Abstract
Objective:
In the REPLENISH trial, women receiving TX-001HR—an oral, softgel capsule, combining 17β-estradiol (E2) and progesterone (E2 mg/P4 mg 1/100, 0.5/100), had significantly improved vasomotor symptoms, while having their endometrium protected from hyperplasia. The objective here was to describe P4 levels sufficient to counteract the potential endometrial effects of 1 or 0.5 mg oral E2 with TX-001HR.
Methods:
In REPLENISH (phase 3; NCT01942668), serum P4, E2, and estrone (E1) levels were characterized in postmenopausal women treated with TX-001HR (E2 mg/P4 mg: 1/100, 0.5/100, [0.5/50, 0.25/50 and placebo not reported here]) at baseline, week 12, and month 12 for P4, and at baseline, weeks 4 and 12, and months 6, 9, and 12 for E2 and E1. In a phase 1 study, pharmacokinetic parameters were assessed after 7 daily doses of oral E2 mg/P4 mg (1/100 and 0.5/100).
Results:
In REPLENISH (n = 1,835), mean P4 levels were 0.39 to 0.55 ng/mL with 100-mg P4 doses; E2 levels were 42.3 to 45.6 pg/mL and 23.0 to 27.4 pg/mL for the 1-mg and 0.5-mg E2 doses, respectively; E1 levels were 214 to 242 pg/mL and 114 to 129 pg/mL for the 1-mg and 0.5-mg E2 doses. In the phase 1 study (n = 40; day 7), mean Cavg for P4 was 0.66 ng/mL with 100-mg P4 doses; E2 was 38.1 pg/mL and 29.2 pg/mL for 1 mg and 0.5 mg E2, respectively; and E1 was 211 and 106 pg/mL for 1 mg and 0.5 mg E2. All three analytes reached steady state within 7 days; accumulation ratios were 1.36 to 1.94.
Conclusions:
P4 levels observed with TX-001HR were similar in the phase 1 and 3 studies, and were associated with no endometrial hyperplasia with either E2 daily dose over 1 year in the REPLENISH phase 3 study, which showed significant improvements in menopausal vasomotor symptoms.
Keywords: Endometrium, Pharmacokinetics, Progesterone, TX-001HR, Vasomotor symptoms
During the menopause transition, decreases in estrogen may cause women to experience vasomotor symptoms (VMS) and genitourinary symptoms of menopause.1 Estrogen therapy has been successfully used for several decades for the management of these menopausal symptoms. However, the prolonged use of unopposed estrogens increases the risk of endometrial hyperplasia2 and endometrial cancer.3,4 In women with a uterus, the addition of a progestin to estrogen therapy has been shown to protect the uterus from estrogen-induced endometrial hyperplasia and endometrial cancer.5,6
Various combinations of estrogen (conjugated equine estrogens [CEEs], ethinyl estradiol, 17β-estradiol [E2]) and synthetic progestogens (norethindrone acetate, drospirenone, norgestimate, medroxyprogesterone acetate [MPA]) products are currently available, including oral and transdermal products. However, oral formulations of E2 and progesterone (P4) in a single capsule have been challenging because of their differences in structure and solubility; as of 2017, no combined oral E2/P4 capsule had been approved by the US Food and Drug Administration (FDA).7 To address this unmet treatment option, clinicians have been prescribing E2 and P4 products separately to their patients. Many women prefer using E2 and P4, as they are bioidentical to the natural hormones produced by the body.8-12 While, up to 21 million US FDA-approved and compounded hormone therapy prescriptions containing P4 were estimated to be filled in the United States per year,13,14 serum P4 levels required for endometrial protection in a continuous-combined regimen have not been well characterized.
An oral hormone therapy (TX-001HR; TherapeuticsMD, Boca Raton, FL) combining natural E2 with P4 in a softgel capsule was investigated for the treatment of moderate to severe VMS in postmenopausal women with a uterus. The phase 3 REPLENISH trial, which evaluated the safety and efficacy of four doses of TX-001HR (E2 mg/P4 mg: 1/100, 0.5/100, 0.5/50, 0.25/50) compared with placebo, found no cases of endometrial hyperplasia or cancer with up to 12 months of continuous use, while significantly reducing the frequency and severity of moderate to severe VMS with the two highest doses.15 In October 2018, the oral 1-mg E2/100-mg P4 dose was approved by the FDA as Bijuva (TherapeuticsMD, Boca Raton, FL).
The primary objective of these analyses was to describe the levels of P4 in the phase 3 REPLENISH trial, which were sufficient to counteract any potential estrogenic effects of 1 or 0.5 mg oral E2 on the endometrial compartment when dosed continuously for 12 weeks. Progesterone levels in a phase 1 study with two of the same doses in REPLENISH were also analyzed. Serum levels of E2 and its main metabolite, estrone (E1) were examined in the phase 3 and phase 1 studies.
METHODS
The serum levels of E2, E1, and P4 with select TX-001HR doses were characterized in two studies: one from a single time point in the phase 3 REPLENISH trial and another from a multitime point phase 1 trial. In both trials, women were administered TX-001HR in the evening with food. Serum hormone levels were quantified at InVentiv Clinical Laboratories, Inc. (Princeton, NJ) using the same validated liquid chromatography or gas chromatography-tandem mass spectrometry (LC/GC-MS/MS) methods.
