Introduction.
Preeclampsia (PE), a hypertensive disease affecting 5–8% of pregnancies,1 is a multisystem disorder, with vascular dysfunction being central to the disease. The heterogeneity of PE’s signs and symptoms has generated controversy regarding whether the cause of PE is mainly related to maternal or placental factors (Supplemental Tables S1, S2).2 Most researchers agree that placental hypoxia and/or oxidative stress plays an important role in the pathophysiology of PE, leading to a cascade of downstream effects.3–5
The maternal cerebral vasculature is highly vulnerable to adverse effects of PE. Short- and long-term cerebrovascular complications of PE include posterior reversible encephalopathy syndrome (PRES), reversible cerebral vasoconstriction syndrome (RCVS), hemorrhagic and ischemic stroke, cerebral small vessel disease, and vascular dementia.6–10 This review summarizes current evidence and recent advances in our understanding of the effects of PE on cerebrovascular disease in women, and outlines gaps in knowledge and directions for future research.
Epidemiology of preeclampsia-associated cerebrovascular disease.
PE is a leading cause of maternal morbidity and mortality worldwide.11 In African-Americans, PE has a higher prevalence and is more likely to be associated with maternal complications, including 3-fold higher mortality rates.12,13 Cerebrovascular disease is the leading cause of maternal mortality in women with PE, with the majority of deaths due to intracerebral hemorrhage (ICH).14–17 In the United States (US), maternal stroke accounted for 7.4 percent of maternal deaths from 2011–2014.18 Rates of antepartum and postpartum stroke, highly associated with PE, increased 47 and 83 percent, respectively, from 1994–1995 to 2006–2007 in the US.19 This has been directly attributed to increasing rates of PE.19,20 PE increases the risk of maternal stroke up to 6-fold.7,21 Risk factors for peripartum stroke in women with PE include older age, African American race, chronic preexisting hypertension, underlying prothrombotic or inflammatory disorders, and infections.22
In the longer term, PE is now recognized by the American Heart Association/American Stroke Association as a sex-specific risk factor for future stroke,23 and guidelines recommend that all women be evaluated for history of PE as part of routine cardiovascular risk assessment. Controlling for other risk factors, a history of PE is associated with a 4-fold increase in risk of developing chronic hypertension,24 a 4-fold increase in heart failure,25 a 3-fold increase in type 2 diabetes,26 and a 2-fold increase in future stroke.25 Women with early-onset PE, diagnosed prior to 34 weeks gestation, are at particularly heightened risk.27 The American College of Obstetrics and Gynecology and the American College of Cardiology recently released a joint statement urging collaboration between obstetrics care providers and primary care providers to identify women during pregnancy who are at elevated risk for future cardiovascular disease, and tailor preventive treatments accordingly.28 PE is still under-recognized as a sex-specific risk factor for future stroke, however, and many women are unaware of their risk.
Preeclampsia and the cerebral vasculature: insights from basic research.
A detailed discussion of the vascular biology of PE is beyond the scope of this review and has been reviewed separately.5 Inflammation, oxidative stress, and hypoxia-induced angiogenic factors including soluble endoglin (sEng) and soluble fms-like tyrosine kinase-1 (sFlt-1), a vascular endothelial growth factor (VEGF) inhibitor, all play important roles in the maternal vascular damage seen in PE.4 PE is unique to human pregnancy, but animal models of PE have been developed, yielding insights into its cerebrovascular effects (Figure 1).
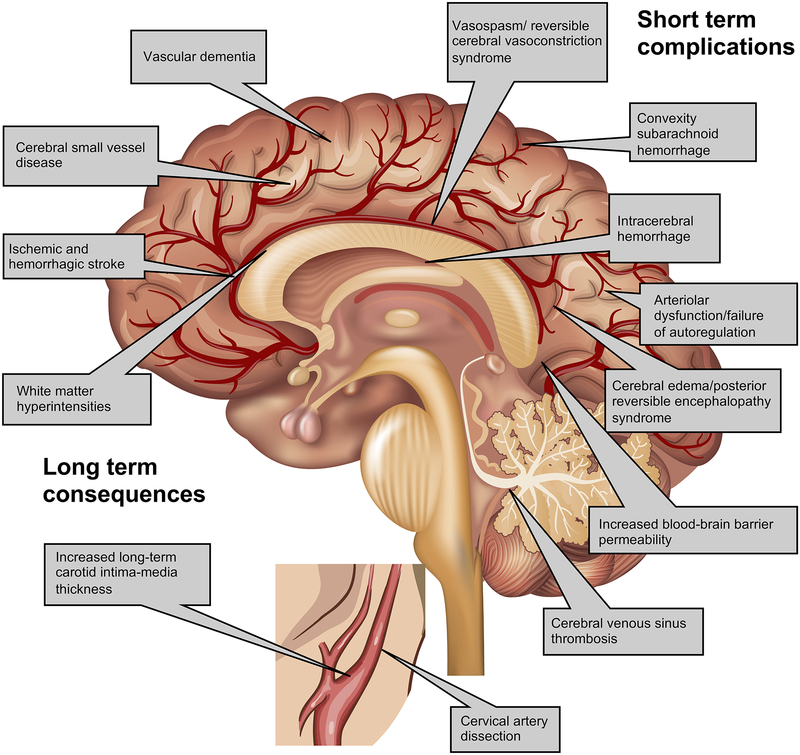
Figure 1. Cerebrovascular effects of preeclampsia.
Legend: Preeclampsia (PE) causes both acute and chronic cerebrovascular disease. In the immediate peripartum period, PE is associated with increased blood-brain barrier permeability, impaired cerebral autoregulation, hypercoagulability and inflammation, resulting in complications such as ischemic and hemorrhagic stroke, posterior reversible encephalopathy syndrome, reversible cerebral vasoconstriction syndrome, and cerebral venous sinus thrombosis. Long term, PE is associated with cerebral small vessel disease including stroke and vascular dementia, as well as increased carotid intima-media thickness.
Cerebrovascular changes in normal pregnancy.
