Abstract
Diabetic retinopathy (DR) is a common eye complication of diabetes, and the pathogenic mechanism of DR is still under investigation. The canonical Wnt signaling pathway is an evolutionarily conserved pathway that plays fundamental roles in embryogenesis and adult tissue homeostasis. Wnt signaling regulates expression of multiple genes that control retinal development and eye organogenesis, and dysregulated Wnt signaling plays pathophysiological roles in many ocular diseases, including DR. This review highlights recent progress in studies of Wnt signaling in DR. We discuss Wnt signaling regulation in the retina and dysregulation of Wnt signaling associated with ocular diseases with an emphasis on DR. We also discuss the therapeutic potential of modulating Wnt signaling in DR. Continued studies in this field will advance our current understanding on DR and contribute to the development of new treatments.
Keywords: Angiogenesis, Diabetes, Inflammation, Oxidative stress, Retinopathy, Wnt signaling
1. Introduction
Diabetic retinopathy (DR), a common microvascular complication of diabetes, is a leading cause of blindness in the working-age population in developed countries. The incidence of diabetes gradually increases every year, and approximately 415 million people in the world have diabetes at the year of 2015 (IDF, 2015). By year of 2040, estimated population with diabetes will reach 642 million (IDF, 2015). It is reported that 9.3% of the population in United States have diabetes and nearly 37% of adults at age of 20 or above may have prediabetes (CDC, 2014). Most patients with type 1 diabetes will eventually develop some degree of DR, and 50–80% patients with type 2 diabetes will develop DR with 20–25 years of diabetes (Curtis, Gardiner, & Stitt, 2009). As a large population of patients with diabetes will eventually develop DR, it posts a major threat to the health of our global population and an economic burden to our health care system. However, the incompletely understood mechanism of DR and the lack of safe and effective therapeutic modalities are major challenges for physicians and researchers in this field.
The canonical Wnt pathway is evolutionarily conserved across species and plays fundamental roles in embryonic development and adult tissue homeostasis (Clevers & Nusse, 2012; MacDonald, Tamai, & He, 2009). The term “Wnt” originates from the combination of “Drosophila wingless (wg)” and murine “Int-1”. Int-1 is an oncogene that induces mammary carcinoma upon inappropriate activation by mouse mammary tumor virus (Nusse & Varmus, 1982). Later, a Drosophila homolog of mouse Int-1, Wg was identified to be a crucial component controlling body patterning of Drosophila during larval development, which led to a significant breakthrough that linked this gene to a novel signal transduction pathway that was conserved across species (Rijsewijk et al., 1987). Followed by characterization of Wg and Int-1, numerous Wnt-related genes and downstream components in vertebrates have been subsequently identified. Thus, the cascade of canonical Wnt signaling was established. In addition, components of the Wnt signaling pathway that are mutated or aberrantly expressed are linked to numerous diseases such as cancer, cardiovascular diseases and bone diseases (Clevers & Nusse, 2012; Malekar et al., 2010). Recently, canonical Wnt signaling has been shown to play fundamental roles in retinal development and eye organogenesis (de Iongh, Abud, & Hime, 2006; Fuhrmann, 2008). Dysregulated canonical Wnt signaling plays a causative role in human ocular diseases, including DR (Chen et al., 2009; de Iongh et al., 2006). The importance of canonical Wnt signaling in DR is increasingly recognized, and interventions to correct dysregulated canonical Wnt signaling are shown to have therapeutic effects in animal models of DR. In this review, we examined the evidence of the involvement of canonical Wnt signaling in DR and the therapeutic potential of suppressing canonical Wnt signaling in DR.
2. Wnt signaling
Wnt proteins are secreted cysteine-rich glycoproteins which serve as ligands and mediate signaling pathways that are involved in cell proliferation, cell migration, cell fate determination and embryogenesis. Binding of the secreted Wnts to specific receptor complexes activates signaling pathways and executes specific instructions to recipient cells. The Wnt-activated signaling pathways can be divided into the canonical Wnt signaling pathway, the non-canonical planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway (Clevers, 2006). The canonical Wnt signaling pathway is the most studied and well-characterized in various diseases including cancer, cardiovascular diseases and ocular diseases. This review focuses on canonical Wnt signaling and its role in ocular diseases, especially in DR.
3. Canonical Wnt signaling
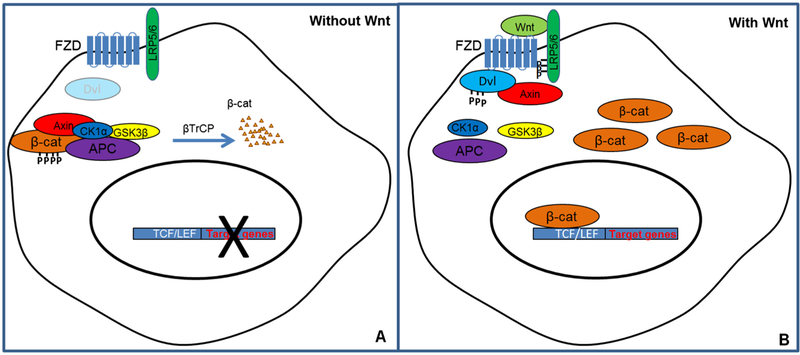
The canonical Wnt signaling pathway is a conserved intracellular signaling pathway comprised of Wnts, frizzled (Fzd) receptors, and co-receptors including low-density lipoprotein receptor-related protein 5/6 (LRP5/6) and an intracellular signaling molecule cascade (Logan & Nusse, 2004; MacDonald et al., 2009). In the absence of Wnt ligand, soluble cytoplasmic β-catenin, a transcription factor, is captured by a scaffold protein Axin that associates with two protein kinases, casein kinase Iα (CKIα) and glycogen synthase kinase 3 beta (GSK3β) as well as adenomatous polyposis coli (APC), which form the destruction complex (Logan & Nusse, 2004). APC is essential for recruiting and anchoring β-catenin, while CKIα and GSK3β mediate the subsequent phosphorylation in the N-terminus of the trapped β-catenin (Zeng et al., 2005). Thus, soluble cytoplasmic β-catenin is constitutively phosphorylated by protein kinases GSK3β and CKIα (Liu et al., 2002). Further, phosphorylated β-catenin is recognized by β-transducin repeats-containing proteins (β-TrCP), which is a component of an ubiquitin-ligase complex that conjugates β-catenin with ubiquitin (Polakis, 2002; Wu & Nusse, 2002). Poly-ubiquitinated β-catenin is then targeted for proteasomal degradation (Aberle, Bauer, Stappert, Kispert, & Kemler, 1997) (Fig. 1A).
Fig. 1.
The canonical Wnt signaling pathway. (A) In the absence of Wnt, β-catenin (β-cat) interacts with the destruction complex formed by a scaffold protein Axin, casein kinase Iα (CKIα) and glycogen synthase kinase 3 beta (GSK-3b) as well as adenomatous polyposis coli (APC), and is constitutively phosphorylated and degraded. (B) In the presence of Wnt, Wnt binds to frizzled receptor (Fzd) and co-receptor low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6). LPR5/6 is phosphorylated, which leads to the recruitment and phosphorylation of Dishevelled (Dvl). Subsequently, Axin is recruited to the receptor complex and the destruction complex is disassembled, blocking the phosphorylation of soluble cytoplasmic β-cat. β-cat is then stabilized and translocates to the nucleus to activate transcription of target genes.
Rapid activation of the canonical Wnt pathway occurs when Wnts bind to cell surface receptor complexes comprising members of the Fzd family and Wnt co-receptors LRP5/6. Studies have suggested the existence of a likely Wnt-Fzd-LRP6 complex in vivo (Bilic et al., 2007; Liu, Bafico, & Aaronson, 2005). The binding of Wnt-Fzd-LPR5/6 leads to the phosphorylation of the cytoplasmic tail of LRP5/6, which plays a crucial role in activation of the canonical Wnt signaling pathway (Goretzki & Mueller, 1998; Li, van Kerkhof, Marzolo, Strous, & Bu, 2001). The Wnt-Fzd-LPR5/6 complex also triggers the recruitment and phosphorylation of Dishevelled (Dvl), which stimulates the formation of LRP5/6 aggregation in the membrane (Bilic et al., 2007). Axin is subsequently recruited to the receptor complex (Fzd-LRP5/6), and the destruction complex is disassembled, which then blocks the phosphorylation of bcatenin and results in β-catenin stabilization (Ikeda et al., 1998). Thus, cytoplasmic β-catenin accumulates and translocates into the nucleus. Nuclear β-catenin then interacts with members of T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) family to activate transcription of Wnt signaling target genes (Behrens et al., 1996; Cavallo et al., 1998; Korinek et al., 1997) (Fig. 1B).
The activation of the canonical Wnt signaling pathway may result in a variety of biological changes through activation of TCF/β-catenin-responsive target genes (Behrens et al., 1996; Cavallo et al., 1998; Korinek et al., 1997). The target genes are involved in many physiological processes, including cell proliferation, differentiation, apoptosis, and cell fate determination (Logan & Nusse, 2004; MacDonald et al., 2009). For a full list of Wnt target genes please refer to the following source (www.stanford/edu/group/nusselab/cgi-bin/wnt/target_genes).