Both trials were conducted in accordance with the regulations of the International Conference on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent before participating in any study-related activities.
Phase 3 REPLENISH trial
The REPLENISH trial (NCT01942668) was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial evaluating the efficacy and safety of daily doses of E2/P4 in nonhysterectomized, postmenopausal women with vasomotor symptoms.15 The trial was conducted at 117 US sites between August, 2013 and October, 2015. The four co-primary endpoints of the trial were changes from baseline to weeks 4 and 12 in the frequency and severity of moderate to severe VMS compared with placebo, and the primary safety endpoint was the incidence of endometrial hyperplasia at 12 months.15
Inclusion and exclusion criteria of the REPLENISH trial were typical of randomized trials evaluating menopausal hormonal therapies and have been described elsewhere.15 Briefly, healthy postmenopausal women, aged 40 to 65 years, ≤34 kg/m2 with a uterus and who were seeking treatment for VMS were eligible to enroll in the REPLENISH study. At baseline, postmenopausal participants had to have a serum E2 level of ≤50 pg/mL with ≥12 months of spontaneous amenorrhea, or ≥6 months of spontaneous amenorrhea with serum follicle-stimulating hormone (FSH) level of >40 mIU/mL, or ≥6 weeks postoperative bilateral oophorectomy. Women were randomized to daily, oral doses of E2 mg/P4 mg (1/100, 0.5/100, 0.5/50, 0.25/50) or placebo for up to 12 months; data for the E2 mg/P4 mg doses 1/100 and 0.5/100 are presented here. To maintain study blinding, a double-dummy technique was used because different doses were different-sized capsules (ie, all women took two capsules).
Serum hormone concentrations of P4, E2, and E1 were measured in all enrolled women. Serum concentrations were assessed at baseline, week 12 and month 12 for P4 and at baseline, weeks 4 and 12, and months 6, 9, and 12 for E2 and E1. Samples were generally collected 9 to 16 hours after the previous evening dose. Mean hormone levels at each time point for TX-001HR doses are reported in this analysis. Only the E2 mg/P4 mg doses (1/100, 0.5/100) that were registered for US FDA approval have been included in this report.
Phase 1 multidose trial
This phase 1, open-label, randomized, parallel-group, multidose trial assessed serum levels of P4, E2, and E1 in healthy postmenopausal women who received TX-001HR (E2 mg/P4 mg: 1/100 and 0.5/100). The study was conducted at one site in 2017. The primary objectives of the study were to describe the single-dose and steady-state pharmacokinetic (PK) parameters after seven daily doses of TX-001HR.
Healthy women (aged 40-65 years; BMI 18-30 kg/m2) were enrolled if they had ≥12 months of spontaneous amenorrhea, ≥6 months of spontaneous amenorrhea with a screening serum FSH >40 mIU/mL; ≥6 weeks postoperative bilateral oophorectomy; or <55 years of age with a history of hysterectomy without bilateral oophorectomy before natural menopause with screening FSH >40 mIU/mL. Menopausal VMS were not assessed in this trial and were not a requirement for enrollment. Exclusion criteria were similar to those of the REPLENISH trial.
Eligible participants were randomized to two E2 mg/P4 mg doses (1/100 or 0.5/100), which were administered orally, once daily for 7 days. Venous blood samples were obtained on day 1 at 60, 30, and 0 minutes before drug administration (average of these time points represent the baseline) and then at 20, 40, 60, and 90 minutes and 2, 3, 4, 6, 8, 12, 18, and 24 hours after drug administration. On day 7, venous blood samples were obtained at 0, 20, 40, 60, and 90 minutes, and at 2, 3, 4, 6, 8, 12, 18, 24, 36, and 48 hours after drug administration.
The PK parameters assessed included maximum serum concentration (Cmax), time to maximum serum concentration (tmax), area under the concentration-time curve during the dosing interval (τ) calculated using the linear trapezoidal method (AUCτ; calculated using linear interpolation between data points) on days 1 and 7, and Cavg at steady state on day 7. Steady state levels were tested with trough concentrations (Ctrough; serum concentration at the end of the dosage interval prior to the next dose) on days 6, 7, and 24 hours after day 7. Hormone accumulation ratios between day 7 and day 1 (AUCτ day 7/AUCτ day 1) were also assessed.
Analytical methods
In both trials, bioanalysis was performed at InVentiv Clinical Laboratories, Inc. (Princeton, NJ). Venous blood samples of 10 mL each were collected without anticoagulant for serum assessment of P4, E2, and E1.
Progesterone was extracted from serum using a liquid-liquid extraction procedure. P4 bioanalysis was performed using a validated LC-MS/MS assay with a lower limit of quantification (LLOQ) of 0.05 ng/mL and upper limit of quantification (ULOQ) of 50 ng/mL. The coefficient of variation (CV) for P4 standards was 4.65% for between-run precision, and the bias was 2.08% for between-run accuracy.