The cerebral circulation has several features that distinguish it from other vascular beds. Chief among these is the neurovascular unit (NVU), which may be conceptualized as a complex of endothelial cells, smooth muscle cells, pericytes, astrocytes, neurons, and extracellular matrix proteins, having multiple specialized functions.29 These include maintaining the structural integrity of the blood-brain barrier (BBB), which maintains the neuronal microenvironment through endothelial tight junctions, transcytosis, and efflux transporters.30 In addition, several cells of the NVU, in particular astrocytes, pericytes and smooth muscle cells, mediate cerebral blood flow at the microvascular level.30–33 This includes regulation of neurovascular coupling, the process by which cerebral blood flow responds at the capillary level to increased neuronal metabolic demand (also known as functional hyperemia);34 and cerebral autoregulation, the mechanism by which the cerebral vasculature regulates cerebral blood flow in response to rapid changes in systemic mean arterial blood pressure over a limited, but wide range (classically, 50 to 150 mmHg), thereby maintaining steady-state cerebral perfusion.35–37
During normal pregnancy, cerebral arterioles undergo outward remodeling and capillary density increases.38 Despite this remodeling, cerebrovascular resistance has been shown to be unchanged under normotensive conditions in healthy pregnant rats, and autoregulation was actually improved.38,39 This finding corroborates small clinical studies of cerebral autoregulation in normotensive pregnant women showing autoregulation in the high-normal range.40–42 VEGF and placental growth factor (PlGF), a VEGF homologue, both of which increase BBB permeability, are both increased in normal pregnancy; however, physiological increases in sFlt-1 during pregnancy, inhibiting both VEGF and PlGF, may prevent the development of vasogenic cerebral edema under non-inflammatory conditions.43 Nevertheless, some evidence suggests that NVU function may be compromised in healthy pregnancy. Cerebral vessels from healthy late-pregnant rats developed a 6-fold increase in BBB permeability after exposure to plasma from healthy pregnant women.44 A recent study demonstrated free hemoglobin in cerebrospinal fluid from normotensive and preeclamptic pregnant women, suggesting abnormal BBB permeability in both groups.45 Pressor-induced acute hypertension caused breakthrough of autoregulation and significant cerebral hyperperfusion in healthy pregnant, but not non-pregnant rats.38,39 Efflux transporters at the BBB in the hippocampus were inhibited in late-pregnant rats, leading to an increase in spontaneous seizures.46 However, tight junction protein expression in the BBB was similar in healthy pregnant and non-pregnant rats.38
Neurovascular dysfunction in preeclampsia.
Multiple studies point to profound dysfunction of the NVU in the setting of PE. Pregnant rats with mechanically-induced placental ischemia had impaired cerebral autoregulation in vivo and increased BBB permeability, demonstrating a direct causal effect of placental ischemia on NVU dysfunction;47 this effect appeared to be mediated by inflammatory cytokines known to be elevated in women with PE.48 Rats fed a high-cholesterol diet to induce experimental PE had impairment of potassium channel-mediated cerebral arteriolar vasodilation and vasoconstriction, critical mechanisms of neurovascular coupling and cerebral autoregulation.49 Pregnant rats infused with sFlt-1 and sEng developed a PE-like syndrome and increased BBB permeability in the postpartum period.50 Exposing rat cerebral vessels to serum from women with PE resulted in an 18-fold increase in BBB permeability; surprisingly, this effect was prevented by VEGF inhibition.44 Placental-ischemic rats had evidence of cerebral edema and neuroinflammation 2 months postpartum, suggesting that the deleterious effects of PE on the NVU persist after delivery.51 The same rats had decreased expression of occludin, a tight junction protein, in the posterior cortex; however, other tight junction proteins in the BBB were unchanged.51 Magnesium, the standard treatment for prevention of eclamptic seizures in women with severe PE, has been shown to reduce brain edema and neuroinflammation in a rat model of eclampsia.52 Neuronal biomarkers were elevated in serum from women with PE during pregnancy and up to a year postpartum, suggesting persistent BBB dysfunction.53,54
A number of clinical studies have borne out these experiments. Women with PE, particularly if they have headache, have been shown to have higher baseline cerebral perfusion pressure, lower cerebrovascular resistance, and decreased vasodilation in response to CO2 inhalation, compared with healthy pregnant women.55–57 Other studies showed impaired dynamic cerebral autoregulation in women with PE.40,58–60 Cerebral perfusion pressure was elevated in women with PE even when hypertension was treated, and correlation between blood pressure and cerebral perfusion pressure was increased, implying impaired autoregulation.61 A neuropathological study of 7 women who died from eclampsia showed cerebral perivascular microhemorrhages and microinfarcts, fibrinoid necrosis, edema and arteriolar vasculopathy in several of the cases; the authors speculated that “breakthrough” of cerebral autoregulation was responsible for the vasculopathy and BBB breakdown.62 In a case series of 28 women with stroke and severe PE or eclampsia, all of the women had pre-stroke systolic blood pressure of 155 mmHg or greater, but only 12.5% of the women had pre-stroke diastolic pressures greater than 110 mmHg, and only 25% had pre-stroke mean arterial pressure over 130 mmHg.63 Of note, 92% of these women had ICH, despite their blood pressure being not necessarily in a “severe” range (systolic greater than 160 mmHg or diastolic >110 mmHg), implying breakthrough of autoregulation below the classic threshold of 150 mmHg.
Interestingly, angiogenic pathways have also been implicated in NVU dysfunction outside of the context of PE.64 Elevated sFlt-1 levels in serum and cerebrospinal fluid of non-pregnant patients with subarachnoid hemorrhage (SAH) predicted cerebral vasospasm,65 and the monoclonal VEGF inhibitor bevacizumab (an analog to sFlt-1) can cause a PE-like syndrome of acute hypertension and vasogenic cerebral edema.66,67 Animal studies have also demonstrated that exogenous VEGF increases blood-brain barrier (BBB) permeability,68 and that this effect is actually inhibited by sFlt-1 during late pregnancy.43 VEGF has recently been shown in an in vitro BBB model to enhance the activation of caveolin-1, a membrane protein involved in BBB transcytosis; in contrast, magnesium decreased caveolin-1 activity and reduced transcellular BBB permeability,69 suggesting that magnesium’s effect on eclampsia prevention could be due to stabilization of the BBB.
Immediate cerebrovascular complications of preeclampsia.