4. Endogenous modulators of canonical Wnt signaling
Canonical Wnt signaling is regulated by multiple mechanisms, such as posttranslational modification of Wnts, antagonists that bind to Wnt proteins or their receptors, and regulation of the Wnt receptors. A number of endogenous modulators of Wnt signaling have been identified. The best-characterized inhibitor of Wnt signaling is dickkopf-related protein 1 (DKK1), which is a secreted protein that negatively regulates Wnt signaling (Niida et al., 2004). DKK1 directly interacts with LRP5/6 and accelerates the internalization of LRP5/6, thus competing with Wnt-Fzd for the binding of LRP5/6 and inhibiting Wnt signaling activation (Mao et al., 2001, 2002). Extracellular ligands such as R-spondins and Norrin are also thought to regulate Wnt signaling. R-spondins directly interacts with Frizzled 8 and LRP5/6 and activates the Wnt signaling pathway (Kim et al., 2006). Norrin, an extracellular protein, specifically interacts with the Frizzled 4 (Fzd-4) receptor and co-receptor LRP5, and activates Wnt signaling (Xu et al., 2004; Ye et al., 2009). Another group of Wnt signaling regulators is secreted Wnt inhibitors, such as Wnt inhibitory factors (WIFs) and secreted frizzled-related proteins (SFRPs). WIFs and SFRPs act as secreted antagonists that directly bind Wnts and block the interaction of Wnt-Fzd, and thus, inhibit Wnt signaling (Bovolenta, Esteve, Ruiz, Cisneros, & Lopez-Rios, 2008; Malinauskas, Aricescu, Lu, Siebold, & Jones, 2011). Studies have also shown that heparin sulfate proteoglycans (HSPG), glycoproteins on the cell surface and in the extracellular matrix, may interact with Wnt proteins and affect Wnt signaling activities (Logan & Nusse, 2004; Rao & Kuhl, 2010). In addition, endostatin, a fragment of collagen XVIII, can block the development of Xenopus induced by β-catenin, indicating that endostatin inhibits Wnt signaling (Hanai et al., 2002). Park et al. reported that pigment epithelium derived factor (PEDF) binds LRP6 and blocks the Wnt-ligand induced LRP6-Fzd receptor dimerization, resulting in Wnt signaling inhibition (Park, Lee et al., 2011). Our recent studies have identified that very low-density lipoprotein receptor (VLDLR) forms a heterodimer with LRP6 and promotes degradation of LRP6, and thus inhibits Wnt signaling (Chen, Hu, Lu, Flannery, & Ma, 2007; Lee et al., 2014).
Recently, the discovery of microRNAs (miRNAs) has revolutionized biomedical research in many areas, as miRNAs have the potential to modulate the expression of the majority of the protein-coding genes. Here, we briefly introduce miRNAs and present evidence for the possible involvement of miRNAs in canonical Wnt signaling regulation. MiRNAs are small (~22nt), single-stranded, non-coding RNA molecules that regulate gene expression at a post-transcriptional level. They bind to the complementary sequences in the 3′ untranslated region (UTR) of target mRNAs and inhibit protein expression of target genes (Filipowicz, Bhattacharyya, & Sonenberg, 2008; Inui, Martello, & Piccolo, 2010; Wahid, Shehzad, Khan, & Kim, 2010). MiRNAs have been shown to play fundamental roles in cellular events such as differentiation, proliferation and apoptosis (He & Hannon, 2004; Wahid et al., 2010). In the past few years, miRNAs have been reported to be involved in development and function of the eye (Loscher et al., 2007, 2008; Ryan, Oliveira-Fernandes, & Lavker, 2006; Xu, 2009; Xu, Witmer, Lumayag, Kovacs, & Valle, 2007). More than 80 miRNAs have been identified in the adult mouse retina using miRNA microarray profiling, and approximately 20 miRNAs are retina-specific (Loscher et al., 2008; Ryan et al., 2006; Xu, 2009; Xu et al., 2007). Moreover, more than 130 miRNAs are found to be associated with eye development (embryonic day (E) 15 and E 18) and several miRNAs (e.g. miR-29b, miR-18b) show significant changes in expressional levels between E 15 and adult age (Hackler, Wan, Swaroop, Qian, & Zack, 2010).
MiRNAs have been shown to regulate components of the Wnt signaling pathway. For example, miR-200a was reported to regulate Wnt signaling in two different ways. First, miR-200a represses mRNAs levels of the E-cadherin repressor proteins ZEB1 and ZEB2, and thus increases the total E-cadherin available for binding to β-catenin and decreases the β-catenin-mediated transcriptional activities (Korpal, Lee, Hu, & Kang, 2008). Second, miR-200a is capable of targeting the β-catenin mRNA directly. In meningioma, down-regulated miR-200a induces over-expression of β-catenin and enhances Wnt/β-catenin activities (Saydam et al., 2009). In addition, a recent study has shown that miR-21 targets the mRNAs of Wnt1 and represses its protein expression (Hashimi et al., 2009). Thatcher et al. reported that Wnt signaling transcription factor LEF1 is directly regulated by miR-203 in zebrafish (Thatcher, Paydar, Anderson, & Patton, 2008). It was reported that miR-8 inhibits Wnt/Wg signaling by targeting Wnt signaling regulators and Wnt signaling components in Drosophila. In addition, mammalian miR-8 may promote adipogenesis through inhibiting Wnt signaling (Kennell, Gerin, MacDougald, & Cadigan, 2008). Moreover, miR-135a and miR-135b directly target Wnt signaling component APC and repress its protein expression, and thus promote Wnt signaling activities by increasing β-catenin levels (Nagel et al., 2008). Our recent study demonstrated that miR-184 negatively modulates Wnt signaling by targeting Wnt co-receptor Frizzled 7 in ischemia-induced retinal neovascularization (Takahashi, Chen, Rajala, & Ma, 2015). Taken together, accumulating evidence suggests that miRNAs are key modulators of Wnt signaling and mediate Wnt-related cellular events.
5. Dysregulated canonical Wnt signaling in ocular diseases
5.1. Diabetic retinopathy
DR, a common microvascular complication of diabetes, is a leading cause of blindness in the working-age population in developed countries. A cascade of microvascular pathology occurs in DR, including leukostasis, pericyte loss, increased number of acellular capillaries, microaneurysms, progressive vascular occlusions, increased vascular permeability, macular edema and the formation of new vessels that ultimately proliferate into the vitreous (Durham & Herman, 2011). According to the vascular progression, DR can be classified into two different stages: non-proliferative DR and proliferative DR (Antonetti, Klein, & Gardner, 2012; Cheung, Mitchell, & Wong, 2010). The presence of microaneurysms, hemorrhages, hard exudates, cotton wool spots, venous dilation and beading, and intra-retinal neovascularization are classical clinical features of non-proliferative DR (Antonetti et al., 2012). Proliferative DR is characterized by neovascularization, pre-retinal hemorrhage, or vitreous hemorrhage (Antonetti et al., 2012). Recent studies have indicated that aberrant activation of Wnt signaling plays a pathogenic role in DR (Chen et al., 2009). Levels of total β-catenin, an essential effector of the canonical Wnt signaling pathway, are elevated in retinal sections of patients with nonproliferative DR compared to those from non-diabetic controls (Chen et al., 2009). In addition, β-catenin levels are up-regulated in two DR animal models, Akita mice and streptozotocin (STZ)-induced diabetic rats, and a rat model of proliferative retinopathy model, oxygen-induced retinopathy (OIR) (Chen et al., 2009). Moreover, the expressional levels of Wnt co-receptor LRP5/6 are also up-regulated in these animal models. To further confirm the causative role of Wnt signaling in DR, a Wnt signaling inhibitor, DKK-1 was intravitreally injected to STZ-induced diabetic rats and OIR rats. DKK-1 reduces retinal inflammation, ameliorates vascular leakage and neovascularization in these animal models (Chen et al., 2009). DKK1 was recently reported to inhibit proliferation and migration of human retinal pigment epithelial (RPE) cells, and the expression of β-catenin and cyclin D1 is decreased in DKK1 over-expressed RPE cells (Zhou, Jiang, Wang, & Xia, 2016). In humans, serum levels of DKK-1 are lower in patients with DR, compared with those from non-diabetic patients or diabetic patients without DR (Qiu et al., 2014). All of the evidence suggests that the DKK-1-related aberrant activation of Wnt signaling plays an important role in DR. Studies have also shown that vitreous samples from patients with proliferative DR have higher levels of LRP6 compared to the nondiabetic controls, and elevated LRP6 levels are correlated with the levels of vascular endothelial growth factor (VEGF) in the vitreous (Gao et al., 2015).
The pathogenic role of Wnt signaling in DR has been further confirmed by other studies (Lee et al., 2012; Zhou et al., 2010). Activation of Wnt signaling by Wnt3A conditioned medium or adenovirus expressing a constitutively active mutant of β-catenin (Ad-S37A) up-regulates the expression of proinflammatory factors, such as VEGF, NF-κB and tumor necrosis factor-alpha (TNF-α), and increases the generation of reactive oxygen species (ROS) in a retinal cell culture model (Zhou et al., 2010). Moreover, intravitreal delivery of Ad-S37A is capable of inducing the expression of VEGF, NF-κB and TNF-α and increasing protein nitrotyrosine levels in the retina of normal rats, suggesting a pathogenic role for aberrant Wnt signaling in the retina (Zhou et al., 2010). In addition, several Wnt signaling inhibitors have shown robust effects on inhibiting Wnt signaling and its downstream target genes and reducing the pathological changes of DR in animal models (Lee et al., 2012; Liu, Zhang et al., 2013; Park, Lee et al., 2011; Wang et al., 2015). The detailed Wnt signaling inhibitors are described later in this review. The over-activated Wnt signaling and potential therapeutic Wnt signaling inhibitors in DR are briefly summarized in Fig. 2.
