Background:
Current decisions on cellular therapies for osteoarthritis are based primarily on clinical experience or on assumptions about preferred cell sourcing. They have not been informed by rigorous standardized measurements of the chondrogenic connective-tissue progenitors (CTP-Cs) or their intrinsic diversity of chondrogenic potential. The goal of this study was to quantitatively define the CTP-Cs resident in cartilage of different grades of osteoarthritis and to compare their concentration, prevalence, and biological potential.
Methods:
Twenty-three patients who had varus malalignment of the knee and were scheduled to undergo elective total knee arthroplasty for idiopathic osteoarthritis and who had grade 1-2 osteoarthritis on the lateral femoral condyle and grade 3-4 osteoarthritis on the medial femoral condyle were recruited for study of the cartilage removed during surgery. CTP-Cs were assayed by a standardized colony-forming-unit assay using automated image-analysis software based on ASTM standard test method F2944-12.
Results:
Cell concentration was significantly greater (p < 0.001) in grade 3-4 cartilage than in grade 1-2 cartilage. The prevalence of CTP-Cs varied widely, but it trended lower in grade 3-4 cartilage than in grade 1-2 samples (p = 0.078). The biological performance of CTP-Cs from grade 1-2 and grade 3-4 cartilage was comparable. Increased cell concentration was a significant predictor of decreased CTP-C prevalence (p = 0.002).
Conclusions:
Although grade 3-4 cartilage showed fewer CTP-Cs than grade 1-2 cartilage, the range of biological performance was comparable, which suggests that either may be used as a source for potent CTP-Cs. However, the biological reason for the heterogeneity of CTP-Cs in cartilage and the biological implications of that heterogeneity are not well understood and require further study.
Clinical Relevance:
In order to improve the efficacy of cartilage cell therapy procedures, it is key to characterize the quality and quantity of the cells and progenitors being administered. Additionally, understanding the heterogeneity in order to select appropriate subsets of populations will improve the rigor of decisions concerning cell sourcing and targeting for pharmacological and cellular therapies.
Cellular therapy for osteoarthritis offers promise; however, outcomes with respect to cartilage repair have been inconsistent1-4. Cartilage-derived cells have been employed in several cell-based treatment strategies. Some of these therapies involve transplantation of freshly isolated cartilage or cartilage-derived cells (for example, osteochondral autograft transfer system, known as OATS)5. Other therapies utilize methods of in vitro culture expansion before transplantation of cartilage-derived cells into a cartilage defect (for example, autologous chondrocyte implantation [ACI])5. These therapies vary with respect to the choice of matrix and dose of cells during transplantation. In general, these cellular therapies use tissues either from a functionally expendable region of normal cartilage or from local diseased cartilage, the debridement of which is necessary for treatment. Currently, decisions are primarily based on clinical experience or assumptions about preferred cell sourcing. They have not been informed by quantitative standardized measurement of the quantity and quality of chondrogenic connective-tissue progenitors (CTP-Cs) that are essential for cartilage repair activities.
Connective-tissue progenitors (CTPs) are present in every connective tissue but are generally low in number, and their verification is not easy6. Colony-forming-unit assay has been used extensively to perform quantitative and functional measurements of the CTPs from different cell sources7-10. CTP-Cs are a heterogeneous population of colony-founding cells, resident in connective tissue, whose progeny can differentiate into a chondrogenic phenotype to contribute to the formation of new cartilage tissue11,12. In the absence of definitive surface markers that can be used to distinguish between cells with and without CTP potential in freshly isolated cells, it is necessary to directly measure function (self-renewal capacity demonstrated by colony formation and their chondrogenic differentiation expression) in order to identify chondrogenic progenitor cells6,13-15. The recently adopted ASTM standard test method F2944-1216 defines reproducible methods that enable quantitative, automated colony analysis and eliminate the challenges of subjective manual counting that have diminished the rigor (accuracy, repeatability, reproducibility, and documentation) of colony analysis in the past17.
In order to provide rigorously standardized information regarding the quality and quantity of chondrogenic progenitors present in different grades of osteoarthritic cartilage, this study was designed to quantitatively define CTP-Cs resident in Outerbridge grades 1 and 2 (grade 1-2) and grades 3 and 4 (grade 3-4) cartilage obtained from the same patient and to compare the cell concentrations, prevalence, and biological potential of the cartilage. The purpose was to contribute to our understanding of cartilage biology and pathophysiology and to improve guidance concerning cell-sourcing decisions for pharmacological and cellular therapies.
Materials and Methods
Recruiting—Inclusion and Exclusion Criteria
This study was approved by the institutional review board of the Cleveland Clinic. Twenty-three patients who had varus malalignment of the knee and were scheduled for elective total knee arthroplasty were recruited. The mean age of the 11 men and 12 women was 60.3 years (range, 37 to 76 years). The inclusion criteria were a diagnosis of idiopathic osteoarthritis, radiographs exhibiting a relatively spared lateral femoral compartment (joint space >2 mm), and disease primarily in the medial compartment. Patients were excluded if they had secondary arthritis related to systemic inflammatory arthritis or if their history included current or previous treatment with systemic glucocorticoids or osteotropic medication, infection, osteonecrosis, neoplasia, prior reconstruction of the anterior cruciate ligament, or known or suspected injury of that ligament.
