Abstract
Pediatric granulomatous arthritis (PGA) refers to two formerly separate entities; autosomal dominant Blau syndrome (BS) and its sporadic phenocopy early-onset sarcoidosis (EOS). In 2001 BS and in 2005 EOS became explained by heterozygous mutations within the gene that encodes nucleotide-binding oligomerization domain-containing protein 2 (NOD2), also called caspase recruitment domain-containing protein 15 (CARD15). NOD2 is a microbe sensor in leukocyte cytosol that activates and regulates inflammation. PGA is characterized by a triad of auto-inflammatory problems (dermatitis, uveitis, and arthritis) in early childhood, which suggests the causal NOD2/CARD15 mutations are activating defects. Additional complications of PGA were recognized especially when NOD2 mutation analysis became generally available. However, in PGA hypercalcemia is only briefly mentioned, and generalized osteosclerosis is not reported although NOD2 regulates NF-κB signaling essential for osteoclastogenesis and osteoclast function.
Herein, we report a 4-year-old girl with PGA uniquely complicated by severe 1,25(OH)2D-mediated hypercalcemia, nephrocalcinosis, and compromised renal function together with radiological and histopathological features of osteopetrosis (OPT). The classic triad of PGA complications was absent although joint pain and an antalgic gait accompanied wrist, knee, and ankle swelling and soft non-tender masses over her hands, knees, and feet. MRI revealed tenosynovitis in her hands and suprapatellar effusions. Synovial biopsy demonstrated reactive synovitis without granulomas. Spontaneous resolution of metaphyseal osteosclerosis occurred while biochemical markers indicated active bone turnover. Anti-inflammatory medications suppressed circulating 1,25(OH)2D, corrected the hypercalcemia, and improved her renal function, joint pain and swelling, and gait. Mutation analysis excluded idiopathic infantile hypercalcemia, type 1, and known forms of OPT, and identified a heterozygous germline missense mutation in NOD2 common in PGA (c.1001G>A, p.Arg334Gln).
Thus, radiological and histological findings of OPT and severe hypercalcemia from apparent extrarenal production of 1,25(OH)2D can complicate NOD2-associated PGA. Although the skeletal findings seem inconsequential, treatment of the hypercalcemia is crucial to protect the kidneys.
Keywords: arthritis, auto-inflammation, Blau syndrome, CARD15, dermatitis, glucocorticoids, granuloma, granulomatous disease, hypercalciuria, methotrexate, neopterin, nephrocalcinosis, nephropathy, NF-κB, NOD2, osteoblast, osteoclast, osteopetrosis, osteosclerosis, sarcoidosis, synovitis, uveitis, vitamin D
I). Introduction:
Extrarenal production of 1,25-dihydroxyvitamin D [1,25(OH)2D, calcitriol] can cause hypercalcemia in at least thirty diseases including cancers (e.g., lymphoma), infections (e.g., tuberculosis), and a number of enigmas (e.g., sarcoidosis).(1) Most, including tuberculosis and sarcoidosis, feature granulomas containing macrophages that express unregulated 1α-hydroxylase, which converts 25(OH)D to 1,25(OH)2D.(1)
Pediatric granulomatous arthritis (PGA), sometimes called autoinflammatory granulomatosis of childhood, typically presents during the first few years of life with the clinical triad of dermatitis, uveitis, and arthritis.(2–6) Beginning in 1985, autosomal dominant Blau syndrome (BS)(7) or Jabs syndrome(8) comprised this disorder,(9) and later early-onset sarcoidosis (EOS) was considered a sporadic phenocopy.(2,4) Subsequently, BS (OMIM #186580)(9) in 2001(10) and EOS (OMIM *609464)(9) in 2005(11,12) were discovered to share mutations within the nucleotide binding domain of nucleotide-binding oligomerization domain-containing protein 2 (NOD2), also called caspase recruitment domain-containing protein 15 (CARD15).(6) NOD2/CARD15 mutation analysis then provided the means to recognize the broader range of PGA manifestations.(13,14) NOD2 in the cytosol of blood leukocytes is a microbe sensor that initiates and regulates inflammation,(6) including by induction of NF-κB translocation to the nucleus for gene transcription.(11,15) This process has positive and negative regulation.(16) Generalized osteosclerosis in keeping with osteoclast (OC) failure causing osteopetrosis (OPT) has not been reported in PGA although NF-κB signaling is crucial for osteoclastogenesis and OC action.(17) Hypercalcemia from extrarenal production of 1,25(OH)2D, presumably by the macrophages within the non-caseating granulomas of PGA, is only briefly mentioned.(14,18)
Herein, we report a young girl with a NOD2 missense mutation that is common in PGA,(19) yet who lacked its classic triad of complications and instead at presentation had severe 1,25(OH)2D-mediated hypercalcemia, nephrocalcinosis, and compromised kidney function as well as radiological and histopathological findings consistent with OPT.(20)
II). Materials and Methods:
A). Case Report:
Our patient’s medical record upon referral described vesicoureteral reflux resulting in recurrent urinary tract infections beginning at age 4 months, and subsequently pyelonephritis that required antibiotic prophylaxis. Her mother said that beginning at age 6 months her daughter had an unusual crawl. At age 16 months, her height was 76th centile, weight 96th centile, and head circumference z-score +3.3. Pneumonia occurred at age 18 months, but we could not review the radiographs.
At age 33 months, her hands, wrists, and lower extremities were radiographed for the first time when her pediatrician observed that joint swelling and subcutaneous bumps had developed there during the previous year. The report mentioned soft tissue swelling over the 2nd – 4th digits of her left hand, but the osteosclerosis present was not reported (see Radiological Findings). Laboratory investigation demonstrated serum alkaline phosphatase (ALP) 215 U/L (< 270 Nl), 25(OH)D 15 ng/ml (30 – 100 Nl), normal lead level, and creatinine (Crt) 0.61 mg/dl that later we considered elevated for age (see below). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were increased: 33 mm/hr (0–10 Nl) and 17 mg/l (< 7.4 Nl), respectively. ANA titer and rheumatoid factor (< 10 IU/ml) were normal. Serum calcium (Ca) had apparently not been measured.
At age 36 months, she was referred to us and had her first Children’s Hospital of Orange County (CHOC) admission for fever, swollen joints, and an antalgic gait. A radiographic skeletal survey demonstrated a diffusely radiodense skeleton featuring osteosclerosis, cortical thickening, and long bone undertubulation consistent with OPT (see Radiological Findings). However, additional laboratory findings were not consistent with OPT, including striking hypercalcemia rather than hypocalcemia(20) with serum Ca 12.7 mg/dl (8.8–10.8 Nl). Circulating intact parathyroid hormone (iPTH) was physiologically suppressed at 5.7 pg/ml (7.5–53.5 Nl), ALP 210 U/L (80–220 Nl), Crt once again 0.6 mg/dl, blood urea nitrogen (BUN) high at 25 mg/dl (5–17 Nl), and globulin and immunoglobulin G (IgG) elevated at 4.4 gm/dl (1.0–4.0 Nl) and 1499 mg/dl (423–1090 Nl), respectively. Urinalysis showed increased protein and 3+ leukocyte esterase. Renal ultrasound demonstrated nephrocalcinosis of moderate severity, both cortical and medullary. Echocardiography, to exclude pericarditis, was normal. Mucopolysaccharide screening was unremarkable. Computed tomography (CT) without contrast showed diffuse calvarial osteosclerosis and diploic thickening, but no hydrocephalus. Mutation analysis of leukocyte DNA (Connective Tissue Gene Tests, Allentown, PA, USA) was negative for 28 genes associated with OPT and other dense bone disorders(20) (i.e., AMER1, ANKH, CA2, CLCN7, COL1A1, CTSK, DLX3, FAM20C, FERMT3, GJA1, HPGD, LEMD3, LRP4, LRP5, MTAP, OSTM1, PLEKHM1, PTDSS1, SLCO2A1, SNX10, SOST, TBXAS1, TCIRG1, TGFB1, TNFRSF11A, TNFRSF11B, TNFSF11, TYROBP). During intravenous hydration, serum Ca decreased from 12.7 to 11.8 mg/dl. Subsequently, oral hydration and a diet low in Ca content was advised, with salmon calcitonin injections reserved for return clinic visits.
