Abstract
Purpose:
Our previous study demonstrated that Mab2F1, a murine monoclonal antibody blocking the Wnt/β-catenin signaling pathway, has beneficial effects on experimental diabetic retinopathy and choroidal neovascularization (NV). The aforementioned antibody has been humanized. This study evaluated effects of the humanized antibody, H1L1, on NV.
Methods:
H1L1 was evaluated in the alkali burn-induced corneal NV rat model. Rats with corneal NV were injected subconjunctivally with Mab2F1 or H1L1 using non-specific mouse or human IgG as controls. Corneal NV and opacity were evaluated using corneal NV area and inflammatory index. Expression of angiogenic and inflammatory factors and components of the Wnt/β-catenin pathway in both the corneas of the animal model and human corneal epithelial (HCE) cells exposed to Wnt3a conditioned medium (WCM) were determined by Western blotting and a luciferase-based promoter assay. Cytotoxicities of these antibodies were evaluated by MTT assay.
Results:
H1L1 reduced the area of corneal NV and opacity, similar to Mab2F1. Both Mab2F1 and H1L1 down-regulated the overexpression of angiogenic and inflammatory factors including VEGF, TNF-α and ICAM-1, and blocked the aberrant activation of the Wnt/β-catenin pathway as shown by down-regulation of phosphorylated LRP6, total LRP6 and non-phosphorylated β-catenin in the cornea of the NV model and cultured HCE cells exposed to WCM. Both antibodies also inhibited the transcriptional activity of β-catenin induced by WCM in HCE cells. No toxic effects of the antibodies were observed in cultured HCE cells.
Conclusions:
H1L1 exhibits anti-angiogenic activities through blocking the Wnt/β-catenin pathway.
Keywords: Cornea, Humanized antibody, Inflammation, Neovascularization, Vision, Wnt pathway, LRP6
1. Introduction
Angiogenesis, the formation of new blood vessels, is closely associated with a number of diseases such as cancer, heart disease, stroke, and ocular neovascularization (NV) (Folkman, 2007; Raimondi et al., 2014). Angiogenesis proceeds through a multistep process including destabilization of the integrated blood vessel, endothelial cell proliferation, migration, and capillary tube formation (Li et al., 2003). Multiple growth factors, their receptors, and intracellular signaling pathways have been identified in each step of angiogenesis (Carmeliet and Collen, 2000; Shibuya, 2014). Therefore, targeting one or more of these pathways may provide novel treatments for angiogenesis.
The Wnt/β-catenin signaling pathway comprises an important intracellular signaling pathway (Klaus and Birchmeier, 2008). Activation of the Wnt/β-catenin signaling pathway occurs when Wnt ligands bind to cell surface receptors frizzled (Fzd) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), resulting in phosphorylation and activation of LRP5/6, consequently increasing the levels of non-phosphorylated β-catenin (non-p-β-catenin). Non-p-β-catenin accumulates in the cytoplasm and is subsequently translocated into the nucleus. In the nucleus, β-catenin forms a complex with members of T cell factor/lymphoid enhancer-binding factors (TCF/LEF), to regulate the transcription of downstream target genes such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), and intracellular adherent molecular-1 (ICAM-1) (Chen and Ma, 2017). The Wnt/β-catenin signaling pathway is involved in numerous aspects of growth and development in many organs and tissues, ranging from cell fate determination, polarity and differentiation to migration, proliferation and function (Moon et al., 2002; Visweswaran et al., 2015). However, the aberrant activation of Wnt/β-catenin signaling pathway plays crucial roles in various diseases including cancer and ocular angiogenic disorders (Chen et al., 2009; Dejana, 2010; Hu et al., 2013; Wang et al., 2015; Zerlin et al., 2008). Inhibition of the Wnt/β-catenin signaling pathway is expected to have beneficial effects on these related diseases. For the past 30 years, therapeutic strategies inhibiting this pathway are increasingly being proposed to aid the fight against these Wnt-related diseases and offer enormous promise (Sebio et al., 2014).
Mab2F1, a murine monoclonal antibody blocking the Wnt/β-catenin signaling pathway through specific binding to the ligand-binding domain of LRP6, was generated in our group (Lee et al., 2012). Previously, we reported that Mab2F1 has anti-angiogenic activities both in vitro and in vivo, showing inhibition of endothelial cell proliferation, migration and tube formation in cell culture models, and suppression of choroidal NV and inflammation in the laser-induced choroidal NV model and ischemia-induced retinal NV in the oxygen-induced retinopathy (OIR) model (Hu et al., 2013; Lee et al., 2012). To develop an antibody-based drug candidate, Mab2F1 was recently humanized and designated H1L1. H1L1 targets the same epitope in LRP6 as the parent antibody, Mab2F1. In the present study, we investigated whether H1L1 has anti-angiogenic activity in an alkali burn-induced corneal NV rat model and further verified whether the beneficial effects are mediated by blocking the Wnt/β-catenin signaling pathway.
2. Materials and methods
2.1. Animals
Sprague-Dawley rats (Male, 8 week-old) were purchased from Charles River (Wilmington, MA). They were housed in a temperature-controlled animal facility with a 12-h light-dark cycle. Food and water were available ad libitum. Animal experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Care, use, and treatment of experimental animals were in strict agreement with the Association of Research in Visual Sciences and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. In all procedures, animals were anesthetized by intramuscular injection of 50 mg kg−1 ketamine hydrochloride mixed with 5 mg kg−1 xylazine (Vedco, St. Joseph, MO, USA).
2.2. In vivo experimental procedure
Rats were randomly assigned into six groups (n = 6 in each group):(1) normal control group, without alkali burn or treatment; (2) alkali burn group, alkali burn without treatment; (3) mouse IgG control group, alkali burn treated with non-specific mouse IgG, as control of the Mab2F1 group; (4) Mab2F1 group, alkali burn treated with Mab2F1; (5) human IgG control group, alkali burn treated with non-specific human IgG, as the control of the H1L1 group; and (6) H1L1 group, alkali burn treated with H1L1. The dose of all treatment agents is 50 μg per 50 μL. Only the right eye of each rat was used. The antibodies were subconjunctivally injected at days 0, 2 and 4 after the injury. Subconjunctival injections were performed with a needle of 30-gauge inserted at the superior bulbar conjunctiva under an operating microscope. Investigators were blinded to the treatments by masked labeling of the drugs, and blinding was continued throughout the experimental phase by the use of randomly assigned numerical identifiers for each rat and labeling as groups 1, 2, 3, 4, 5 and 6 to mask the treatment. The clinical evaluations of corneal NV and inflammatory index were performed on days 4, 6 and 8, and the corneas were dissected for Western blot analysis on day 8 after the injury.