Procurement of Cartilage for Study
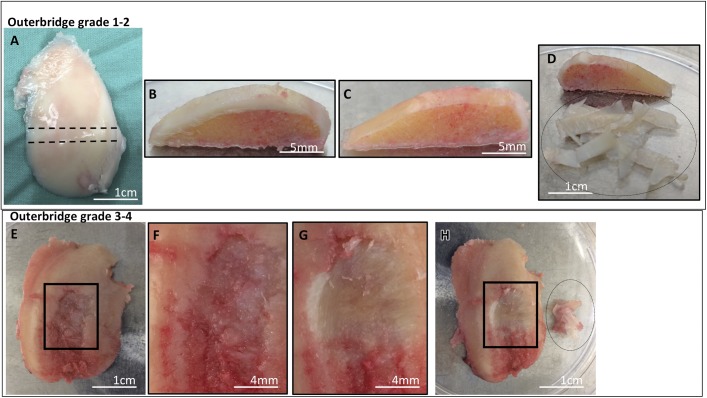
During the arthroplasty procedure, a tangential osteochondral specimen of the lateral femoral condyle and the medial femoral condyle was harvested with the initial distal femoral cut. Samples of Outerbridge grade 1-2 cartilage were obtained from the lateral femoral condyle (Figs. 1-A, 1-B, and 1-C), and samples of grade 3-4 cartilage were obtained from the medial femoral condyle (Figs. 1-E, 1-F, and 1-G). Grade 1-2 includes cartilage with an intact surface (grade 1) and minimal fibrillation (grade 2), and grade 3-4 cartilage includes cartilage with fissures to subchondral bone18,19. Within 1 hour after it had been obtained, the cartilage tissue was separated from the subchondral bone with a scalpel blade and was diced (Figs. 1-D and 1-H).
Fig. 1.
Figs. 1-A through 1-H Representative images showing procurement of cartilage samples from Outerbridge grade 1-2 and grade 3-4 cartilage. Cartilage was collected from patients who had varus malalignment of the knee and underwent total knee arthroplasty. For grade 1-2 cartilage, cartilage from the lateral femoral condyle with a thickness of ≥2 mm was collected (Fig. 1-A), and an osteochondral arch was cut in the coronal plane from the load-bearing portion of the condyle (Fig. 1-B). Using a scalpel blade, cartilage tissue was sliced and separated until a zone of calcified cartilage was reached (Fig. 1-C). The separated cartilage tissue (inside the dotted-line oval) was weighed and enzymatically digested to collect cells for culture (Fig. 1-D). The weight of cartilage obtained in this representative sample of grade 1-2 cartilage was 380 mg. For grade 3-4 samples, cartilage from the medial femoral condyle that exhibited focal lesions in at least a 1-cm2 region of the condyle (Fig. 1-E) and showed fissures to subchondral bone (Fig. 1-F) was obtained from the same patient. Using a scalpel blade, cartilage tissue was carefully separated from the region of the lesion until a zone of calcified cartilage was reached (Fig. 1-G). The separated cartilage tissue (inside the dotted-line oval) was weighed and enzymatically digested to collect cells for culture (Fig. 1-H). The weight of cartilage obtained in this representative sample of grade 3-4 cartilage was 180 mg.
Analysis of Colony Formation
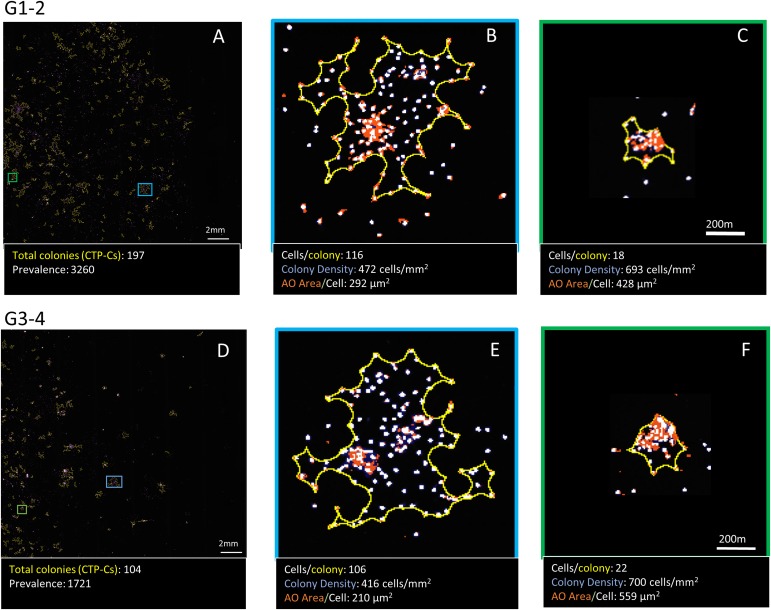
The wet weight of the separated cartilage tissue was measured. Cells were placed in 2-dimensional culture using the protocol described previously10. Briefly, cells were enzymatically isolated using collagenase type II (6,000 U/mL) and dispase (3 U/mL) for 3 hours in a 37°C water bath, filtered, centrifuged, and counted. They were then placed in Lab-Tek chamber slides (plating density, 24,000 cells/cm2) (Thermo Fisher Scientific) for culture in chondrogenic media: DMEM-F12 (Dulbecco modified Eagle medium and nutrient mixture F12) with 5 mM glucose, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% insulin-transferrin-selenium-ethanolamine (ITS-X; Gibco), 10-7 M dexamethasone, 360 μg/mL L-glutamine, 1 mM proline, 250 μg/mL L-ascorbic acid, and 10 ng/mL TGF-β (transforming growth factor-beta)10. Automated quantitative analysis of colony-forming units was performed using Colonyze image-analysis software (owned by the Cleveland Clinic) to quantify and characterize CTPs derived from grade 1-2 and grade 3-4 cartilage on day 610,16. Slides were stained with bis-benzimide, which stains nuclei, and acridine orange (AO), which stains sulfated glycosaminoglycans in the extracellular matrix around cells. The slides then were processed to count all of the colonies in the Lab-Tek chamber, to determine CTP prevalence (where the number of colonies indicates the number of CTPs) and concentration. The biological potential of the CTPs was quantified by measuring individual colony metrics (Figs. 2-A through 2-F).