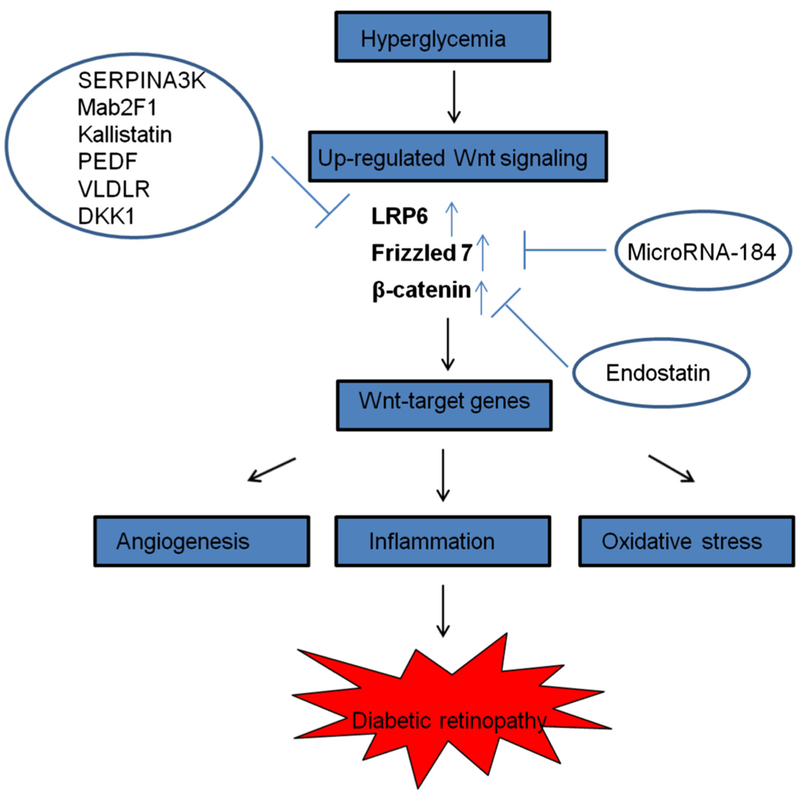
Fig. 2.
Over-activated canonical Wnt signaling in DR and potential therapeutic inhibitors of canonical Wnt signaling for DR. In diabetic conditions, hyperglycemia induces the up-regulation of Wnt signaling in the retina. Levels of Wnt signaling components, low-density lipoprotein receptor-related protein 6 (LRP6), Frizzled 7 and β-catenin are up-regulated, and the Wnt signaling downstream target genes are over-expressed, which results in angiogenesis, inflammation and increased oxidative stress in diabetic retina and leads to DR. Wnt signaling inhibitors with therapeutic potential – SERPINA3K: Serine proteinase inhibitors 3K, PEDF: Pigment epithelium-derived factor, VLDLR: very low-density lipoprotein receptor, DKK1: dickkopf-related protein 1. Mab2F1: an anti-LRP6 monoclonal antibody which was generated in our laboratory.
5.2. Age related macular degeneration (AMD)
AMD is one of the leading causes of blindness among people aged 60 and over (Lim, Mitchell, Seddon, Holz, & Wong, 2012). Patients with AMD develop central vision loss, which is caused by degeneration and/or atrophy of both photoreceptors and the retinal pigment epithelium in the macula. AMD occurs in two phases, in which non-exudative (dry) AMD precedes exudative (wet) AMD (Lim et al., 2012). The pathological features of “dry” AMD include the presence of drusen and pigment disruption, but without blood vessel leakage and neovascularization. “Wet” AMD is characterized by the formation of choroidal neovascularization (CNV), in which pathological blood vessels grow from the choriocapillaris through Bruch’s membrane into the sub-pigment epithelial or sub-retinal space. Aberrant Wnt signaling is found to be involved in AMD in patients and animal models (Hu et al., 2013; Tuo et al., 2015; Zhou et al., 2010). Haines et al. investigated genetic linkage and allelic association for developing AMD in patients using a family-based associated dataset, and found that LRP6, VEGF and VLDLR show clear evidence for linkage and association with AMD, suggesting a role for Wnt signaling in the pathogenesis of AMD (Haines et al., 2006). A recent study has also shown that Wnt signaling component LRP6 and Wnt signaling target genes Cyclin1, c-Myc, Axin2 and VEGF are elevated in the retina from both “dry” and “Wet” AMD patients (Tuo et al., 2015). Levels of kallistatin, a negative Wnt signaling regulator, are decreased in the plasma of AMD patients (including “dry” and “Wet” AMD) (Tuo et al., 2015), further supporting the role of Wnt signaling in AMD. Moreover, the inhibition of Wnt signaling using a monoclonal antibody (Mab2F1) blocking LRP6 confers beneficial effects in a mouse dry AMD model (Tuo et al., 2015). Furthermore, levels of Wnt signaling components and Wnt signaling target genes were over-expressed in laser-induced CNV, an animal model for “wet” AMD (Hu et al., 2013). Inhibition of Wnt signaling using a the Mab2F1 antibody attenuates laser-induced CNV and vascular leakage (Hu et al., 2013), suggesting that Wnt signaling plays a pathogenic role in “wet” AMD.
5.3. Familial exudative vitreoretinopathy (FEVR)
FEVR is a hereditary disease characterized by hypovascularization of the peripheral retina leading to fibrovascular mass lesions (Criswick & Schepens, 1969). Clinically, it manifests symptoms with variable severities. Mild forms are asymptomatic, while in severe cases, compensatory growth of retinal blood vessels may cause retinal detachment and blindness. FEVR displays several forms of inheritance, such as autosomal dominant, autosomal recessive, and X-linked (Li et al., 1992; van Nouhuys, 1982). The autosomal dominant form of FEVR is the most common (Li et al., 1992; van Nouhuys, 1982). There are three identified autosomal dominant loci: EVR1 (11q13-q23), EVR3 (11p12–13) and EVR4 (11q13-q23) (de Iongh et al., 2006). Mapping of EVR1 mutation in the chromosome 11q13-q23 has defined deletion within the Wnt receptor Fzd-4 gene, which is estimated to occur in 20% of FEVR cases (Robitaille et al., 2002; Toomes et al., 2004). In addition, analysis of gene mutations in 3 families with autosomal recessive FEVR leads to the identification of 3 mutations in the LRP5 gene (R570Q, R752G and E136K) (Jiao, Ventruto, Trese, Shastry, & Hejtmancik, 2004). One study has also reported that a mutation in Norrin may associate with FEVR though mapping of X-linked FEVR kindreds (EVR2) in one family, although other studies in X-linked FEVR families failed to confirm the mutations in Norrin (Fullwood et al., 1993). In addition, the mutations of Fzd-4 or LRP5 in mouse models generally recapitulate the eye phenotypes in human FEVR (Gong et al., 2001; Xu et al., 2004).
5.4. Norrie disease
Norrie disease is an X-linked genetic disorder characterized by retinal dysplasia, hearing loss, intellectual disability and blindness (Berger, 1998). Mutations in Norrin, a secreted protein, have been found to attribute to Norrie disease (Chen et al., 1993). Research studies have shown that Norrin, a non-Wnt ligand, can bind to Fzd-4 with high affinity and activate the canonical Wnt signaling pathway through Norrin-Fzd-4-LRP5 interaction (Xu et al., 2004).
5.5. Retinitis pigmentosa
Retinitis pigmentosa is a group of inherited eye diseases with progressive degeneration of photoreceptors, which causes severe vision impairment (Ferrari et al., 2011). Studies have demonstrated that multiple secreted frizzle related proteins (SFRP-1, 2, 5) are associated with photoreceptor degeneration and apoptosis in retinitis pigmentosa (Jones, Jomary, Grist, Stewart, & Neal, 2000a). In the degenerating retinas, SFRPs are found to be present in the inner limiting membrane, Bruch’s membrane and surviving photoreceptors (Garcia-Hoyos et al., 2004; Jones, Jomary, Grist, Stewart, & Neal, 2000b). However, the role of Wnt signaling in retinitis pigmentosa needs to be further confirmed as no direct evidence has been obtained to show that SFRPs modulate the Wnt signaling in these degenerating retinas, and thus, contribute to retinitis pigmentosa.
6. Mechanisms of canonical Wnt signaling involvement in diabetic retinopathy
Among Wnt signaling-related ocular diseases, DR is the most common, and its pathogenic mechanism remains unclear. We and others have demonstrated that dysregulated Wnt signaling may be associated with unbalanced oxidative stress, pathological angiogenesis and inflammation, and thus contributes to the pathogenesis of DR (Fig. 2).
6.1. Canonical Wnt signaling and oxidative stress
Oxidative stress is defined as an imbalance between the formation and removal of highly reactive molecules, which includes reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Maritim, Sanders, & Watkins, 2003). Oxidative stress increases retinal inflammation, causes mitochondrial and endothelial cell dysfunction, and activates glial cells, thus contributing to vascular defects and neurodegeneration in DR (Kowluru & Chan, 2007). Recent studies have demonstrated that Wnt signaling may be implicated in the balance or regulation of oxidative stress. A recent study identified that Dvl, a Wnt signaling component, binds to the thioredoxin-like protein nucleoredoxin (NRX), a regulator of the cellular redox balance, and the interaction between Dvl and NRX is regulated by oxidative stress (Funato, Michiue, Asashima, & Miki, 2006). Over-expression of NPX inhibits Wnt signaling in cultured cells, whereas knockdown of NRX constitutively activates Wnt signaling and enhances the expression of endogenous Wnt target genes (Funato et al., 2006). In addition, Essers et al. reported that β-catenin directly binds to Forkhead box O (FOXO), a transcription factor negatively regulated by the insulin signaling pathway, and enhances FOXO transcriptional activity in cultured cells (Essers et al., 2005). Loss of β-catenin reduces the activity of FOXO, shortens the life span of C. elegans and decreases their resistance to oxidative stress, suggesting that an important role of β-catenin in oxidative stress through regulating FOXO function. Recently, canonical Wnt signaling has been shown to have protective effects on oxidative stress-induced liver cell apoptosis through modulating the level of FoxO3 (Tao et al., 2013). Moreover, Lento et al. reported that Wnt signaling was activated in hematopoietic stem and progenitor cells exposed to radiation, which helped to suppress the formation of ROS. In an β-catenin knockout mouse model, hematopoietic stem and progenitor cells lost the ability to suppress oxidative stress and could not counteract the toxic effects induced by radiation, and thus they were unable to regenerate or recover after radiation (Lento et al., 2014). Taken together, the evidence indicates the delicate regulation of Wnt signaling is important for cells or tissues to maintain their physiological levels of ROS.
