Abstract
Background:
Knee osteoarthritis risk is high after anterior cruciate ligament reconstruction (ACLR) and arthroscopic meniscal surgery, and higher among individuals who undergo both. Although osteoarthritis development is multifactorial, altered walking mechanics may influence osteoarthritis progression. The purpose of this study was to compare gait mechanics after ACLR among participants who had undergone no medial meniscal surgery, partial medial meniscectomy, or medial meniscal repair.
Methods:
This was a secondary analysis of data collected prospectively as part of a clinical trial. Sixty-one athletes (mean age of 21.4 ± 8.2 years) who had undergone primary ACLR participated in the study when they achieved impairment resolution (5.3 ± 1.7 months postoperatively), including minimal to no effusion, full knee range of motion, and ≥80% quadriceps-strength symmetry. Participants were classified by concomitant medial meniscal treatment: no involvement or nonsurgical management of a small, stable tear; partial meniscectomy; or meniscal repair. Participants underwent comprehensive walking analyses. Joint contact forces were estimated using a previously validated, electromyography-driven musculoskeletal model. Variables were analyzed using a mixed-model analysis of variance with group and limb comparisons (α = 0.05); group comparisons of interlimb differences in measurements (surgical minus contralateral limb) were performed to determine significant interactions.
Results:
The participants in the partial meniscectomy group walked with a higher peak knee adduction moment (pKAM) in the surgical versus the contralateral limb as compared with those in the meniscal repair group and those with no medial meniscal surgery (group difference for partial versus repair: 0.10 N-m/kg-m, p = 0.020; and for partial versus none: 0.06 N-m/kg-m, p = 0.037). Participants in the repair group walked with a smaller percentage of medial to total tibiofemoral loading in the surgical limb compared with both of the other groups (group difference for repair versus partial: −12%, p = 0.001; and for repair versus none: −7%, p = 0.011). The participants in the repair group loaded the medial compartment of the surgical versus the contralateral limb 0.5 times body weight less than did the participants in the partial meniscectomy group.
Conclusions:
Participants in the partial meniscectomy group walked with higher pKAM and shifted loading toward the medial compartment of the surgical limb, while participants in the repair group did the opposite, walking with lower pKAM and unloading the surgical limb relative to the contralateral limb. These findings may partially explain the conflicting evidence regarding pKAM after ACLR and the elevated risk for osteoarthritis (whether from overloading or underloading) after ACLR with concomitant medial meniscectomy or repair.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
An estimated 250,000 anterior cruciate ligament (ACL) ruptures occur annually within the United States1. ACL ruptures are traumatic injuries, and thus, other knee structures are often involved2. The co-occurrence of meniscal injuries with ACL rupture is especially common2-6, with a rate of >61% among individuals undergoing primary ACL reconstruction (ACLR)2. During or after ACLR, concomitant meniscal tears may be treated nonsurgically or surgically via partial meniscectomy or meniscal repair. Regardless of how the meniscus is treated, however, the risk for developing knee osteoarthritis is elevated greatly after ACLR with concomitant meniscal tear compared with ACLR when both the lateral and medial menisci are intact4,5,7-10. Knee osteoarthritis may be especially common in the medial tibiofemoral compartment11,12. Therefore, the investigation of factors associated with its development and progression is a critical step to improving treatment options and rehabilitation strategies for this debilitating disease.
While the development and progression of knee osteoarthritis is multifactorial, alterations in walking gait mechanics are associated with the early development of osteoarthritis after ACLR13-15 and with knee osteoarthritis progression and severity16-19. Several biomechanical variables may be of particular interest when discussing medial tibiofemoral osteoarthritis development. Loading of the medial compartment of the tibiofemoral joint is likely of chief importance because it encompasses all factors contributing to compressive joint loading; moreover, medial compartment unloading during walking 6 months after ACLR has been associated with radiographic evidence of knee osteoarthritis 5 years postoperatively14. Evaluating the proportion of medial compartment to total tibiofemoral joint loading is also important because it shows the degree to which joint loading is concentrated in the medial compartment versus distributed across the medial and lateral compartments. Directly measuring medial compartment or total tibiofemoral loading, however, is not feasible, and thus, musculoskeletal modeling approaches are necessary to estimate joint loading. Many studies are limited to using kinetic variables as surrogates for joint loading, in part because of the complexity of musculoskeletal modeling. While both sagittal and coronal-plane kinetics contribute to joint loading20, the knee adduction moment is likely the most widely reported kinetic variable implicated in the development of knee osteoarthritis.