Estradiol and E1 were extracted from serum using a solid-phase extraction procedure. Simultaneous E2 and E1 bioanalysis was performed using a validated GC-MS/MS. For E2, the LLOQ was 2.0 pg/mL and the ULOQ was 500 pg/mL. For E1, the LLOQ was 5.0 pg/mL and the ULOQ was 1000 pg/mL. The CV for E2 standards was 3.51% for between-run precision and the bias was 0.00% for between-run accuracy, and for E1 standards the CV was 5.37% for between-run precision and the bias was 2.50% for between-run accuracy.
Statistical analyses
For both studies, baseline and demographic variables were descriptively summarized by treatment group. Descriptive and statistical analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC). Data for both studies presented here are unadjusted for baseline values. In the REPLENISH trial, sample values estimated as below the limit of quantification were replaced by the LLOQ for that analyte, as determined during method validation (P4, 0.05 ng/mL; E2, 2.0 pg/mL; E1, 5.0 pg/mL). In the phase 1 study, sample values estimated as below the limit of quantification were replaced by a “0” when calculating the PK parameters, except for when calculating AUCτ which has embedded or trailing values and therefore those values were treated as “missing.” Statistical analyses comparing PK measurements between days 1 and 7 were not performed, and were reported descriptively. Dose proportionality for E2 was analyzed using the following power model: Ln(Y) = alpha + beta ∗ ln(dose) + error, where beta (slope) measures the proportionality between doses; beta = 0 implies dose independence and beta = 1 implies dose proportionality.
RESULTS
Phase 3 REPLENISH trial
A total of 1,845 women were randomized to the study; 1,835 women took one full dose of the study drug and were included in the safety population. Of these, 415 women were randomized to 1 mg E2/100 mg P4, 424 women to 0.5 mg E2/100 mg P4 (with 996 randomized to the remaining doses and placebo; results not included in this report). Of the 1,835 women, 1,275 (69.5%) completed the 12-month study. Overall, baseline demographic characteristics of women randomized to the different E2/P4 doses (Table 1) were not different between treatment groups, including overall mean age (54.6 years) and BMI (26.6 kg/m2). The majority (67%) were white and 31% were African American. Baseline serum P4 levels were below level of quantification (0.05 ng/mL) for the majority of women. Mean baseline serum levels were 6.1 pg/mL for E2 and 23.3 pg/mL for E1.
TABLE 1.
Demographics and baseline characteristics for participants dosed with 1 mg or 0.5 mg E2 combined with 100 mg P4
| REPLENISH trial | Phase 1 study | |||
| Characteristics | 1 mg E2/100 mg P4 | 0.5 mg E2/100 mg P4 | 1 mg E2/100 mg P4 | 0.5 mg E2/100 mg P4 |
| N | 415 | 424 | 20 | 20 |
| Age, y | 54.7 ± 4.4 | 54.5 ± 4.5 | 57.8 ± 5.7 | 56.6 ± 4.7 |
| Race, n (%) | ||||
| White | 271 (65.3) | 281 (66.3) | 16 (80.0) | 17 (85.0) |
| African American | 134 (32.3) | 136 (32.1) | 4 (20.0) | 3 (15.0) |
| Othera | 10 (2.4) | 7 (1.6) | 0 | 0 |
| BMI, kg/m2 | 26.8 ± 4.1 | 26.7 ± 4.3 | 25.1 ± 2.5 | 26.4 ± 2.9 |
| Time since menopause, y | 5.8 ± 4.9 | 6.0 ± 5.1 | 10.0 ± 5.0 | 6.5 ± 3.7 |
| Bilateral oophorectomy, n (%) | 4 (1.0) | 6 (1.4) | 4 (20.0) | 1 (5.0) |
| Hysterectomy, n (%) | 0b | 0b | 6 (30.0) | 3 (15.0) |
| Baseline hormone values | ||||
| Progesterone, ng/mL | 0.056 ± 0.024 | 0.065 ± 0.150 | 0.006 ± 0.015 | 0.034 ± 0.107 |
| Estradiol, pg/mL | 6.3 ± 6.6 | 6.5 ± 7.2 | 6.0 ± 7.6 | 20.4 ± 41.1 |
| Estrone, pg/mL | 23.3 ± 12.6 | 23.3 ± 12.1 | 18.9 ± 7.6 | 32.0 ± 43.7 |
Data shown as mean ± SD, unless stated otherwise.
BMI, body mass index; E2, 17β-estradiol; P4, progesterone; SD, standard deviation.
aOther includes other, Asian, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and unknown.
bAll study participants of the REPLENISH trial were required to have an intact uterus.
Over the 12-month trial, mean levels for P4 were 0.39 to 0.55 ng/mL for the 100-mg P4 doses (Fig. 1A). For E2, mean serum levels were 42.3 to 45.6 pg/mL for the 1-mg E2 dose and 23.0 to 27.4 pg/mL for the 0.5-mg E2 dose (Fig. 1B). For E1, mean serum levels were 214 to 242 pg/mL for the dose containing 1 mg E2 and 114 to 129 pg/mL for the dose containing 0.5 mg E2 (Fig. 1C). A dose response was observed for E2 and E1 with hormone levels remaining consistent over time for each treatment.