2.3. Corneal NV induced by alkali burn
Corneal NV was induced in adult Sprague Dawley rats as described previously with minor modifications (Wang et al., 2014). Briefly, after the animals were anesthetized, a piece of round filter paper (3.5 mm in diameter), soaked in 1 N NaOH, was applied onto the center of the corneal surface for 30 s. Then the ocular surface was rinsed with 30 mL of PBS solution.
2.4. Evaluation of corneal NV
Corneal NV was quantified as described previously (Zhou et al., 2014). Briefly, rats were anesthetized, and images of corneal NV were captured under an operating microscope. The cornea was divided into 4 quarters. The vessel length of each quarter was measured using a vernier caliper. The area (A) of corneal NV was calculated using the following equation: (π = 3.1416; i refer to each quarter of the cornea; L is the length of the selected vessel; and R is the radius of rat cornea).
2.5. Evaluation of corneal inflammatory index
The corneal opacity and edema was evaluated using the inflammatory index as described previously (Laria et al., 1997; Zhou et al., 2014). Briefly, the inflammatory index was evaluated under operating microscope measured based on the following parameters: ciliary hyperemia (0 = no ciliary hyperemia; 1 = present < 1 mm; 2 = present between 1 and 2 mm; 3 = present > 2 mm); central corneal edema (0 = no edema; 1 = present with visible iris details; 2 = present without visible iris details; 3 = present without visible pupil); and peripheral corneal edema (0 = no edema; 1 = present with visible iris details; 2 = present without visible iris details; 3 = present with no visible iris). The final inflammatory index was calculated using the sum of scores of these three parameters which was divided by a factor of 9.
2.6. Antibody preparation
Mab2F1 was raised using a recombinant peptide comprising the E1E2 domain of LRP6 as described previously (Hu et al., 2013; Lee et al., 2012). 2F1 was humanized using a proven method derived from Dr. Cary Queen’s work at Protein Design Labs (Queen, 1989) (manuscript in preparation) that retains the epitope binding sequences of the mouse antibody. The humanized variable light chain (VL) was fused to the human kappa constant region, and the humanized variable heavy VH region was fused to the human IgG2 Fc.
The antibody H1L1 was made by co-transfecting expression vectors encoding the heavy and light chains in 293F cells (Invitrogen, Carlsbad, CA) using PEI. Subsequently, the antibody was purified by affinity chromatography using protein G (Thermo Scientific, Barrington, IL, USA) and concentrated using Centricon filter centrifugation (EMD Millipore). Then the purified antibodies were dialyzed against PBS. The antibodies were tested using a chromogenic LAL Assay (Lonza) to ensure low levels of endotoxin. Using ARPE19 TOPFLASH cells, the Wnt inhibitory activity of the purified antibody was examined by TOPFLASH assay, a luciferase-based promoter activity assay as described previously (Lee et al., 2012). The nonspecific mouse IgG and human IgG were purchased from Sigma (Saint Louis, MO).
2.7. Human corneal epithelial (HCE) cells culture
The SV-40 immortalized HCE cell line (10.014 pRSV-T; American Type Culture Collection, Manassas, VA) was maintained in Keratinocyte Serum-Free Medium (KSFM) (17005042, Gibco, Grand Island, NY, USA) supplemented with 0.05 mg mL−1 bovine pituitary extract, 5 ng mL−1 recombinant human epidermal growth factor, 0.005 mg mL−1 insulin, 500 ng mL−1 hydrocortisone at 37°C and 5% CO2. The culture medium was changed every 2 days. Cells from passage 5 to 7 after purchase were used for the experiments. In an individual experiment, cells of the same passage were used.
For studying the effects of Mab2F1 and H1L1 on activation of the Wnt/β-catenin signaling pathway in HCE, HCE cells at 70% confluency were pre-treated with Mab2F1, H1L1, mouse IgG or human IgG for 2 h. Then the cells were incubated with 10% Wnt3a cell conditioned medium (WCM) for 24 h, with the L cell conditioned medium (LCM) as the controls. Thereafter, the cells were lysed and prepared for Western blot analysis.
2.8. MTT assay
The viability of HCE cells was determined using MTT assay. Briefly, HCE cells were seeded in 96-well plates at a density of 1 × 104 cells per well in KSFM cultured conditions for 24 h. The cells were incubated with Mab2F1 (25–100 μg mL−1), or H1L1 (25–100 μg mL−1), with the same volume of mouse IgG and human IgG as controls for the desired time. Thereafter, 20 μL MTT was added to the culture medium and incubated for another 4 h. The absorbance was recorded at a wavelength of 570 nm using a microplate reader (Pelkin Elmer Victor 3®1420 Multi label count Wallac, Shelton, CT, USA).
2.9. Western blot analysis
Proteins were extracted from the corneas or cells with cold RIPA buffer, and concentrations were measured using a BCA assay kit (Pierce, Rockford, IL). Equal amounts (50 μg per lane) of total protein were subjected to electrophoresis on 8% SDS polyacrylamide gel electrophoresis and then electro-transferred onto nitrocellulose membranes. After blocking with 10% fat-free milk for 1 h, the membranes were incubated with primary antibodies overnight at 4°C, and then incubated with the HRP-conjugated secondary antibody for 1 h. The specific bands were visualized by enhanced chemiluminescence re-agents (Pierce, Rockford, IL, USA), recorded by the transilluminator (ChemiDox XRS; Bio-Rad, USA) and quantified by densitometry using Image J and normalized by β-actin. Primary antibodies were the rabbit antibodies against phosphorylated LRP6 (pLRP6) (Cell Signaling Technology, Cat#2568), total LRP6 (Santa Cruz, Cat#sc-15399), nonphospho-β-catenin (Cell Signaling Technology, Cat#8814), VEGF (Abcam, Cat#ab46154), ICAM-1 (Abcam, Cat#ab27536), and the mouse antibody against β-actin antibody (Sigma-Aldrich, Cat#A5441).