Acute cerebrovascular disorders, including PRES, RCVS, ischemic and hemorrhagic stroke, and cerebral venous sinus thrombosis (CVST), are dreaded complications of PE which can result in permanent maternal disability or death. The risk of acute cerebrovascular disease in pregnancies complicated by PE is as high as 1 in 500 deliveries;22 by comparison, the overall risk of pregnancy-related acute cerebrovascular disease is approximately 30 per 100,000 deliveries.70
Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome.
PRES is a syndrome of vasogenic edema and BBB breakdown, affecting both cortical and subcortical structures and all regions of the brain. There is a predilection for the parietal and occipital lobes, sometimes resulting in visual disturbances or cortical blindness. In severe cases PRES may result in coma, status epilepticus, and ICH. PRES can be seen in patients with acute hypertension outside of pregnancy, particularly in patients with renal dysfunction or those taking calcineurin inhibitors for immunosuppression. Up to 98% of women with eclampsia have radiological evidence of PRES, leading some to question whether eclampsia constitutes “obstetric PRES.”71,72 However, PE-associated PRES may have unique features, including a higher prevalence of headache, less frequently altered mental status, and possibly a better prognosis.8
RCVS was first described in postpartum women by Call and Fleming,73 but can occur in non-pregnant patients of both sexes. Presenting with a sudden, severe “thunderclap” headache, RCVS causes vasospasm of the arteries of the circle of Willis, and can be associated with ischemic stroke and non-aneurysmal SAH, usually over the cerebral convexities. While angiographically, RCVS looks similar to primary central nervous system vasculitis, pathologically there is no inflammatory infiltrate and the disorder usually has a transient and monophasic course.74 Unlike vasculitis, RCVS is not steroid responsive; in fact, steroids may worsen the course of both PRES and RCVS.75,76
While the term “reversible” in both PRES and RCVS implies a benign course, both disorders can lead to devastating consequences (Figure 2). Some authors consider both vasculopathies to be on a continuum with PE, as the three disorders share multiple features and often overlap.77 PRES and RCVS occur most often in the postpartum period, sometimes with little warning and no signs of PE during the pregnancy. However, elevated sFlt-1 to PlGF ratio, a marker for PE, was reported in the serum of a woman with postpartum RCVS and PRES who had no hypertension during pregnancy.78
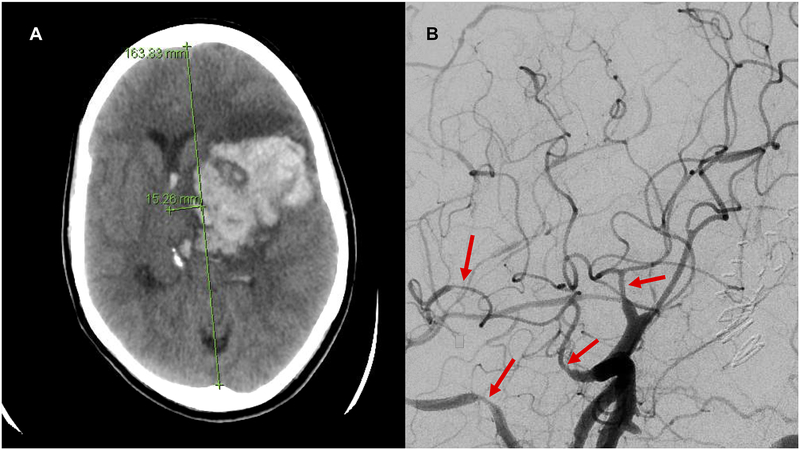
Figure 2. Intracerebral hemorrhage in a postpartum woman with preeclampsia.
Legend: A 32-year-old woman presented 4 days postpartum with hypertension and severe headache, found to have severe preeclampsia. Computed tomography of the head without contrast (A) showed left sided intracerebral hemorrhage with cerebral herniation. Cerebral angiogram (B) showed diffuse segmental vasospasm of the proximal and distal large cerebral arteries. Brain biopsy showed no evidence of vasculitis. She was diagnosed with the reversible cerebral vasoconstriction syndrome.
Arterial ischemic stroke in preeclampsia.
Arterial ischemic stroke (AIS) occurs when occlusion of a cerebral artery results in infarction of the central nervous system. AIS in women with PE can occur through multiple mechanisms. Peripartum cardiomyopathy is highly associated with PE79 and can cause acute systolic heart failure and arrhythmias, both of which can lead to cardioembolic AIS, particularly in the setting of PE-related hypercoagulability. Severe vasospasm from RCVS can cause hypoperfusion distal to the point of spasm, resulting in AIS.80 PE-related hypercoagulability can also provoke in situ thrombosis in cerebral vessels, particularly in cases of eclampsia or the HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome.62,81,82
Cervical artery dissections (carotid or vertebral) have been reported in association with PE, which can cause AIS either through occlusion of the cervical vessel or distal embolization of clot from the false lumen at the site of the dissection.83–85 It is not known whether PE increases the risk of cervical artery dissection, but PE has a well-established association with spontaneous coronary artery dissection,86 and PE-associated aortic dissections have also been reported.87,88
Migraine, particularly migraine with aura, increases the risk of AIS in pregnancy; epidemiological studies have found an 8- to 30-fold increase in risk of AIS in pregnant migraineurs.89 Migraine has also been associated with PE in multiple studies: a systematic review found odds ratios for PE of 1.08 to 3.5 in migraineurs,89 and some authors have speculated that there may be shared pathophysiology of endothelial dysfunction, platelet activation and altered vasoreactivity.90 However, PE may also be a confounder in the relationship between migraine and pregnancy-related AIS.
Subarachnoid and intracerebral hemorrhage in preeclampsia.