FIG. 1.
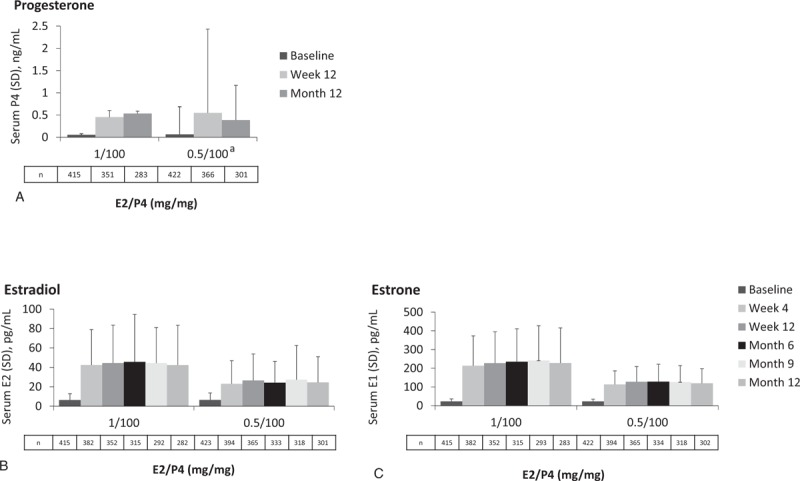
The REPLENISH trial: unadjusted mean hormone concentrations for progesterone (A), estradiol (B), and estrone (C) at baseline and up to 12 months. aThe variation observed in the 0.5-mg E2/100-mg P4 group was driven by one sample (outlier). E1, estrone; E2, 17β-estradiol; P4, progesterone; SD, standard deviation.
Phase 1 multidose trial
In all, 40 participants were randomized into the phase 1 study; 20 in each dose group. Among them, 37 women (92.5%) completed the study; 3 women who were randomized to 0.5 mg E2/100 mg P4 discontinued early (withdrew [n = 2] and lost to follow-up [n = 1]). All 40 randomized participants received at least one dose of study drug and had sufficient data to calculate at least one PK parameter. Baseline demographics (Table 1) were not different between the two treatment groups, including overall mean age (57.2 years) and BMI (25.7 kg/m2). The majority were white (82.5%), followed by African American (17.5%).
Baseline serum P4 levels were below level of quantification (0.05 ng/mL) for the vast majority of women. Mean baseline serum levels were 0.02 ng/mL for P4, 13.2 pg/mL for E2, and 25.5 pg/mL for E1. Women randomized to the 0.5-mg E2/100-mg P4 dose had higher hormone baseline values than those randomized to the 1-mg E2/100-mg P4 dose (Table 1).
Mean serum concentrations for P4, E2, and E1 on day 1 and day 7 are shown in Fig. 2. A summary of unadjusted PK parameters for all three hormones are reported in Table 2. For P4, mean Cmax ranged from 4.4 to 11.3 ng/mL, mean Cavg ranged from 0.53 to 0.77 ng/mL, and mean AUCτ ranged from 12.5 to 18.2 h ng/mL on day 7 with the two 100-mg P4 formulations. Both doses containing 100 mg P4 had an accumulation ratio (AUCτ day 7: AUCτ day 1) of ∼1.4.
FIG. 2.
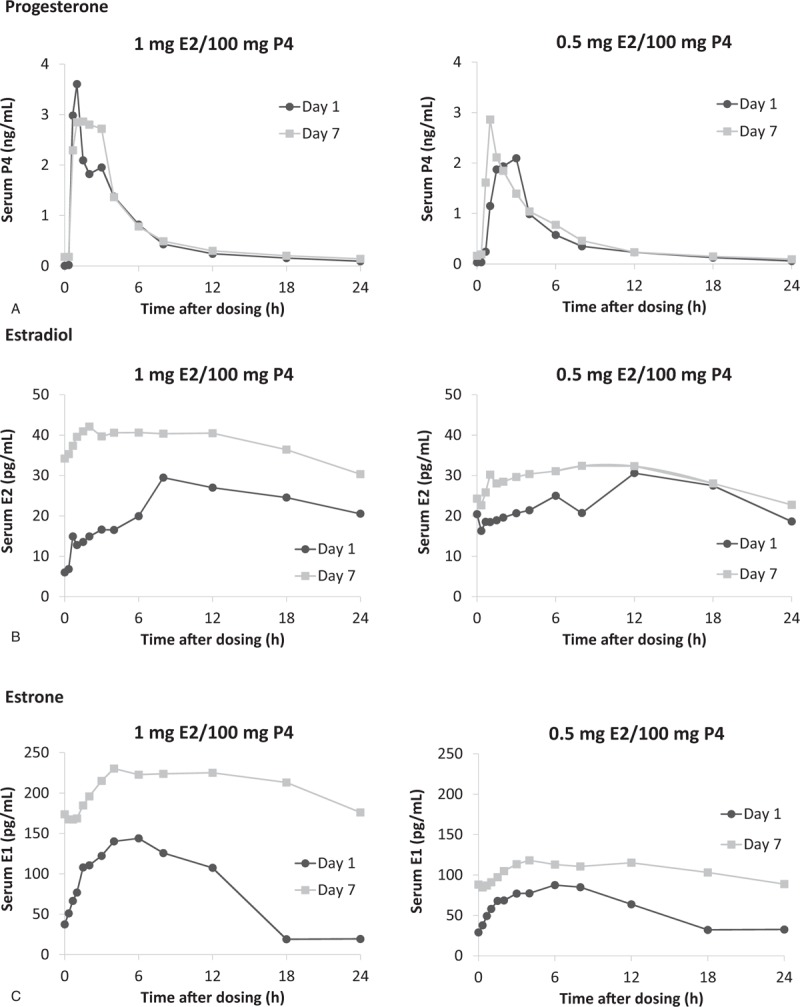
Phase 1 multidose study findings: unadjusted mean serum concentrations for progesterone (A), estradiol (B), and estrone (C) for 1 mg E2/100 mg P4 and 0.5 mg E2/100 mg P4 on day 1 (n = 20) and day 7 (n = 17). E1, estrone; E2, 17β-estradiol; P4, progesterone.