Fig. 2.
Figs. 2-A through 2-F Automated colony-forming-unit assay using Colonyze image analysis to measure prevalence and colony metrics. Representative images of a 10-mm2 region of a 2-cm2 Lab-Tek chamber slide stained with bis-benzimide and acridine orange (AO) from grade 1-2 and grade 3-4 cartilage are shown (Figs. 2-A and 2-D). The total number of colonies in the chamber was quantified, using the automated image analysis, to determine PCTP-C (total number of CTPs per million cells plated; number of colonies indicates number of CTPs) from grade 1-2 and grade 3-4 cartilage. Colony metrics including number of cells per colony, colony density, and AO area/cell were determined for all of the colonies. Two representative types of colonies—large and less dense (Figs. 2-B and 2-E) and small and more dense (Figs. 2-C and 2-F)—obtained from grade 1-2 cartilage (top panel) and grade 3-4 cartilage (bottom panel) are shown, along with their respective metrics: number of cells per colony, colony density, and AO area/cell.
Statistical Analysis
The outcome variables for the analysis included (1) cell concentration (total cells obtained after enzymatic digestion per milligram of tissue), (2) CTP prevalence (PCTP-C) (CTPs per million cells plated), (3) CTP concentration (CTPs per milligram of tissue, calculated as the product of cell concentration and PCTP-C), (4) number of cells per colony, (5) colony density (number of cells per colony divided by the area of the colony), and (6) AO area/cell (total AO-stained area per colony divided by number of cells per colony). These variables were transformed to Gaussian distributions. Generalized linear models were fit, where the dependent variable was 1 of these outcome variables and the independent variables were age, sex, cell concentration, tissue source, and 2-way interaction of cell concentration and tissue source. Generalized estimating equations were used to account for the clustered data. Median values and the widths of the interquartile ranges (IQRs) were determined. A significance level of 0.05 was used overall, with the Holm method used to control the family-wise error rate.
Results
Nucleated Cell Concentration (Cells per Milligram of Wet Weight of Tissue)
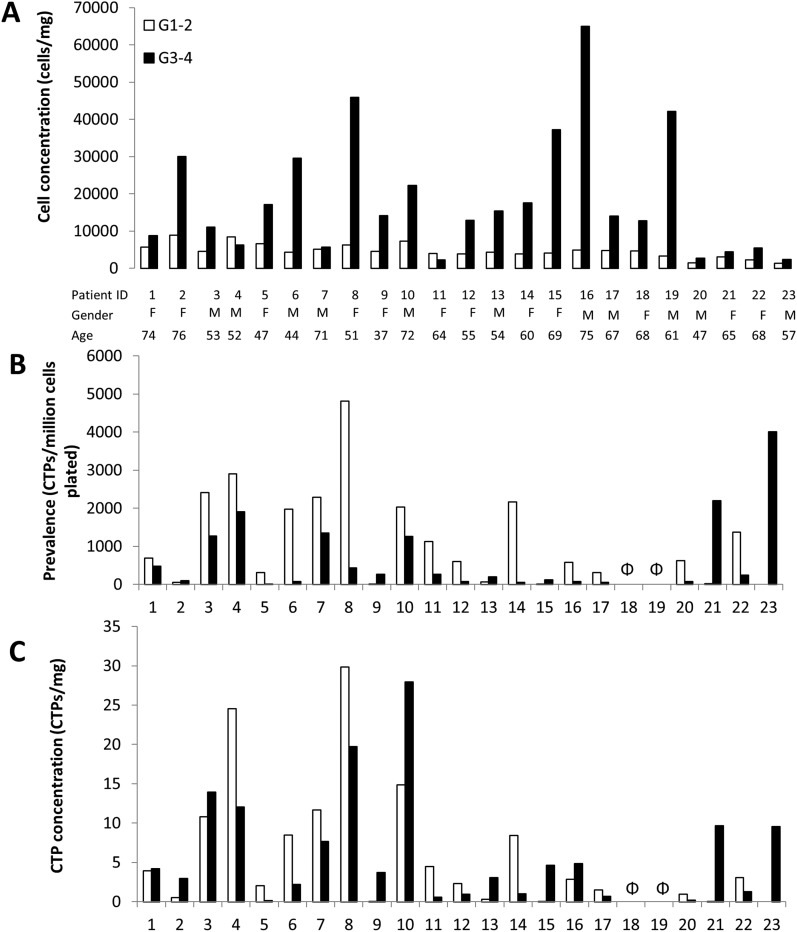
Cell concentration for grade 1-2 cartilage (median 4,490; IQR 1,860) was significantly less than it was for grade 3-4 cartilage (median 14,050; IQR 19,865) (p < 0.001) (Table I). Wide variation was seen between the patients with respect to cell concentration (Fig. 3-A).