However, abnormal Wnt signaling may lead to excessive oxidative stress in tissues, thereby resulting in detrimental effects. A recent study found that aberrant Wnt signaling is causatively related with intestinal tumorigenesis induced by oxidative stress in an oncogenic mouse model (Isoda et al., 2014). Dysregulated Wnt signaling has also been shown to increase oxidative stress in DR. Liu et al. reported that nitrosative stress induced by peroxynitrite (PN), 4-hydroxynonenal and high glucose activates Wnt signaling and increases the expression of VEGF and intercellular adhesion molecule-1 (ICAM-1) in RPE cells, whereas inhibition of nitrosative stress suppresses Wnt signaling and reduces expression of VEGF and inflammatory mediator ICAM-1(Liu, Li et al., 2013). Further, the treatment of uric acid, a PN scavenger, ameliorates diabetes-induced Wnt signaling activation and reduces the inflammation and vascular leakage in the retina of a STZ-induced diabetic rat model (Liu, Li et al., 2013). In bovine retinal capillary endothelial cells (RCECs), high glucose induces the activation of Wnt signaling, while aminoguanidine, which is known to have antioxidant activity, blocks high glucose-induced Wnt signaling activation (Chen et al., 2009). Additionally, the Wnt signaling inhibitor DKK1 can reduce ROS generation induced by TNF-α and high glucose, suggesting that Wnt signaling may regulate oxidative stress in DR (Chen et al., 2009). Furthermore, SERPINA3K, a negative Wnt signaling regulator, significantly decreases ROS generation and up-regulates the expression and activity of manganese super-oxide in hypoxic retinal cells (Zhang, Hu, & Ma, 2009). Cumulatively, these findings suggest that Wnt signaling may both contribute to and be induced by oxidative stress.
6.2. Canonical Wnt signaling and angiogenesis
Canonical Wnt signaling plays important roles in the development and differentiation of the retinal vasculature (Chen et al., 2011; Ye et al., 2009). In physiological angiogenesis, canonical Wnt signaling is critical in regulating endothelial cell proliferation and maintaining vessel stability during vascular formation via regulating Dll4/Notch signaling (Corada et al., 2010). Canonical Wnt signaling controls blood-brain barrier (BBB) formation in the central nervous system, including blood-retina barrier in the retina (Liebner et al., 2008). The null mutations of the Wnt signaling components, Fzd-4, LRP5, LEF-1 and Norrin, induce similar vascular alterations in the murine retina, which suggests a role of Wnt signaling in development of the retinal vascular network. For example, Fzd-4 deletion in mice leads to defective intra-retinal vasculature, abnormal vascular patterning and intraocular hemorrhages (Xu et al., 2004). LRP5 knockout mice show delayed retinal vascular development. In addition, retinal vessels are diluted and tortuous, with abnormal aggregations of endothelial cells in the retina of adult LRP5 knockout mice (Chen et al., 2011).
VEGF, a major pro-angiogenic factor in physiological and pathological angiogenesis, has been identified as a downstream target gene of canonical Wnt signaling (Zhang, Gaspard, & Chung, 2001). VEGF plays an important role in growth of new retinal blood vessels and contributes to the pathogenesis of proliferative DR. It was reported that VEGF levels in aqueous humor and vitreous fluid of diabetic patients were correlated with the severity of DR (Noma et al., 2002). Studies have shown that VEGF increases the phosphorylation of tight-junction proteins such as occludin and Zonula Occluden-1 (ZO-1) (Antonetti, Barber, Hollinger, Wolpert, & Gardner, 1999). In addition, VEGF promotes endothelial cell proliferation by activating mitogen-activated protein (MAP), upregulating the phosphatidylinositol 3-kinase (PI3)/Akt pathway after the induction of vascular endothelial growth factor receptor 2 (Ferrara, 2004). Our group has found that activation of Wnt signaling increases VEGF levels in the retina of diabetic animal models, while suppression of Wnt signaling down-regulates the expression of VEGF, suggesting that Wnt signaling mediates VEGF expression in the diabetic retina (Chen et al., 2009). A recent study has reported that retinal levels of Wnt receptor Fzd-4 and Wnt co-receptor LRP5 are up-regulated in a mouse model of OIR, and loss of LRP5 inhibits the pathological neovascularization in OIR, suggesting that Wnt signaling may regulate pathological neovascularization in animal models of proliferative retinopathy (Chen et al., 2011).
6.3. Canonical Wnt signaling and inflammation
Inflammation is a biological response of tissues to injury which is involved in multiple cell types and molecular mediators. Acute inflammation is a protective response which helps to alleviate the cause of cellular injury and initiate tissue repair. However, chronic inflammation is detrimental to tissues, which induces tissue damage and structural destruction with fibrosis and angiogenesis. Chronic inflammation induces a variety of physiological and molecular abnormalities that have been found in retinas and/or vitreous humor from diabetic patients and diabetic animal models (Adamis & Berman, 2008; Joussen et al., 2004). For example, levels of proinflammatory factors such as TNF-α, IL-8, IL-6, monocyte chemoattractant protein-1(MCP-1), endothelin-1, VEGF and ICAM-1 are increased in the vitreous of patients with proliferative DR (Adamiec-Mroczek, Oficjalska-Mlynczak, & Misiuk-Hojlo, 2010; Huang et al., 2011). Increased levels of IL-1β, TNF-α and IL-6 in diabetic retinas are associated with the BRB breakdown, retinal leukostasis and retinal cell apoptosis (Adamis & Berman, 2008). In addition, leukocyte-endothelial cell adhesion and leukostasis are increased in patients with DR (Chibber, Ben-Mahmud, Chibber, & Kohner, 2007), which is accompanied by elevated levels of inflammatory cytokines (van Hecke et al., 2005).
Wnt signaling activation has been shown to contribute to inflammation in many tissues, including the retina. Halleskog et al. reported that levels of β-catenin are increased in microglia associated with neuroinflammation under pathogenic conditions such as Alzheimer’s disease (Halleskog et al., 2011). Wnt3A activates the canonical Wnt signaling pathway in cultured mouse microglial cells, increases levels of proinflammatory cytokines and induces the release of inflammatory mediators IL-6, IL-12 and TNF-α (Halleskog et al., 2011). A recent study has also shown that the inflammatory cytokines IL-6 and TNF-α activate Wnt signaling and impair preadipocyte differentiation and lipid accumulation in vitro (Gustafson & Smith, 2006). In addition, Over-activated Wnt signaling is also associated with increased ICAM-1, TNF-α and VEGF in the retina and suppression of Wnt signaling reduces the expression of ICAM-1, TNF-α and VEGF, suggesting a proinflamma-tory role of Wnt signaling in the retina (Chen et al., 2009; Park, Jin, Hu, Zhou, & Ma, 2011).
7. Animal models for studying canonical Wnt signaling regulation in the retina
Wnt reporter mice have been widely used to evaluate Wnt signaling activation and locate Wnt-responsive cells. BATGAL mice are reporter mice expressing the β-galactosidase gene under the control of Wnt signaling (Maretto et al., 2003). Similarly, TOPGAL mice are known to express the β-galactosidase gene under the control of β-catenin/TCF-inducible promoter (DasGupta & Fuchs, 1999). The Axin2-lacZ mouse is another commonly used reporter mouse for evaluation of canonical Wnt signaling activity in which the lacZ transgene is knocked into the exon 2 of Axin2, a direct target gene regulated by canonical Wnt signaling (Jho et al., 2002). Al Alam et al. have compared the β-galactosidase expression patterns upon Wnt signaling activation among these three reporter mice in murine lung after naphthalene injury (Al Alam et al., 2011). They found that β-galactosidase, which is already highly expressed in normal lung, is induced in the bronchial epithelium in BATGAL and TOPGAL mice, while the expression of β-galactosidase is increased in the bronchial epithelium of injured lungs in Axin2-lacZ mice (Al Alam et al., 2011). Thus, Axin2-lacZ mice are the most reliable Wnt reporter line compared with the other two lines to assess Wnt signaling activities in naphthalene induced lung injury.
Several animal models of DR have increased Wnt signaling activities in the retina. For example, the Akita mouse is a type 1 diabetic animal model, which carries a mutation in the insulin 2 gene and has progressive loss of β-cell function and significant hyperglycemia (Wang et al., 1999). Akita mice develop early features of DR at 3–4 months of age, such as increased vascular permeability and increased acellular capillaries (Barber et al., 2005). Chen et al. reported that levels of β-catenin were elevated in the retina of Akita mice at 4 months of age (Chen et al., 2009). STZ-induced diabetic rats have been the most commonly used type 1 diabetic animal model for DR due to an easy induction process and the abundant previous reports on the phenotypes (Robinson, Barathi, Chaurasia, Wong, & Kern, 2012). Chen et al. also found that STZ-induced diabetic rats have over-activated Wnt signaling in the retina 1 month after the onset of diabetes (Chen et al., 2009). Retinal Wnt signaling is also over-activated in the mouse and rat models of OIR (Chen et al., 2009; Takahashi et al., 2015). The OIR model is a model of ischemia-induced retinal neovascularization and commonly used as a proliferative retinopathy model (Stahl et al., 2010). Wnt signaling components, phosphorylated LDLR-related protein 6 (p-LRP6), non-phosphorylated β-catenin (non-β-catenin) and total β-catenin levels are increased along with elevated VEGF levels in the retina of these three models (Chen et al., 2009). Further, eyecups from rat and mouse models of laser-induced CNV have elevated levels of the Wnt signaling pathway components (p-LRP6, total LPR6 and non-p-β-catenin) and Wnt target genes (VEGF, c-myc and cyclin D1) (Hu et al., 2013). Taken together, these models will allow us to investigate the pathologic mechanisms of Wnt signaling upregulation in the retina and increase our knowledge of Wnt signaling regulation in ocular diseases.