Altered walking patterns are often present in individuals after isolated ACLR18,21-30 or isolated arthroscopic partial meniscectomy31,32. After isolated ACLR, smaller sagittal-plane knee angles, excursions, and moments are demonstrated during walking22,25,26,28-30. Alterations in coronal-plane knee gait mechanics after ACLR, however, have been less consistently reported. Among individuals who have undergone ACLR, previous studies typically have found similar33-35 or smaller34,36-38 peak knee adduction moments (pKAMs) in the surgical limb compared with the uninvolved (contralateral) limb or control limbs, but conflicting evidence exists18. In contrast, after isolated arthroscopic partial meniscectomy, the pKAM and impulse were seen to increase from preoperatively to 12 months after surgery31. These findings suggest that meniscectomy may lead to an opposite pattern of coronal-plane kinetics and joint loading compared with what is more commonly found after ACLR.
While studies investigating the effect of ACLR on gait mechanics may include individuals with meniscal pathology33, the effect of concomitant meniscal tear and surgical intervention involving the meniscus has not been thoroughly investigated. The purpose of the current study was to compare knee mechanics and joint loading during level walking among participants who had undergone ACLR and had had no medial meniscal surgery (minimal to no tear), had partial medial meniscectomy, or had medial meniscal repair. We hypothesized that there would be differences in coronal-plane gait mechanics and medial tibiofemoral compartment loading according to medial meniscal treatment among participants after ACLR.
Materials and Methods
Participants
This was a secondary analysis of data collected prospectively as part of a clinical trial (ClinicalTrials.gov identifier: NCT01773317). Institutional review board approval was obtained, and all participants provided informed consent prior to study enrollment.
Data were collected at the University of Delaware between October 2011 and December 2016. Sixty-one athletes (mean age [and standard deviation] of 21.4 ± 8.2 years) who had undergone primary ACLR participated in the study after physical therapy and impairment resolution (5.3 ± 1.7 months postoperatively). Impairment resolution was operationally defined as minimal to no effusion39, full and symmetrical knee range of motion, a quadriceps strength index of at least 80%, and the initiation of a running progression40-43. Individuals were excluded if (1) they did not participate regularly (>50 hours/year) in Level-I or II sports (i.e., sports involving jumping, cutting, and/or pivoting, such as basketball, football, baseball, or racket sports)44,45, (2) the duration of time since undergoing ACLR was <3 months or >10 months, (3) they had previously undergone ACLR and/or had a history of serious lower-extremity injury to either limb, or (4) the knee had an osteochondral defect of >1 cm2. Participants were classified according to concomitant medial meniscal pathology and intervention, on the basis of operative reports. The 3 mutually exclusive categories were no involvement or nonsurgical management of a small, stable tear (“none”; n = 37); partial meniscectomy (“partial”; n = 12); or meniscal repair (“repair”; n = 12).
Motion Analysis Testing
Participants underwent motion analysis during over-ground walking at a self-selected speed maintained to ±5% across trials. Kinematic data were captured at 120 Hz using an 8-camera motion-capture system (VICON; Oxford Metrics) and 39 retroreflective markers and shells affixed to the lower extremities bilaterally. Kinetic data were captured at 1,080 Hz using an embedded force platform (Bertec); joint moments were calculated via inverse dynamics using commercial software (Visual3D; C-Motion). Surface electromyography (EMG; Motion Lab Systems) was also performed bilaterally at 1,080 Hz at 7 muscle sites per limb that cross the knee joint: the medial and lateral aspects of the gastrocnemius, the medial and lateral sides of the hamstring, the vastus medialis, the vastus lateralis, and the rectus femoris. Skin preparation, electrode placement, and filtering were performed as previously described46. EMG signals were normalized to maximum values obtained during maximum volitional isometric contractions or dynamic trials (whichever was greater).
Musculoskeletal Modeling
Joint contact forces were estimated using a previously validated, patient-specific musculoskeletal model47,48. Anthropometric measurements were used to scale the model individually for each subject. Five walking trials per limb were used for musculoskeletal modeling. Muscle parameters were adjusted within physiological norms via simulated annealing to match the EMG-driven sagittal-plane knee moment to the sagittal knee moment derived from inverse dynamics. Using these tunings, 3 predicted trials per limb were selected by minimizing the root mean squared error and maximizing the r2 values of the 2 sagittal knee moment curves. A frontal-plane-moment balancing algorithm was used subsequently to estimate the distribution of tibiofemoral loading to the medial and lateral compartments49.
Quadriceps Strength Index
Quadriceps femoris strength was assessed for both lower extremities of each participant. Participants were seated securely in the chair of an electromechanical dynamometer (Biodex Medical Systems), with their knees flexed to 90°. Testing was performed isometrically using an electrical burst superimposition technique50,51. The contralateral limb was tested first, followed by the surgical (ACLR) limb; approximately 3 trials per limb were recorded. The highest volitionally achieved values for each limb were used to calculate the quadriceps strength index (QI = ACLR/contralateral × 100%).