Fig. 3.
Figs. 3-A, 3-B, and 3-C Variability between patients in terms of cell concentration (Fig. 3-A), prevalence (Fig. 3-B), and mean CTP concentration (Fig. 3-C) in grade 1-2 cartilage, represented by white bars, and grade 3-4 cartilage, represented by black bars. Each patient is consistently represented with the same ID in all 3 plots, to allow comparison for the output parameters. No CTP-Cs were obtained for Patients 18 and 19 from either grade 1-2 or grade 3-4 cartilage (indicated by Φ).
TABLE I.
Quantitative Characterization of Grade 1-2 and Grade 3-4 Cartilage with Respect to Cell Concentration and Population of CTP-Cs*
| Metrics for Cell and CTP Quantification | Grade 1-2 Cartilage | Grade 3-4 Cartilage | P Value |
| Cell concentration (cells/mg) | 4,490 (1,860) | 14,050 (19,865) | <0.001 |
| CTP-C prevalence (PCTP-C) (CTPs/million cells plated) | 811 (1,754) | 232 (927) | 0.078 |
| CTP-C concentration (CTPs/mg wet weight) | 2 (7) | 3 (7) | 0.737 |
CTP = connective tissue progenitor, and CTP-C = chondrogenic CTP. Values are reported as the median for samples with non-zero prevalence, with the width of the interquartile range in parentheses. P < 0.05 indicates significance.
Sex was not a significant predictor of cell concentration: for men, the median was 5,385 and the IQR was 9,710 and for women, the median was 6,200 and the IQR was 10,070 (p = 0.533). Similarly, age was not a significant predictor: for patients <50 years old, the median was 658 and the IQR was 9,810; for patients 50 to 59 years old, the median was 6,245 and the IQR was 7,870; for patients 60 to 69 years old, the median was 4,500 and the IQR was 9,815; and for patients ≥70 years old, the median was 8,055 and the IQR was 6,520 (p = 0.258).
CTP Prevalence, PCTP-C (CTPs per Million Cells Plated)
PCTP-C trended lower in grade 3-4 cartilage (median, 232; IQR, 927) than in grade 1-2 cartilage (median, 811; IQR, 1,754) (p = 0.078) (Table I). These median values can be stated as a prevalence of 1 CTP per 1,233 cells and 1 CTP per 4,310 cells for grade 1-2 and grade 3-4 cartilage, respectively. Wide variation between individuals was found for PCTP-C in both grade 1-2 and grade 3-4 cartilage (Fig. 3-B).
For grade 1-2 and grade 3-4 cartilage, the age of the patient was a significant predictor of PCTP-C (p = 0.013). Our data suggest that as age increases, prevalence decreases, with the exception in this study of patients between 50 and 59 years old, who had a higher prevalence than patients <50 years old. Sex was not a significant predictor (p = 0.211).
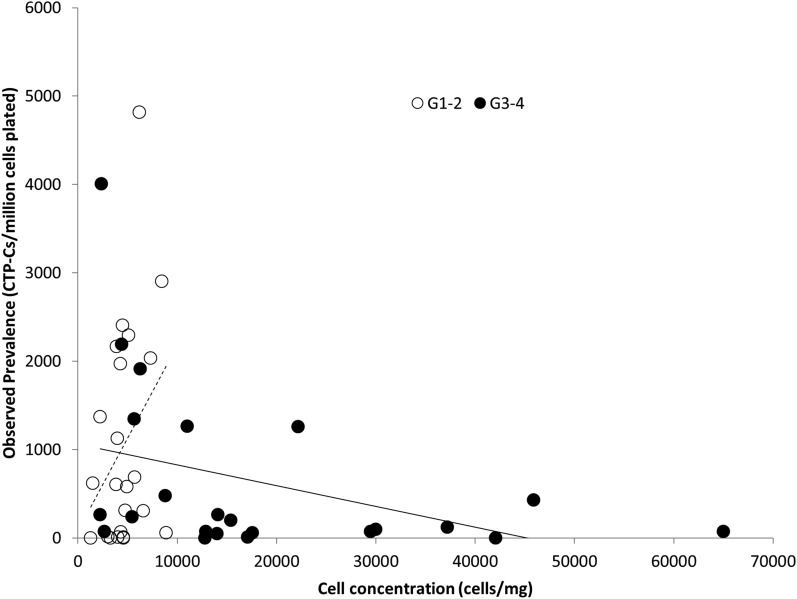
Cell concentration was also a significant predictor of PCTP-C (p = 0.002). In grade 1-2 cartilage, a higher value for cell concentration was associated with a higher value for PCTP-C. In grade 3-4 cartilage, the opposite was true: a higher value for cell concentration was associated with a lower value for PCTP-C (estimated correlation, −0.30) (Fig. 4).
Fig. 4.
Cell concentration and CTP-C prevalence in grade 1-2 cartilage (hollow circles) and grade 3-4 cartilage (black circles) showed significant correlation (p < 0.05). Note that the cell concentration obtained from grade 1-2 cartilage was always <10,000 cells/mg, whereas for grade 3-4 cartilage the cell concentration range had a wide spread.