TABLE 2.
Phase 1 study—unadjusted PK parameters for progesterone, estradiol, and estrone on days 1 and 7
| Progesterone | Estradiol | Estrone | |||||
| Parameters (units) | 1 mg E2/100 mg P4 | 0.5 mg E2/100 mg P4 | Parameters (units) | 1 mg E2/100 mg P4 | 0.5 mg E2/100 mg P4 | 1 mg E2/100 mg P4 | 0.5 mg E2/100 mg P4 |
| Day 1 | (n = 20) | (n = 20) | Day 1 | (n = 20) | (n = 20) | (n = 20) | (n = 20) |
| AUCτ, h ng/mL | 14.3 ± 9.9 | 10.6 ± 9.5 | AUCτ, h pg/mL | 543 ± 250 | 559 ± 695 | 2,860 ± 883 | 1,754 ± 836 |
| Cmax, ng/mL | 6.5 ± 6.2 | 3.8 ± 3.2 | Cmax, pg/mL | 37.6 ± 35.5 | 33.8 ± 48.6 | 171 ± 67.3 | 99.2 ± 54.5 |
| tmax, h | 2.2 ± 1.5 | 2.5 ± 1.9 | tmax, h | 10.0 ± 6.8 | 11.1 ± 7.2 | 11.1 ± 5.8 | 11.8 ± 5.8 |
| Day 7 | (n = 20) | (n = 17) | Day 7 | (n = 20) | (n = 17) | (n = 20) | (n = 17) |
| AUCτ, h ng/mL | 18.2 ± 15.5 | 12.5 ± 10.9 | AUCτ, h pg/mL | 910 ± 339 | 699 ± 567 | 5,046 ± 2,155 | 2,538 ± 1,170 |
| Cavg, ng/mL | 0.77 ± 0.64 | 0.53 ± 0.45 | Cavg, pg/mL | 38.1 ± 14.2 | 29.2 ± 23.7 | 211 ± 90.1 | 106 ± 48.9 |
| Cmax, ng/mL | 11.3 ± 23.1 | 4.4 ± 5.7 | Cmax, pg/mL | 48.2 ± 15.8 | 37.2 ± 28.7 | 257 ± 101 | 131 ± 56.2 |
| tmax, h | 2.6 ± 1.5 | 2.9 ± 2.3 | tmax, h | 5.6 ± 5.6 | 5.9 ± 4.4 | 5.5 ± 3.5 | 8.5 ± 4.9 |
| Accumulation ratio | 1.44 ± 0.95 | 1.36 ± 0.73 | Accumulation ratio | 1.81 ± 0.65 | 1.94 ± 1.96 | 1.72 ± 0.41 | 1.54 ± 0.25 |
All data expressed as mean ± SD. Accumulation ratio = AUCτ day 7/AUCτ day 1.
E2, 17β-estradiol; P4, progesterone.
Statistical analysis of the 0.5-mg and 1-mg E2 doses for E2 PK parameters, AUCτ, and Cmax, on days 1 and 7 showed that the observed results were dose-dependent, although not dose-proportional. Both doses had an accumulation ratio of ∼1.9 for E2. Similarly, day 7 mean Cavg for E1 was 211 pg/mL for the 1-mg E2 dose and 106 pg/mL for the 0.5-mg E2 dose. The accumulation ratio for E1 was ∼1.6 for both doses.
Steady states for P4, E2, and E1 were shown by consistent Ctrough levels from predose day 6 through 24 h postdose day 7 (Table 3), and thus, steady state was achieved within 1 week of daily dosing regardless of dose administered.
TABLE 3.