8. Targeting canonical Wnt signaling in diabetic retinopathy
Because Wnt signaling is over-activated in DR and contributes to retinal inflammation and angiogenesis, suppression of over-activated Wnt signaling offers a new therapeutic strategy for DR. Several studies carried out by our group and others have shown that inhibition of Wnt signaling has beneficial effects on DR. Here we summarize several Wnt signaling inhibitors that have been tested in the retina of diabetic animal models and have displayed therapeutic potential in DR (Table 1).
Table 1.
Potential canonical Wnt signaling inhibitors on DR.
| Name | Tested Animal Models | Main Function | Target | Citations |
|---|---|---|---|---|
| SERPINA3K | OIR in mice | Anti-oxidant | LRP6 | Zhang et al. (2009), Zhang, Zhou etal. (2010) |
| STZ-induced diabetes in rats | Anti-inflammation | |||
| Anti-fibrosis | ||||
| Kallistatin | OIR, STZ-induced diabetes, Akita in kallistatin-transgenic mice | Anti-inflammation | LRP6 | Liu, Zhang et al. (2013) |
| Anti-angiogenesis | ||||
| PEDF | OIR, CNV in PEDF transgenic mice | Anti-inflammation | LRP6 | Park, Lee et al. (2011) |
| OIR, STZ-induce diabetes in rats | Anti-angiogenesis | |||
| VLDLR | OIR in mice | Anti-angiogenesis | LRP6 | Chen et al. (2007), |
| Alkali burn-induced corneal neovascularization in rats | Wang etal. (2015) | |||
| MiRNA-184 | OIR model in mice | Anti-angiogenesis | Fzd-7 | Takahashi etal. (2015) |
| Mab2Fl* | OIR and STZ-induced diabetes in rats | Anti-inflammation Anti-angiogenesis | LRP6 | Lee et al. (2012) |
| DKK1 | OIR and STZ-induced diabetes in rats | Anti-inflammation | LRP6 | Chen et al. (2009) |
| Anti-angiogenesis | ||||
| Endostatin | Transgenic mice with inducible expression of VEGF | Anti-angiogenesis | β-Catenin | Hanai et al. (2002), Takahashi et al. (2003) |
PEDF: Pigment epithelium–derived factor; VLDLR: Very low-density lipoprotein receptor; miRNA-184: microRNA-184; DKK1: Dickkopf-related protein 1; LRP6: Low-density lipoprotein receptor-related protein 6; Fzd-7: frizzled 7; OIR: oxygen-induced retinopathy, STZ: streptozotocin.
Mab2F1 is an anti-LRP6 monoclonal antibody which was generated in our lab.
8.1. Serine proteinase inhibitors (SERPIN) – SERPINA3K and kallistatin
SERPINA3K is a specific inhibitor of tissue kallikrein with anti-inflammatory and anti-angiogenic activities (Chao, Tillman, Wang, Margolius, & Chao, 1986). SERPINA3K binds to the extracellular domain of Wnt co-receptor LRP6 and blocks LRP6 dimerization with the Fzd receptor, thus inhibiting Wnt signaling (Zhang, Abreu et al., 2010). Studies have shown that retinal levels of SERPINA3K were decreased in STZ-induced diabetic rats (Hatcher, Ma, Chao, Chao, & Ottlecz, 1997), indicating that decreased SERPINA3K may play a role in DR. In addition, SERPINA3K reduces DR by suppressing Wnt signaling and inhibiting the expression of connective tissue growth factor and fibronectin in STZ-induced diabetic rats (Zhang, Zhou, & Ma, 2010). Intravitreal injection of SERPINA3K reduces retinal inflammation and oxidative stress in a mouse OIR model (Zhang et al., 2009).
Kallistatin, a member of the serine proteinase inhibitor super-family, has been found to be an endogenous inhibitor of Wnt signaling (Liu, Zhang et al., 2013). Kallistatin binds to the extracellular domain of Wnt co-receptor LRP6 with high affinity and inhibits the Wnt signaling pathway (Liu, Zhang et al., 2013). Levels of Kallistatin are significantly decreased in the vitreous fluid of patients with DR (Jenkins et al., 2010), indicating that Kallistatin plays a role in DR. Kallistatin-transgenic (TG) mice have high retinal levels of Kallistatin and display normal retinal structure and functions under normal condition (Liu, Zhang et al., 2013). In the OIR model, however, Kallistatin-TG mice have reduced areas of retinal neovascularization and alleviated retinal inflammation compared with WT mice (Liu, Zhang et al., 2013). Neuroinflammation and vascular leakage are decreased in the retina of Kallistatin-TG X Akita mice relative to Akita mice, suggesting a protective role of Kallistatin against DR. Further, the study using diabetic Kallistatin-TG mice crossed with BATGAL reporter mice provides direct evidence that diabetes induces Wnt signaling upregulation, which is attenuated by Kallistatin over-expression in Kallistatin-TG mice (Liu, Zhang et al., 2013).
8.2. Pigment epithelium-derived factor (PEDF)
PEDF, a serine proteinase inhibitor, has displayed multiple functions, such as anti-angiogenesis, anti-oxidation, anti-fibrosis and anti-inflammation in various diseases (Dawson et al., 1999; Matsuoka, Ogata, Minamino, & Matsumura, 2006; Tsao, Ho, Chen, & Cheng, 2006; Zhang et al., 2006). PEDF knockout mice have elevated Wnt signaling in the retina compared with WT mice, and overexpression of PEDF in transgenic mice attenuates Wnt signaling activation induced by retinal ischemia, suggesting that PEDF functions as an inhibitor of the canonical Wnt pathway (Park, Lee et al., 2011). PEDF inhibits Wnt signaling activity through high-affinity binding of LRP6. Binding of PEDF with LRP6 blocks Wnt ligand-induced LRP6-Fzd receptor dimerization and inhibits Wnt signaling activation (Park, Lee et al., 2011). PEDF plays an important role in DR and modulating PEDF levels has shown therapeutic potential for DR. PEDF levels are decreased in aqueous and vitreous fluid of patients with DR and DR animal models (Boehm, Lang, Feldmann et al., 2003; Boehm, Lang, Volpert et al., 2003, Cohen, Hud, Shea, & Shearman, 2008). Intravitreal injection of PEDF significantly reduces vascular permeability and ameliorates retinal inflammation in STZ-induced diabetes and OIR, suggesting an anti-inflammatory role of PEDF in DR (Zhang et al., 2006). In addition, intravitreal administration of purified recombinant human PEDF inhibits retinal neovascularization in a mouse OIR model, indicating the anti-angiogenic effect of PEDF on ischemia-induced neovascularization (Duh et al., 2002). PEDF transgenic (TG) mice with OIR have developed smaller areas of retinal neovascularization compared with those in WT OIR mice (Park, Jin et al., 2011). In addition, over-expression of PEDF using PEDF-TG mice attenuates retinal inflammation and reduces vascular leakage in OIR (Park, Jin et al., 2011). Accordingly, PEDF knockout mice demonstrate exacerbated hyperoxia-mediated vessel obliteration in OIR relative to WT control mice (Huang, Wang, Sorenson, & Sheibani, 2008). Furthermore, a protective role of PEDF is also observed in a laser-induced CNV model (Park, Jin et al., 2011). PEDF-TG mice develop smaller CNV lesions and have lower levels of retinal inflammatory factors compared with those in WT mice after laser photocoagulation.
8.3. Very low-density lipoprotein receptor (VLDLR)
VLDLR is a transmembrane receptor that belongs to the low-density lipoprotein receptor (LDLR) family (Dieckmann, Dietrich, & Herz, 2010). VLDLR is bound by a variety of ligands, such as lipoproteins, proteinases, proteinase-inhibitor complexes, vita-mins, reelin and other macromolecules, to mediate lipid metabolism and other biological functions (Dieckmann et al., 2010; Hussain, Strickland, & Bakillah, 1999). VLDLR knockout (VLDLR−/−) mice are viable and fertile (Frykman, Brown, Yamamoto, Goldstein, & Herz, 1995), but display abnormal intra-retinal and sub-retinal neovascularization, indicating that VLDLR plays a critical role in retinal angiogenesis (Hu et al., 2008; Jiang, Hu, Meng, Gao, & Qiao, 2009; Li et al., 2007). In the retina of VLDLR−/− mice, Wnt signaling is over-activated, subsequently promoting the production of VEGF and inflammatory factors and ultimately resulting in pathological angiogenesis and inflammation in the retina (Chen et al., 2007). VLDLR heterodimerizes with Wnt co-receptor LRP6 through its extracellular domain, resulting in internalization and degradation of LRP6, and consequently, Wnt signaling inhibition (Lee et al., 2014). Nanoparticles containing the N-terminus of VLDLR (VLDLR-N nanoparticles) inhibit endothelial cell proliferation, migration and tube formation in cultured endothelial cells (Wang et al., 2015). Meanwhile, VLDLR-N nanoparticles suppress over-activation of Wnt signaling and reduce retinal neovascularization in a mouse OIR model (Wang et al., 2015), indicating the therapeutic potential of VLDLR in proliferative retinopathy. Interestingly, VLDLR is found to release its soluble extracellular domain (sVLDLR-N) into extracellular space, and sVLDLR-N alone is capable of inhibiting Wnt signaling both in vitro and in vivo, and may mediate intercellular regulation of Wnt signaling (Chen, Takahashi, Oka, & Ma, 2016; Lee et al., 2014; Magrane, Casaroli-Marano, Reina, Gafvels, & Vilaro, 1999). Levels of sVLDLR-N in the plasma were decreased in both type 1 and type 2 diabetic mouse models (Chen et al., 2016), suggesting that reduced levels of sVLDLR-N in the plasma may result in Wnt signaling activation and thus contribute to diabetic complications.