2.10. Luciferase assay
HCE cells were seeded in 24-well plates and transfected with 0.25 mg TOPFLASH (TCF reporter plasmid; Firefly luciferase) and 0.05 mg pRL-TK (pRL reporter plasmid; Renilla luciferase) constructs using lipofectamine 2000. After 4 h, the cells were incubated with fresh media for 24 h. The cells were pre-treated with Mab2F1 or H1L1 at concentration of 25 μg mL−1, with the same amounts of mouse and human IgG as controls for 2 h, and then incubated with 10% WCM, with the LCM as the control for 16 h. Thereafter, the cells were lysed and luciferase activity was measured using a dual luciferase assay kit (Promega, Madison, WI, USA) following the manufacturer’s protocol. Renilla reniformis luciferase activity was measured to normalize transfection effciency. All experiments were performed at least in triplicate.
2.11. Statistical analysis
The analysis was performed with SPSS 17.0 for Windows (Chicago, IL, USA). Data were expressed as mean ± SEM, and analyzed using One-way or Two-way analysis of variance test (ANOVA), followed by the Bonferroni post hoc analysis to compare the differences between the two groups. Tests were two-tailed, and the level of probability (P) was set at P < 0.05 for all experiments.
3. Results
3.1. Inhibitory effects of Mab2F1 and H1L1 on angiogenesis in alkali burn-induced corneal NV
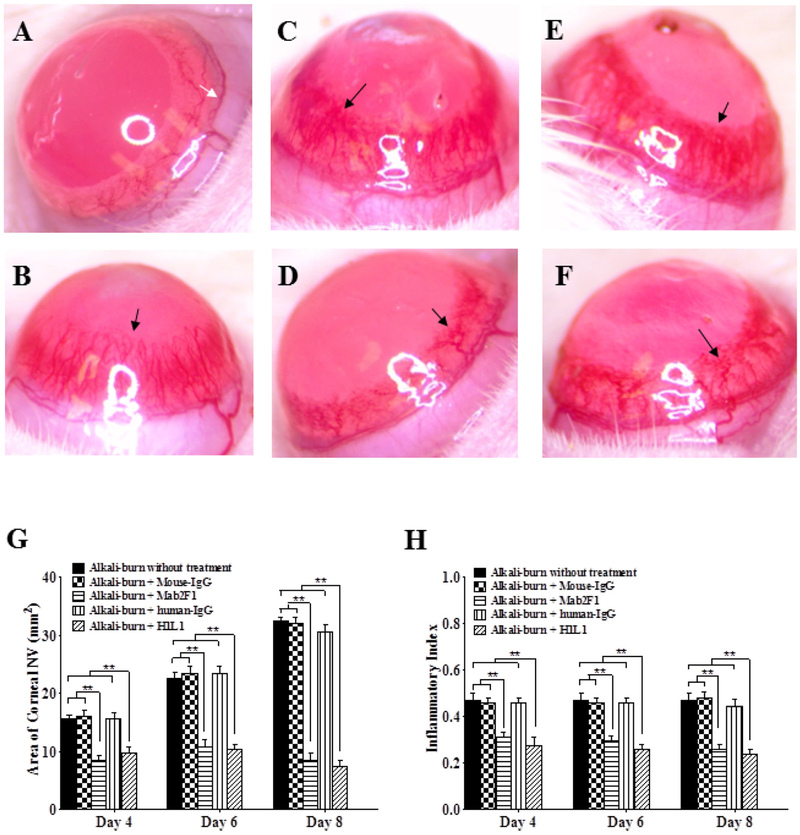
The corneal angiogenesis model was used to examine the anti-angiogenic activity of H1L1. On day 8 after alkali burn, significant NV was observed in the corneas of control rats with alkali burn without treatment, alkali burn treated with isotype control mouse IgG or human IgG, while there was significantly decreased NV in the alkali burn rats treated with Mab2F1 or H1L1 human monoclonal antibody (Fig. 1A–F). Quantification of the corneal NV area showed that Mab2F1 and H1L1 both significantly decreased corneal NV areas on days 4, 6 and 8 after the injury, while non-specific mouse and human IgG had no significant effects at any of the time points (Fig. 1G). Alkali burn also induced apparent edema and opacity of the cornea as demonstrated by the increase of inflammatory index (Fig. 1H), which was significantly reduced by Mab2F1 and H1L1 treatment on days 4, 6 and 8 after the injury, while control mouse IgG and human IgG had no effect at these time points. These results indicated that H1L1 has anti-angiogenic activities in this in vivo angiogenesis model, with activities similar to those of murine antibody Mab2F1.
Fig. 1.
Mab2F1 and H1L1 Inhibit Corneal Neovascularization (NV) and Opacity Induced by Alkali Burn. A–F: Representative images of the cornea on day 8 after alkali burn. A: Normal cornea without alkali burn; B: Alkali burn without treatment; C: Alkali burn with non-specific mouse IgG treatment; D: Alkali burn with Mab2F1 treatment; E: Alkali burn with non-specific human IgG treatment; F: Alkali burn with H1L1 treatment. White arrow indicated initial vessels boundary of corneal limbus; black arrow indicated corneal NV. G: Quantification of corneal NV areas on days 4, 6 and 8 after the injury. H: Quantification of the inflammatory index on days 4, 6 and 8 after the injury. Data were presented as Mean ± SEM (n = 6 per group) and analyzed by One-way analysis of variance test, followed by the Bonferroni post hoc analysis to compare the differences between the two groups. **P < 0.01, versus alkali-burn without treatment group; ## P < 0.01, Mab2F1 group versus mouse IgG group; ξξ P < 0.01, H1L1 group versus human IgG group.