However, follow-up was inconsistent and ten weeks later serum Ca was 12.8 mg/dl, now explained by elevated circulating 1,25(OH)2D at 166 pg/ml (24–86 Nl). Her serum iPTH remained suppressed at 4.2 pg/ml. Bone marrow biopsy at a posterior iliac spine showed few OCs and osteoblasts (OBs), considered in keeping with her hypercalcemia and secondary hypoparathyroidism. No granulomas were observed (see Histopathological Findings). Magnetic resonance imaging (MRI) revealed mild periaortic adenopathy as well as “decreased marrow signal” within her osteosclerotic spine that together with the findings from the radiographic bone survey supported a type of OPT. Four months later, serum Ca was 12.6 mg/dl, iPTH 5.6 pg/ml (16.2–63 Nl), 25(OH)D 33 ng/ml, and 1,25(OH)2D 152 pg/ml.
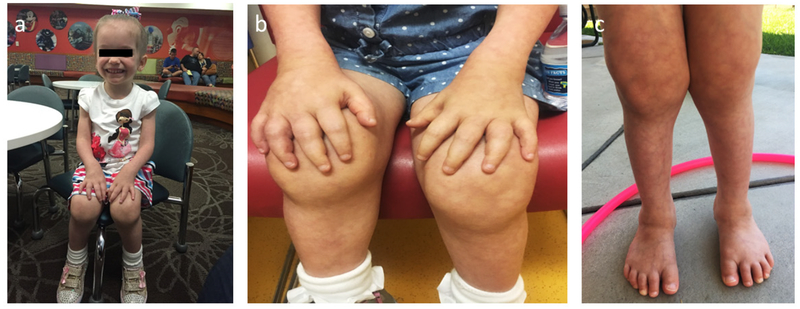
At nearly 4 years-of-age, inpatient investigation at the Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, St. Louis, MO, USA (Research Center) followed informed written consent. She was provided her restricted intake of dietary Ca estimated to be 450 mg daily (RDA 1000 mg/day). Physical examination showed a broad forehead and widely spaced teeth, diffuse cutis marmorata, but no evidence of dermatitis or uveitis (Figure 1A). There were swollen wrists, knees, and ankles seemingly with synovial thickening but without other signs of arthritis. Soft, spongy, non-tender, non-erythematous masses were present over the dorsum of her hands, knees, and feet. Decreased mobility of her tapered fingers was noted at the interphalangeal and metacarpophalangeal joints. Fasting serum Ca was 13.6 mg/dl, marked hypercalciuria was present despite her low Ca diet, and further testing confirmed compromised renal function (Table 1). Hepatitis C and HIV screening were negative. Angiotensin converting enzyme (ACE) was normal, but ESR and CRP remained elevated. Infantile hypercalcemia, type 1(21,22) (OMIM #143880)(9) was excluded by normal CYP24A1 sequencing and copy number analysis (Fulgent Diagnostics, Temple City, CA), verifying results from our research laboratory (see Mutation Analyses). Review of her bone and marrow histology revealed changes in keeping with OPT (see Histopathological Findings). However, against the possibility of OPT and supported by the mutation analyses, serum markers of bone turnover carboxy-terminal collagen crosslinks and osteocalcin were elevated, and serum bone-specific ALP and urine deoxypyridinoline were normal not low. Furthermore, creatine kinase and lactate dehydrogenase were low not high,(23,24) and tartrate resistant acid phosphatase was normal not elevated.(24) Bone mineral density assessed using DXA was elevated, but skeletal radiographs showed the metaphyseal osteosclerosis had resolved (see Radiological Findings), something that rarely occurs spontaneously among the OPTs.(25–27)
Figure 1: Patient’s Principal Physical Findings: A. Before Anti-Inflammatory Treatment:
a) At 4 years-of-age, there is sparse hair, a broad forehead, and widely spaced teeth.
b) Swelling (but no heat or tenderness) involves her fingers, hands, wrists, and knees. Palpable, soft, spongy, non-tender, and non-erythematous lesions are over the dorsum of her hands, knees, and feet.
c) Her skin shows diffuse cutis marmorata, but no dermatosis in keeping with PGA.
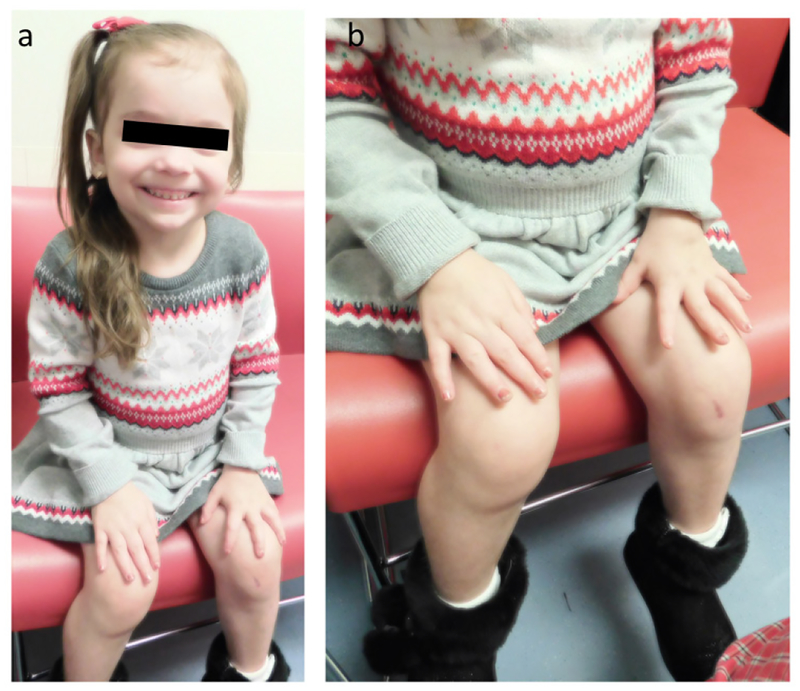
B. During Anti-Inflammatory Treatment:
After 6 months of medical treatment, swelling of her (a) fingers, hands, and wrists and (b) knees is markedly improved.