Variables of Interest
Primary variables of interest included the peak knee adduction moment (pKAM) and peak medial compartment contact force (pMCCF). Secondary variables of interest were the peak knee flexion angle (pKFA) and moment (pKFM), the peak knee adduction angle (pKAA), and the percentage of medial to total joint contact force at the pKFA (medial to total loading). (Medial to total loading comparisons were made at the pKFA because of its general temporal coincidence with peak tibiofemoral joint loading and to standardize across participants46,52.) Moments were normalized by body mass × height, while joint contact forces were normalized by body weight, to allow for comparisons among participants53. Gait speed and the quadriceps strength index were also compared across the groups.
Statistical Analyses
Statistical analyses were conducted using SPSS (version 24.0; IBM). Demographic characteristics were analyzed using 1-way analysis of variance (ANOVA) and chi-square tests of proportions. Peak variables during gait were analyzed using a 3 × 2 mixed-model ANOVA with group (none versus partial versus repair) and limb (surgical versus contralateral) comparisons (α = 0.05). Post-hoc t tests were conducted using the least-significant-difference method; between-group comparisons of interlimb differences in measurements (surgical minus contralateral limb) were performed, with 95% confidence intervals (CIs) and effect sizes (Cohen d value)54 calculated, to identify significant interactions.
Results
There were no differences among the groups with respect to demographic characteristics, with the exception of body mass index (BMI) (Table I). BMI was higher in both the partial meniscectomy group (post-hoc p = 0.049) and the meniscal repair group (post-hoc p = 0.031) compared with the group with no involvement or nonsurgical management of a small, stable tear (“none”). However, BMI did not differ between the partial and repair groups (post-hoc p = 0.872). The quadriceps strength index and gait speed did not differ among the 3 groups. The vast majority of subjects in each group participated in Level-I sports prior to injury, and pre-injury sports level (Level I versus II) was similar across the groups.
TABLE I.
Demographic Characteristics, Quadriceps Strength Index, and Gait Speed
| Group by Medial Meniscal Treatment* | ||||
| Variable | None (N = 37) | Partial (N = 12) | Repair (N = 12) | P Value |
| Sex† | 0.200 | |||
| Female | 19 | 7 | 3 | |
| Male | 18 | 5 | 9 | |
| Age‡ (yr) | 21.0 ± 7.9 | 23.7 ± 11.4 | 20.3 ± 4.8 | 0.539 |
| BMI‡ (kg/m2) | 24.9 ± 3.1 | 27.1 ± 3.5 | 27.4 ± 3.5 | 0.034 |
| Pre-injury sport level† | 0.675 | |||
| Level I | 34 | 10 | 11 | |
| Level II | 3 | 2 | 1 | |
| Graft type† | 0.780 | |||
| Allograft | 9 | 4 | 2 | |
| BPTB | 8 | 1 | 3 | |
| Hamstring autograft | 20 | 7 | 7 | |
| Lateral meniscal treatment*† | 0.221 | |||
| None | 20 | 3 | 8 | |
| Partial | 12 | 7 | 4 | |
| Repair | 5 | 2 | 0 | |
| No. of weeks after ACLR‡ | 24.0 ± 8.1 | 22.5 ± 5.0 | 19.0 ± 4.8 | 0.114 |
| Quadriceps strength index‡ (%) | 91.9 ± 9.6 | 93.4 ± 8.9 | 90.9 ± 6.7 | 0.799 |
| Gait speed‡ (m/s) | 1.54 ± 0.12 | 1.50 ± 0.14 | 1.56 ± 0.07 | 0.542 |
None = no involvement or nonsurgical management of a small, stable tear; partial = partial meniscectomy; and repair = meniscal repair.
The values are given as the number of participants. Level-I sports involve jumping, pivoting, and hard cutting (e.g., basketball, football, soccer), and Level-II sports involve lateral motion but less jumping or hard cutting than Level-I sports (e.g., baseball/softball, racket sports, skiing). BPTB = bone-patellar tendon-bone autograft.
The values are given as the mean and the standard deviation. Body mass index (BMI) was higher in both the partial (post-hoc p = 0.049) and repair (post-hoc p = 0.031) groups compared with none but did not differ between the partial and repair groups (post-hoc p = 0.872). ACLR = anterior cruciate ligament reconstruction.