8.4. MicroRNA-184
MiRNA-184 is an eye-specific miRNAs and abundantly expressed in the cornea, lens and retina (Ryan et al., 2006). Our previous study reported that miRNA-184 is significantly down-regulated in the retina of OIR mice (Takahashi et al., 2015). Based on bioinformatic analysis, miRNA-184 targets several components of Wnt signaling such as Wnt receptor (Frizzled-3, Frizzled-7) and Wnt ligands (Wnt-9, Wnt-16) (Shen et al., 2008; Takahashi et al., 2015). In the retina of a mouse OIR model, miR-184 modulates Wnt signaling by targeting Wnt receptor Fzd-7 (Takahashi et al., 2015). Intravitreal delivery of miRNA-184 mimic decreases retinal levels of Wnt signaling components (p-LRP6, non-p-β-catenin) and VEGF in ischemia-induced retinal neovascularization, suggesting the therapeutic potential of targeting miR-184 in proliferative retinopathy (Takahashi et al., 2015). In addition, decreased levels of miR-184 in the cornea may result in activation of Wnt signaling and lead to corneal neovascularization (Zong et al., 2016).
8.5. Anti-LRP6 monoclonal antibody
Levels of LRP6, a co-receptor of Wnt signaling, are increased in the retina of DR animal models (Chen et al., 2009). Inhibition of Wnt signaling by Mab2F1 has shown therapeutic potential on DR (Lee et al., 2012). Mab2F1 is a mouse monoclonal antibody against the E1E2 domain of LRP6. In retinal endothelial cells, Mab2F1 inhibits high-glucose induced Wnt signaling upregulation and suppresses the expression of inflammatory factors (VEGF, ICAM-1 and TNF-α) induced by high glucose (Lee et al., 2012). Moreover, Mab2F1 inhibits the migration and tube formation of endothelial cells (Lee et al., 2012). Intravitreal delivery of Mab2F1 reduces vascular leakage, inhibits retinal inflammation and ameliorates retinal neovascularization in the OIR model. In addition, intravitreal delivery of Mab2F1 reduces vascular leakage and inhibits retinal inflammation in a STZ-induced diabetic rat model (Lee et al., 2012). Mab2F1 has also displayed therapeutic effects in laser-induced CNV and in retinal degeneration in a mouse model of dry AMD (Hu et al., 2013; Tuo et al., 2015).
8.6. Other Wnt antagonists in DR
Intravitreal delivery of Wnt antagonist DKK1 attenuates inflammation and reduces vascular leakage in STZ-induced diabetic rats (Chen et al., 2009). In addition, intravitreal injection of DKK1 in OIR rats results in significant reductions of vascular leakage and neovascularization in the retina (Chen et al., 2009). Endostatin, a fragment of collagen XVIII with anti-proliferative and anti-angiogenic properties, is a potential anti-angiogenic drug for tumors and is currently undergoing the phase II clinical trials. The levels of endostatin in aqueous humor and vitreous fluid of diabetic patients were significantly correlated with the severity of DR (Noma et al., 2002), suggesting a possible pathogenic role of endostatin in DR. Prior findings demonstrate that endostatin inhibits Wnt signaling by promoting the degradation of β-catenin via a novel GSK3-independent mechanism (Hanai et al., 2002). Intravitreal delivery of endostatin into transgenic mice with inducible expression of VEGF alleviates VEGF-induced retinal vascular leakage and retinal neovascularization (Takahashi et al., 2003). Behl et al. reviewed the possible therapeutic applications of endostatin in DR and suggested that endostatin may have anti-angiogenic effects on DR through downregulation of inhibitor of differentiation/DNA synthesis (Id) 1 and Id3, inhibition of matrix metalloproteinases and suppression of VEGF expression (Behl & Kotwani, 2015).
9. Crosstalk of canonical Wnt signaling with other signaling pathways
The interactions of canonical Wnt signaling with other signaling pathways such as NF-κB signaling, Notch signaling, and hippo signaling have been reported, which play essential roles in maintaining tissue homeostasis, and aberrant regulation of signaling pathways may lead to many human diseases, such as cancer. For instance, Du and Geller (2010) have comprehensively summarized the cross-regulation of Wnt signaling and NF-κB signaling from different aspects in the cancer field, including the interactions directly or indirectly involved components or target genes of both signaling pathways. Meanwhile, crosstalks between Wnt signaling and Notch signaling have also been reviewed in the fields of cancer and cardiovascular biology (Caliceti, Nigro, Rizzo, & Ferrari, 2014; Morris & Huang, 2016). In general, Wnt signaling and Notch signaling have opposing effects on cell-fate determination during embryogenesis (Hayward, Kalmar, & Arias, 2008). The balance between Wnt signaling and Notch is tightly controlled and critical for proper vascular development (Hayward et al., 2008). Further, crosstalk of Wnt signaling and hippo signaling has been reported by Heallen et al. (2011). In the mice with inactivated Hippo pathway components, they found that a subset of Wnt target genes was up-regulated in the developing mouse heart, indicating crosstalk of Wnt signaling with hippo signaling (Heallen et al., 2011). Several review articles were published to report the cross-regulation of Wnt signaling and hippo signaling (Kim & Jho, 2014; Morris & Huang, 2016; Varelas et al., 2010). However, few documented evidence indicated the presence of cross-regulation of Wnt signaling and other signaling pathways in retinal diseases. Since previous studies have shown that NF-κB signaling, Notch signaling and hippo signaling play important roles in the developing retina and retinal diseases, it is possible that such cross-regulation in cancer cells is also present in retina, and the disturbance of network among these signaling pathways may lead to retinal diseases.
10. Conclusions and future directions
Canonical Wnt signaling is involved in the pathogenesis of DR and other retinal diseases, including AMD, FEVR, Norrie disease and retinitis pigmentosa. The mechanisms underlying the involvement of canonical Wnt signaling in DR may be associated with unbalanced oxidative stress, over-expressed angiogenic and inflammatory factors. Wnt signaling reported mice, diabetic animal models (Akita mice and STZ-induced diabetic mice) and mouse or rat models of OIR are recommended animal models for studying canonical Wnt signaling in the retina under physiological or pathological conditions. Suppression of canonical Wnt signaling by inhibiting Wnt signaling components, such as LRP6, Frizzled receptor and β-catenin, has shown beneficial effects in animal models of DR. Thus, blockade of Wnt signaling is expected to be effective on DR in patients who are not responsive to currently available therapies. As more and more attention has been focused on Wnt signaling, our understanding of its role in DR will be further advanced. More efforts are needed to explore mechanism of aberrant Wnt signaling activation in DR and to develop effective strategies to correct the aberrant Wnt signaling. Recently, the crosstalk of Wnt signaling with other signaling pathways has raised great attention, and the investigation of the integral role of these signaling pathways in ocular diseases will be a promising research direction in the future.
Acknowledgments
This study was supported by the US National Institutes of Health (NIH) grants (EY012231, EY018659, EY019309 and GM104934), a grant from Oklahoma Center for the Advancement of Science & Technology (OCAST; HR16–041) to JXM and a fellowship from American Heart Association (AHA; 14PRE20460229) to QC. We thank Dr. Elizabeth Moran at Boston Children’s Hospital for her critical review of this manuscript. The authors declared no conflicts of interest.
References
- Aberle H, Bauer A, Stappert J, Kispert A, & Kemler R (1997). Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO Journal, 16(13), 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamiec-Mroczek J, Oficjalska-Mlynczak J, & Misiuk-Hojlo M (2010). Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: Analysis of vitreous samples. Cytokine, 49(3), 269–274. [DOI] [PubMed] [Google Scholar]
- Adamis AP, & Berman AJ (2008). Immunological mechanisms in the pathogenesis of diabetic retinopathy. Seminars Immunopathology, 30(2), 65–84. [DOI] [PubMed] [Google Scholar]
- Al Alam D, Green M, Tabatabai Irani R, Parsa S, Danopoulos S, Sala FG, et al. (2011). Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One, 6(8), e23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, & Gardner TW (1999). Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. Journal of Biological Chemistry, 274(33), 23463–23467. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, & Gardner TW (2012). Diabetic retinopathy. New England Journal of Medicine, 366(13), 1227–1239. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, et al. (2005). The Ins2Akita mouse as a model of early retinal complications in diabetes. Investigative Ophthalmology & Visual Science, 46(6), 2210–2218. [DOI] [PubMed] [Google Scholar]
- Behl T, & Kotwani A (2015). Possible role of endostatin in the antiangiogenic therapy of diabetic retinopathy. Life Sciences, 135, 131–137. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, & Birchmeier W (1996). Functional interaction of beta-catenin with the transcription factor LEF-1. Nature, 382(6592), 638–642. [DOI] [PubMed] [Google Scholar]
- Berger W (1998). Molecular dissection of Norrie disease. Acta Anatomical (Basel),162(2–3), 95–100. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, & Niehrs C (2007). Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science, 316(5831), 1619–1622. [DOI] [PubMed] [Google Scholar]
- Boehm BO, Lang G, Feldmann B, Kurkhaus A, Rosinger S, Volpert O, et al. (2003). Proliferative diabetic retinopathy is associated with a low level of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor. A pilot study. Hormone and Metabolic Research, 35(6), 382–386. [DOI] [PubMed] [Google Scholar]
- Boehm BO, Lang G, Volpert O, Jehle PM, Kurkhaus A, Rosinger S, et al. (2003). Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia, 46(3), 394–400. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, & Lopez-Rios J (2008). Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. Journal of Cell Science, 121(Pt 6), 737–746. [DOI] [PubMed] [Google Scholar]
- Caliceti C, Nigro P, Rizzo P, & Ferrari R (2014). ROS, Notch, and Wnt signaling pathways: Crosstalk between three major regulators of cardiovascular biology. Biomedical Research International, 2014, 318714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, et al. (1998). Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature, 395(6702), 604–608. [DOI] [PubMed] [Google Scholar]
- CDC (2014). National diabetes statistics report.