Table 1:
Patient Biochemistry and Hematology at Research Center*
| Test | Units | Value | Normal Range | |
|---|---|---|---|---|
| Blood: | Calcium (Total) | mg/dL | 13.6 | 9.0 – 10.1 |
| Calcium, Ionized | mg/dL | 6.8 | 4.5 – 5.3 | |
| Phosphorus | mg/dL | 6.1 | 4.3 – 5.4 | |
| Uric Acid | mg/dL | 7.4 | 2.2 – 4.7 | |
| Creatinine | mg/dL | 0.7 | 0.5 – 1.1 | |
| Blood urea Nitrogen | mg/dL | 34 | 8 – 18 | |
| Glucose | mg/dL | 70 | 70 – 105 | |
| Sodium | mmol/L | 141 | 136 – 145 | |
| Potassium | mmol/L | 4.9 | 3.5 – 5.1 | |
| Chloride | mmol/L | 105 | 97 – 107 | |
| Total protein | g/dL | 8 | 6.3 – 8.7 | |
| Albumin | g/dL | 3.3 | 3.6 – 5.2 | |
| Aspartate Transaminase | U/L | 17 | 0 – 47 | |
| Alanine Transaminase | U/L | 14 | 20 – 42 | |
| Lactate Dehydrogenase | U/L | 141 | 184–341 | |
| Creatine Kinase | U/L | 15 | 24 – 173 | |
| Bone and Mineral Homeostasis: | ||||
| Alkaline Phosphatase (ALP) | U/L | 197 | 172 – 405 | |
| Bone ALP**** | U/L | 67 | 0 – 189 | |
| Parathyroid Hormone (Intact) | pg/mL | 4 | 15 – 65 | |
| Parathyroid hormone-related peptide | pmol/L | <1.1 | <2.0 | |
| 25(OH)D‡‡ | ng/mL | 24 | 30 – 100 | |
| 1,25(OH)2D*** | pg/mL | 108 | 20 – 79 | |
| Osteocalcin+ | ng/mL | 317 | 44 – 130 | |
| CTX++ | ng/mL | 3.1 | 0.5 – 1.8 | |
| TRAP# | IU | 14.7 | 6.3 – 26.7 (age ≤ 0–18 years) | |
| Inflammation: | ||||
| Erythrocyte Sedimentation Rate | mm/hr | 55 | 0 – 20 | |
| C-Reactive Protein | mg/dL | 1.8 | 0.0 – 0.9 | |
| Hepatitis C and HIV Screening | Negative | |||
| Angiotension Converting Enzyme | U/L | 99 | 22 – 108 | |
| Cell Counts: | ||||
| White cells | k/uL | 13.3 | 5.0 – 15.5 | |
| Hemoglobin | g/dL | 11.5 | 11.5 – 13.5 | |
| Hematocrit | % | 36.6 | 34.0 – 40.0 | |
| Platelets | k/uL | 514 | 140 – 440 | |
| Urine: | Calcium/Creatinine | mg/g | 1337 – 1506 | 75 – 250 |
| Creatinine Clearance | ml/min/1.73 m2 | 39 and 49 | 79 – 187 | |
| **Deoxypyridinoline/Crt | nmol/nmol Crt | 35 | 2 – 41 |
Shriners Hospital for Children, St. Louis, MO, USA (Dade Behring Dimension Xpand instrument: Siemens Health Care Diagnostics, Inc., Los Angeles, CA, USA).
Osteocalcin (OCN: Kit #LKON1; Siemens Health Care Diagnostics).
Bone-specific ALP (BAP) by ELISA (Quidel Comp., San Diego, CA, USA)
CTX (β-CrossLaps/serum, Roche Diagnostics, Indianapolis, IN, USA)
Urine Free Deoxypyridinoline (DPD: Immulite 1000 Pyrilinks–D Kit; Siemens Medical Solutions Diagnostics Ltd., Lianberis, Gwynedd, UK)
Tartrate-resistant acid phosphatase (TRAP-5b) (Kit #8033, Quidel; Los Angeles, CA, USA)
25(OH)D (Immunodiagnostic Systems, 25-hydroxyvitamin D EIA; Gaithersburg, MD, USA)
1,25(OH)D (Lab Corp, Burlington, MD, USA)
She then returned to CHOC for further rheumatological evaluation. Renal ultrasound showed unchanged increased echogenicity of the cortex and renal pyramids consistent with cortical and medullary nephrocalcinosis (see Radiological Findings). Rheumatoid factor was now positive at 25 IU/ml (< 14 Nl) and serum IgG remained elevated at 1,719 mg/dl (423–1090 Nl). ACE was now high at 102 U/L (8–53 Nl) consistent with granulomatous disease. Serum neopterin (Lab Corp, San Pedro, CA, USA), a product of monocytes/macrophages and marker of immune system activation,(28) was markedly elevated at 21.6 ng/ml (< 2.5 Nl). Then, a gene panel for auto-inflammatory diseases (Fulgent Diagnostics, Temple City, CA, USA) revealed a heterozygous germline change (c.1001G>A, p.Arg334Gln) in NOD2 common in PGA,(14) which we then verified (see Mutation Analyses).
B). Treatment:
Prednisolone therapy, 1 mg/kg/day, assessed five weeks later showed her serum Ca corrected from 12.8 to 9.8 mg/dl (8.8 – 10.8 Nl), 1,25(OH)2D was suppressed from 200 to 13 pg/ml (24–86 Nl), and ESR normalized from 40 to 9 mm/hr (0–20 Nl). Also, her serum Crt decreased from 0.9 to 0.6 mg/dl (0.5–0.8 Nl), but returned to 0.9 mg/dl although a spot urine Ca/Crt ratio was normal. MRI several weeks later revealed tenosynovitis in her hands and wrists, joint fluid, synovitis, and synovial cysts in the knees (see Radiological Findings). The prednisolone, having improved her pain and mobility, was then tapered weekly to 0.5 mg/kg/day. She began maintenance adalimumab (Humira®) (20 mg every 2 weeks), oral methotrexate (10 mg every week), and leucovorin. Synovial biopsy of her left knee after 2.5 months of medical treatment showed reactive changes but no granulomas (see Histopathologic Findings). Prednisolone was then tapered off due to hypertension. Despite the adalimumab and methotrexate therapy and although circulating 1,25(OH)2D was reported low at 16 pg/mL, serum Ca increased to 12.6 mg/dl. ESR had remained normal at 17 mm/hr, serum CRP increased to 62.3 mg/l with no clear infectious etiology, Crt was 0.8 mg/dl, and a spot urine Ca/Crt ratio was markedly elevated at 0.85. While methotrexate treatment continued, prednisolone (0.5 mg/kg/day) was restarted and then tapered off over 2 weeks. Because adalimumab response was unsatisfactory, she was switched after a 2-week washout period to infliximab (Remicade®) (7.5 mg/kg/dose) at an interval of every 2 weeks for 3 doses, and then monthly thereafter. Currently, she receives methotrexate weekly and methylprednisolone (15 mg/kg/dose) and infliximab monthly. Her most recent serum Ca was 10 mg/dl, 1,25(OH)2D 11 pg/ml, Crt 0.7 mg/dl, ESR 17 mm/hr, and spot urine Ca/Crt ratio was normal. Her hand and knee swelling, joint synovitis, gait, and mobility are remarkably improved (Figure 1B). Amlodipine for hypertension was reduced from 1.8 to 1.7 ml bid (~0.1 mg/kg/dose). Follow-up radiographs of her hands, wrists, and knees showed a dense band of osteosclerosis, attributable to the methotrexate at provisional zones of calcification. Methotrexate can microfracture newly formed endochondral bone (see Radiological Findings).(29,30)
C). Mutation Analyses:
The two principal findings from the commercial mutation analyses of CYP24A1 and NOD2/CARD15 were verified in our research laboratory using a new sample of patient leukocyte DNA. Our primers and PCR conditions are available on request.
1). CYP24A1:
All 11 coding exons and adjacent mRNA splice junctions of CYP24A1 underlying idiopathic infantile hypercalcemia, type 1 (OMIM #143880)(9) were PCR amplified and Sanger sequenced using primers we designed with ExonPrimer from the University of California, Santa Cruz, CA, USA, Genome Browser (http://genome.ucsc.edu/). DNA sequence was generated using an Applied Biosystems 3130 (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed by visual examination of electropherograms and by aligning patient and control sequences using Sequencher software (Gene Codes Corp., Ann Arbor, MI, USA).
2). NOD2/CARD15:
Our patient’s NOD2/CARD15 mutation in exon 4 was searched for using PCR amplification, Sanger sequencing, and primers we designed.