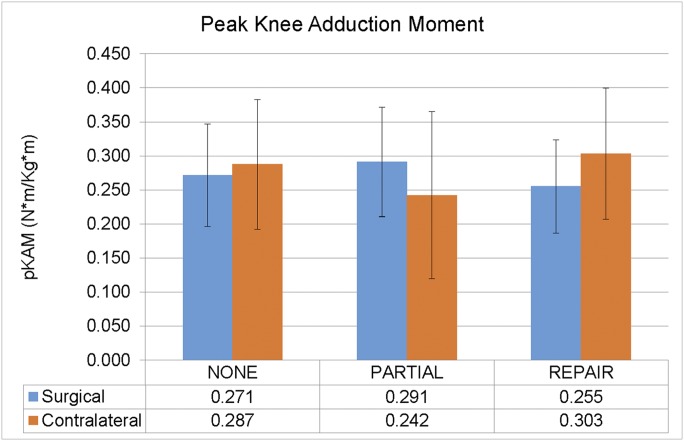
There were 3 biomechanical variables that demonstrated significant interaction effects between group (partial versus repair versus none) and limb (surgical versus contralateral). There was a group-by-limb interaction effect (p = 0.010) for the peak knee adduction moment, pKAM, characterized by differing responses between the partial meniscectomy and meniscal repair groups with respect to the surgical (i.e., ACLR) versus contralateral (i.e., uninvolved) limb (Fig. 1). We found that the pKAM during walking was significantly higher in the surgical versus the contralateral limb in the partial group compared with both the repair group and those with no medial meniscal surgery (Table II). In contrast, the pKAM during walking tended to be relatively lower in the surgical versus the contralateral limb in the group that underwent repair compared with no medial meniscal surgery.
Fig. 1.
An interaction effect was found for the peak knee adduction moment (pKAM) (p = 0.010). Note that pKAM during walking for the partial medial meniscectomy group was greater in the surgical (ACLR) versus the contralateral limb, while pKAM was lesser during walking for the surgical versus the contralateral limb in the medial meniscal repair group. Among those who did not undergo medial meniscal surgery (“none”), pKAM during walking was similar between the 2 limbs. The error bars indicate 1 standard deviation above and below the mean.
TABLE II.
Group Comparisons of Interlimb Differences (Surgical Minus Contralateral Limb) for Peak Knee Adduction Moment
| pKAM |
|||
| Group Comparison* | Difference (95% CI)† (N-m/kg-m) | Cohen D Value | P Value |
| Partial vs. repair | 0.10 (0.02 to 0.18) | 1.03 | 0.020‡ |
| Partial vs. none | 0.06 (0.00 to 0.13) | 0.71 | 0.037‡ |
| Repair vs. none | −0.03 (−0.09 to 0.02) | 0.38 | 0.262 |
Partial = partial medial meniscectomy, repair = medial meniscal repair, and none = no medial meniscal surgery.
CI = confidence interval. Positive values indicate that the group listed first walked with greater surgical versus contralateral limb peak knee adduction moment (pKAM) compared with the second group; negative values indicate lesser relative pKAM.
Significant (p < 0.05).
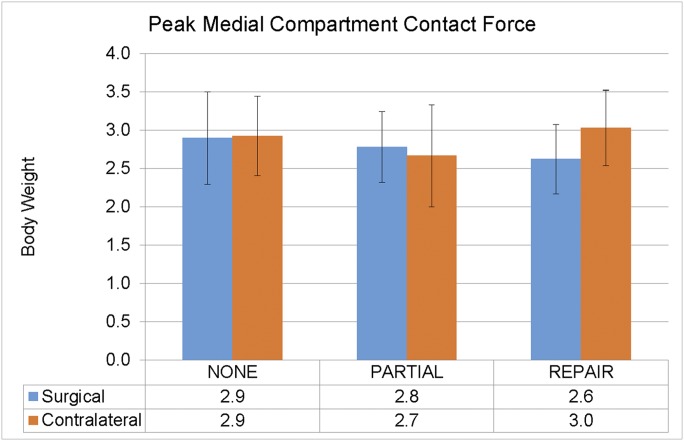
We found no interaction effect (p = 0.112) or main effect of the limb (p = 0.259) for peak medial compartment contact force, pMCCF, but participants in the repair group walked with meaningful55 underloading in the surgical limb (Fig. 2). There was also a pronounced difference between groups (Cohen d = 0.99) in interlimb measurements for pMCCF loading: participants in the repair group loaded the medial compartment in the surgical versus the contralateral limb 0.5 times body weight (95% CI, 0.1 to 1.0 times body weight) less than did participants in the partial group.
Fig. 2.
A pronounced group difference (Cohen d = 0.99) in interlimb loading was found: participants in the meniscal repair group loaded the medial compartment in the surgical (ACLR) versus contralateral limb 0.5 times body weight (95% CI, 0.1 to 1.0 times body weight) less than did those in the partial meniscectomy group. None = no medial meniscal surgery. The error bars indicate 1 standard deviation above and below the mean.