Phase 1 study—unadjusted steady-state (Ctrough) hormone levels
| Progesterone, ng/mL | Estradiol, pg/mL | Estrone, pg/mL | ||||
| Ctrough | 1 mg E2/100 mg P4 (n = 20) | 0.5 mg E2/100 mg P4 (n = 17) | 1 mg E2/100 mg P4 (n = 20) | 0.5 mg E2/100 mg P4 (n = 17) | 1 mg E2/100 mg P4 (n = 20) | 0.5 mg E2/100 mg P4 (n = 17) |
| Day 6 (predose) | 0.15 ± 0.13a | 0.16 ± 0.14b | 28.4 ± 12.1 | 23.1 ± 36.3 | 171.5 ± 81.6 | 88.4 ± 38.4 |
| Day 7 (predose) | 0.18 ± 0.15 | 0.16 ± 0.14 | 34.2 ± 17.4 | 24.2 ± 21.5 | 173.7 ± 83.0 | 88.1 ± 40.6 |
| Day 7 (24-h postdose) | 0.15 ± 0.11c | 0.12 ± 0.07d | 30.3 ± 12.9 | 22.7 ± 18.7 | 176.0 ± 85.3 | 88.8 ± 50.5 |
All data expressed as mean ± SD.
E2, 17β-estradiol; P4, progesterone; SD, standard deviation.
an = 18.
bn = 15.
cn = 19.
dn = 14.
DISCUSSION
Oral doses of 100 mg P4 were sufficient to counteract potential estrogenic stimulation of the endometrium with 1 mg or 0.5 mg of oral E2 for over 12 months, as shown in the REPLENISH trial.15 Serum P4 levels averaged 0.39 to 0.55 ng/mL with the 100-mg P4 doses in the phase 3 study, generally 9 to 16 hours after dosing. Similar concentrations were observed in the phase 1 study, with serum P4 levels averaging 0.53 to 0.77 ng/mL with the 100-mg P4 doses after 7 days of treatment at 8 to 12 hours after dosing. While these trough levels of P4 are lower than luteal phase levels in ovulatory women, these values are significantly higher than the generally undetectable baseline levels of postmenopausal and sufficient to ensure endometrial protection.
There are few PK data using micronized P4. In our phase 1 study, Cmax P4 levels were 11.3 ng/mL for 1-mg E2/100-mg P4 doses and 4.4 ng/mL for 0.5 mg E2/100 mg P4 after 7 days of daily treatment. One older study reported a Cmax of 17.3 ng/mL after only 5 days of oral 100 mg P4 (assessment method not reported),16 whereas another reported a Cmax of 6.5 ng/mL after 1 day of oral 100 mg P4 (analyzed by radioimmunoassay).17 Another small study only examined levels over time and found P4 levels of 1.16 ng/mL after about 2 weeks (analyzed by competitive binding immunoenzymatic assay).18 While this latter value is more similar to our data within several hours of dosing, there are clearly differences in assay methodology between these studies.
Historically, very high oral doses of crystalline P4 were needed to have biological effects due to low absorption.19 Better absorption was achieved with micronized P4 particles suspended in an oil-based formulation.19 Creating a product that combines E2 and P4 in a single oral pill has also been challenging due to the differing structures and solubilities of E2 and P4, and could have resulted in poor P4 bioavailability. The unique formulation of TX-001HR allows E2/P4 to be combined in a single softgel capsule, while retaining pharmacologically effective absorption of both E2 and P4 as shown in this report.
The REPLENISH trial is the first randomized controlled trial, powered to detect endometrial hyperplasia to show that continuous daily use of oral 100 mg P4 with 0.5 or 1 mg E2 can protect postmenopausal women from endometrial hyperplasia.15 Multiple clinical trials have demonstrated that sequential use of oral 200 mg P4 for 12 to 14 days each cycle may offer protection against estrogen-induced endometrial hyperplasia.2,20-22 The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial was the first randomized controlled trial to evaluate the potential protective effect of P4 on the endometrium from estrogen-induced stimulation. While the PEPI study was not fully powered to demonstrate endometrial protection, women taking 200 mg P4 cyclically for 12 days plus 0.625 mg CEE for up to 36 months had rates of endometrial hyperplasia similar to that of placebo.2 On the contrary, two European observational studies—the Etude Epidémiologique Auprès de Femmes de l’Education Nationale (E3N) and the European Prospective Investigation into Cancer and Nutrition (EPIC) trials—reported an increased risk of endometrial cancer with P4 use.23,24 The risk of endometrial cancer in these studies was not analyzed by type of dose or timing (sequential or continuous), and therefore their conclusions may be of limited applicability.23,24 Also, it was postulated by Gompel25 that because the risk of endometrial cancer was similar between the E2-only and the E2 with P4 treatments in those studies, which is at odds with the results of other randomized controlled trials or observational cohort studies, that it might have been caused by a lack of compliance with P4, which was not present in any oral combination, and was taken separately from the E2. In contrast, a French study using percutaneous E2 and micronized P4 continuously for 21 to 25 days detected no hyperplasia and reported that the endometria was generally inactive on biopsy.26 It has been recognized that, with respect to endometrial protection, the duration of progestogen treatment is more important than the dose, but that the dose, regimen, route of administration, and ratio of estrogen to P4 are also important factors.27
It has been shown that relatively low doses of P4 can inhibit endometrial glandular mitotic activity, and that this is related to the duration of treatment, requiring at least 9 days or more of exposure.28 It has also been argued that using larger doses of P4 on a sequential basis and causing full secretory transformation of the endometrium are not necessary and does not provide additional benefit for the prevention of hyperplasia.28 Constant doses of P4, as the ones observed in these trials, could down-regulate endometrial estrogen receptors and turn off mitotic activity and proliferation. Most of the participants in the REPLENISH trial had an atrophic endometrium after 1 year of therapy.15 Based on these results, the continuous daily exposure of P4 appears to be sufficient to oppose the action of E2 on the endometrium, despite P4's shorter duration of elevated serum concentration. One hypothesis that could explain this is that the effects of P4 on gene expression at the tissue level outlast its measurable serum level.