CTP-C Concentration (CTPs per Milligram of Tissue)
CTP-C concentration was not significantly different between grade 1-2 and grade 3-4 cartilage (p = 0.737). For grade 1-2, the median was 2 and the IQR was 7; for grade 3-4 cartilage, the median was 3 and the IQR was 7 (Fig. 3-C) (Table I).
Neither sex nor age was a significant predictor of CTP-C concentration (p = 0.148 and p = 0.166, respectively).
Analysis of Biological Potential
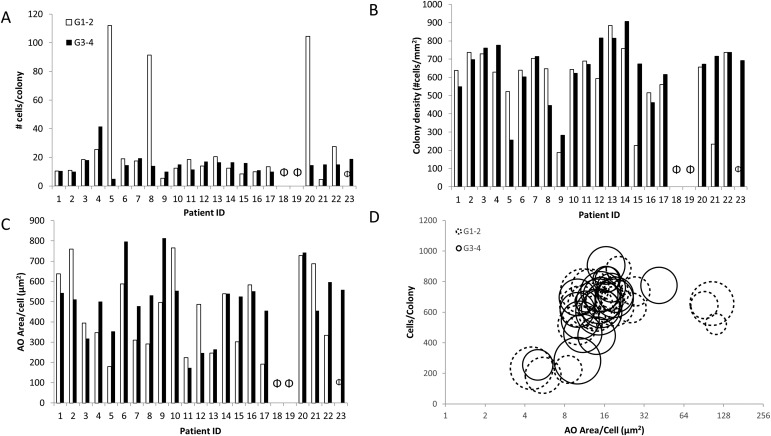
No significant differences between grade 1-2 cartilage and grade 3-4 samples were found for any of the colony metrics (Figs. 5-A through 5-D), which are summarized in Table II. Additionally, large heterogeneity in CTP-C populations was found between and within individuals.
Fig. 5.
Figs. 5-A through 5-D Variation seen in the CTP-C population derived from grade 1-2 and grade 3-4 cartilage with respect to number of cells per colony (Fig. 5-A), colony density (Fig. 5-B), and AO area/cell (acridine-orange-stained area per colony divided by number of cells per colony) (Fig. 5-C). No CTP-Cs were obtained for Patients 18 and 19 from either grade 1-2 or grade 3-4 cartilage (indicated by Φ). A bubble plot with all 3 parameters plotted for all 23 patients for CTP-Cs derived from grade 1-2 and grade 3-4 cartilage (Fig. 5-D). Solid-line bubbles indicate grade 1-2 and broken-line bubbles indicate grade 3-4. Bubble size represents AO area/cell.
TABLE II.
Quantitative Characterization of CTP-C Populations Derived from Grade 1-2 and Grade 3-4 Cartilage with Respect to their Biological Potential*
| CTP Characterization Based on Colony Metrics | Grade 1-2 Cartilage | Grade 3-4 Cartilage | P Value |
| Proliferation potential (bis-benzimide staining as nuclear marker) | |||
| No. of cells per colony | 15 (11) | 14 (7) | 0.42 |
| Migration potential (no. of cells in the colony per colony area) | |||
| Colony density (cells/mm2) | 638 (471) | 672 (275) | 0.50 |
| Differentiation potential (AO staining as GAG-ECM marker) | |||
| AO area/cell (μm2) | 347 (364) | 511 (236) | 0.78 |
CTP = connective-tissue progenitor, CTP-C = chondrogenic CTP, and GAG-ECM = glycosaminoglycan-extracellular matrix. Values are reported as the median for samples with non-zero prevalence, with the width of the interquartile range in parentheses. P < 0.05 indicates significance.
Cell Proliferation
The total number of cells per colony in grade 1-2 cartilage (median, 15; IQR, 11) and grade 3-4 cartilage (median, 14; IQR, 7) was not significantly different (p = 0.420) (Fig. 5-A). These values correspond to median doubling times (log2 [number of cells per colony]/number of days in culture) of 37 and 38 hours for grade 1-2 and grade 3-4 cartilage-derived CTP-Cs, respectively.
Age was a significant predictor of total number of cells per colony (p = 0.005): older patients had significantly fewer cells per colony than younger patients. Sex was not a significant predictor (p = 0.689).
Colony density, a metric related to cell-cell affinity and migration in each colony, was not significantly different (p > 0.05) between grade 1-2 cartilage (median, 638 cells/mm2; IQR, 471 cells/mm2) and grade 3-4 samples (median, 672 cells/mm2; IQR, 275 cells/mm2) (Fig. 5-B). However, colony density varied widely, indicating large heterogeneity between the CTP-Cs in each sample.
Differentiation
Relative assessments of glycosaminoglycans in the extracellular matrix, as determined by AO area/cell in a colony, was not significantly different (p = 0.78) between grade 1-2 cartilage (median, 347 µm2; IQR, 364 µm2) and grade 3-4 cartilage (median, 511 µm2; IQR, 236 µm2) (Fig. 5-C).
Increasing age was associated with a moderate increase in glycosaminoglycans in the extracellular matrix (p = 0.059). Cells derived from patients between 50 and 69 years old had lower values for glycosaminoglycans in the extracellular matrix area per cell than patients ≥70 years. Sex was not a significant predictor of production of glycosaminoglycans in the extracellular matrix by CTP-Cs (p = 0.55). Figure 5-D is a bubble plot indicating the correlations among all of the 3 colony metrics measured for the 23 patients whose cartilage was studied.