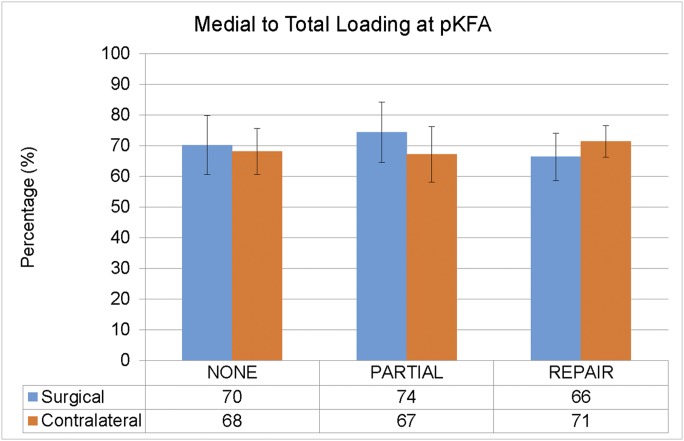
We found a group-by-limb interaction effect (p = 0.025) for the percentage of medial to total joint contact force at the peak knee flexion angle, pKFA (medial to total loading). Similar to our findings for the pKAM, participants in the repair group walked with a relatively lesser amount of medial to total loading in the surgical versus the contralateral limb (Fig. 3). In contrast, participants in the partial group walked with a relatively greater amount of medial to total loading in the surgical versus the contralateral limb. Those in the repair group shifted loading away from the medial compartment of the surgical limb compared with the partial group and those who did not undergo medial meniscal surgery (Table III). The participants in the partial group tended to walk with relatively more loading distributed to the surgical limb medial compartment compared with those with no medial meniscal surgery.
Fig. 3.
An interaction effect was found for the percentage of medial to total loading at the peak knee flexion angle (pKFA) (p = 0.025). Note the relative shifts toward and away from the surgical (ACLR) limb medial compartment in the partial and repair groups, respectively. None = no medial meniscal surgery. The error bars indicate 1 standard deviation above and below the mean.
TABLE III.
Group Comparisons of Interlimb Differences (Surgical Minus Contralateral Limb) for Medial to Total Loading*
| Medial to Total Loading |
|||
| Group Comparison* | Difference (95% CI)† | Cohen D Value | P Value |
| Partial vs. repair | 12% (5% to 19%) | 1.50 | 0.001‡ |
| Partial vs. none | 5% (−1% to 11%) | 0.58 | 0.089 |
| Repair vs. none | −7% (−13% to −2%) | 0.88 | 0.011‡ |
Partial = partial medial meniscectomy, repair = medial meniscal repair, and none = no medial meniscal surgery.
CI = confidence interval. Positive values indicate that the group listed first walked with greater surgical versus contralateral limb medial to total loading compared with the second group; negative values indicate lesser relative medial to total loading.
Significant (p < 0.05).
We also found a group-by-limb interaction effect (p = 0.023) for the peak knee adduction angle, pKAA (Table IV). The pKAA during walking was relatively greater in the surgical versus the contralateral limb for participants in the partial group compared with those who did not undergo medial meniscal surgery (p = 0.041). There were, however, no significant differences between the repair group and either of the other groups.
TABLE IV.
Group Comparisons of Interlimb Differences (Surgical Minus Contralateral Limb) for Peak Knee Adduction Angle
| pKAA | |||
| Group Comparison* | Difference (95% CI)† | Cohen D Value | P Value |
| Partial vs. repair | 1.8° (−2.0° to 5.6°) | 0.40 | 0.334 |
| Partial vs. none | 2.4° (0.1° to 4.7°) | 0.70 | 0.041‡ |
| Repair vs. none | −0.6° (−2.9° to 1.6°) | 0.18 | 0.582 |
Partial = partial medial meniscectomy, repair = medial meniscal repair, and none = no medial meniscal surgery.
CI = confidence interval. Positive values indicate that the group listed first walked with greater surgical versus contralateral limb peak knee adduction angle (pKAA) compared with the second group; negative values indicate lesser relative pKAA, or more abduction.
Significant (p < 0.05).
There were main effects of the limb for both the peak knee flexion angle, pKFA (p < 0.001), and the peak knee flexion moment, pKFM (p < 0.001); however, these differences were moderated by the quadriceps strength index (controlling for QI, main effect of limb p = 0.637 and p = 0.794, respectively). Pooling across groups, participants walked with a smaller pKFA (mean interlimb difference [95% CI], −2.3° [−4.2° to −0.4°], Cohen d = 0.43) and pKFM (−0.09 [−0.14 to −0.04] N-m/kg-m, d = 0.65) in the surgical versus the contralateral limb.
Discussion
The purpose of this study was to determine if meniscal treatment influences walking mechanics after ACLR. Our findings suggest that coronal-plane gait mechanics and tibiofemoral joint loading patterns differ among patients who undergo ACLR plus medial meniscal repair compared with ACLR plus partial medial meniscectomy. Our hypothesis, that there would be different loading patterns according to meniscal treatment, was supported: the repair group walked with a smaller peak knee adduction moment, pKAM, and shifted loading away from the medial compartment in the surgical versus the contralateral limb, while the partial group walked with a higher pKAM and shifted loading toward the medial compartment in the surgical limb. In contrast, the group with no medial meniscal surgery walked with relatively symmetrical medial compartment loading profiles.