The combination of P4 with E2 in the same capsule did not inhibit the effects of E2 on VMS relief. TX-001HR containing 1 mg or 0.5 mg of E2 combined with 100 mg P4 in the REPLENISH trial significantly improved the frequency and/or severity of moderate to severe VMS as early as 3 weeks and up to 12 weeks.15 There is also evidence that progestogens alone can be effective for treatment of VMS at higher doses.29 In the REPLENISH trial, E2 serum levels averaged 42.3 to 45.6 pg/mL for the 1-mg E2/100-mg P4 dose and 23.0 to 27.4 pg/mL for the 0.5-mg E2/100-mg P4 dose over 12 months. Similar values for E2 were also observed in the phase 1 study, with serum levels averaging 38.1 pg/mL for the 1-mg E2/100-mg P4 dose and 29.2 pg/mL for the 0.5-mg E2/100-mg P4 dose on day 7, with steady-state levels achieved within 7 days.
TX-001HR is the first oral formulation that combines bioidentical E2 and P4 in a single softgel capsule being evaluated for the treatment of moderate to severe VMS in postmenopausal women with a uterus. Combining both E2 and P4 in a single capsule may facilitate compliance and convenience to the patient,30 but may also increase efficacy and safety while reducing the incidence of withdrawal bleeding.15,31,32 Additionally, various studies have suggested that bioidentical P4 may have a better side effect profile than synthetic progestogens regarding breast cancer33,34 and venous thromboembolism.35
Some of the limitations of these studies include the fact that the populations were mostly healthy, which may limit the applicability of the results to the general population. Also, while demographic characteristics were similar between the two treatment groups of the phase 1 study, hormone levels appeared somewhat different at baseline.
CONCLUSIONS
In the REPLENISH study, levels of P4 were associated with endometrial protection from E2 administered at 1 mg or 0.5 mg daily over 12 months, and levels of E2 were associated with improvement in moderate to severe VMS frequency and severity by week 3.15 Similar P4, E2, and E1 levels were observed in this phase 1 PK study and the phase 3 REPLENISH trial, with steady state levels of the three hormones achieved within 7 days. Continuous dosing with 100 mg P4 appears to be adequate to counteract the potential estrogenic effects of 0.5 mg or 1 mg E2 on the endometrium. The evaluations reported here help to further our understanding of levels of P4 required for endometrial protection when dosed in a continuous combined regimen with E2 in postmenopausal hormone therapy.
Acknowledgment
The authors acknowledge the medical writing assistance provided by Dominique J. Verlaan, PhD, of Precise Publications, LLC (Bedminster, New Jersey).
Footnotes
Funding/support: TherapeuticsMD (Boca Raton, FL) sponsored the studies and supported the medical writing assistance of Dominique J. Verlaan, PhD of Precise Publications, LLC (Bedminster, NJ).
Financial disclosure/conflicts of interest: Dr Lobo has received research grants from TherapeuticsMD and has served as a consultant (in the past 3 years) to Allergan, AMAG, JDS Therapeutics, Mithra, Pfizer, Teva, and TherapeuticsMD. Dr Liu consults for Allergan, Bayer Healthcare, Pfizer, and TherapeuticsMD; and has received research support (paid to UH Cleveland Medical Center) from AbbVie, Allergan, Bayer Healthcare, Ferring, and Palatin. Dr Stanczyk consults for Agile Therapeutics, Dr Reddy's Laboratories, Mithra, Pantarhei, and TherapeuticsMD. Dr Constantine consults to multiple pharmaceutical companies including, but not limited to, TherapeuticsMD, and has stock options from TherapeuticsMD. Dr Pickar consults for Pfizer, Shionogi, and TherapeuticsMD; and has stock options with TherapeuticsMD. Drs Shadiack, Bernick, and Mirkin are employees of TherapeuticsMD with stock/stock options. Dr Bernick is also a Board member of TherapeuticsMD.