- Chao J, Tillman DM, Wang MY, Margolius HS, & Chao L (1986). Identification of a new tissue-kallikrein-binding protein. Biochemical Journal, 239(2), 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, & Craig IW (1993). A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nature Genetics, 5(2), 180–183. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lu K, Flannery JG, & Ma JX (2007). Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. Journal of Biological Chemistry, 282(47), 34420–34428. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, et al. (2009). Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. American Journal of Pathology, 175(6), 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, et al. (2011). Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation, 124(17), 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Takahashi Y, Oka K, & Ma JX (2016). Functional differences of very-low-density lipoprotein receptor splice variants in regulating Wnt signaling. Molecular and Cellular Biology, 36(20), 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, & Wong TY (2010). Diabetic retinopathy. Lancet, 376(9735), 124–136. [DOI] [PubMed] [Google Scholar]
- Chibber R, Ben-Mahmud BM, Chibber S, & Kohner EM (2007). Leukocytes in diabetic retinopathy. Current Diabetes Review, 3(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Clevers H (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127(3), 469–480. [DOI] [PubMed] [Google Scholar]
- Clevers H, & Nusse R (2012). Wnt/beta-catenin signaling and disease. Cell, 149(6),1192–1205. [DOI] [PubMed] [Google Scholar]
- Cohen MP, Hud E, Shea E, & Shearman CW (2008). Vitreous fluid of db/db mice exhibits alterations in angiogenic and metabolic factors consistent with early diabetic retinopathy. Ophthalmic Research, 40(1), 5–9. [DOI] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, et al. (2010). The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Developmental Cell, 18(6), 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswick VG, & Schepens CL (1969). Familial exudative vitreoretinopathy.American Journal of Ophthalmology, 68(4), 578–594. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Gardiner TA, & Stitt AW (2009). Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye (London), 23(7), 1496–1508. [DOI] [PubMed] [Google Scholar]
- DasGupta R, & Fuchs E (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development, 126(20), 4557–4568. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, & Bouck NP (1999). Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science, 285(5425), 245–248. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Abud HE, & Hime GR (2006). WNT/Frizzled signaling in eye development and disease. Frontiers Biosciences, 11, 2442–2464. [DOI] [PubMed] [Google Scholar]
- Dieckmann M, Dietrich MF, & Herz J (2010). Lipoprotein receptors – An evolutionarily ancient multifunctional receptor family. Biology Chemical, 391(11), 1341–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, & Geller DA (2010). Cross-regulation between Wnt and NF-kappaB signaling pathways. Forest Immunopathology Diseases Therapeutics, 1(3), 155–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh EJ, Yang HS, Suzuma I, Miyagi M, Youngman E, Mori K, et al. (2002). Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Investigative Ophthalmology & Visual Science, 43(3), 821–829. [PubMed] [Google Scholar]
- Durham JT, & Herman IM (2011). Microvascular modifications in diabetic retinopathy. Current Diabetes Reports, 11(4), 253–264. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, & Korswagen HC (2005). Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science, 308(5725), 1181–1184. [DOI] [PubMed] [Google Scholar]
- Ferrara N (2004). Vascular endothelial growth factor: Basic science and clinical progress. Endocrine Reviews, 25(4), 581–611. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, & Parmeggiani F (2011). Retinitis pigmentosa: Genes and disease mechanisms. Current Genomics, 12(4), 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, & Sonenberg N (2008). Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nature Reviews Genetics, 9(2), 102–114. [DOI] [PubMed] [Google Scholar]
- Frykman PK, Brown MS, Yamamoto T, Goldstein JL, & Herz J (1995). Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America, 92(18), 8453–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S (2008). Wnt signaling in eye organogenesis. Organogenesis, 4(2),60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood P, Jones J, Bundey S, Dudgeon J, Fielder AR, & Kilpatrick MW (1993). X linked exudative vitreoretinopathy: Clinical features and genetic linkage analysis. British Journal of Ophthalmology, 77(3), 168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato Y, Michiue T, Asashima M, & Miki H (2006). The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nature Cell Biology, 8(5), 501–508. [DOI] [PubMed] [Google Scholar]
- Gao X, Ma K, Lu N, Xu Y, Hong T, & Peng X (2015). Elevated LRP6 levels correlate with vascular endothelial growth factor in the vitreous of proliferative diabetic retinopathy. Molecular Vision, 21, 665–672. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hoyos M, Cantalapiedra D, Arroyo C, Esteve P, Rodriguez J, Riveiro R, et al. (2004). Evaluation of SFRP1 as a candidate for human retinal dystrophies. Molecular Vision, 10, 426–431. [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell, 107(4), 513–523. [DOI] [PubMed] [Google Scholar]
- Goretzki L, & Mueller BM (1998). Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochemical Journal, 336(Pt2), 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, & Smith U (2006). Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. Journal of Biological Chemistry, 281(14), 9507–9516. [DOI] [PubMed] [Google Scholar]
- Hackler L Jr., Wan J, Swaroop A, Qian J, & Zack DJ (2010). MicroRNA profile of the developing mouse retina. Investigative Ophthalmology & Visual Science, 51(4), 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, et al. (2006). Functional candidate genes in age-related macular degeneration: Significant association with VEGF, VLDLR, and LRP6. Investigative Ophthalmology & Visual Science, 47(1), 329–335. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, et al. (2011). WNT signaling in activated microglia is proinflammatory. Glia, 59(1), 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai J, Gloy J, Karumanchi SA, Kale S, Tang J, Hu G, et al. (2002). Endostatin is a potential inhibitor of Wnt signaling. Journal of Cell Biology, 158(3), 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimi ST, Fulcher JA, Chang MH, Gov L, Wang S, & Lee B (2009). MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood, 114(2), 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher HC, Ma JX, Chao J, Chao L, & Ottlecz A (1997). Kallikrein-binding protein levels are reduced in the retinas of streptozotocin-induced diabetic rats. Investigative Ophthalmology & Visual Science, 38(3), 658–664. [PubMed] [Google Scholar]
- Hayward P, Kalmar T, & Arias AM (2008). Wnt/Notch signalling and information processing during development. Development, 135(3), 411–424. [DOI] [PubMed] [Google Scholar]
- He L, & Hannon GJ (2004). MicroRNAs: Small RNAs with a big role in gene regulation. Nature Reviews Genetics, 5(7), 522–531. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, & Martin JF (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science, 332(6028), 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, & Ma JX (2013). Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Investigative Ophthalmology & Visual Science, 54(1), 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, & Qiao X (2008). Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s retinal angiomatous proliferation. Investigative Ophthalmology & Visual Science, 49(1), 407–415. [DOI] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, & Vinores SA (2011). TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investigative Ophthalmology & Visual Science, 52(3), 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Wang S, Sorenson CM, & Sheibani N (2008). PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Experimental Eye Research, 87(3), 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MM, Strickland DK, & Bakillah A (1999). The mammalian low-density lipoprotein receptor family. Annual Review of Nutrition, 19, 141–172. [DOI] [PubMed] [Google Scholar]
- IDF (2015). IDF diabetes atlas (7th ed.). [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, & Kikuchi A (1998). Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO Journal, 17(5), 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M, Martello G, & Piccolo S (2010). MicroRNA control of signal transduction.Nature Reviews Molecular Cell Biology, 11(4), 252–263. [DOI] [PubMed] [Google Scholar]
- Isoda T, Nakatsu Y, Yamauchi K, Piao J, Yao T, Honda H, et al. (2014). Abnormality in Wnt signaling is causatively associated with oxidative stress-induced intestinal tumorigenesis in MUTYH-null mice. International Journal of Biological Sciences, 10(8), 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AJ, McBride JD, Januszewski AS, Karschimkus CS, Zhang B, O’Neal DN, et al. (2010). Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. Journal of Angiogenesis Research, 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, & Costantini F (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology, 22(4), 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Hu W, Meng H, Gao H, & Qiao X (2009). Loss of VLDL receptor activates retinal vascular endothelial cells and promotes angiogenesis. Investigative Ophthalmology & Visual Science, 50(2), 844–850. [DOI] [PubMed] [Google Scholar]
- Jiao X, Ventruto V, Trese MT, Shastry BS, & Hejtmancik JF (2004). Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. American Journal of Human Genetics, 75(5), 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, & Neal MJ (2000a). Altered expression of secreted frizzled-related protein-2 in retinitis pigmentosa retinas. Investigative Ophthalmology & Visual Science, 41(6), 1297–1301. [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, & Neal MJ (2000b). Modulated expression of secreted frizzled-related proteins in human retinal degeneration. NeuroReport, 11(18), 3963–3967. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. (2004). A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB Journal, 18(12), 1450–1452. [DOI] [PubMed] [Google Scholar]
- Kennell JA, Gerin I, MacDougald OA, & Cadigan KM (2008). The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proceedings of the National academy of Sciences of the United States of America, 105(40), 15417–15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, & Jho EH (2014). Cross-talk between Wnt/beta-catenin and Hippo signaling pathways: A brief review. BMB Reports, 47(10), 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, et al. (2006). R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle, 5(1), 23–26. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science, 275(5307), 1784–1787. [DOI] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, & Kang Y (2008). The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. Journal of Biological Chemistry, 283(22), 14910–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, & Chan PS (2007). Oxidative stress and diabetic retinopathy.Experimental Diabetes Research, 2007, 43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, & Ma JX (2012). Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes, 61(11), 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Shin Y, Cheng R, Park K, Hu Y, McBride J, et al. (2014). Receptor heterodimerization as a novel mechanism for the regulation of Wnt/beta-catenin signaling. Journal of Cell Science, 127(22), 4857–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, et al. (2014). Loss of beta-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes & Development, 28(9), 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Huang Z, Kingsley R, Zhou X, Li F, Parke DW 2nd, & Cao W (2007). Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: An animal model of retinal angiomatous proliferation. Archives of Ophthalmology, 125(6), 795–803. [DOI] [PubMed] [Google Scholar]