The distinct gait strategies of the participants in the partial and repair groups may help explain their elevated risk for posttraumatic osteoarthritis4,5,7-10, although for different reasons: overloading versus underloading14-16,56. While overloading has traditionally been associated with osteoarthritis (as may be the case for the partial participants), underloading the medial compartment (as is the case with repair participants) after ACLR has been associated with future osteoarthritis development14. Wellsandt and colleagues found lower surgical limb loading in the medial tibiofemoral compartment 6 months after ACLR among participants who subsequently developed radiographic evidence of osteoarthritis 5 years after ACLR14. Similarly, Pietrosimone et al. found that lesser biomechanical loading of the surgical versus the contralateral limb 6 months after ACLR was associated with higher levels of biochemical markers indicative of harmful joint metabolism15. Therefore, patients after ACLR with concomitant partial medial meniscectomy or medial meniscal repair may each benefit from different, targeted interventions to restore symmetry in the medial tibiofemoral compartment during walking. Interventions could be developed to gradually increase loading among those with combined ACLR and meniscal repair and decrease loading among those with combined ACLR and partial meniscectomy.
Our findings for both the partial and repair groups, while interesting, are not surprising. Previous studies noted varying results for pKAM among participants after ACLR18,33-38 but did not control for medial meniscal pathology. Thorlund et al. reported that the pKAM and impulse increased in the surgical versus the contralateral limb from before to 12 months after arthroscopic partial meniscectomy (without ACLR)31. Although we did not have preoperative measures in the present study, pKAM was greater in the surgical versus the contralateral limb of the participants in the partial group compared with both the repair group and “none.” In contrast, those in the repair group not only walked with relatively lesser pKAM and medial to total loading compared with the partial group but also walked with meaningful peak medial compartment contact force, pMCCF, underloading in the surgical versus the contralateral limb. Patients undergoing meniscal repair (with or without ACLR) often have weight-bearing restrictions for upward of 4 to 6 weeks postoperatively57, whereas arthroscopic meniscectomy rarely has weight-bearing precautions. All participants in the present study who underwent meniscal repair had protected weight-bearing restrictions ranging from non-weight-bearing to weight-bearing-as-tolerated with the knee locked in full extension for 4 weeks. It is plausible that during this period of off-loading following meniscal repair, and in the subsequent months of rehabilitation, patients learn to shift loading away from the medial compartment in the surgical limb and toward the lateral compartment and/or contralateral limb. This explanation could, at least in part, explain why participants in the repair group in the present study shifted loading away from the medial compartment in the surgical limb compared with those in the other groups.
There are some limitations to consider when evaluating and interpreting the findings of the present study. We did not control for the location of medial meniscal tear. Surgical decision-making regarding the selection of repair versus meniscectomy is, however, based largely on the location (i.e., vascular versus avascular zone58) and the extent of the meniscal tear; thus, it is unclear whether the location or extent of the pathology, or the quality of surgical intervention itself, had greater impact on the results. To a large degree, however, it does not matter if the cause is the initial injury or iatrogenic, as the implications for rehabilitation remain either way. We also did not control for lateral meniscal pathology, graft type, or patient sex; there were no differences, however, among the groups for any of these variables. Moreover, by not controlling for these variables, our findings may be more generalizable to individuals after ACLR. The musculoskeletal modeling approach also comes with limitations; it estimates values that cannot be measured in vivo without a device like an instrumented knee prosthesis. The modeling approach is both patient-specific and previously validated and thus provides informative estimations of values that cannot be measured. The present study lacks long-term follow-up; the time frame assessed, however, may be critical for understanding the risk of early osteoarthritis development14 at a time when patients are still undergoing rehabilitation. Treatments to target gait impairments could be developed at this relatively early stage to potentially mitigate future osteoarthritis risk.
In conclusion, our results suggest that concomitant medial meniscal tear and treatment may influence walking mechanics after ACLR. Those who underwent partial medial meniscectomy walked with a higher pKAM and shifted loading toward the medial compartment in the surgical limb while those who had meniscal repair did the opposite, walking with a lower pKAM and unloading the surgical versus contralateral limb. These findings may help to explain the conflicting evidence regarding pKAM after ACLR and the elevated risk for osteoarthritis after ACLR with concomitant medial meniscectomy or repair.
Acknowledgments
Note: The authors thank Martha Callahan, Angela H. Smith, and the Delaware Rehabilitation Institute for their assistance with patient recruitment, and Amelia Arundale, Kathleen Cummer, P. Michael Eckrich, Georgia Gagianas, Celeste Dix, and Naoaki Ito for their assistance with data collection and processing.
Footnotes
Investigation performed at the University of Delaware, Newark, Delaware
Disclosure: Funding was provided by the National Institutes of Health, including the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of General Medical Sciences: R01-AR048212, R37-HD037985, R01-HD087459, P30-GM103333, U54-GM104941, and T32-HD00749. This work was also supported in part by a Promotion of Doctoral Studies (PODS)–Level I Scholarship from the Foundation for Physical Therapy (J.J.C.) and a University Doctoral Fellowship Award from the University of Delaware, Newark, Delaware (J.J.C.). The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/E774).