Hemorrhagic stroke, comprising ICH and SAH, can occur either spontaneously or secondary to rupture of vascular lesions such as aneurysms, arteriovenous malformations (AVMs), or moyamoya vasculopathy. Data conflict regarding whether pregnancy increases the risk of aneurysm or AVM rupture,91–93 and no studies have evaluated the effect of PE on risk of rupture. Interestingly, VEGF is increased in brain AVMs, and VEGF inhibition with sFlt-1 decreased brain AVM severity in animal studies.94 Theoretically, this could lead to progression of brain AVMs during normal pregnancy; conversely, high serum levels of sFlt-1 in PE could ameliorate this effect. A case series of 19 pregnancy-associated hemorrhagic strokes showed that none of the women with ruptured vascular lesions had PE, whereas 46% of the women with primary spontaneous ICH or non-aneurysmal SAH had PE.95
Moyamoya vasculopathy is a progressive steno-occlusive disorder of the terminal internal carotid arteries, leading to the formation of fragile collaterals which are prone to rupture, especially in the setting of hypertension. Moyamoya-related ICH in association with PE has been reported.96,97
While secondary ICH or SAH can occur in women with PE, hemorrhages without an underlying vascular lesion are seen more often, usually in the peripartum and postpartum period.95 In non-pregnant patients, spontaneous ICH is usually related to cerebral small vessel disease due to chronic hypertension or cerebral amyloid angiopathy. Spontaneous ICH can also be seen with coagulopathies. Similarly, the vascular pathophysiology of ICH in PE may also be due to arteriolar dysfunction, with compromised autoregulation unable to compensate for acute hypertension;49 this could be aggravated by PE-related coagulopathy. Non-aneurysmal SAH, usually over the cerebral convexities, can be a feature of PE-related RCVS, and portends a better prognosis compared to aneurysmal SAH.6 Unfortunately, few studies have provided radiological or pathological details of primary hemorrhagic strokes seen in PE.62,98
Cerebral venous sinus thrombosis in preeclampsia.
Normal pregnancy causes changes in the coagulation system that contribute to an increased risk of venous thrombotic events, including CVST, particularly in the puerperium.99–101 These changes include higher production of procoagulant coagulation factors, including factors V, VII, VIII, IX, X, and von Willebrand factor; decrease in protein S levels, and acquired activated protein C resistance; and placentally-produced plasminogen activator inhibitors, which decrease endogenous tissue plasminogen activator activity.102 PE causes hypercoagulability, systemic inflammation, platelet activation and endothelial injury, all of which predispose to thrombosis and increase the risk of CVST.103,104 Occurring most often postpartum, CVST presents insidiously with headache, and is often mistaken for post-dural puncture headache or migraine. Clots can propagate rapidly, leading to venous congestion in the adjacent brain tissue with catastrophic consequences including venous infarction, hemorrhage, and increased intracranial pressure. Cesarean section and infections, both of which are more common in women with PE, increase the risk of postpartum CVST.105
Long term effects of preeclampsia.
The effects of PE on the maternal brain appear to continue for years past the initial injury. Women with a history of remote PE had 3 times higher odds of having abnormally high carotid intima-media thickness,106 a marker of subclinical atherosclerosis highly associated with future stroke. Women with a history of PE have increased white matter hyperintensities on brain MRI,9,107 a marker of cerebral small vessel disease that is highly associated with stroke and dementia (Figure 3). Prior PE is an independent risk factor for future stroke in women, particularly in middle age.27,108–110 Multiple studies have suggested an association between PE and cognitive decline;111–113 this association was recently confirmed in a prospective population-based study showing an increased risk of vascular dementia, but not Alzheimer disease, in women with a history of PE.10 No randomized trials have been conducted for primary prevention of cerebrovascular disease in women with a history of PE, but a recent prospective cohort study showed a possible benefit of aspirin for stroke risk reduction in this population.114
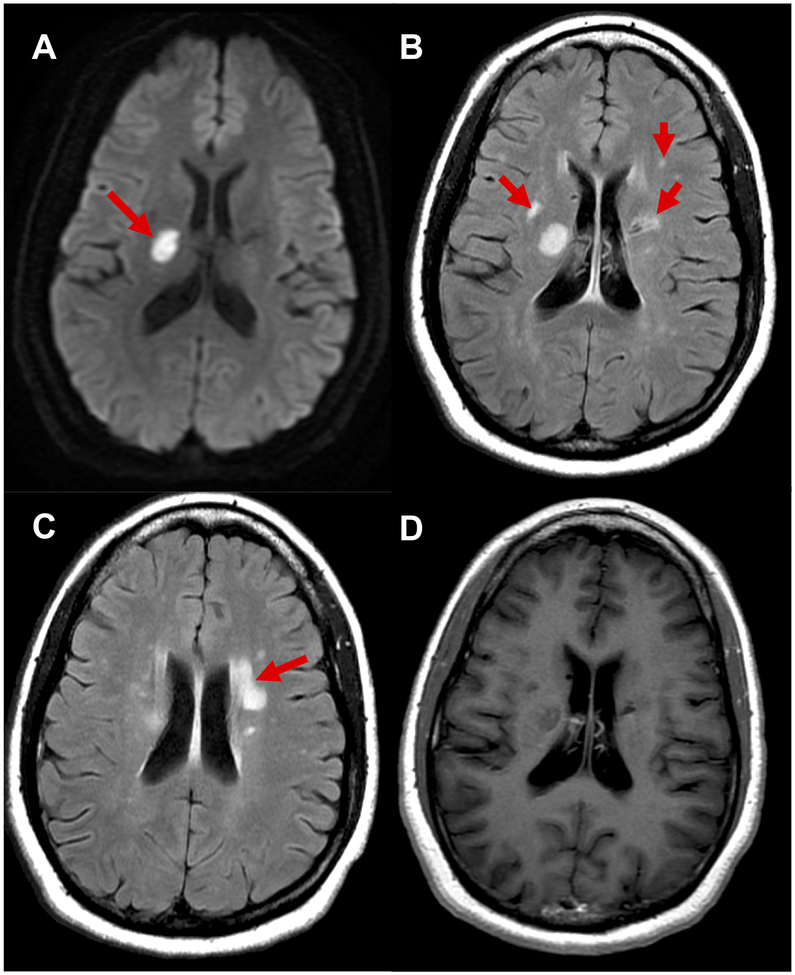
Figure 3. Long term cerebral small vessel disease after preeclampsia.
Legend: A 47-year-old woman with diabetes, hypertension, active tobacco use and history of preterm preeclampsia presented with left sided weakness and cognitive complaints. Magnetic resonance imaging of the brain with and without contrast showed an acute lacunar infarct in the right internal capsule on diffusion weighted images (A), patchy subcortical white matter hyperintensities on fluid-attenuated inversion recovery sequences (B, C), and no contrast enhancement (D). Additional studies including lumbar puncture showed no evidence of demyelinating disease or other inflammatory etiology. She was diagnosed with ischemic stroke and white matter changes due to cerebral small vessel disease.