D). Radiological Studies:
All radiological images (radiographs, renal sonography, DXA, CT, MRI) spanning patient ages 21/4 to 41/2 years were reviewed.
E). Bone, Marrow, and Synovial Histopathology Studies:
Additional sections of both biopsy specimens were examined. The core posterior iliac spine biopsy had been performed when the patient was 3–1/3 years-of-age, before anti-auto-inflammatory treatment began and without prior tetracycline “labeling”. The specimen was decalcified, paraffin-embedded, and then stained with H&E and toluidine blue. The synovial biopsy of a knee had been performed at 4 years-of-age (after medical therapy had begun using prednisolone, adalimumab, and methotrexate).
F). Literature Review:
We comprehensively reviewed the literature concerning BS, EOS, and PGA beginning with reports in the 1980s. Our search especially sought any precedent for hypercalcemia and osteosclerosis in PGA by including among the key words 1,25(OH)2D, osteopetrosis, etc.
III). Results:
A). Mutation Analyses:
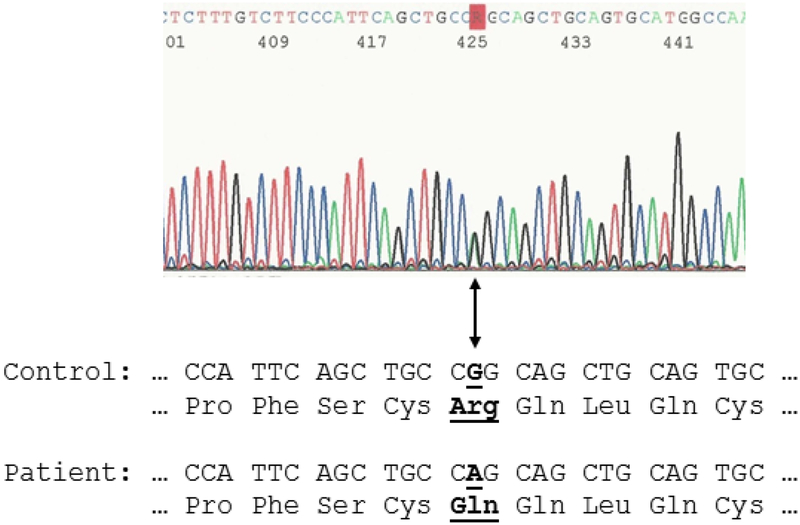
We determined that our patient did not carry a CYP24A1 defect, and confirmed that she was heterozygous for a common NOD2 mutation causing PGA (c.1001G>A, p.Arg334Gln) (Figure 2).
Figure 2: Patient’s NOD2 mutation.
Sanger-sequencing of peripheral blood leukocyte DNA revealed a heterozygous missense mutation (c.1001G>A, p.Arg334Gln) in exon 4 of NOD2. The “R” in the electropherogram localizes both nucleotides, heterozygous G and heterozygous A, at cDNA position #1001 (amino acid residue #334). The DNA and amino acid residue sequence (below) show the consensus sequences for a control and the patient’s mutation (bold type, underline, and arrow identify the mutant nucleotide and amino acid change).
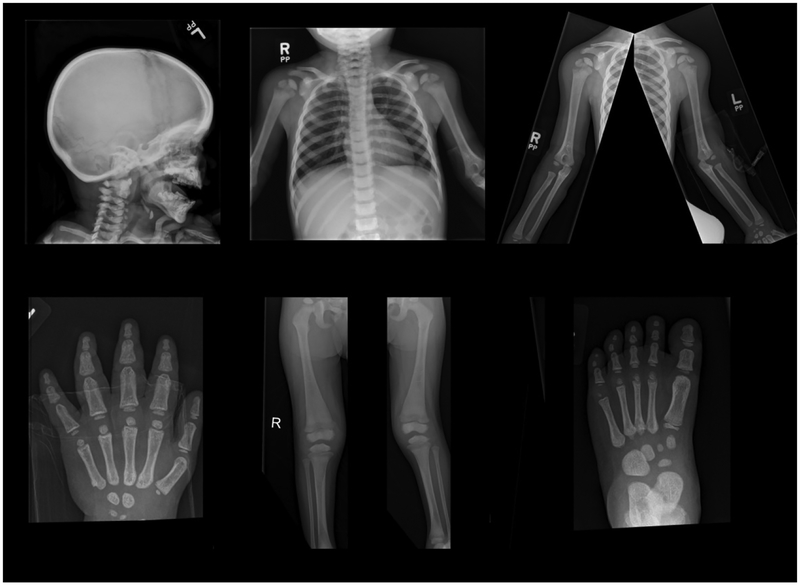
B). Radiological Findings:
At 2–3/4 years-of-age, the patient’s earliest radiographs of the hands, wrists, and lower extremities showed diffuse osteosclerosis with very minimal metaphyseal sparing. At age 3 years, her skeleton was diffusely osteosclerotic with cortical and diploic thickening and showed failure of tubulation, together consistent with OPT (Figure 3A). The epiphyses, in particular at the knees and ankles, were very osteosclerotic, while the metaphyses were less dense. CT of her head showed a thick sclerotic cranial vault, but a normal appearing brain. MRI examination of the entire osteosclerotic spine showed marrow replacement featuring a decreased marrow signal. This was consistent with OPT given the findings of the radiographic bone survey.
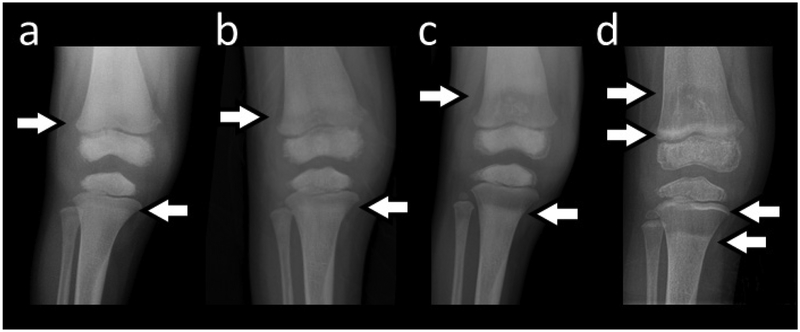
Figure 3: Skeletal Radiographic Findings:
A) At age 3 years, there is diploic thickening of the skull. Generalized osteosclerosis includes the carpal and tarsal bones, and especially the epiphyses, and there is cortical thickening. Undertubulation of major long bones accompanies minimal sparing of the sclerosis in the metaphyses.
B) Sequential anteroposterior radiographs of our patient’s right knee depict the gradual resolution of the osteosclerosis, and in particular in the metaphyses, over ages 2 years 9 months, 3 years, and 3 years 9 months. Notably, the undertubulation of the distal femur did not correct.
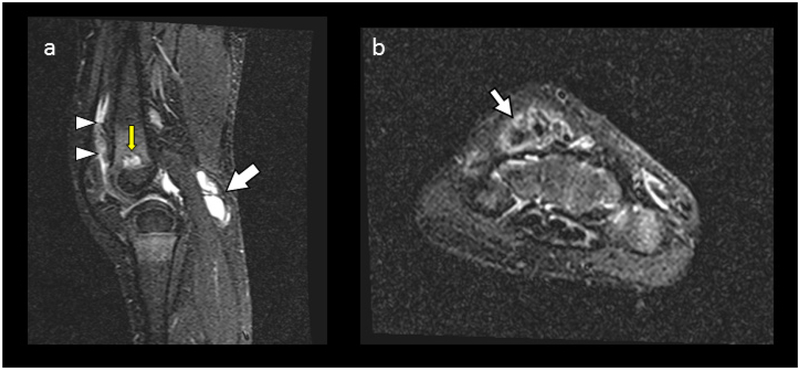
C) MRI of Knees And Wrists:
a) At age 4 years, Short-Tau-Inversion Recovery Sequence (STIR), Sagittal MR image (lateral) of the knee reveals joint fluid and synovial thickening from synovitis (arrowheads), a Baker’s cyst (arrow), cystic areas in the metaphyses at the physeal lines (yellow arrow), and red marrow replacement in the diaphysis by sclerotic bone. Normally, the metaphyses contain red marrow. However, no bone or joint cartilage destruction is present.
b) STIR axial right wrist shows tenosynovitis (arrow) and edema.