3.2. Mab2F1 and H1L1 attenuate the overexpression of angiogenic and inflammatory factors in rat cornea with alkali burn
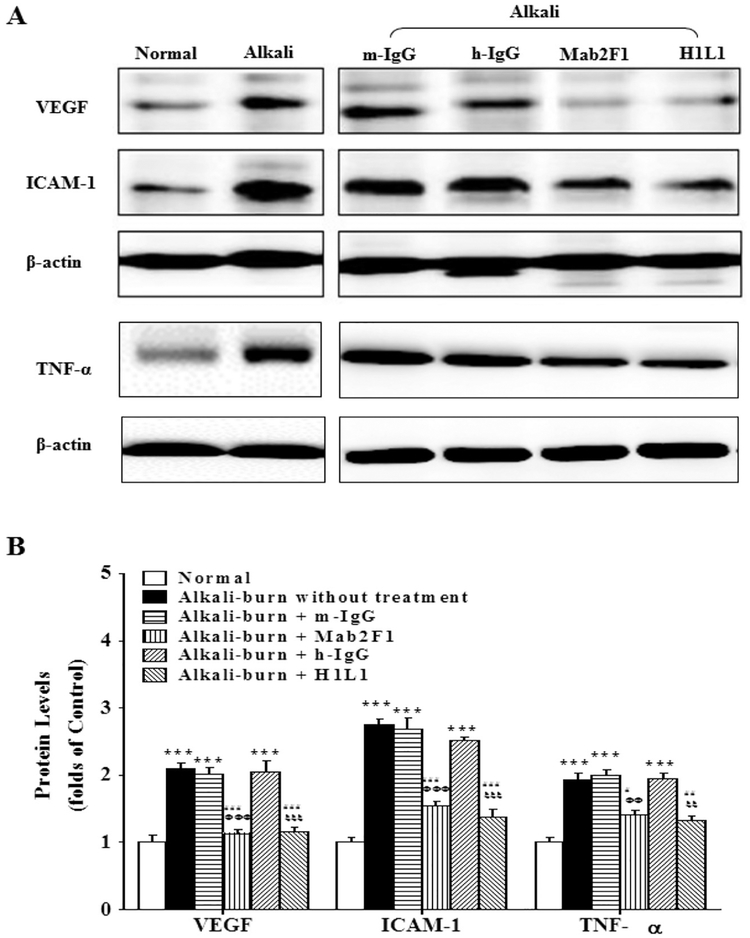
To investigate the mechanism underlying the anti-angiogenic effects of Mab2F1 and H1L1 in the cornea, we measured pro-angiogenic and pro-inflammatory factors VEGF, TNF-α, and ICAM-1. As shown by Western blot analysis, alkali burn significantly induced overexpression of VEGF, TNF-α and ICAM-1 on day 8 after alkali burn, compared to control corneas (Fig. 2). Mab2F1 and H1L1, but not the nonspecific mouse and human IgG, significantly down-regulated levels of VEGF, TNF-α and ICAM-1 (Fig. 2) in the corneas with alkali burn. These results demonstrate that the beneficial effects of Mab2F1 and H1L1 on corneal NV are through downregulating overexpression of angiogenic and inflammatory factors.
Fig. 2.
Mab2F1 and H1L1 downregulate the overexpression of angiogenic and inflammatory factors in the rat corneas with alkali burn. A: Western blot analysis of VEGF, TNF-α and ICAM-1. B: VEGF, TNF-α and ICAM-1 levels were semi-quantified by densitometry and normalized to β-actin levels. Data were presented as mean ± SEM (n = 3 per group) and analyzed by Two-way ANOVA, followed by the Bonferroni post hoc analysis to compare the differences between the two groups. **P < 0.01, versus normal group. # P < 0.05, versus alkali burn without treatment; ## P < 0.01, versus alkali-burn without treatment. ΦΦ P < 0.01, Mab2F1 group versus mouse IgG (m-IgG) group. ξ P < 0.05, H1L1 group versus human IgG group (h-IgG); ξξ P < 0.01, H1L1 group versus h-IgG group.
3.3. Mab2F1 and H1L1 block the abnormal activation of the Wnt/β-catenin signaling pathway in alkali burn-induced corneal NV
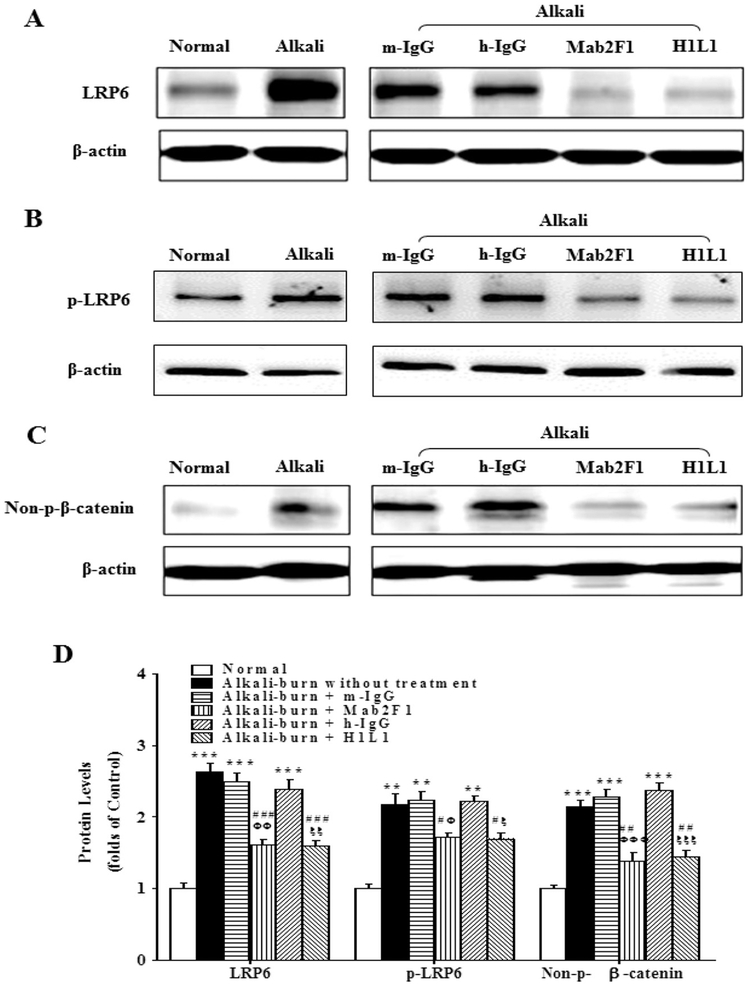
To determine whether the inhibitory effects of Mab2F1 and H1L1 on corneal NV are through blocking activation of the Wnt/β-catenin signaling pathway, we measured components of the Wnt/β-catenin signaling pathway including p-LRP6, total LRP6 and non-p-β-catenin levels by Western blot analysis 8 days after alkali burn. As shown in Fig. 3, levels of p-LRP6, LRP6 and non-p-β-catenin were significantly increased in the rat corneas with alkali burn, compared to that of normal control rats. Mab2F1 and H1L1 treatment decreased levels of p-LRP6, LRP6 and non-p-β-catenin in the corneas with alkali burn, while mouse and human IgG had no such effect. The results indicated that Mab2F1 and H1L1 attenuate abnormal activation of Wnt/β-catenin signaling in the rat cornea with alkali burn-induced NV.
Fig. 3.