Gaps in knowledge and future directions.
Many questions remain regarding the effects of PE on the maternal brain, and there is an urgent need for more basic, translational and clinical research in this area.
Characterizing molecular mechanisms of neurovascular unit dysfunction in preeclampsia.
PE clearly causes NVU dysfunction, but the cellular mechanisms are not well understood. While measures of cerebral hemodynamics have been shown to be disrupted in PE, it remains uncertain whether BBB dysfunction in PE is the cause, or the effect, of disrupted cerebral autoregulation. In other words, does impaired autoregulation lead to hyperperfusion at blood pressures below the threshold of normal autoregulatory limits, thus overcoming the BBB due to increased cerebral perfusion pressure? Or does increased BBB permeability permit leakage of toxic factors into the brain parenchymal microenvironment, causing neuroinflammation, impairment of neurovascular signaling and subsequent loss of autoregulation? What is the role of VEGF and VEGF inhibitors in the development of PE-related BBB dysfunction, and how does neuroinflammation interact with these factors? A complex and as-yet poorly characterized interaction between inflammatory cytokines and BBB and autoregulatory impairment may underlie PE-related NVU dysfunction, leading to both immediate and long-term cerebrovascular disease.
Predicting cerebrovascular complications in the immediate postpartum period.
Most maternal strokes occur in the first 2 weeks postpartum, often after women have been discharged home following delivery. While risk factors associated with postpartum stroke have been identified, there are currently no biomarkers or screening tools to predict which women with PE may be at higher risk for developing this rare, but disastrous complication. Future research is needed to develop screening tools to identify which women are at highest risk of postpartum stroke. The role of genetics and/or genomics in PE-associated cerebrovascular complications remains unexplored.
Identifying and preventing long-term sequelae of preeclampsia.
Increasing evidence supports that PE is independently associated with long-term cerebrovascular disease in women.10,25,114 More uncertain is whether PE is an early marker for higher baseline risk in susceptible women, or whether PE itself causes lasting vascular damage in the brain. Regardless of whether PE is a marker or a precipitant of cerebrovascular disease, there is a vital need to develop early preventive strategies. Not least of these is education of the public and the medical community regarding the need to engage women with their health after a pregnancy complicated by PE. PE is not currently incorporated into cardiovascular risk calculators, and most women are unaware of their increased risk. Stroke is now the third leading cause of death in US women,115 and cerebrovascular disease is increasingly recognized as a major contributor to dementia. Clinical trials are needed to test strategies for the primary and secondary prevention of cerebrovascular disease in women after PE. Establishing robust cross-disciplinary collaborations between researchers in maternal medicine, cardiovascular disease and vascular neuroscience will be critical to future efforts to reduce the burden of cerebrovascular disease in women after PE.
Supplementary Material
Funding:
Dr. Miller receives funding from a National Institutes of Health National Center for Advancing Translation Sciences KL2 TRANSFORM Career Development Award (5KL2TR001874–03) and from the Gerstner Family Foundation Louis V. Gerstner Scholars program.
Footnotes
Disclosures: Dr. Miller receives personal compensation from medicolegal consulting related to maternal stroke.
REFERENCES
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best practice & research. Clinical obstetrics & gynaecology. 2011;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 2.Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Current opinion in obstetrics & gynecology. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 3.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. [DOI] [PubMed] [Google Scholar]
- 4.Tannetta D, Sargent I. Placental Disease and the Maternal Syndrome of Preeclampsia: Missing Links? Current hypertension reports. 2013;15(6):590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulopoulou S Maternal Vascular Physiology in Preeclampsia. Hypertension. 2017;70(6):1066–1073. [DOI] [PubMed] [Google Scholar]
- 6.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, Fonarow GC, Schwamm LH, Kuklina EV, George MG. Patient Characteristics and Outcomes After Hemorrhagic Stroke in Pregnancy. Circulation. Cardiovascular quality and outcomes. 2015;8(6 suppl 3):S170–S178. [DOI] [PubMed] [Google Scholar]
- 7.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol 2015;125(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: Association With Posterior Reversible Encephalopathy Syndrome and Stroke. Stroke. 2018:STROKEAHA.117.018416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siepmann T, Boardman H, Bilderbeck A, Griffanti L, Kenworthy Y, Zwager C, McKean D, Francis J, Neubauer S, Yu GZ, Lewandowski AJ, Sverrisdottir YB, Leeson P. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017;88(13):1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. The Lancet. Global health. 2014;2(6):e323–33. [DOI] [PubMed] [Google Scholar]
- 12.Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phillips WR, Phipps MG, Silverstein M, et al. Screening for Preeclampsia. JAMA. 2017;317(16):1661–7. [DOI] [PubMed] [Google Scholar]
- 13.Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, Mueller A, Shaefi S, Scavone B, Kociol RD, Talmor D, Rana S. Racial Disparities in Comorbidities, Complications, and Maternal and Fetal Outcomes in Women With Preeclampsia/eclampsia. Hypertension in pregnancy. 2015;34(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics & Gynecology. 2001;97(4):533–538. [DOI] [PubMed] [Google Scholar]
- 15.Moodley J Maternal deaths due to hypertensive disorders in pregnancy. Best practice & research. Clinical obstetrics & gynaecology. 2008;22(3):559–567. [DOI] [PubMed] [Google Scholar]
- 16.Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. European journal of obstetrics, gynecology, and reproductive biology. 2013;171(2):266–270. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa J, Ikeda T, Sekizawa A, Tanaka H, Nakata M, Murakoshi T, Katsuragi S, Osato K, Ishiwata I, Kinoshita K, Maternal Death Exploratory Committee, Japan Association of Obstetricians and Gynecologists. Maternal Death Due to Stroke Associated With Pregnancy-Induced Hypertension. Circulation Journal. 2015;79(8):1835–1840. [DOI] [PubMed] [Google Scholar]
