwent Prokera placement at the Scheie Eye Institute from August 2012 to August 2016 were reviewed retrospectively. Patients with SS had previously been diagnosed using the American-European Consensus Group (AECG) criteria or the American College of Rheumatology (ACR)/Sjögren’s International Collaborative Clinical Alliance (SICCA) criteria.12–13 Prokera insertion was performed in the office by one of two clinicians (SEO, MMG) at the Scheie Eye Institute. The indication for placement was ocular surface disease that was refractory to standard treatment.
Symptoms were evaluated subjectively at each visit, when patients were asked to describe their ocular discomfort. Visual acuity was assessed at each visit using a Snellen Chart. Ocular surface staining was evaluated using the NEI grading scale with fluorescein (SEO) or the Oxford scale with fluorescein and lissamine green (MMG). The eye with worse signs and symptoms was selected as the treatment eye.
The timing of placement was at the discretion of the treating physician. Informed written consent for retention of the Prokera was obtained from all patients. Each CAM was inserted under topical anesthesia with 0.5% proparacaine hydrochloride eye drops and placed ensuring that the amniotic membrane covered the cornea and most of the bulbar conjunctiva. Tolerance to CAM insertion was assessed at the same visit by asking the patient whether they experienced any pain or foreign body sensation once the topical anesthesia had worn off. Patients returned to the office for removal of the ring once the CAM had dissolved and for subsequent follow-up.
At each visit, patient symptoms based on subjective report were recorded and slit-lamp examination of the ocular surface was performed. Visual acuity was also measured. Data was collected from the medical record regarding patient demographics, CAM retention time, mean follow-up time, time to recurrence of symptoms, and changes in symptoms and signs. Relapse was defined as subjectively worsening symptoms of dry eye disease or objectively worsening corneal or conjunctival staining.
Visual acuity was converted to logMAR for analysis. Statistical significance with regard to change in visual acuity, corneal and conjunctival staining was evaluated using a paired t test. A P value of <0.05 was considered significant.
Results
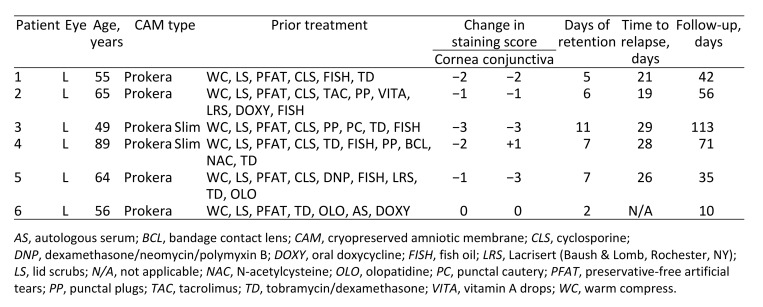
A total of 6 patients met inclusion criteria; all were female. Mean age was 62.5 ± 13.0 years (range, 49–86 years). Coincidentally, all 6 eyes chosen for CAM placement were left eyes, because this was the worse eye for each patient. No patients underwent bilateral CAM placement, and none had CAM placed more than once during the study period. Preintervention mean visual acuity was 20/25; there was no significant change after CAM removal (P = 0.32). Relevant clinical data are summarized in Table 1. All patients were on topical medications at the time of the study with 4 patients (66.7%) using topical cyclosporine 0.05% twice daily and 3 (50%) using dexamethasone 0.1% twice daily. All patients had previously failed conservative treatment with artificial tears, warm compresses, and eyelid scrubs. Two patients (33%) had previously taken doxycycline 100 mg twice daily for 1 month and had minimal benefit.
Table 1.
Demographic information, prior therapy, retention times, and follow-up in Sjögren syndrome patients who underwent insertion of self-retained cryopreserved amniotic membrane
Of the 6 patients, 4 were symptomatic from DED prior to insertion of CAM and complained of irritation, itchiness, dryness, or grittiness. The duration of CAM placement ranged from 2 to 11 days (mean, 6.3 days). After removal of the ring, all symptomatic patients (4/4) reported improvement in symptoms. Furthermore, after the membrane dissolved, there was improvement in slit-lamp examination findings of corneal and conjunctival staining in 83% of patients (5/6). Corneal staining scores significantly improved from a mean of 1.83 points to a mean of 0.33 points (average reduction, 1.5 points [P = 0.0172]) at first visit after the membrane dissolved. Conjunctival staining scores improved from a mean of 1.83 points to 0.5 points (average of 1.33-point reduction [P = 0.1019]) at the first visit after the membrane dissolved. There was no change in staining scores of the contralateral eye during the study period with an average of 1.50 points for both corneal and conjunctival staining before and after treatment (P = 0.50).
Foreign body sensation and blurred vision were reported by 3 patients with insertion of the CAM. The ring was removed in 1 patient after 48 hours because of foreign body sensation and mucopurulent discharge; the patient was lost to follow-up after 10 days. This patient experienced increased photophobia and foreign body sensation after removal of the CAM but had a stable score of 2 points for corneal and conjunctival staining before and after treatment.
All patients who completed therapy (5/5) experienced a relapse in their signs and symptoms within 1 month of removal of the CAM, with an average time to relapse of 24.6 days (range, 19–29). Mean follow-up time was 54.5 days (range, 10–113).