References
- 1.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006. September;34(9):1512-32. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt RWB, Inacio MCS, Bellevue KD, Schepps AL, Maletis GB. Isolated ACL versus multiple knee ligament injury: associations with patient characteristics, cartilage status, and meniscal tears identified during ACL reconstruction. Phys Sportsmed. 2017. September;45(3):323-8. Epub 2017 May 4. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007. October;35(10):1756-69. Epub 2007 Aug 29. [DOI] [PubMed] [Google Scholar]
- 4.Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009. July;37(7):1434-43. [DOI] [PubMed] [Google Scholar]
- 5.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P, Dubrana F. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009. August;16(4):239-44. Epub 2008 Dec 20. [DOI] [PubMed] [Google Scholar]
- 6.Hagino T, Ochiai S, Senga S, Yamashita T, Wako M, Ando T, Haro H. Meniscal tears associated with anterior cruciate ligament injury. Arch Orthop Trauma Surg. 2015. December;135(12):1701-6. Epub 2015 Aug 19. [DOI] [PubMed] [Google Scholar]
- 7.Smith MV, Nepple JJ, Wright RW, Matava MJ, Brophy RH. Knee osteoarthritis is associated with previous meniscus and anterior cruciate ligament surgery among elite college American football athletes. Sports Health. 2017. May-Jun;9(3):247-51. Epub 2016 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones MH, Spindler KP. Risk factors for radiographic joint space narrowing and patient reported outcomes of post-traumatic osteoarthritis after ACL reconstruction: Data from the MOON cohort. J Orthop Res. 2017. July;35(7):1366-74. Epub 2017 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, Beck N, van Leeuwen J, Crossley KM. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol. 2015. April;67(4):946-55. [DOI] [PubMed] [Google Scholar]
- 10.Leiter JRS, Gourlay R, McRae S, de Korompay N, MacDonald PB. Long-term follow-up of ACL reconstruction with hamstring autograft. Knee Surg Sports Traumatol Arthrosc. 2014. May;22(5):1061-9. Epub 2013 Apr 18. [DOI] [PubMed] [Google Scholar]
- 11.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014. May;42(5):1049-57. Epub 2014 Mar 18. [DOI] [PubMed] [Google Scholar]
- 12.Cantin O, Lustig S, Rongieras F, Saragaglia D, Lefèvre N, Graveleau N, Hulet C; Société Française de Chirurgie Orthopédique et Traumatologique. Outcome of cartilage at 12years of follow-up after anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res. 2016. November;102(7):857-61. Epub 2016 Aug 17. [DOI] [PubMed] [Google Scholar]
- 13.Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017. March;35(3):625-33. Epub 2016 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016. January;44(1):143-51. Epub 2015 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, Luc-Harkey BA, Ulici V, Marshall SW, Jordan JM, Spang JT. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017. October Oct;35(10):2288-97. Epub 2017 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009. February;91(Suppl 1):95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma L. Osteoarthritis year in review 2015: clinical. Osteoarthritis Cartilage. 2016. January;24(1):36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009. May;43(5):366-70. Epub 2008 Nov 28. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013. January;21(1):10-5. Epub 2012 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manal K, Gardinier E, Buchanan TS, Snyder-Mackler L. A more informed evaluation of medial compartment loading: the combined use of the knee adduction and flexor moments. Osteoarthritis Cartilage. 2015. July;23(7):1107-11. Epub 2015 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med. 2013. June;41(6):1310-8. Epub 2013 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capin JJ, Zarzycki R, Arundale A, Cummer K, Snyder-Mackler L. Report of the primary outcomes for gait mechanics in men of the ACL-SPORTS trial: secondary prevention with and without perturbation training does not restore gait symmetry in men 1 or 2 years after ACL reconstruction. Clin Orthop Relat Res. 2017. October;475(10):2513-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011. July 7;44(10):1948-53. Epub 2011 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Gait mechanics 2 years after anterior cruciate ligament reconstruction are associated with longer-term changes in patient-reported outcomes. J Orthop Res. 2017. March;35(3):634-40. Epub 2016 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Stasi S, Hartigan EH, Snyder-Mackler L. Sex-specific gait adaptations prior to and up to 6 months after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2015. March;45(3):207-14. Epub 2015 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White K, Logerstedt D, Snyder-Mackler L. Gait asymmetries persist 1 year after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2013. July 25;1(2):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartigan E, Lawrence M, Murray T, Shaw B, Collins E, Powers K, Townsend J. Biomechanical profiles when towing a sled and wearing a weighted vest once cleared for sports post-ACL reconstruction. Sports Health. 2016. September;8(5):456-64. Epub 2016 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxby DJ, Bryant AL, Modenese L, Gerus P, Killen BA, Konrath J, Fortin K, Wrigley TV, Bennell KL, Cicuttini FM, Vertullo C, Feller JA, Whitehead T, Gallie P, Lloyd DG. Tibiofemoral contact forces in the anterior cruciate ligament-reconstructed knee. Med Sci Sports Exerc. 2016. November;48(11):2195-206. [DOI] [PubMed] [Google Scholar]