- 18.CDC ed. Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention: Reproductive Health; 2016. [Google Scholar]
- 19.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42(9):2564–2570. [DOI] [PubMed] [Google Scholar]
- 20.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? The American Journal of Obstetrics & Gynecology. 2002;186(2):198–203. [DOI] [PubMed] [Google Scholar]
- 22.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert LR, Marshall RS, Elkind MSV, Willey JZ. Risk Factors for Pregnancy-Associated Stroke in Women With Preeclampsia. Stroke. 2017;48(7):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Pina IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leslie MS, Briggs LA. Preeclampsia and the Risk of Future Vascular Disease and Mortality: A Review. Journal of midwifery & women’s health. 2016;61(3):315–324. [DOI] [PubMed] [Google Scholar]
- 25.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation. Cardiovascular quality and outcomes. 2017;10(2):e003497. [DOI] [PubMed] [Google Scholar]
- 26.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive Pregnancy Disorders and Subsequent Cardiovascular Morbidity and Type 2 Diabetes Mellitus in the Mother. Hypertension. 2009;53(6):944–951. [DOI] [PubMed] [Google Scholar]
- 27.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK, American Heart Association and the American College of Obstetricians and Gynecologists. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137(24):e843–e852. [DOI] [PubMed] [Google Scholar]
- 29.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brayden JE, Li Y, Tavares MJ. Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. Journal of Cerebral Blood Flow & Metabolism. 2012;33(2):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamner JW, Tan CO. Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke. 2014;45(6):1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, Filosa JA. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(21):8245–8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthiaume A-A, Hartmann DA, Majesky MW, Bhat NR, Shih AY. Pericyte Structural Remodeling in Cerebrovascular Health and Homeostasis. Frontiers in Aging Neuroscience. 2018;10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nature neuroscience. 2016;19(6):771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiological reviews. 1959;39(2):183–238. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. The American journal of physiology. 1998;274(1 Pt 2):H233–41. [DOI] [PubMed] [Google Scholar]
- 37.Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. Journal of applied physiology. 2012;113(8):1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipolla MJ, Sweet JG, Chan S-L. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. Journal of Applied Physiology. 2011;110(2):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cipolla MJ, Bishop N, Chan SL. Effect of Pregnancy on Autoregulation of Cerebral Blood Flow in Anterior Versus Posterior Cerebrum. Hypertension. 2012;60(3):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Veen TR, Panerai RB, Haeri S, Griffioen AC, Zeeman GG, Belfort MA. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet Gynecol. 2013;122(5):1064–1069. [DOI] [PubMed] [Google Scholar]
- 41.van Veen TR, Panerai RB, Haeri S, van den Berg PP, Zeeman GG, Belfort MA. Cerebral autoregulation in pregnancies complicated by diabetes and overweight. Diabetes & vascular disease research. 2015;12(5):377–380. [DOI] [PubMed] [Google Scholar]
- 42.Janzarik WG, Ehlers E, Ehmann R, Gerds TA, Schork J, Mayer S, Gabriel B, Weiller C, Prompeler H, Reinhard M. Dynamic cerebral autoregulation in pregnancy and the risk of preeclampsia. Hypertension. 2014;63(1):161–166. [DOI] [PubMed] [Google Scholar]
- 43.Schreurs MPH, Houston EM, May V, Cipolla MJ. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(1):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56(5):1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Berg CB, Duvekot JJ, Güzel C, Hansson SR, de Leeuw TG, Steegers EAP, Versendaal J, Luider TM, Stoop MP. Elevated levels of protein AMBP in cerebrospinal fluid of women with preeclampsia compared to normotensive pregnant women. PROTEOMICS - Clinical Applications. 2016;11(1–2):1600082–10. [DOI] [PubMed] [Google Scholar]
- 46.Johnson AC, Hammer ES, Sakkaki S, Tremble SM, Holmes GL, Cipolla MJ. Inhibition of blood-brain barrier efflux transporters promotes seizure in pregnant rats: Role of circulating factors. Brain, behavior, and immunity. 2018;67:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, Ryan MJ. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiological reports. 2014;2(8):e12134–e12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-α. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;309(11):R1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson AC, Cipolla MJ. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvascular Research. 2018;119:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace K, Bean C, Bowles T, Spencer S-K, Randle W, Kyle PB, Shaffery J. Hypertension, Anxiety, and Blood-Brain Barrier Permeability Are Increased in Postpartum Severe Preeclampsia/Hemolysis, Elevated Liver Enzymes, and Low Platelet Count Syndrome Rats. Hypertension. 2018;72(4):946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clayton AM, Shao Q, Paauw ND, Giambrone AB, Granger JP, Warrington JP. Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy. Brain, behavior, and immunity. 2018;70:376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Han X, Yang J, Bao J, Di X, Zhang G, Liu H. Magnesium Sulfate Provides Neuroprotection in Eclampsia-Like Seizure Model by Ameliorating Neuroinflammation and Brain Edema. Molecular neurobiology. 2017;54(10):7938–7948. [DOI] [PubMed] [Google Scholar]
- 53.Bergman L, Zetterberg H, Kaihola H, Hagberg H, Blennow K, Akerud H. Blood-based cerebral biomarkers in preeclampsia: Plasma concentrations of NfL, tau, S100B and NSE during pregnancy in women who later develop preeclampsia - A nested case control study. Motta A, ed. PloS one. 2018;13(5):e0196025–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergman L, Akerud H, Wikström A-K, Larsson M, Naessen T, Akhter T. Cerebral Biomarkers in Women With Preeclampsia Are Still Elevated 1 Year Postpartum. American journal of hypertension. 2016;29(12):1374–1379. [DOI] [PubMed] [Google Scholar]