Mab2F1 and H1L1 Suppress Abnormal Activation of Wnt/β-Catenin Signaling Pathway in the Cornea with Alkali Burn. A–C: Western blot analysis of total LRP6 (A), p-LRP6 (B), non-p-β-catenin (C). D: The results of Western blotting were semi-quantified by densitometry and normalized to β-actin levels. Data were presented as Mean ± SEM (n = 3 per group) and analyzed by Two-way ANOVA, followed by the Bonferroni post hoc analysis to compare the differences between the two groups. ** P < 0.01, versus normal group. # P < 0.05, versus alkali burn without treatment; ## P < 0.01, versus alkali-burn without treatment. Φ P < 0.05, Mab2F1 group versus mouse IgG group (m-IgG); ΦΦ P < 0.01; Mab2F1 group versus m-IgG group; ξ P < 0.05, H1L1 group versus human IgG (h-IgG) group; ξξ P < 0.01, H1L1 group versus h-IgG group.
3.4. Mab2F1 and H1L1 suppress activation of the Wnt/β-catenin signaling pathway and expression of angiogenic and inflammatory factors induced by Wnt ligand in HCE cells
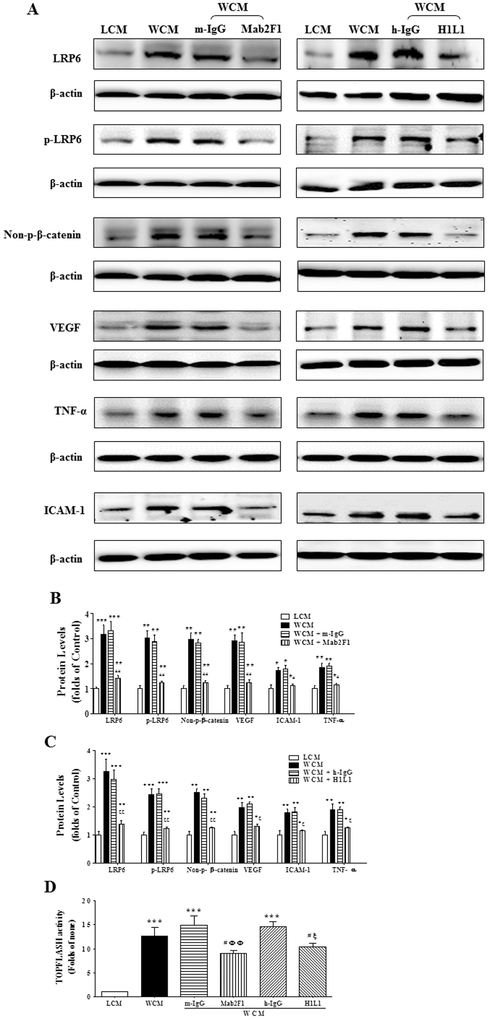
To verify that Mab2F1 and H1L1 attenuate abnormal activation of Wnt/β-catenin signaling pathway in vitro, SV40 immortalized HCE cells, a cell line derived from human normal corneal epithelial cells, one of most commonly used cell model of corneal epithelial cells for studies of cellular signaling and molecular pathways (Adhikary et al., 2008; Liu et al., 2007; Rajalakshmy et al., 2014; Roy et al., 2015; Zheng et al., 2015), were exposed to Wnt ligand to activate Wnt/β-catenin signaling pathway. WCM, a conditioned medium containing Wnt3a, a ligand of the Wnt/β-catenin signaling pathway, has been well characterized (Shibamoto et al., 1998) and is commonly used for the activation of the Wnt/β-catenin signaling pathway (Chen et al., 2016; Oh et al., 2014; Park et al., 2016; Wei et al., 2015; Zhou et al., 2010). WCM activated Wnt/β-catenin signaling in HCE cells as shown by the increases of total LRP6, p-LRP6 and non-p-β-catenin (Fig. 4A–C). Mab2F1 and H1L1 efficiently inhibited the WCM-induced increase of LRP6 levels in a concentration-dependent manner (Suppl. Fig. 1). These two antibodies also attenuated the WCM-induced increases of p-LRP6 and non-p-β-catenin levels (Fig. 4A–C). In contrast, nonspecific mouse IgG and human IgG had no effect on LRP6, p-LRP6 and non-p-β-catenin (Fig. 4A–C). Moreover, the result showed that WCM upregulated the expression of angiogenic and inflammatory factors including VEGF, TNF-α, and ICAM-1 which are known target genes of Wnt/β-catenin signaling pathway (Fig. 4A–C), while Mab2F1 and H1L1 both attenuated the overexpression of these angiogenic and inflammatory factors (Fig. 4A–C). These results suggest that activation of the Wnt/β-catenin signaling pathway in HCE cells plays a pathologic role in the development of corneal NV, and Mab2F1 and H1L1 inhibit the development of NV by blocking the Wnt/β-catenin signaling pathway in HCE cells. Further, luciferase-based promoter activity assay confirmed that WCM significantly induced transcriptional activity of β-catenin, and Mab2F1 and H1L1 both attenuated the Wnt ligand-induced transcriptional activity of β-catenin in HCE cells (Fig. 4D).
Fig. 4.
Inhibition of Wnt Ligand-induced Activation of Wnt Signaling and over-expression of inflammatory factors by Mab2F1 and H1L1 in human corneal epithelial (HCE) cells. A: HCE cells were pre-treated with or without the Mab2F1, H1L1, mouse IgG (m-IgG) or human IgG (h-IgG) at the concentration of 25 μg mL−1 for 2 h, and then incubated with 10% Wnt3a conditioned medium (WCM), with L cell conditioned medium (LCM) as the controls, for 24 h. Levels of total LRP6, p-LRP6, Non-p-β-catenin, VEGF, TNF-α, and ICAM-1 were measured by Western blot analysis. B & C: The results of Western blotting were semi-quantified by densitometry and normalized to β-actin levels. D: HCE cells were transfected with the TOPFLASH plasmids for 4 h, and then were pre-treated with Mab2F1 or H1L1 at the concentration of 25 μg mL−1, with the same amount of m-IgG and h-IgG as controls for 2 h, and then incubated with 10% WCM, with the LCM as the control, for 16 h. TOPFLASH activity was measured using Luciferase assay and expressed as the firefly/renilla ratio relative to that of LCM. Data were presented as Mean ± SEM (n = 6 per group) and analyzed by Two-way ANOVA, followed by the Bonferroni post hoc analysis to compare the differences between the two groups. ** P < 0.01, versus LCM group; *** P < 0.001, versus LCM group. # P < 0.05, versus WCM group; ## P < 0.01, versus WCM group. ΦΦ P < 0.01, WCM + Mab2F1 versus WCM + m-IgG group. ξ P < 0.05, WCM + H1L1 group versus WCM + h-IgG group; ξξ P < 0.01, WCM + H1L1 group versus WCM + h-IgG group.