REFERENCES
- 1.Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Writing Group for the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. JAMA 1996; 275:370–375. [DOI] [PubMed] [Google Scholar]
- 3.Mack TM, Pike MC, Henderson BE, et al. Estrogens and endometrial cancer in a retirement community. N Engl J Med 1976; 294:1262–1267. [DOI] [PubMed] [Google Scholar]
- 4.Thom MH, Studd JW. Oestrogens and endometrial hyperplasia. Br J Hosp Med 1980; 23:506.508-509, 511-513. [PubMed] [Google Scholar]
- 5.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev 1997; 18:502–519. [DOI] [PubMed] [Google Scholar]
- 6.Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two-year substudy results. Fertil Steril 2003; 80:1234–1240. [DOI] [PubMed] [Google Scholar]
- 7.Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B. Pharmacokinetics of the first combination 17beta-estradiol/progesterone capsule in clinical development for menopausal hormone therapy. Menopause 2015; 22:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002; 288:321–333. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians, Gynecologists. Compounded bioidentical menopausal hormone therapy. Committee Opinion No. 532. Obstet Gynecol 2012; 120:411–415. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Kaunitz AM. Menopause management: getting clinical care back on track. N Engl J Med 2016; 374:803–806. [DOI] [PubMed] [Google Scholar]
- 11.Gass ML, Stuenkel CA, Utian WH, et al. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause 2015; 22:1276–1284. [DOI] [PubMed] [Google Scholar]
- 12.Fishman JR, Flatt MA, Settersten RA., Jr Bioidentical hormones, menopausal women, and the lure of the “natural” in U.S. anti-aging medicine. Soc Sci Med 2015; 132:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirkin S. Evidence on the use of progesterone in menopausal hormone therapy. Climacteric 2018; 21:346–354. [DOI] [PubMed] [Google Scholar]
- 14.TherapeuticsMD. TXMD overview November 2017. Available at: https://ir.therapeuticsmd.com/static-files/c2e7f9d1-d150-4b4d-8703-e4f5c362169c Accessed January 7, 2018. [Google Scholar]
- 15.Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol–progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2018; 132:161–170. [DOI] [PubMed] [Google Scholar]
- 16.Prometrium® (Progesterone, USP) Prescribing Information. St. Petersburg, FL: Catalent Pharma Solutions; 2011. [Google Scholar]
- 17.Simon JA, Robinson DE, Andrews MC, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril 1993; 60:26–33. [PubMed] [Google Scholar]
- 18.Sood R, Warndahl RA, Schroeder DR, et al. Bioidentical compounded hormones: a pharmacokinetic evaluation in a randomized clinical trial. Maturitas 2013; 74:375–382. [DOI] [PubMed] [Google Scholar]
- 19.Ruan X, Mueck AO. Systemic progesterone therapy: oral, vaginal, injections and even transdermal? Maturitas 2014; 79:248–255. [DOI] [PubMed] [Google Scholar]
- 20.Pelissier C, Maroni M, Yaneva H, Brin S, Peltier-Pujol F, Jondet M. Chlormadinone acetate versus micronized progesterone in the sequential combined hormone replacement therapy of the menopause. Maturitas 2001; 40:85–94. [DOI] [PubMed] [Google Scholar]
- 21.Van Gorp T, Neven P. Endometrial safety of hormone replacement therapy: review of literature. Maturitas 2002; 42:93–104. [DOI] [PubMed] [Google Scholar]
- 22.Moyer DL, Felix JC, Kurman RJ, Cuffie CA. Micronized progesterone regulation of the endometrial glandular cycling pool. Int J Gynecol Pathol 2001; 20:374–379. [DOI] [PubMed] [Google Scholar]
- 23.Allen NE, Tsilidis KK, Key TJ, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol 2010; 172:1394–1403. [DOI] [PubMed] [Google Scholar]
- 24.Fournier A, Dossus L, Mesrine S, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992-2008. Am J Epidemiol 2014; 180:508–517. [DOI] [PubMed] [Google Scholar]
- 25.Gompel A. Progesterone, progestins and the endometrium in perimenopause and in menopausal hormone therapy. Climacteric 2018; 21:321–325. [DOI] [PubMed] [Google Scholar]
- 26.Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimal micronized progesterone dose. A multicenter study. Maturitas 1994; 19:103–115. [DOI] [PubMed] [Google Scholar]
- 27.Stanczyk FZ, Paulson RJ, Roy S. Percutaneous administration of progesterone: blood levels and endometrial protection. Menopause 2005; 12:232–237. [DOI] [PubMed] [Google Scholar]
- 28.Moyer DL, de Lignieres B, Driguez P, Pez JP. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril 1993; 59:992–997. [DOI] [PubMed] [Google Scholar]
- 29.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med 1994; 331:347–352. [DOI] [PubMed] [Google Scholar]
- 30.Ellerington MC, Whitcroft SI, Whitehead MI. HRT: developments in therapy. Br Med Bull 1992; 48:401–425. [DOI] [PubMed] [Google Scholar]
- 31.Sutton SS, Hardin JW, Bramley TJ, D'Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care 2016; 22:242–248. [PubMed] [Google Scholar]
- 32.Coca A, Agabiti-Rosei E, Cifkova R, Manolis AJ, Redon J, Mancia G. The polypill in cardiovascular prevention: evidence, limitations and perspective - position paper of the European Society of Hypertension. J Hypertens 2017; 35:1546–1553. [DOI] [PubMed] [Google Scholar]
- 33.Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among post-menopausal women in France. PLoS One 2013; 8:e78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol 2008; 26:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol 2010; 30:340–345. [DOI] [PubMed] [Google Scholar]