- 55.Belfort MA, Saade GR, Grunewald C, Dildy GA, Abedejos P, Herd JA, Nisell H. Association of cerebral perfusion pressure with headache in women with pre-eclampsia. Br J Obstet Gynaecol. 1999;106(8):814–821. [DOI] [PubMed] [Google Scholar]
- 56.Riskin-Mashiah S, Belfort MA, Saade GR, Herd JA. Cerebrovascular reactivity in normal pregnancy and preeclampsia. Obstet Gynecol 2001;98(5 Pt 1):827–832. [PubMed] [Google Scholar]
- 57.Riskin-Mashiah S, Belfort MA. Preeclampsia is associated with global cerebral hemodynamic changes. Journal of the Society for Gynecologic Investigation. 2005;12(4):253–256. [DOI] [PubMed] [Google Scholar]
- 58.Oehm E, Reinhard M, Keck C, Els T, Spreer J, Hetzel A. Impaired dynamic cerebral autoregulation in eclampsia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003;22(4):395–398. [DOI] [PubMed] [Google Scholar]
- 59.Oehm E, Hetzel A, Els T, Berlis A, Keck C, Will H-G, Reinhard M. Cerebral hemodynamics and autoregulation in reversible posterior leukoencephalopathy syndrome caused by pre-/eclampsia. Cerebrovascular diseases (Basel, Switzerland). 2006;22(2–3):204–208. [DOI] [PubMed] [Google Scholar]
- 60.van Veen TR, Panerai RB, Haeri S, Singh J, Adusumalli JA, Zeeman GG, Belfort MA. Cerebral autoregulation in different hypertensive disorders of pregnancy. American Journal of Obstetrics and Gynecology. 2015;212(4):513.e1–7. [DOI] [PubMed] [Google Scholar]
- 61.Sonneveld MJ, Brusse IA, Duvekot JJ, Steegers EAP, Grune F, Visser GH. Cerebral perfusion pressure in women with preeclampsia is elevated even after treatment of elevated blood pressure. Acta obstetricia et gynecologica Scandinavica. 2014;93(5):508–511. [DOI] [PubMed] [Google Scholar]
- 62.Richards A, Graham D, Bullock R. Clinicopathological study of neurological complications due to hypertensive disorders of pregnancy. J Neurol Neurosurg Psychiatry. 1988;51(3):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and Severe Preeclampsia and Eclampsia: A Paradigm Shift Focusing on Systolic Blood Pressure. Obstet Gynecol. 2005;105(2):246–254. [DOI] [PubMed] [Google Scholar]
- 64.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovascular research. 2011;89(3):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griessenauer CJ, Chua MH, Hanafy KA, Baffour YT, Chen R, LeBlanc RH3, Patel AS, Salem M, Karumanchi SA, Xu D, Thadhani R, Ogilvy CS, Thomas AJ. Soluble Fms-like tyrosine kinase-1 (sFlt-1) and risk of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World neurosurgery. 2017. [DOI] [PubMed] [Google Scholar]
- 66.Cross SN, Ratner E, Rutherford TJ, Schwartz PE, Norwitz ER. Bevacizumab-mediated interference with VEGF signaling is sufficient to induce a preeclampsia-like syndrome in nonpregnant women. Reviews in obstetrics & gynecology. 2012;5(1):2–8. [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo P-Y, Chen X-L, Liu Y-W, Xiao C-L, Liu C-Y. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PloS one. 2014;9(7):e102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang S, Xia R, Jiang Y, Wang L, Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PloS one. 2014;9(2):e86407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu D, Su Y, Fu B, Xu H. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-β Transcytosis. 2018:1–14. [DOI] [PubMed] [Google Scholar]
- 70.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert LR, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. International Journal of Stroke. 2017;12(7):687–697. [DOI] [PubMed] [Google Scholar]
- 71.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, LaMarca B, Martin JNJ. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. The American Journal of Obstetrics & Gynecology. 2013;208(6):468.e1–6. [DOI] [PubMed] [Google Scholar]
- 72.Postma IR, Slager S, Kremer HPH, de Groot JC, Zeeman GG. Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet Gynecol Surv. 2014;69(5):287–300. [DOI] [PubMed] [Google Scholar]
- 73.Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19(9):1159–1170. [DOI] [PubMed] [Google Scholar]
- 74.Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP, Caviness VS. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Annals of Neurology. 2016;79(6):882–894. [DOI] [PubMed] [Google Scholar]
- 75.Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88(3):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, Knobel A, Gupta A, Navi BB. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. Journal of the Neurological Sciences. 2017;380:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feske SK, Singhal AB. Cerebrovascular disorders complicating pregnancy. Continuum (Minneapolis, Minn.). 2014;20(1 Neurology of Pregnancy):80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singhal AB, Kimberly WT, Schaefer PW, Hedley-Whyte ET. Case records of the Massachusetts General Hospital. Case 8–2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. Cabot RC, Harris NL, Shepard J-AO, Rosenberg ES, Cort AM, Ebeling SH, Peters CC, eds. N Engl J Med. 2009;360(11):1126–1137. [DOI] [PubMed] [Google Scholar]
- 79.Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2013;62(18):1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fugate JE, Wijdicks EFM, Parisi JE, Kallmes DF, Cloft HJ, Flemming KD, Giraldo EA, Rabinstein AA. Fulminant postpartum cerebral vasoconstriction syndrome. Archives of neurology. 2012;69(1):111–117. [DOI] [PubMed] [Google Scholar]
- 81.Soh Y, Yasuhi I, Nakayama D, Ishimaru T. A case of postpartum cerebellar infarction with hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. Gynecologic and obstetric investigation. 2002;53(4):240–242. [DOI] [PubMed] [Google Scholar]
- 82.Altamura C, Vasapollo B, Tibuzzi F, Novelli GP, Valensise H, Rossini PM, Vernieri F. Postpartum cerebellar infarction and haemolysis, elevated liver enzymes, low platelet (HELLP) syndrome. Neurological Sciences. 2005;26(1):40–42. [DOI] [PubMed] [Google Scholar]
- 83.Borelli P, Baldacci F, Vergallo A, Del Dotto P, Lucetti C, Nuti A, Bonuccelli U. Bilateral thalamic infarct caused by spontaneous vertebral artery dissection in pre-eclampsia with HELLP syndrome: a previously unreported association. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2012;21(8):914, e9–10. [DOI] [PubMed] [Google Scholar]
- 84.Finley A, Rogers B, Richards TJ, Vogel H. Postpartum vertebral artery dissection. BMJ case reports. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shanmugalingam R, Reza Pour N, Chuah SC, Vo TM, Beran R, Hennessy A, Makris A. Vertebral artery dissection in hypertensive disorders of pregnancy: a case series and literature review. BMC pregnancy and childbirth. 2016;16(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous Coronary Artery Dissection Associated With Pregnancy. Journal of the American College of Cardiology. 2017;70(4):426–435. [DOI] [PubMed] [Google Scholar]