3.5. Mab2F1 and H1L1 exhibit no toxic effects on corneal epithelial cells
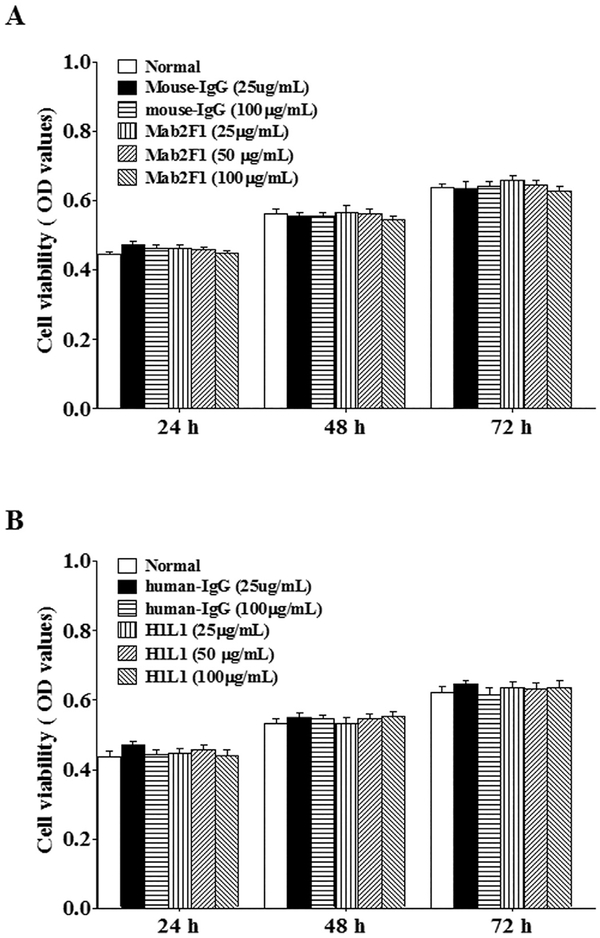
We investigated whether Mab2F1 and H1L1 affect the viability of normal HCE cells. HCE cells cultured under normal conditions were treated with various concentrations of Mab2F1 and H1L1, with nonspecific mouse and human IgG as controls, respectively. The cell viability was measured by MTT assay at 24, 48 and 72 h after the treatment. The results demonstrated that H1L1 or Mab2F1, as well as mouse and human IgG, did not alter cell viability (Fig. 5A & B), suggesting that Mab2F1 and H1L1 have no severe toxic effects on HCE cells.
Fig. 5.
No Toxic effects of Mab2F1 and H1L1 on HCE cells. The cultured HCE cells were treated with Mab2F1 or H1L1 (25–100 μg mL−1), with mouse IgG or human IgG as the control for 24 h, then the cell viability was measured using MTT assay. Data were presented as Mean ± SEM (n = 6 per group) and analyzed by One-way ANOVA. The differences in cell viability among the groups were not statistically significant at any of the time points (all P > 0.05).
4. Discussion
This study is the first to show that a humanized antibody blocking Wnt/β-catenin signaling, H1L1, has anti-angiogenic activities in a rat model of corneal NV, and that the efficacy of this antibody is comparable to that of its parent mouse antibody, Mab2F1. Further, this study indicates that H1L1, as well as Mab2F1, inhibits overexpression of angiogenic and inflammatory factors including VEGF, TNF-α and ICAM-1 through blocking abnormal activation of Wnt/β-catenin signaling in the corneal NV model and in a cultured corneal epithelial cell line. Moreover, these antibodies showed no detectable toxic effects on cultured corneal epithelial cells. These observations demonstrated the potential of this novel humanized antibody as a drug candidate for the treatment of neovascular disorders.
Over the past several decades, VEGF signaling has been identified as a central player in angiogenesis (Azar, 2006; Ng et al., 2006), and humanized antibodies that target VEGF such as bevacizumab are a leading clinical therapy to suppress pathological angiogenesis (Chang et al., 2012; Culy, 2005; Ferrara et al., 2004). However, mono-therapeutic blocking the VEGF signaling pathway is not sufficient to inhibit angiogenesis in all patients. In addition, anti-VEGF antibodies are associated with severe side effects, including bleeding, proteinuria, hypertension, stroke, gastrointestinal perforation, and neuronal cell death (Arriaga and Becerra, 2006; Gordon and Cunningham, 2005; Raimondi et al., 2014). These concerns support the demand for the development of novel therapeutics with non-VEGF targets. To date, many other proangiogenic factors have been identified to be involved in pathological angiogenesis, and humanized monoclonal antibodies, such as Olaratumab targeting human PDGF/PDGF receptor signaling, Nesvacumab targeting Ang/Tie signaling, Rilotumumab targeting HGF/c-Met signaling and IBI303 targeting TNF-α (Kong et al., 2017; Liu et al., 2015), have been developed. Abnormal activation of Wnt/β-catenin signaling plays an important pathogenic role in pathological angiogenesis (Chen et al., 2009). However, the anti-angiogenic effects of the humanized antibody against the Wnt/β-catenin signaling pathway have not been reported previously.
Our recent study showed that murine monoclonal antibody Mab2F1, designed to specifically bind the ElE2 domain of LRP6, a co-receptor of the Wnt pathway, has potent anti-angiogenic activities in animal models (Hu et al., 2013; Lee et al., 2012). This antibody recognizes LRP6 of multiple species including the human, bovine, rat and mouse (Lee et al., 2012). Mab2F1 cannot be used for a human therapy due to its murine origin, as antibodies of murine origin may have a potential human anti-mouse (HAMA) immune response (Miller et al., 1983; Schroff et al., 1985). Therefore, we humanized Mab2F1 using the PDL technology. H1L1 conserves the epitope-binding sequences of Mab2F1. In this study, we evaluated the effects of H1L1 on angiogenesis using the alkali burn-induced corneal NV model, a widely used NV model (Hsu et al., 2015) and chose day 8 after the injury as the endpoint of evaluation because of corneal NV peaks at 7–10 days in this model. We found that H1L1 exhibited potent anti-angiogenic activities, and the efficacies are similar to that of its parent antibody, Mab2F1, in this model. These results are consistent with our previous studies showing the effects of Mab2F on the choroidal NV (Hu et al., 2013) and ischemia-induced retinal NV (Lee et al., 2012) in animal models. This finding suggests that H1L1 is a promising drug candidate for the treatment of neovascular diseases including corneal NV.