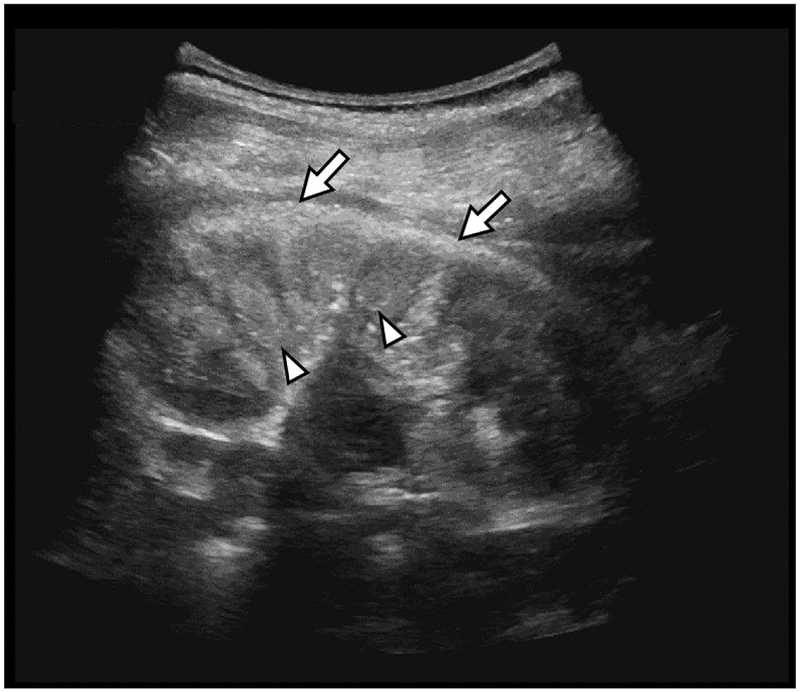
D) Renal Ultrasound:
At nearly 4 years-of-age, nephrocalcinosis involves the cortex (arrows) and medullary pyramids (arrowheads).
At nearly age 4 years, the generalized osteosclerosis was unchanged except the metaphyseal osteosclerosis had resolved (Figure 3B). The newly formed endochondral bone was no longer radiodense (Supplementary Figure 1). Bone mineral density assessed using DXA (Hologic Discovery A, Waltham, MA, USA) was 0.932 gm/cm2 at the lumbar spine (z-score +6.3), and 0.894 gm/cm2 at the left total hip (age-appropriate normal range not available). MR examination after medical treatment began, demonstrated tenosynovitis in the hands and wrists, while in the knees there was synovitis, joint fluid, and synovial cysts, including a Baker’s (popliteal) cyst (Figure 3C). Cortical and medullary nephrocalcinosis was revealed by sonography (Figure 3D).
Notably, the changes typical of PGA(2,3) were absent in her hands and wrists, with no contractures, camptodactyly, dysplastic first carpal row, abnormal distal radial epiphyses, plump distal ulna, thin diaphysis of the second metacarpal, or epiphyseal overgrowth. However, her MR findings of tenosynovitis and synovitis were similar to PGA, but articular cartilage destruction was absent.
The most recent radiographs of her hands and knees, at age 41/2 years, showed the osteosclerosis to be progressively decreasing. The metaphyses were no longer osteosclerotic. The increased density at the provisional zones of calcification at the knee was considered to be a result of the methotrexate treatment. Undertubulation remained (Figure 3B).(29,30)
Our patient’s father, said perhaps to have “dense bones”, was not evaluated, but the patient’s mutational analysis was negative for heritable osteosclerosis.
C). Bone, Marrow, and Synovial Histopathology:
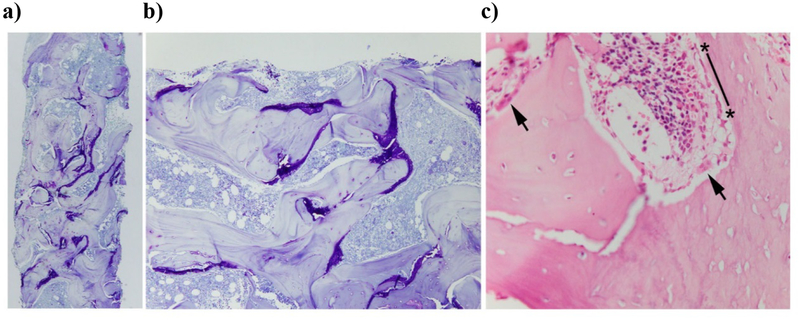
The bone marrow biopsy before anti-inflammatory treatment showed trabeculae that were increased numerically and thick although the cortex was not expanded (Figure 4). Cartilage was present in large amounts mainly in the deeper zones of trabeculae away from the cortex. Its distribution, located mainly on the surface rather than within the center of trabeculae, was unusual. Trabeculae deeper in the specimen had relatively few OBs or OCs on their surface but, where present, these cells were normal in morphology and close to the cortex. The deep trabeculae had few cells, but nearer the cortex the cells were present and appeared normal. No granulomas were identified.
Figure 4: Bone Core Biopsy at Age 3 Years (Before Anti-inflammatory Treatment).
a) Large amounts of unresorbed cartilage (purple) are present, particularly in the deep trabecular bone rather than near the cortex (at top left) (toluidine blue stain X 40).
b) Cartilage is present primarily on the surface of trabeculae, rather than central as in typical osteopetrosis (toluidine blue stain X 100).
c) Normal-appearing OBs and OCs are near the cortex, likely reflecting more recently deposited bone In contrast, relatively few OBs and OCs are present deep in the trabecular bone (not shown) (H & E stain X 400)
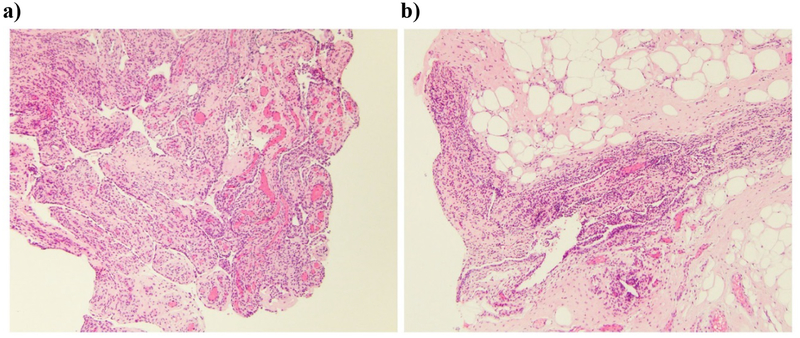
The synovial specimen, obtained after commencement of medical therapy, showed proliferative synovitis with mild chronic inflammation, primarily composed of lymphocytes. No granulomas were identified (Figure 5).
Figure 5: Synovial Biopsy During Anti-inflammatory Treatment.
Synovial specimens show a) proliferative synovitis and b) areas of lymphocytic infiltration, but no granulomas are present. Both images H&E x 100.