- 87.Huang J, Liu H, Ding Y-L. Two cases of acute aortic dissection following preeclampsia in non-Marfan patients. Chin Med J (Engl). 2012;125(11):2073–2075. [PubMed] [Google Scholar]
- 88.Winiszewski H, Pili-Floury S, Capellier G, Piton G, Besch G. Type B Aortic Dissection Diagnosed by Left-Sided Transthoracic Ultrasonography in a Woman With Preeclampsia. A&A practice. 2018;11(6):169–171. [DOI] [PubMed] [Google Scholar]
- 89.Wabnitz A, Bushnell C. Migraine, cardiovascular disease, and stroke during pregnancy: systematic review of the literature. Cephalalgia. 2015;35(2):132–139. [DOI] [PubMed] [Google Scholar]
- 90.Facchinetti F, Sacco A. Preeclampsia and migraine: a prediction perspective. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2018;39(Suppl 1):79–80. [DOI] [PubMed] [Google Scholar]
- 91.Porras JL, Yang W, Philadelphia E, Law J, Garzon-Muvdi T, Caplan JM, Colby GP, Coon AL, Tamargo RJ, Huang J. Hemorrhage Risk of Brain Arteriovenous Malformations During Pregnancy and Puerperium in a North American Cohort. Stroke. 2017;48(6):1507–1513. [DOI] [PubMed] [Google Scholar]
- 92.Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27(6):855–65– discussion 865–6. [DOI] [PubMed] [Google Scholar]
- 93.Liu X-J, Wang S, Zhao Y-L, Teo M, Guo P, Zhang D, Wang R, Cao Y, Ye X, Kang S, Zhao J-Z. Risk of cerebral arteriovenous malformation rupture during pregnancy and puerperium. Neurology. 2014;82(20):1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu W, Shen F, Mao L, Zhan L, Kang S, Sun Z, Nelson J, Zhang R, Zou D, McDougall CM, Lawton MT, Vu TH, Wu Z, Scaria A, Colosi P, et al. Soluble FLT1 Gene Therapy Alleviates Brain Arteriovenous Malformation Severity. Stroke. 2017;48(5):1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller EC, Sundheim KM, Willey JZ, Boehme AK, Agalliu D, Marshall RS. The Impact of Pregnancy on Hemorrhagic Stroke in Young Women. Cerebrovascular diseases (Basel, Switzerland). 2018;46(1–2):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun JC, Yakimov M, al-Badawi I, Honey CR. Hemorrhagic moyamoya disease during pregnancy. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2000;27(1):73–76. [DOI] [PubMed] [Google Scholar]
- 97.Yoshimatsu J, Ikeda T, Katsuragi S, Minematsu K, Toyoda K, Nagatsuka K, Naritomi H, Miyamoto S, Iihara K, Yamamoto H, Ohno Y. Factors contributing to mortality and morbidity in pregnancy-associated intracerebral hemorrhage in Japan. The journal of obstetrics and gynaecology research. 2014;40(5):1267–1273. [DOI] [PubMed] [Google Scholar]
- 98.Lappin JM, Darke S, Duflou J, Kaye S, Farrell M. Fatal Stroke in Pregnancy and the Puerperium. Stroke. 2018;49(12):3050–3053. [DOI] [PubMed] [Google Scholar]
- 99.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: How can high-risk women be identified? American Journal of Obstetrics and Gynecology. 2002;186(2):198–203. [DOI] [PubMed] [Google Scholar]
- 100.Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. The Lancet. 2010;375(9713):500–512. [DOI] [PubMed] [Google Scholar]
- 101.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370(14):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenner B Haemostatic changes in pregnancy. Thrombosis research. 2004;114(5–6):409–414. [DOI] [PubMed] [Google Scholar]
- 103.Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, Bock F, Nazir S, Huebner H, Mertens PR, Fischer K-D, Zenclussen AC, Offermanns S, Aharon A, Brenner B, et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood. 2016;128(17):2153–2164. [DOI] [PubMed] [Google Scholar]
- 104.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstetrics & Gynecology. 2005;106(3):509–516. [DOI] [PubMed] [Google Scholar]
- 105.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274–1282. [DOI] [PubMed] [Google Scholar]
- 106.Garovic VD, Milic NM, Weissgerber TL, Mielke MM, Bailey KR, Lahr B, Jayachandran M, White WM, Hodis HN, Miller VM. Carotid Artery Intima-Media Thickness and Subclinical Atherosclerosis in Women With Remote Histories of Preeclampsia: Results From a Rochester Epidemiology Project-Based Study and Meta-analysis. Mayo Clinic Proceedings. 2017;92(9):1328–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiegman MJ, Zeeman GG, Aukes AM, Bolte AC, Faas MM, Aarnoudse JG, de Groot JC. Regional distribution of cerebral white matter lesions years after preeclampsia and eclampsia. Obstetrics & Gynecology. 2014;123(4):790–795. [DOI] [PubMed] [Google Scholar]
- 108.Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, Giles WH, Kittner SJ. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke. 2006;37(4):1055–1059. [DOI] [PubMed] [Google Scholar]
- 109.Canoy D, Cairns BJ, Balkwill A, Wright FL, Khalil A, Beral V, Green J, Reeves G, Million Women Study C. Hypertension in pregnancy and risk of coronary heart disease and stroke: A prospective study in a large UK cohort. International journal of cardiology. 2016;222:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ, Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation. 2017;135(6):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Postma IR, Bouma A, de Groot JC, Aukes AM, Aarnoudse JG, Zeeman GG. Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: A follow-up study in women after hypertensive disorders of pregnancy. Journal of clinical and experimental neuropsychology. 2016;38(5):585–598. [DOI] [PubMed] [Google Scholar]
- 112.Fields JA, Garovic VD, Mielke MM, Kantarci K, Jayachandran M, White WM, Butts AM, Graff-Radford J, Lahr BD, Bailey KR, Miller VM. Preeclampsia and cognitive impairment later in life. American Journal of Obstetrics and Gynecology. 2017;217(1):74.e1–74.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elharram M, Dayan N, Kaur A, Landry T, Pilote L. Long-Term Cognitive Impairment After Preeclampsia: A Systematic Review and Meta-analysis. Obstet Gynecol 2018;132(2):355–364. [DOI] [PubMed] [Google Scholar]
- 114.Miller EC, Boehme AK, Chung NT, Wang SS, Lacey JV, Lakshminarayan K, Zhong C, Woo D, Bello NA, Wapner R, Elkind MSV, Willey JZ. Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology. 2019;92(4):e305–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.National Center for Health Statistics. Health, United States, 2017: With special feature on mortality. cdc.gov. 2018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.