Colony Heterogeneity
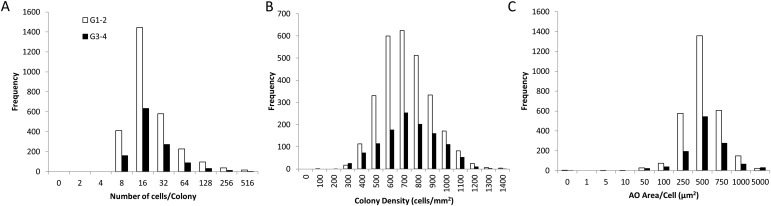
The histogram distribution including all 1,195 colonies assessed was essentially the same for the colonies derived from grade 1-2 and grade 3-4 cartilage (Figs. 6-A, 6-B, and 6-C). The only difference was in the frequency of occurrence of colonies, which was reduced by 2 to 3 times in grade 3-4 cartilage compared with grade 1-2 cartilage due to the strong trend for reduced prevalence in grade 3-4 samples.
Fig. 6.
Figs. 6-A, 6-B, and 6-C Histograms representing magnitude of variation between CTP-Cs obtained from grade 1-2 and grade 3-4 cartilage for different colony metrics: number of cells per colony (Fig. 6-A), colony density (Fig. 6-B), and AO area/cell (Fig. 6-C).
The large heterogeneity among CTP-Cs is further illustrated in Appendix Figure E-1, where all of the 1,195 colonies are plotted in 3 dimensions with respect to number of cells per colony, colony density, and AO area/cell.
Discussion
To our knowledge, this study is the first to quantitatively compare and characterize the cell and CTP-C population in Outerbridge grade 1-2 and grade 3-4 cartilage using systematic and reproducible methods for automated assay of colony-forming units in accordance with ASTM standard test method F2944-12. We found that compared with grade 1-2 cartilage, the value for cell concentration in grade 3-4 cartilage was significantly greater; however, the value for PCTP-C was lower. In terms of proliferation and differential potential, CTP-Cs in both grade 1-2 and grade 3-4 cartilage performed similarly.
The study design allowed paired comparison of cartilage in different stages of osteoarthritic degeneration from the same patient and harvested from the same joint, enabling control of the chemical milieu of the joint fluid. However, this study has several limitations. First, we cannot exclude the possibility that some of the differences we found were influenced by anatomical and/or mechanical medial-to-lateral differences in cartilage20,21. However, other studies have suggested that there are no differences in cell concentration or in potential for proliferation and differentiation of in vitro culture-expanded cells between the lateral and medial femoral condyles of healthy young individuals22,23. The second limitation is that the characterization of chondrogenic differentiation in this initial study was purposefully limited to the simple method of AO staining. Future studies may benefit from the addition of more extracellular-matrix markers for chondrogenic differentiation.
Our analysis suggests that more advanced stages of osteoarthritis are associated with measurable change in the number of cells and progenitors. We found that the mean value for nucleated cell concentration in grade 3-4 cartilage was 3.1-fold greater than in grade 1-2 cartilage, indicating that progression of osteoarthritis is associated with increased cell concentration. Some increase in cell concentration must be the result of proliferation of chondrocytes, as described in histopathological changes during osteoarthritis (cloning)24,25. Depending on the sampling and tissue preparation, an increased cell concentration could also be a contribution of local inflammatory cells, synovial overgrowth from pannus, or stromal cells associated with vascular invasion of cartilage tissue26-31. However, the sampling methods in this study avoided the inclusion of cartilage near the synovial interface where pannus might be present.
Our data also indicate that in early phases of osteoarthritis (grade 1-2), as cell concentration increases, PCTP-C also increases, suggesting activation of quiescent chondrocytes that now function as chondrogenic progenitors, as characterized by their ability to self-renew and proliferate. However, in cartilage in the late stages of osteoarthritis (grade 3-4), we found that as cell concentration increases, PCTP-C decreases (Fig. 4). This provides an insight into how disease progression may affect progenitors resident in cartilage. This decrease in PCTP-C represents a failure of self-renewal of the CTP-Cs13,32, implying that if a CTP-C divides in late osteoarthritis it does not always retain at least 1 daughter cell that maintains the CTP-C phenotype of biological potential for further cell division. As a result, PCTP-C in the tissue is potentially depleted through the process of activation into the cell cycle. Alternatively, PCTP-C could also be depleted without CTP-C activation through the loss of a so-called stem-cell niche, possibly associated with degeneration of the extracellular matrix within cartilage30 resulting in either death or dysfunction of CTP-Cs and rendering them incapable as progenitors.
This analysis suggests that the biological potential of the CTP-Cs present in cartilage tissue changes in association with disease progression and that these differences would be manifested in the in vitro performance. Several previous studies have assessed the proliferation and differentiation potential of cartilage-derived cells (for example, passage 1 or 2 to confluence)33,34. They have generally reported that culture-expanded cells from osteoarthritic cartilage are associated with lower proliferation rates34 and lower expression of differentiation markers26,35. However, these studies assessed the performance of culture-expanded cells at a time when the initial heterogeneity of the tissue-derived population is no longer present or observable. These methods do not encompass the diversity in the progenitors’ milieu derived from primary culture, and they deploy cells in a competition for dominance where only the progeny of the most rapidly proliferating clones are represented in the population that is available for study. Gradually increasing awareness of the importance of understanding the heterogeneity, clonal isolation, and characterization of cells is evident in recent literature9,36-39. For clinical applications, in order to improve the efficacy of cartilage cell therapy procedures, it is key to characterize the quality of the cells being administered.