IV). Discussion:
Our patient’s PGA is explained by a heterozygous missense mutation in NOD2 (c.1001G>A, p.Arg334Gln) that is expected at residue #334 to change the most commonly altered amino acid in this disorder.(19) Yet, she lacked the classic triad of PGA complications (dermatitis, uveitis, arthritis), and instead uniquely manifested severe hypercalcemia together with radiographic and histological findings in keeping with OPT. This conundrum is discussed below.
A). Pediatric Granulomatous Arthritis:
There are two forms of pediatric sarcoidosis.(31) Older affected children manifest, usually at 12–15 years-of-age,(32) the findings of adult patients such as malaise, fever, lymphadenopathy, and respiratory symptoms. Those much younger typically show the classic triad of PGA.(13) The term “PGA” emerged after 2005 when sporadic EOS was discovered to share with heritable BS identical mutations within NOD2.(2,33) Consequently, in 2017, EOS was “transferred” to BS within OMIM.(9)
Now, based upon positive NOD2 mutation analysis, a broader phenotypic range is appreciated for PGA, and several reviews, grounded in positive mutation analysis, are published.(13,14,34) In one overview from 2009, Rosé et al(14) reported the majority of 45 such patients manifested the classic triad of clinical complications, yet 12 had “atypical” features sometimes including a granulomatous glomerular and interstitial nephritis. Nevertheless, this review(14) and others(2) do not mention generalized osteosclerosis or hypercalcemia in PGA.
B). Hypercalcemia in PGA:
Although PGA is associated with nephritis,(14) our patient’s renal impairment seemed readily attributable instead to damage from her severe hypercalcemia and hypercalciuria leading to cortical and medullary nephrocalcinosis. To understand her 1,25(OH)2D-mediated hypercalcemia, we first excluded by mutation analysis idiopathic infantile hypercalcemia, type 1.(21) In this disorder, hypercalcemia reflects diminished catabolism of 1,25(OH)2D to 1,24,25(OH)3D.(21,35) Instead, her signs, symptoms, and initial laboratory findings suggested presence of granulomatous disease known to cause hypercalcemia, including among children.(1,36) PGA was then identified by positive NOD2 mutation analysis, although she represented a variant lacking the classical triad of findings. Granulomas were not found in her bone marrow or synovium that could explain the hypercalcemia, yet markedly elevated circulating neopterin also indicated their presence somewhere. In PGA, granulomas are commonly identified in the associated dermatitis, but our patient’s skin did not show this complication, and therefore was not biopsied. Granulomas in PGA have been found in liver,(37) kidney;(37) a lymph node and tonsil,(38) esophagus, synovial cyst, tendon,(39) and elsewhere. In classic sarcoidosis, skeletal granulomas typically involve the marrow or cortex, and aberrant vitamin D and Ca metabolism occurs in up to 50% of patients.(40) Macrophages within granulomas can contain unregulated 1α-hydroxylase that activates vitamin D by hydroxylating 25(OH)D to 1,25(OH)2D.(1) In 2004, studying typically older pediatric patients with sarcoidosis in Denmark, Hoffmann et al(32) reported hypercalcemia affected ~30% of these individuals and was sometimes severe and associated with nephrocalcinosis. Among 48 such children, three < age 5 years demonstrated hypercalcemia, and therefore were likely examples of PGA although NOD2 mutation analysis was not performed.(32)
In 2010, Borzutzky et al(34) reviewed the NOD2-associated diseases and reported an infant with EOS, including the classical clinical triad, with elevated serum Ca of 13.0 mg/dl together with bilateral symmetrical prominent diaphyseal periosteal reaction illustrated in the tibial diaphyses. The hypercalcemia responded to anti-inflammatory medication, but the outcome of the periostitis was not mentioned.(34)
In 2011, Wong et al(41) noted hypercalciuria and small renal stones in an 18 year-old woman with EOS, and in 2016 Gedalia et al(38) mentioned hypercalcemia in a patient with EOS. However, for either patient, NOD2 mutation analysis was not performed.
Finally, NOD2 mutation-positive (c.2123G>A) BS with 1,25(OH)2D-mediated hypercalcemia was reported briefly in 2016 in an abstract by Teng Lo et al(18) concerning a 5-year-old affected girl. Her serum Ca was 13.4 mg/dl (8.4–10.2 Nl), iPTH physiologically suppressed at 6 pg/mL (15–65 Nl), 1,25(OH)2D inappropriately normal at 64 pg/mL (15–75 Nl), 25(OH)D low at 13 ng/mL (20–50 Nl), and PTH-related peptide undetectable. Her hypercalcemia corrected with prednisolone and methotrexate therapy. Notably, a radiographic skeletal survey was reported as normal.(18)
C). Generalized Osteosclerosis in PGA:
Although it seems unlikely, perhaps in previous instances of PGA any generalized osteosclerosis was overlooked, went unreported, corrected spontaneously, or resolved with conventional medical treatment. Our patient’s earliest radiographs suggested OPT because diffuse osteosclerosis and hyperostosis accompanied impaired tubulation (modeling) of her long bones.(20) Additionally, her bone marrow biopsy showed the histopathological hallmark of all OPTs; i.e., calcified primary spongiosa persisting following endochondral bone formation.(20) Scattered radiodense lesions do occur in adult sarcoidosis, but bone involvement is rarely reported in early-onset childhood sarcoidosis.(42)
In fact, remarkable correction of our patient’s metaphyseal osteosclerosis occurred while her treatment was only a Ca-restricted diet. However, this improvement was not accompanied by red marrow signal suggesting true recovery on the MRI. We elected not to biopsy a representative metaphysis for histological information. Why and how this correction occurred is an enigma, but we note that early attempts to treat OPT did involve such dietary Ca restriction.(20) It seems unlikely that her diffuse osteosclerosis resulted from suppressed OC action due to 1,25(OH)2D-mediated hypercalcemia and secondary hypoparathyroidism, because this is not a feature of idiopathic infantile hypercalcemia.(9) Instead, 1,25(OH)2D binding to the vitamin D receptor can, through NF-κB signaling, enhance OC-mediated bone resorption.(1) In fact, when first assessed, her bone turnover markers were not suppressed. Serum total and bone-specific ALP and TRAP as well as urine DPD were normal, and serum OCN and CTX were elevated. Also, hypercalcemia would be paradoxical in OPT, as hypocalcemia is anticipated when there is OC failure.(20) Negative mineral balance explaining her hypercalcemia seemed untenable, because she was growing and therefore in positive Ca balance. Notably, correction of her osteosclerosis has to date been selectively metaphyseal.
In some ultra-rare situations of rapid skeletal remodeling, OPT can be mimicked radiographically. For example, in 2014 we reported(43) radiographic OPT in an 11-year-old boy with a parasellar giant cell granuloma whose bone turnover markers and histopathological findings instead revealed accelerated bone turnover. In 2016, we reported a neonate with radiographic OPT and discovered the first mutation involving the five-member NF-κB complex (a heterozygous loss-of-function missense defect in RELA encoding p65).(44) This mutation decreased activation of NF-κB in vitro, yet no cartilage remained trapped within the primary calcified spongiosa. Instead of OPT, a net increase in bone formation seemed to explain the osteosclerosis.(44) Our patient’s initial skeletal findings of OPT seem difficult to reconcile with NOD2/CARD15 pathophysiology in PGA.(13) Missense mutations in NOD2 in PGA are considered activating defects. Phenotype/genotype correlation, considered more likely with gain-of-function mutation, has been reported in PGA.(13,33) However, our patient’s unresorbed primary spongiosa was in keeping with OC failure sometime early in her clinical course. Perhaps, NF-κB activity was somehow suppressed early on, but recovered restoring bone remodeling.