- 29.Hall M, Stevermer CA, Gillette JC. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture. 2012. May;36(1):56-60. Epub 2012 Feb 5. [DOI] [PubMed] [Google Scholar]
- 30.Pratt KA, Sigward SM. Knee loading deficits during dynamic tasks in individuals following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017. June;47(6):411-9. Epub 2017 May 12. [DOI] [PubMed] [Google Scholar]
- 31.Thorlund JB, Holsgaard-Larsen A, Creaby MW, Jørgensen GM, Nissen N, Englund M, Lohmander LS. Changes in knee joint load indices from before to 12 months after arthroscopic partial meniscectomy: a prospective cohort study. Osteoarthritis Cartilage. 2016. July;24(7):1153-9. Epub 2016 Feb 2. [DOI] [PubMed] [Google Scholar]
- 32.Mononen ME, Jurvelin JS, Korhonen RK. Effects of radial tears and partial meniscectomy of lateral meniscus on the knee joint mechanics during the stance phase of the gait cycle—A 3D finite element study. J Orthop Res. 2013. August;31(8):1208-17. Epub 2013 Apr 9. [DOI] [PubMed] [Google Scholar]
- 33.Varma RK, Duffell LD, Nathwani D, McGregor AH. Knee moments of anterior cruciate ligament reconstructed and control participants during normal and inclined walking. BMJ Open. 2014. June 4;4(6):e004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster KE, Feller JA. The knee adduction moment in hamstring and patellar tendon anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2012. November;20(11):2214-9. Epub 2011 Dec 24. [DOI] [PubMed] [Google Scholar]
- 35.Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2012. April;46(5):355-9. Epub 2011 Apr 20. [DOI] [PubMed] [Google Scholar]
- 36.Patterson MR, Delahunt E, Caulfield B. Peak knee adduction moment during gait in anterior cruciate ligament reconstructed females. Clin Biomech (Bristol, Avon). 2014. February;29(2):138-42. Epub 2013 Dec 4. [DOI] [PubMed] [Google Scholar]
- 37.Webster KE, Feller JA, Wittwer JE. Longitudinal changes in knee joint biomechanics during level walking following anterior cruciate ligament reconstruction surgery. Gait Posture. 2012. June;36(2):167-71. Epub 2012 Apr 1. [DOI] [PubMed] [Google Scholar]
- 38.Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013. February 1;46(3):515-20. Epub 2012 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther. 2009. December;39(12):845-9. [DOI] [PubMed] [Google Scholar]
- 40.Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012. July;42(7):601-14. Epub 2012 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament- specialized post-operative return-to-sports (ACL-SPORTS) training: a randomized control trial. BMC Musculoskelet Disord. 2013. March 23;14(14):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capin JJ, Khandha A, Zarzycki R, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J Orthop Res. 2017. September;35(9):1894-901. Epub 2016 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capin JJ, Behrns W, Thatcher K, Arundale A, Smith AH, Snyder-Mackler L. On-ice return-to-hockey progression after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017. May;47(5):324-33. Epub 2017 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hefti F, Müller W, Jakob RP, Stäubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-34. [DOI] [PubMed] [Google Scholar]
- 45.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994. Sep-Oct;22(5):632-44. [DOI] [PubMed] [Google Scholar]
- 46.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Altered loading in the injured knee after ACL rupture. J Orthop Res. 2013. March;31(3):458-64. Epub 2012 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J Biomech Eng. 2013. February;135(2):021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004. November;20(4):367-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009. October 16;42(14):2294-300. Epub 2009 Jul 31. [DOI] [PubMed] [Google Scholar]
- 50.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995. August;77(8):1166-73. [DOI] [PubMed] [Google Scholar]
- 51.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR., 3rd Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994. April;76(4):555-60. [DOI] [PubMed] [Google Scholar]
- 52.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Gait and neuromuscular asymmetries after acute anterior cruciate ligament rupture. Med Sci Sports Exerc. 2012. August;44(8):1490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: a comparison of two techniques. J Biomech. 2003. April;36(4):599-603. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- 55.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Minimum detectable change for knee joint contact force estimates using an EMG-driven model. Gait Posture. 2013. September;38(4):1051-3. Epub 2013 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudhari AMW, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008. February;40(2):215-22. [DOI] [PubMed] [Google Scholar]
- 57.VanderHave KL, Perkins C, Le M. Weightbearing versus nonweightbearing after meniscus repair. Sports Health. 2015. Sep-Oct;7(5):399-402. Epub 2015 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998. August;193(Pt 2):161-78. [DOI] [PMC free article] [PubMed] [Google Scholar]