The analysis performed in this study is different by intent. Understanding the heterogeneity of CTP-Cs in native tissues required analysis at a time (day 6) when the differences in performance of individual colony-founding CTP-Cs can still be observed and objectively quantified (see Appendix Fig. E-1). The current study assessed 1,195 colonies from the cartilage of 23 patients using automated large-field-of-view image analysis in accordance with the ASTM standard test method16. Using this approach, we found no statistical difference in overall diversity of biological performance of the progeny of CTP-Cs from Outerbridge grade 1-2 and grade 3-4 cartilage. From a practical perspective, we found no evidence supporting loss of mean function among remaining CTP-Cs during the progression of osteoarthritic disease. Moreover, we found that harvested grade 1-2 cartilage and grade 3-4 cartilage appear to be comparable as sources for CTP-Cs, with adjustment for changes in overall cell concentration and prevalence.
This analysis cannot, of course, be extrapolated to predict long-term performance. However, it does enable the opportunity to cluster the CTP-Cs into groups based on biological behavior and to design strategies that manage or exploit the initial heterogeneity to selectively enhance the control and performance of culture-expanded clonal populations—populations that might otherwise be inaccessible using conventional methods of polyclonal mixing. Future work will focus on characterizing and defining these subgroups and ultimately testing their performance in vivo in immunodeficient animal models.
Patient variables, including age, sex, body mass index, genetic background, and health history, play a critical role in tissue health and function40,41. We found that increasing age was significantly associated with a decrease in PCTP-C (except for patients 50 to 59 years old) and proliferation potential. There was no evidence that the sex of the donor influenced cell concentration, PCTP-C, or CTP-C performance. Previous studies examining age and sex as independent variables to predict the prevalence of colony-forming cells in human cartilage have not been conclusive42,43. However, our initial study was not designed or powered to systematically explore the effects of individual differences in genetics or systemic biology.
In conclusion, cell concentration increased significantly in cartilage with more severe osteoarthritis (grade 3-4). Additionally, increased cell concentration in Outerbridge grade 3-4 cartilage was a significant predictor of lower PCTP-C. Although the CTP-Cs decreased in number, the biological performance of the CTP-Cs, assessed based on proliferation potential and AO-stained area of glycosaminoglycans in the extracellular matrix, was comparable in grade 1-2 and grade 3-4 cartilage. This suggests that either grade 1-2 or grade 3-4 cartilage can be used as a source for CTP-Cs. Analysis of the heterogeneity of CTP-C-derived colonies suggests that CTP-Cs are not just 1 population of cells with large variation, but are better defined as a heterogeneous mixture of colony-founding populations. An improved understanding of the heterogeneity of CTP-Cs and the opportunity to quantify and selectively isolate subsets will advance our understanding of cartilage biology and pathophysiology. It will also improve the rigor of cell sourcing and targeting decisions for pharmacological and cellular therapies.
Appendix
A figure showing a 3-dimensional plot of the heterogeneous distribution of all of the colonies obtained from the 23 patients is available with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/E931).
Footnotes
Investigation performed at the Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
Disclosure: This work was supported by a National Institute of Health grant (R01AR063733) awarded to G.F.M. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had financial relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/E930).
References
- 1.Richardson JB, Wright KT, Wales J, Kuiper JH, McCarthy HS, Gallacher P, Harrison PE, Roberts S. Efficacy and safety of autologous cell therapies for knee cartilage defects (autologous stem cells, chondrocytes or the two): randomized controlled trial design. Regen Med. 2017. July;12(5):493-501. Epub 2017 Jun 21. [DOI] [PubMed] [Google Scholar]
- 2.Stoltz JF, de Isla N, Li YP, Bensoussan D, Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N, Reppel L, He Y. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int. 2015;2015:734731 Epub 2015 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piuzzi NS, Ng M, Chughtai M, Khlopas A, Ng K, Mont MA, Muschler GF. The stem-cell market for the treatment of knee osteoarthritis: a patient perspective. J Knee Surg. 2018. July;31(6):551-6. Epub 2017 Jul 24. [DOI] [PubMed] [Google Scholar]
- 4.Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, Muschler GF. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016. September 21;98(18):1511-21. [DOI] [PubMed] [Google Scholar]
- 5.Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014. May;6(3):265-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002. February;(395):66-80. [DOI] [PubMed] [Google Scholar]
- 7.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004. May;50(5):1522-32. [DOI] [PubMed] [Google Scholar]
- 8.Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011. April 15;13(2):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, Lewis H, Roberts S, Shaw HM, Dudhia J, Fairclough J, Briggs T, Archer CW. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010. October 14;5(10):e13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantripragada VP, Bova WA, Boehm C, Piuzzi NS, Obuchowski NA, Midura RJ, Muschler GF. Progenitor cells from different zones of human cartilage and their correlation with histopathological osteoarthritis progression. J Orthop Res. 2018. June;36(6):1728-38. Epub 2017 Dec 29. [DOI] [PubMed] [Google Scholar]
- 11.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001. January;19(1):117-25. [DOI] [PubMed] [Google Scholar]
- 12.Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res Int. 2014;2014:951512 Epub 2014 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015. April;11(4):206-12. Epub 2014 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004. July;86-A(7):1541-58. [DOI] [PubMed] [Google Scholar]
- 15.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003. May;48(5):1315-25. [DOI] [PubMed] [Google Scholar]
- 16.ASTM International. ASTM F2944-12 Standard test method for automated colony forming unit (CFU) assays—image acquisition and analysis method for enumerating and characterizing cells and colonies in culture. 2012. https://www.astm.org/Standards/F2944.htm. Accessed 2018 Jun 1.