Discussion
In this case series, we found that the use of self-retained CAM in SS patients refractory to standard therapy was well tolerated and improved the health of the ocular surface, leading to decreased signs or symptoms of ocular dryness in all patients. Similarly, in a prior study, multilayer sutured amniotic membrane transplantation was shown to be beneficial in patients with SS and nontraumatic corneal perforations or deep corneal ulcers with a descemetocele.5
Recently, Cheng and colleagues11 reported success using CAM in a patient with immune-mediated DED in the setting of rheumatoid arthritis. It is likely that Prokera is helpful in treating ocular surface disease in SS patients because of the anti-inflammatory properties of amniotic membrane.1–3,14 Inflammation has been shown to play a central role in causing ocular surface damage found in patients with SS.3 For example, patients with SS have been found to have increased tear film concentration of matrix metalloproteinase 9 (MMP-9), a molecule related to the inflammatory process responsible for increased corneal epithelial desquamation, corneal surface irregularity, and ocular surface damage.15
The anti-inflammatory properties of amniotic membrane have been demonstrated in other disease models of corneal inflammation. For example, amniotic membrane has been shown to cause decreased expression of MMP-9 in a mouse model of herpes simplex viral keratitis.16 In vivo studies have shown that heavy chain–hyaluronic acid (HC-HA)/PTX3 purified from human amniotic membrane suppresses inflammatory responses by modulating both the innate and adaptive immune responses.17 Therefore, it is possible that in SS patients CAM decreases inflammatory mediators such as MMP-9 and suppresses the local immune response, thereby decreasing inflammation on the ocular surface.
There are conflicting reports regarding the duration of treatment effects for the use of CAM in treating the ocular surface.3,4,7 The Dry Eye Amniotic Membrane (DREAM) Study recently evaluated the effect of CAM on patients with various types of dry eye disease and determined that CAM was effective for moderate-to-severe dry eye disease. In that study, the majority of patients experienced a reduction in dry eye workshop scores for up to 3 months.7 However, in contrast, Suri and colleagues found a shorter treatment effect with approximately 18% of eyes with neurotrophic keratopathy (NK) experiencing a recurrence within 15 days.4 All of our patients began to experience recurrence of both signs and symptoms of dry eye disease within 1 month of CAM removal. The differences in treatment duration effects may be due to the various underlying pathophysiology of each ocular surface disease. For example, SS involves inflammation and infiltration of the lacrimal glands, whereas other types of DED, such as NK, originate from the ocular surface. Because amniotic membranes are placed directly onto the cornea, they may have less of an effect on the lacrimal glands. Further studies are needed to determine whether repeated use of Prokera could generate a more lasting effect in SS patients.
Our study was limited by its retrospective design, small number of patients, and relatively short follow-up. A standardized symptom grading scale was not administered at each visit, which limited the ability to quantify symptom improvement. Future prospective studies using a standardized questionnaire, such as the Ocular Surface Disease Index, at each visit would be helpful.
To our knowledge, this is the first case series to evaluate the use of Prokera in SS. Future larger prospective studies that include a control group would be helpful in evaluating the efficacy of the use of CAM in SS patients. Nevertheless, our results suggest that self-retained CAM may be a viable therapeutic option in patients with SS who have ocular surface disease refractory to standard DED therapies.
Literature Search
PubMed was searched on January 24, 2019, without date restriction, for English-language results, using the following terms singly and in combination: Sjögren syndrome AND Prokera AND dry eye.
References
- 1.Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009;54:686–96. doi: 10.1016/j.survophthal.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem. 2014;289:13531–42. doi: 10.1074/jbc.M113.525287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AMS, Zhao D, Chen R, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14:56–63. doi: 10.1016/j.jtos.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39:341–347. doi: 10.1097/ICL.0b013e3182a2f8fa. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AMS, Tighe S, Sheha H, Tseng SCG. Adjunctive role of self-retained cryopreserved amniotic membrane in treating immune-related dry eye disease. Int Ophthalmol. 2018;38:2219–22. doi: 10.1007/s10792-017-0708-y. [DOI] [PubMed] [Google Scholar]
- 6.Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16:132–8. doi: 10.1016/j.jtos.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677–81. doi: 10.2147/OPTH.S162203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal B, Bowman SJ, Fox PC, et al. Primary Sjögren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9:240–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Pfister RR, Murphy GE. Corneal ulceration and perforation associated with Sjögren’s syndrome. Arch Ophthalmol. 1980;98:89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- 12.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiboski SC, Shiboski CH, Criswell L, et al. Sjögren’s International Collaborative Clinical Alliance (SICCA) Research Groups American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–34. [PubMed] [Google Scholar]
- 15.Aragona P, Aguennouz M, Rania L, et al. Matric metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology. 2015;122:62–71. doi: 10.1016/j.ophtha.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Heiligenhaus A, Li HF, Yang Y, Wasmuth S, Steuhl KP, Bauer D. Transplantation of amniotic membrane in murine herpes stromal keratitis modulates matrix metalloproteinases in the cornea. Invest Ophthalmol Vis Sci. 2005;46:4079–85. doi: 10.1167/iovs.05-0192. [DOI] [PubMed] [Google Scholar]
- 17.He H, Tan Y, Duffort S, Perez VL, Tseng SC. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthalmol Vis Sci. 2014;55:1647–56. doi: 10.1167/iovs.13-13094. [DOI] [PMC free article] [PubMed] [Google Scholar]