- Li Y, Muller B, Fuhrmann C, van Nouhuys CE, Laqua H, Humphries P, et al. (1992). The autosomal dominant familial exudative vitreoretinopathy locus maps on 11q and is closely linked to D11S533. American Journal of Human Genetics, 51(4), 749–754. [PMC free article] [PubMed] [Google Scholar]
- Li Y, van Kerkhof P, Marzolo MP, Strous GJ, & Bu G (2001). Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: Implication for receptor-mediated endocytosis. Molecular and Cellular Biology, 21(4), 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. Journal of Cell Biology, 183(3), 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, & Wong TY (2012). Age-related macular degeneration. Lancet, 379(9827), 1728–1738. [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, & Aaronson SA (2005). The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Molecular and Cellular Biology, 25(9), 3475–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, et al. (2013). Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxidants & Redox Signaling, 18(10), 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. (2002). Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell, 108(6), 837–847. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang B, McBride JD, Zhou K, Lee K, Zhou Y, et al. (2013). Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes, 62(12), 4228–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, & Nusse R (2004). The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology, 20, 781–810. [DOI] [PubMed] [Google Scholar]
- Loscher CJ, Hokamp K, Kenna PF, Ivens AC, Humphries P, Palfi A, & Farrar GJ (2007). Altered retinal microRNA expression profile in a mouse model of retinitis pigmentosa. Genome Biology, 8(11), R248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher CJ, Hokamp K, Wilson JH, Li T, Humphries P, Farrar GJ, & Palfi A (2008). A common microRNA signature in mouse models of retinal degeneration. Experimental Eye Research, 87(6), 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, & He X (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell, 17(1), 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Casaroli-Marano RP, Reina M, Gafvels M, & Vilaro S (1999). The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell. FEBS Letters, 451(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, … Hardt SE (2010). Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension, 55(4), 939–945. [DOI] [PubMed] [Google Scholar]
- Malinauskas T, Aricescu AR, Lu W, Siebold C, & Jones EY (2011). Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nature Structural & Molecular Biology, 18(8), 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. (2002). Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature, 417(6889), 664–667. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, & Niehrs C (2001). LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature, 411(6835), 321–325. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, et al. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National academy of Sciences of the United States of America, 100(6), 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, & Watkins JB 3rd, (2003). Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology, 17(1), 24–38. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Ogata N, Minamino K, & Matsumura M (2006). Expression of pigment epithelium-derived factor and vascular endothelial growth factor in fibrovascular membranes from patients with proliferative diabetic retinopathy. Japanese Journal of Ophthalmology, 50(2), 116–120. [DOI] [PubMed] [Google Scholar]
- Morris SL, & Huang S (2016). Crosstalk of the Wnt/beta-catenin pathway with other pathways in cancer cells. Genes & Diseases, 3(1), 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, … Agami R (2008). Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Research, 68(14), 5795–5802. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. (2004). DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene, 23(52), 8520–8526. [DOI] [PubMed] [Google Scholar]
- Noma H, Funatsu H, Yamashita H, Kitano S, Mishima HK, & Hori S (2002). Regulation of angiogenesis in diabetic retinopathy: Possible balance between vascular endothelial growth factor and endostatin. Archives of Ophthalmology, 120(8), 1075–1080. [DOI] [PubMed] [Google Scholar]
- Nusse R, & Varmus HE (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Park K, Jin J, Hu Y, Zhou K, & Ma JX (2011). Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. American Journal of Pathology, 178(2), 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Lee K, Zhang B, Zhou T, He X, Gao G, et al. (2011). Identification of a novel inhibitor of the canonical Wnt pathway. Molecular and Cellular Biology, 31(14), 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2002). Casein kinase 1: A Wnt’er of disconnect. Current Biology, 12(14),R499–r501. [DOI] [PubMed] [Google Scholar]
- Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X, … Liu Z (2014). Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye (London), 28(4), 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TP, & Kuhl M (2010). An updated overview on Wnt signaling pathways: A prelude for more. Circulation Research, 106(12), 1798–1806. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, & Nusse R (1987). The Drosophila homolog of the mouse mammary oncogene Int-1 is identical to the segment polarity gene wingless. Cell, 50(4), 649–657. [DOI] [PubMed] [Google Scholar]
- Robinson R, Barathi VA, Chaurasia SS, Wong TY, & Kern TS (2012). Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Disease Model Mechanisms, 5(4), 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, et al. (2002). Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature Genetics, 32(2), 326–330. [DOI] [PubMed] [Google Scholar]
- Ryan DG, Oliveira-Fernandes M, & Lavker RM (2006). MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Molecular Vision, 12, 1175–1184. [PubMed] [Google Scholar]
- Saydam O, Shen Y, Wurdinger T, Senol O, Boke E, James MF, et al. (2009). Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Molecular and Cellular Biology, 29(21), 5923–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yang X, Xie B, Chen Y, Swaim M, Hackett SF, & Campochiaro PA (2008). MicroRNAs regulate ocular neovascularization. Molecular Therapy, 16(7), 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, et al. (2010). The mouse retina as an angiogenesis model. Investigative Ophthalmology & Visual Science, 51(6), 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Chen Q, Rajala RV, & Ma JX (2015). MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Letters, 589(10), 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Saishin Y, Saishin Y, Silva RL, Oshima Y, Oshima S, et al. (2003). Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB Journal, 17(8), 896–898. [DOI] [PubMed] [Google Scholar]
- Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, & Sylvester KG (2013). Wnt/beta-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. Journal of Biological Chemistry, 288(24), 17214–17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher EJ, Paydar I, Anderson KK, & Patton JG (2008). Regulation of zebrafish fin regeneration by microRNAs. Proceedings of the National academy of Sciences of the United States of America, 105(47), 18384–18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Scott S, Mackey DA, Craig JE, Appukuttan B, et al. (2004). Spectrum and frequency of FZD4 mutations in familial exudative vitreoretinopathy. Investigative Ophthalmology & Visual Science, 45(7), 2083–2090. [DOI] [PubMed] [Google Scholar]
- Tsao YP, Ho TC, Chen SL, & Cheng HC (2006). Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sciences, 79(6), 545–550. [DOI] [PubMed] [Google Scholar]
- Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu F, et al. (2015). Wnt signaling in age-related macular degeneration: Human macular tissue and mouse model. Journal of Translational Medicine, 13, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM, et al. (2005). Inflammation and endothelial dysfunction are associated with retinopathy: The Hoorn Study. Diabetologia, 48(7), 1300–1306. [DOI] [PubMed] [Google Scholar]
- van Nouhuys CE (1982). Dominant exudative vitreoretinopathy and other vascular developmental disorders of the peripheral retina. Documenta Ophthalmologica, 54(1–4), 1–414. [DOI] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, et al. (2010). The Hippo pathway regulates Wnt/beta-catenin signaling. Developmental Cell, 18(4), 579–591. [DOI] [PubMed] [Google Scholar]
- Wahid F, Shehzad A, Khan T, & Kim YY (2010). MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochimica et Biophysica Acta, 1803(11), 1231–1243. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cheng R, Lee K, Tyagi P, Ding L, Kompella UB, et al. (2015). Nanoparticle-mediated expression of a wnt pathway inhibitor ameliorates ocular neovascularization. Arteriosclerosis Thrombosis and Vascular Biology, 35(4), 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, et al. (1999). A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Journal of Clinical Investigation, 103(1), 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, & Nusse R (2002). Ligand receptor interactions in the Wnt signaling pathway in Drosophila. Journal of Biological Chemistry, 277(44), 41762–41769. [DOI] [PubMed] [Google Scholar]
- Xu S (2009). MicroRNA expression in the eyes and their significance in relation to functions. Progress in Retinal and Eye Research, 28(2), 87–116. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. (2004). Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell, 116(6), 883–895. [DOI] [PubMed] [Google Scholar]
- Xu S, Witmer PD, Lumayag S, Kovacs B, & Valle D (2007). MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. Journal of Biological Chemistry, 282(34), 25053–25066. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, et al. (2009). Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell, 139(2), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. (2005). A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature, 438 (7069), 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, et al. (2010). Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proceedings of the National academy of Sciences of the United States of America, 107(15), 6900–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gaspard JP, & Chung DC (2001). Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Research, 61(16), 6050–6054. [PubMed] [Google Scholar]
- Zhang B, Hu Y, & Ma JX (2009). Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Investigative Ophthalmology & Visual Science, 50(8), 3943–3952. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, & Ma JX (2006). Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB Journal, 20(2), 323–325. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhou KK, & Ma JX (2010). Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes, 59(7), 1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, & Ma JX (2010). The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Investigative Ophthalmology & Visual Science, 51(9), 4371–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jiang J, Wang S, & Xia X (2016). DKK1 inhibits proliferation and migration in human retinal pigment epithelial cells via the Wnt/beta-catenin signaling pathway. Experimental and Therapeutic Medicine, 12(2), 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong R, Zhou T, Lin Z, Bao X, Xiu Y, Chen Y, et al. (2016). Down-regulation of microRNA-184 is associated with corneal neovascularization. Investigative Ophthalmology & Visual Science, 57(3), 1398–1407. [DOI] [PubMed] [Google Scholar]