- 17.Powell K, Kwee E, Nutter B, Herderick E, Paul P, Thut D, Boehm C, Muschler G. Variability in subjective review of umbilical cord blood colony forming unit assay. Cytometry B Clin Cytom. 2016. November;90(6):517-24. Epub 2016 May 24. [DOI] [PubMed] [Google Scholar]
- 18.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961. November;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 19.Uhl M, Allmann KH, Ihling C, Hauer MP, Conca W, Langer M. Cartilage destruction in small joints by rheumatoid arthritis: assessment of fat-suppressed three-dimensional gradient-echo MR pulse sequences in vitro. Skeletal Radiol. 1998. December;27(12):677-82. [DOI] [PubMed] [Google Scholar]
- 20.Scott CEH, Nutton RW, Biant LC. Lateral compartment osteoarthritis of the knee: Biomechanics and surgical management of end-stage disease. Bone Joint J. 2013. April;95-B(4):436-44. [DOI] [PubMed] [Google Scholar]
- 21.Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017. April;44(4):410-7. Epub 2017 Mar 1. [DOI] [PubMed] [Google Scholar]
- 22.Quinn TM, Hunziker EB, Häuselmann HJ. Variation of cell and matrix morphologies in articular cartilage among locations in the adult human knee. Osteoarthritis Cartilage. 2005. August;13(8):672-8. [DOI] [PubMed] [Google Scholar]
- 23.Stenhamre H, Slynarski K, Petrén C, Tallheden T, Lindahl A. Topographic variation in redifferentiation capacity of chondrocytes in the adult human knee joint. Osteoarthritis Cartilage. 2008. November;16(11):1356-62. Epub 2008 May 12. [DOI] [PubMed] [Google Scholar]
- 24.Mantripragada VP, Piuzzi NS, Zachos T, Obuchowski NA, Muschler GF, Midura RJ. Histopathological assessment of primary osteoarthritic knees in large patient cohort reveal the possibility of several potential patterns of osteoarthritis initiation. Curr Res Transl Med. 2017. November;65(4):133-9. Epub 2017 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum. 2010. August;62(8):2206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA, Martin JA. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012. November;64(11):3626-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Hu C, Yu S, Yan J, Peng H, Ouyang HW, Tuan RS. Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1β/nerve growth factor signaling. Arthritis Res Ther. 2015. November 17;17(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009. April 3;4(4):324-35. [DOI] [PubMed] [Google Scholar]
- 29.Mantripragada VP, Piuzzi NS, Zachos T, Obuchowski NA, Muschler GF, Midura RJ. High occurrence of osteoarthritic histopathological features unaccounted for by traditional scoring systems in lateral femoral condyles from total knee arthroplasty patients with varus alignment. Acta Orthop. 2018. April;89(2):197-203. Epub 2017 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011. February 24;21:202-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006;74:371-403. [DOI] [PubMed] [Google Scholar]
- 32.Muschler GF, Midura RJ, Nakamoto C. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. J Biomed Biotechnol. 2003;2003(3):170-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008. December 30;105(52):20641-6. Epub 2008 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agar G, Blumenstein S, Bar-Ziv Y, Kardosh R, Schrift-Tzadok M, Gal-Levy R, Fischler T, Goldschmid R, Yayon A. The chondrogenic potential of mesenchymal cells and chondrocytes from osteoarthritic subjects: a comparative analysis. Cartilage. 2011. January;2(1):40-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002. March;46(3):704-13. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Zheng H, Buckwalter JA, Martin JA. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthritis Cartilage. 2014. September;22(9):1318-26. Epub 2014 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stich S, Loch A, Park SJ, Häupl T, Ringe J, Sittinger M. Characterization of single cell derived cultures of periosteal progenitor cells to ensure the cell quality for clinical application. PLoS One. 2017. May 31;12(5):e0178560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ando W, Kutcher JJ, Krawetz R, Sen A, Nakamura N, Frank CB, Hart DA. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage. Cytotherapy. 2014. June;16(6):776-88. Epub 2014 Feb 12. [DOI] [PubMed] [Google Scholar]
- 39.Sengers BG, Dawson JI, Oreffo ROC. Characterisation of human bone marrow stromal cell heterogeneity for skeletal regeneration strategies using a two-stage colony assay and computational modelling. Bone. 2010. February;46(2):496-503. Epub 2009 Oct 8. [DOI] [PubMed] [Google Scholar]
- 40.Karsdal MA, Byrjalsen I, Bay-Jensen AC, Henriksen K, Riis BJ, Christiansen C. Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis—the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation. BMC Musculoskelet Disord. 2010. June 17;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady K, Dickinson SC, Hollander AP. Changes in chondrogenic progenitor populations associated with aging and osteoarthritis. Cartilage. 2015. April;6(2)(Suppl):30S-5S. Epub 2015 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008. March;129(3):163-73. Epub 2007 Dec 17. [DOI] [PubMed] [Google Scholar]
- 43.Dexheimer V, Mueller S, Braatz F, Richter W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS One. 2011;6(8):e22980 Epub 2011 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]