D). NOD2 Pathophysiology:
Exchange of the arginine residue at position #334 accounts for > 80% of individuals with PGA(12,13,19,33) and, as in our patient, the substituted amino acid is often glycine.(14) Residue #334 occupies the central nucleotide binding and oligomerization domain of NOD2 having ATPase activity.(12,13) Nevertheless, it is unknown precisely how such PGA mutations impact ATPase hydrolysis or cause conformational changes that affect NF-κB activation. In 2010, Borzutzky et al(34) reviewed the complex role of NOD2 in innate immunity, including as a intracellular receptor for bacterial lipopolysaccharides(6) by interacting with bacterial muramyl dipeptide,(34) and then accelerating translocation of NF-κB into the nucleus for transcription of various mediators of the inflammatory response. NF-κB is a key transcription factor for osteoclastogenesis as well as OC activation and function,(14,17) including when the vitamin D receptor is activated by 1,25(OH)2D binding.(1) Hence, a gain-of-function mutation in NOD2 that activates NF-κB would not be expected to cause OPT, but perhaps instead cause rapid skeletal remodeling that still could resemble OPT radiographically.(43–45) Whether modifying genes, infections, or environmental factors condition the process is unknown. We anticipate, based upon her metaphyseal changes and active bone remodeling, that our patient’s histopathological findings of OPT will fully resolve. Perhaps crosstalk between inflammatory and bone cells initially compromised NF-κB mediated OC function leading transiently to a form of autoimmune OPT. OPT caused by immune deficiencies is recognized,(46,47) and neutralizing autoantibodies that deactivate osteoprotegerin (OPG) seemed to explain an autoimmune type of osteoporosis.(48)
Understandably, the auto-inflammatory nature of PGA suggested NOD2 gain-of-function underlying PGA.(11,12,49–50) Defects within the nucleotide binding domain of NOD2 would act through receptor-interacting protein kinase 2, and then the MAP kinase and NF-κB pathways. However, gain-of-function of NOD2 in PGA was challenged in 2017 by Ong et al,(37) who found in peripheral blood monocytic cells obtained from affected family members with BS impaired production of IL-6, TNFα, NF-κB, IL-1β, and INF-γ. We note that INF-γ1b (Actimmune™) is used to treat malignant OPT prior to hematopoietic marrow cell transplantation.(51,52) In fact, the etiology and pathogenesis of pediatric granulomatous disease seems especially complicated(13) because it can apparently occur despite wild-type NOD2, and mutations in NOD2 can be clinically silent.(14) Thus, our patient’s radiological and histopathological changes of OPT and then correction of her metaphyseal osteosclerosis remain an enigma. The osteosclerosis that recently appeared at her zones of provisional calcification is likely a result of methotrexate treatment. Follow-up of our patient will show if her skeletal changes resolve.(25–27) Fortunately, they have not been associated with complications.
E). Treatment:
Therapy for PGA has been glucocorticosteroid administration with consideration of other immunosuppressives such as methotrexate, azathioprine, and mycophenolate mofetil.(34) Infliximab(53) and thalidomide(54) have been used recently. Experience with our patient suggests hypercalcemia responds to control of the disease process that leads to granulomatous disease and elevated circulating 1,25(OH)2D levels, and that the skeletal changes do not cause complications.
F). Conclusions:
For our patient with a unique variant of PGA, a prompt and correct diagnosis was important because early on her radiographic findings were concerning for OPT which would require further evaluation and treatment.(20,51) Her NOD2 missense mutation is common in PGA and established this diagnosis. Her hypercalcemia was important because it caused significant renal compromise. Assessing PGA patients for hypercalciuria, that would likely precede hypercalcemia, might be an especially sensitive screening procedure. Historical chart reviews and detailed prospective studies are now needed to clarify the prevalence and impact of generalized osteosclerosis and hypercalcemia in PGA.
Supplementary Material
V). Acknowledgements:
Our report reflects the skill and dedication of the nursing, laboratory, and dietary staff of the Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, St. Louis, MO, USA. We thank Deborah Wenkert, M.D. for alerting us to reference #18. We are grateful to Yousef Abu-Amer, PhD for constructive review of our manuscript. Margaret Husky and Shenghui Duan sequenced NOD2 and CYP24A1.
Funded In Part By: Shriners Hospitals for Children, The Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund and The Hypophosphatasia Research Fund at The Barnes-Jewish Hospital Foundation.
Footnotes
Presented In Part At: The American Society for Bone and Mineral Research 2017 Annual Meeting, September 8–11, 2017, Denver, CO, USA [J Bone Miner Res 32 (Suppl 1): S297, 2017].
Disclosures: The authors have no relevant conflicts of interest.
V) References:
- 1.Larner DP, Adams JS, Hewison M. “Regulation of Renal and Extrarenal 1α-Hydroxylase”. In Vitamin D (4th Ed). Feldman D (ed). Elsevier; (Academic Press, San Diego, CA: ) pp 117–137, 2018. [Google Scholar]
- 2.Caso F, Galozzi P, Costa L, Sfriso P, Cantarini L, Punzi L. Autoinflammatory granulomatous diseases: from Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open. July 20;1(1):e000097, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters CH, Maes A, Foley KP, Bertin J, Rosé CD. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr Rheumatol Online J. 2014. August 6;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosé CD, Pans S, Casteels I, Anton J, Bader-Meunier B, Brissaud P, Cimaz R, Espada G, Fernandez-Martin J, Hachulla E, Harjacek M, Khubchandani R, Mackensen F, Merino R, Naranjo A, Oliveira-Knupp S, Pajot C, Russo R, Thomée C, Vastert S, Wulffraat N, Arostegui JI, Foley KP, Bertin J, Wouters CH. Blau syndrome: cross-sectional data from a multicentre study of clinical, radiological and functional outcomes. Rheumatology (Oxford) 54: 1008–16, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Yao Q, Shen M, McDonald C, Lacbawan F, Moran R, Shen B. NOD2-associated autoinflammatory disease: a large cohort study. Rheumatology (Oxford). 54: 1904–12, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Watanabe T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunology 4: 484–495, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau EB. Familial granutomatous arthritis, iritis, and rash. J Pediatr. 107: 689–93, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Jabs DA, Houk JL, Bias WB, Arnett FC. Familial granulomatous synovitis, uvenitis, and cranial neuropathies. Am J Med. 78: 801–4, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD), World Wide Web URL: http://omim.org/. (January 1, 2018).