As for the mechanism of action, both in vivo and in vitro results in this study showed that H1L1, as well as Mab2F1, downregulated overexpression of angiogenic and inflammatory factors including VEGF, TNF-α, and ICAM-1, and blocking abnormal activation of the Wnt/β-catenin signaling pathway. VEGF is known to plays an important role in angiogenesis including corneal NV as a key pro-angiogenic and proinflammatory factor (Bressler, 2009; Horsley and Kahook, 2010). TNF-α is a commonly accepted key inflammatory factor, playing a pathogenic role in NV (Fornoni et al., 2008; Michalova and Lim, 2008; Navarro-Gonzalez and Mora-Fernandez, 2008; Sakimoto et al., 2009). ICAM-1 plays a critical role in regulating leukocyte adherence, migration and accumulation at sites of inflammation (Carmona et al., 2008). Increased ICAM-1 expression can lead to massive leukocyte infiltration which in turn generate proangiogenic cytokines including VEGF (Philipp and Gottinger, 1993). The Wnt/β-catenin signaling pathway is known to up-regulate angiogenic and inflammatory factors including VEGF, TNF-α, and ICAM-1 (Masckauchan and Kitajewski, 2006; Simo et al., 2006). It is likely that the anti-NV effects of Mab2F1 and H1L1 can be at least partially ascribed to the down-regulation of angiogenic and inflammatory factors via blocking the Wnt/β-catenin signaling pathway. Our result is particularly significant because the Wnt/β-catenin signaling pathway regulates multiple inflammatory and angiogenic factors in addition to VEGF. H1L1 has the promise as an alternative or combination treatment with anti-VEGF antibodies for pathological angio-genesis as it inhibits overexpression of multiple angiogenic factors as well as VEGF.
It is essential that a therapeutic agent for corneal NV should not affect the viability of corneal epithelial cells. The Wnt/β-catenin signaling pathway is involved in development in normal organs and tissues (Moon et al., 2002; Visweswaran et al., 2015). Thus, the present study evaluated possible toxic effects of Mab2F1 and H1L1 on HCE cells. Mab2F1 and H1L1 exhibited no detectable effects on viability of cultured HCE cells. In addition, we observed no side effects in the animal models such as irritation, corneal epithelial defects, corneal ulcers, or conjunctival melting after the administration of these antibodies. These results suggest that the antibodies themselves do not affect corneal epithelial cell viability.
In conclusion, we report here generation of a novel humanized monoclonal antibody blocking the Wnt/β-catenin signaling pathway, H1L1, and demonstrate that this antibody has anti-angiogenic activities using a corneal NV model, supporting its potential as a drug candidate to treat angiogenesis. Further investigation is warranted to determine the safety profile in vivo, additive or synergistic effects with other antiangiogenic agents, and beneficial effects on other diseases associated with abnormal activation of the Wnt/β-catenin signaling pathway.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants (EY018659, EY019309, GM104934, GM122744), a Juvenile Diabetes Research Foundation (JDRF) grant (2-SRA-2014–147-Q-R), and an Oklahoma Center for the Advancement of Science and Technology (OCAST) grant (HR16–041).
Abbreviations:
- Ang
angiopoietin
- DR
diabetic retinopathy
- EGF
epidermal growth factor
- HCE
human corneal epithelium
- HGF
- ICAM-1
intracellular adherent molecular-1
- LCM
L cell conditioned medium
- LRP5/6
low-density lipoprotein receptor-related protein 5/6
- NV
neovascularization
- non-p-β-catenin
non-phosphorylated β-catenin
- OIR
oxygen-induced retinopathy
- PDGF
platelet-derived growth factor
- TCF
T cell factors
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- WCM
Wnt3a cell conditioned medium
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mvr.2018.03.011.
Conflict of interest disclosures
J-x Ma, J. Larrick and A. Mendelson have equity ownership in Wntgen LLC, which owns patents covering Wnt pathway antibodies. Other authors have no conflicts of interest.
References
- Adhikary G, Sun Y, Pearlman E, 2008. C-Jun NH2 terminal kinase (JNK) is an essential mediator of toll-like receptor 2-induced corneal inflammation. J. Leukoc. Biol 83 (4), 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga Y, Becerra CR, 2006. Adverse effects of bevacizumab and their management in solid tumors. Supportive Cancer Ther 3 (4), 247–250. [DOI] [PubMed] [Google Scholar]
- Azar DT, 2006. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American ophthalmo-logical society thesis). Trans. Am. Ophthalmol. Soc 104, 264–302. [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, 2009. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology 116 (10 Suppl), S15–23. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D, 2000. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N. Y. Acad. Sci 902, 249–262 (discussion 262–244). [DOI] [PubMed] [Google Scholar]
- Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S, 2008. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 111 (5), 2640–2646. [DOI] [PubMed] [Google Scholar]
- Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT, 2012. Corneal neovascularization: an anti-VEGF therapy review. Surv. Ophthalmol 57 (5), 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Fu Q, Li D, Wu Y, Sun S, Zhang X, 2016. Wnt3a suppresses Pseudomonas aeruginosa-induced inflammation and promotes bacterial killing in macrophages. Mol. Med. Rep 13 (3), 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, et al. , 2009. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol 175 (6), 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma JX, 2017. Canonical Wnt signaling in diabetic retinopathy. Vis. Res 139, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culy C, 2005. Bevacizumab: antiangiogenic cancer therapy. Drugs Today 41 (1), 23–36. [DOI] [PubMed] [Google Scholar]
- Dejana E, 2010. The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res 107 (8), 943–952. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W, 2004. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov 3(5), 391–400. [DOI] [PubMed] [Google Scholar]
- Folkman J, 2007. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov 6 (4), 273–286. [DOI] [PubMed] [Google Scholar]
- Fornoni A, Ijaz A, Tejada T, Lenz O, 2008. Role of inflammation in diabetic nephropathy. Curr. Diabetes Rev 4 (1), 10–17. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Cunningham D, 2005. Managing patients treated with bevacizumab combination therapy. Oncology 69 (Suppl. 3), 25–33. [DOI] [PubMed] [Google Scholar]