- 10.Miceli-Richard C, Lesage S, Rybojab M, Prieur AM, Manouvrier-Han S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet. 29: 19–20, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara I, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-κB activation: common genetic etiology with Blau syndrome. Blood 105: 1195–1197, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Rose CD, Doyle TM, McIlvain-Simpson G, Coffman JE, Rosenbaum JT, Davey MP, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 32: 373–5, 2005. [PubMed] [Google Scholar]
- 13.Rose CD, Martin TM and Wouters Ch. Blau syndrome revisited. Curr Opin Rheumatol 23: 411–418, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Rosé CD, Aróstegui JI, Martin TM, Espada G, Scalzi L, Yagüe J, Rosenbaum JT, Modesto C, Cristina Arnal M, Merino R, García-Consuegra J, Carballo Silva MA, Wouters CH. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: study of an international registry and a national cohort in Spain. Arthritis Rheum. 60: 1797–803, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB*. Journal of Biological Chemistry, 276: 4812–4818, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Boyle JP, Parkhouse R, and Monie TP. Insights into the molecular basis of the NOD2 signalling pathway. Open Biol 4: 140178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novack DV: Role of NF-κB in the skeleton. Cell Res 21(1): 169–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng Lo HB, Wampler Muskardin TL, Tebben PJ. Blau syndrome: an unusual cause of hypercalcemia (Abstract). Proceedings Endocrine Society’s 98th Annual Meeting and Expo, April 1–4, 2016, Boston, MA. [Google Scholar]
- 19.Takeuchi Y, Shigemura T, Kobayashi N, Kaneko N, Iwasaki T, Minami K, Kobayashi K, Masumoto J, Agematsu K. Early diagnosis of early-onset sarcoidosis: a case report with functional analysis and review of the literature. Clin Rheumatol 36: 1189–96, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Whyte MP: Sclerosing Bone Disorders In “Primer On Metabolic Bone Diseases and Disorders of Mineral Metabolism,” (9th Ed). American Society for Bone and Mineral Research, Wiley-Blackwell, (in press), 2018 [Google Scholar]
- 21.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Stokes VJ, Nielsen MF, Hannan FM, Thakker RV: Hypercalcemic disorders in children. J Bone Miner Res. 32: 2157–2170, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whyte MP, Chines A, Silva DP, Landt Y, Ladenson JH: Creatine kinase brain isoenzyme (BB-CK) presence in serum distinguishes osteopetroses among the sclerosing bone disorders. J Bone Miner Res 11: 1438–43, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Whyte MP, Kempa LG, McAlister WH, Zhang F, Mumm S, Wenkert D: Elevated serum lactate dehydrogenase isoenzymes and aspartate transaminase distinguish Albers-Schőnberg disease (chloride channel 7 deficiency osteopetrosis) among the sclerosing bone disorders. J Bone Miner Res 25: 2515–26, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Sly WS, Whyte MP, Sundaram V, Tashian R, Hewett-Emmett D, Vainsel M, Al–Mosawi M, Gruskin A, Sakati N, Ohlsson A, Guibaud P: Carbonic anhydrase II deficiency in twelve families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. NEJM 313: 139–45, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Whyte MP, Murphy WA, Fallon MD, Sly WS, Teitelbaum SL, McAlister WH, Avioli LV: Osteopetrosis, renal tubular acidosis, and basal ganglia calcification in three sisters. American Journal of Medicine 69: 64–74, 1980. [DOI] [PubMed] [Google Scholar]
- 27.Whyte MP, Wenkert D, McAlister WH, Novack D, Nenninger AR, Mumm S: Dysosteosclerosis presents as an “osteoclast-poor” form of osteopetrosis: comprehensive investigation of a 3-year-old girl and literature review. J Bone Miner Res 25: 2527–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 3: 175–87, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ragab AH, French RT, Vietti TJ: Osteoporotic fractures secondary to methotrexate therapy of acute leukemia in remission. Cancer 25: 580–585, 1970 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AM: Methotrexate osteopathy. Skeletal Radiol 11: 13–6, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Leigh MW: Sarcoidosis In “Nelson Textbook of Pediatrics” (18th Ed) Kliegman RM, Behrman RE, Jenson HB, Stanton BF (eds). Sunders (Elsevier), Philadelphia, PA; pp 1035–1036, 2007. [Google Scholar]
- 32.Hoffmann AL, Milman N, Byg K-E. Childhood sarcoidosis in Denmark 1979–1994; incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr 93: 30–36, 2004 [PubMed] [Google Scholar]
- 33.Kanazawa N. Sarcoidosis and autoinflammation. Inflammation and Regeneration 31: 66–71, 2011 [Google Scholar]
- 34.Borzutzky A, Fried A, Chou J, Bonilla FA, Kim S, Dedeoglu F. NOD2-associated diseases: bridging innate immunity and autoinflammation. Clinical Immunology 134, 251–261, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocrine Reviews 37: 521–547, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong LTC, Nachbur U, Rowczenio D, Ziegler JB, Fischer E, Lin MW. A novel nucleotide oligomerisation domain 2 mutation in a family with Blau syndrome: phenotype and function. Innate Immunity 23:578–583, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Gedalia A, Khan TA, Shetty AK, Dimitriades VR, Espinoza LR. Childhood sarcoidosis: Louisiana experience. Clin Rheumatol 35: 1879–1884, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Yotsumoto S, Takahashi Y, Takei S, Shimada S, Miyata K, Kanzaki T. Early onset sarcoidosis masquerating as juvenile rheumatoid arthritis. J Am Acad Dermatol 43: 969–71, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Iannuzzi MC. “Sarcoidosis”. In “Goldman’s Cecil Textbook of Medicine” (25th Ed). Goldman L, Schafer AW (eds) W.B. Saunders Co., Philadelphia; pp 603–608, 2016. [Google Scholar]
- 41.Wong L-S, Ho J-C, Yen Y-T. Early-onset childhood sarcoidosis: a case report. Dermatologica Sinica 29 125–128, 2011 [Google Scholar]
- 42.Taybi H, Radiology of Syndromes and Metabolic Disorders (2nd Ed) Year Book Medical Publishers, Inc. Chicago, 1983, p 349. [Google Scholar]
- 43.Whyte MP, Madson KL, Mumm S, McAlister WH, Novack DV, Blair JC, Helliwell TR, Stolina M, Abernethy LJ, Shaw NJ. Rapid skeletal turnover in a radiographic mimic of osteopetrosis. J Bone Miner Res 29: 2601–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frederiksen AL, Larsen MJ, Brusgaard K, Novack DV, Knudsen PJT, Schrøder HD, Qiu W, Eckhardt C, McAlister WH, Kassem M, Mumm S, Frost M, Whyte MP: Neonatal high bone mass with first mutation of the NF-κB complex: heterozygous de novo missense (p.Asp512Ser) RELA (Rela/p65). Journal of Bone and Mineral Research, 31: 163–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke K, Sul O-J, Chung S-W, Suh J-H, Chol H-S. Lack of NOD2 attenuates ovariectomy-induced bone loss via inhibition of osteoclasts. Journal of Endocrinology 235: 85–96, 2017. [DOI] [PubMed] [Google Scholar]
- 46.Villa A, Vezzoni P, Frattini A. Osteopetroses and immunodeficiencies in humans. Curr Opin Allergy Clin Immunol 6: 421–27, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Whyte MP: “Mendelian Disorders of RANKL/OPG/RANK/NF-κB Signaling”. In “Genetics of Bone Biology and Skeletal Disease” Thakker RV, Whyte MP, Eisman J, Igarashi T (eds). Elsevier (Academic Press), San Diego, CA, pp 453–68, 2018. [Google Scholar]
- 48.Riches PL, McRorie E, Fraser WD, Determann C, van’t Hof R, Ralston SH. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med 361: 1459–65, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Zurek B, Proell M. Wagner RN, Schwarzenbacher R, Kufer TA. Mutational analysis of human NOD1 and NOD2 NACHT domains reveals different modes of activation. Innate Immun 18: 100–111, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Kambe N, Nishikomori R and Kanazawa N. The cytosolic pattern-recognition receptor Nod2 and inflammatory granulomatous disorders. J Dermatol Sci 39: 71–80, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, Ghosh P, Moran A, Predergast MM, Tromp G, Williams CJ, Inohara N, Núñez G. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J 23: 1587–1597, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam I, Gray AK, Acton D, Gerard-O’Riley RL, Reilly AM, Econs MJ. Interferon gamma, but not calcitriol improves the osteopetrotic phenotypes in ADO2 mice. J Bone Miner Res. 30: 2005–13, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Key LL Jr, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL. Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med. 332: 1594–9, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Yasui K, Yashiro M, Tsuge M, Manki A, Takemoto K, Yamamoto M, Morishima T. Thalidomide dramatically improves the symptoms of early-onset sarcoidosis/Blau syndrome. Arthritis & Rheumatism; 62: 250–57, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.