- Horsley MB, Kahook MY, 2010. Anti-VEGF therapy for glaucoma. Curr. Opin. Ophthalmol 21 (2), 112–117. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Chang HM, Lin TC, Hung KH, Chien KH, Chen SY, et al. , 2015. Corneal neovascularization and contemporary antiangiogenic therapeutics. J. Chin. Med. Assoc 78 (6), 323–330. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma JX, 2013. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest. Ophthalmol. Vis. Sci 54 (1), 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W, 2008. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8 (5), 387–398. [DOI] [PubMed] [Google Scholar]
- Kong DH, Kim MR, Jang JH, Na HJ, Lee S, 2017. A review of anti-angiogenic targets for monoclonal antibody cancer therapy. Int. J. Mol. Sci 18 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laria C, Alio JL, Ruiz-Moreno JM, 1997. Combined non-steroidal therapy in experimental corneal injury. Ophthalmic Res 29 (3), 145–153. [DOI] [PubMed] [Google Scholar]
- Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, et al. , 2012. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 61 (11), 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK, 2003. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol 170 (6), 3369–3376. [DOI] [PubMed] [Google Scholar]
- Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, et al. , 2007. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp. Eye Res 84 (3), 599–609. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang G, Zhang J, Xing K, Dai L, Cheng L, et al. , 2015. Anti-TNF-alpha monoclonal antibody reverses psoriasis through dual inhibition of inflammation and angiogenesis. Int. Immunopharmacol 28 (1), 731–743. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Kitajewski J, 2006. Wnt/frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology 21, 181–188. [DOI] [PubMed] [Google Scholar]
- Michalova K, Lim L, 2008. Biologic agents in the management of inflammatory eye diseases. Curr Allergy Asthma Rep 8 (4), 339–347. [DOI] [PubMed] [Google Scholar]
- Miller RA, Oseroff AR, Stratte PT, Levy R, 1983. Monoclonal antibody therapeutic trials in seven patients with T-cell lymphoma. Blood 62 (5), 988–995. [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N, 2002. The promise and perils of Wnt signaling through beta-catenin. Science 296 (5573), 1644–1646. [DOI] [PubMed] [Google Scholar]
- Navarro-Gonzalez JF, Mora-Fernandez C, 2008. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol 19 (3), 433–442. [DOI] [PubMed] [Google Scholar]
- Ng YS, Krilleke D, Shima DT, 2006. VEGF function in vascular pathogenesis. Exp. Cell Res 312 (5), 527–537. [DOI] [PubMed] [Google Scholar]
- Oh S, Gwak J, Park S, Yang CS, 2014. Green tea polyphenol EGCG suppresses Wnt/beta-catenin signaling by promoting GSK-3beta- and PP2A-independent beta-catenin phosphorylation/degradation. Biofactors 40 (6), 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim JH, Kim JE, Song GY, Zhou W, Goh SH, et al. , 2016. Cytotoxic activity of aeroplysinin-1 against colon cancer cells by promoting beta-catenin degradation. Food Chem. Toxicol 93, 66–72. [DOI] [PubMed] [Google Scholar]
- Philipp W, Gottinger W, 1993. Leukocyte adhesion molecules in diseased corneas.Invest. Ophthalmol. Vis. Sci 34 (8), 2449–2459. [PubMed] [Google Scholar]
- Raimondi C, Fantin A, Lampropoulou A, Denti L, Chikh A, Ruhrberg C, 2014. Imatinib inhibits VEGF-independent angiogenesis by targeting neuropilin 1-dependent ABL1 activation in endothelial cells. J. Exp. Med 211 (6), 1167–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmy AR, Malathi J, Madhavan HN, 2014. HCV core and NS3 proteins mediate toll like receptor induced innate immune response in corneal epithelium. Exp. Eye Res 128, 117–128. [DOI] [PubMed] [Google Scholar]
- Roy S, Marla S, Praneetha DC, 2015. Recognition of Corynebacterium pseudo-diphtheriticum by toll-like receptors and up-regulation of antimicrobial peptides in human corneal epithelial cells. Virulence 6 (7), 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimoto T, Yamada A, Sawa M, 2009. Release of soluble tumor necrosis factor receptor 1 from corneal epithelium by TNF-alpha-converting enzyme-dependent ecto-domain shedding. Invest. Ophthalmol. Vis. Sci 50 (10), 4618–4621. [DOI] [PubMed] [Google Scholar]
- Schroff RW, Foon KA, Beatty SM, Oldham RK, Morgan AC Jr., 1985. Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res 45 (2), 879–885. [PubMed] [Google Scholar]
- Sebio A, Kahn M, Lenz HJ, 2014. The potential of targeting Wnt/beta-catenin in colon cancer. Expert Opin. Ther. Targets 18 (6), 611–615. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S, 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3 (10), 659–670. [DOI] [PubMed] [Google Scholar]
- Shibuya M, 2014. VEGF-VEGFR signals in health and disease. Biomol. Ther 22 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C, 2006. Angiogenic and anti-angiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev 2 (1), 71–98. [DOI] [PubMed] [Google Scholar]
- Visweswaran M, Pohl S, Arfuso F, Newsholme P, Dilley R, Pervaiz S, et al. , 2015. Multi-lineage differentiation of mesenchymal stem cells - to Wnt, or not Wnt. Int. J. Biochem. Cell Biol 68, 139–147. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cheng R, Lee K, Tyagi P, Ding L, Kompella UB, et al. , 2015Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler. Thromb. Vasc. Biol 35 (4), 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhao H, Ma JX, Xu X, 2014. Inhibition of pathological corneal neovascularization by a small peptide derived from human apolipoprotein (a) Kringle V. Cornea 33 (4), 405–413. [DOI] [PubMed] [Google Scholar]
- Wei L, Ding L, Mo MS, Lei M, Zhang L, Chen K, et al. , 2015. Wnt3a protects SHSY5Y cells against 6-hydroxydopamine toxicity by restoration of mitochondria function. Transl. Neurodegener 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M, Julius MA, Kitajewski J, 2008. Wnt/frizzled signaling in angiogenesis.Angiogenesis 11 (1), 63–69. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Ren Y, Reinach PS, Xiao B, Lu H, Zhu Y, et al. , 2015. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp. Eye Res 134, 133–140. [DOI] [PubMed] [Google Scholar]
- Zhou T, Chen L, Huang CH, Lin Z, Zong R, Zhu C, et al. , 2014. Serine proteinase inhibitor SERPINA3K suppresses corneal neovascularization via inhibiting Wnt signaling and VEGF. Invest. Ophthalmol. Vis. Sci 55, 4863–4872. [DOI] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, et al. , 2010. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 51 (9), 4371–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.