Abstract
In two multi-center cohort studies of 2,912 infants hospitalized for bronchiolitis during 2007–2014, the five most common pathogens were RSV (76.5%), rhinovirus (23.8%), coronavirus (6.9%), adenovirus (6.4%), and human metapneumovirus (6.0%). Hospitalization months significantly differed for these common pathogens (P≤0.01), except for coronavirus (P=0.30). There was significant heterogeneity in temporal patterns by region in RSV-A and -B (both P<0.001).
Keywords: respiratory syncytial virus, rhinovirus, bronchiolitis, children, epidemiology
BACKGROUND
Bronchiolitis is an important public health problem in the U.S. Indeed, bronchiolitis is the leading cause of infant hospitalizations, accounting for 130,000 hospitalizations with a direct cost of at least $550 million each year.1 Respiratory syncytial virus (RSV) is the most common causative pathogen of bronchiolitis, and has been used to define bronchiolitis cohorts for decades.2 In recent years, the advent of molecular techniques has revealed a diverse group of respiratory pathogens related to severe bronchiolitis (i.e., bronchiolitis that requires hospitalization).2
Understanding the epidemiology of respiratory pathogens in infants with severe bronchiolitis is essential for developing effective prophylactic (e.g., immunoprophylaxis) and treatment (e.g., antiviral agents) strategies. Prior active and passive surveillance efforts have reported temporal patterns of common respiratory viruses in the general population3–6 and children (<5 years of age) with acute respiratory infections (ARIs).7–9 However, most studies have focused on a single pathogen (e.g., RSV) and had a limited geographic diversity. Furthermore, no study has comprehensively investigated the geographic and temporal patterns of respiratory pathogens in infants with bronchiolitis, including infants with severe bronchiolitis–a population with high morbidity.
To address this knowledge gap, we analyzed data from two prospective cohort studies of U.S. children hospitalized with bronchiolitis. Specifically, we examined the epidemiology of respiratory pathogens among infants with severe bronchiolitis and investigated potential differences between these pathogens according to U.S. region and hospitalization month.
METHODS
The present analysis combines data from two multi-center, multi-year prospective cohort studies of infants with severe bronchiolitis. Using a similar protocol, one study enrolled subjects at 16 sites during three consecutive bronchiolitis season (from November 1 through March 31) in 2007–2010 – the 30th Multicenter Airway Research Collaboration (MARC-30), while the other enrolled subjects at 17 sites from November 1 to April 30 in 2011–2014 – MARC-35. The details of study design, setting, participants, and methods of data collection are described in Supplemental Digital Content 1. The institutional review board at each of the participating hospitals approved the studies. Written informed consent was obtained from the parent or guardian.
In brief, investigators collected clinical data and nasopharyngeal aspirates within 24 hours of hospitalization by using a standardized protocol. Identification of respiratory pathogens was performed by using singleplex or duplex two-step real-time PCR (rtPCR) at Baylor College of Medicine (Houston, TX, USA). Real-time reverse transcriptase-PCR was used for the detection of RNA respiratory viruses, including RSV (types A and B), rhinovirus, coronaviruses (NL-63, OC-43, HKU1, and 229E), human metapneumovirus (hMPV), parainfluenza viruses (types 1, 2 and 3), enteroviruses, and influenza viruses (types A and B, and 2009 novel H1N1). rtPCR was also used for the detection of DNA pathogens which included adenovirus, human bocavirus type 1, Mycoplasma pneumoniae, and Bordetella pertussis.
In the current analysis, we analyzed the data of infants (<1 year of age) from MARC-30 and MARC-35. We compared the likelihood of having each of the five most common pathogens (with a detection likelihood of >5%) between the U.S. census regions (Northeast, Midwest, South, West) by using chi-squared test. To examine the region-×-month (compilation of study years) interactions with regard to the likelihood of having each virus, we used random-effects model adjusting for hospitalization year and patient clustering within hospitals, as well as likelihood ratio test.
RESULTS
In MARC-30, of 3,910 children with severe bronchiolitis eligible to the study, 2,207 children (age <2 years) were enrolled (56.4%). In MARC-35, of 1,228 infants with severe bronchiolitis eligible to the study, 1,016 infants were enrolled (82.7%). In both studies, the enrolled and non-enrolled children were not significantly different in age and sex (both P>0.05). The overall analytic cohort comprised 2,912 infants (age <1 year) with severe bronchiolitis from all four U.S. regions: 1,896 infants from MARC-30 and 1,016 infants from MARC-35. The median age was 3.2 (IQR 1.6–6.1) months, 40.5% were female, and 39.4% were non-Hispanic white. Of these, 16.9% were admitted to an ICU and 7.1% underwent mechanical ventilation (see table, Supplemental Digital Content 2). Overall, 66.2% had a single respiratory pathogen infection while 28.9% had a coinfection by ≥2 respiratory pathogens; no respiratory pathogens were detected in 4.9%. Among infants with coinfection, RSV plus rhinovirus infection was most common (46.2% [13.3% of analytic cohort]). The five most common respiratory pathogens were detected in 92.8% of the analytic cohort. Specifically, RSV was the most commonly detected pathogen (overall 76.5%; RSV-A 48.6%; RSV-B 28.5%), followed by rhinovirus (23.8%), coronavirus (6.9%), adenovirus (6.4%), and hMPV (6.0%) (see figure, Supplemental Digital Content 3). Approximately two-thirds of RSV-A and RSV-B bronchiolitis were caused by single respiratory pathogen (see figure, Supplemental Digital Content 4). The likelihood of infection with RSV-A and -B, four species of coronavirus, and hMPV significantly varied across the study years (all P<0.05; see table, Supplemental Digital Content 5).
Across the four U.S. regions, while there was no significant difference in the likelihood of overall RSV infection (P=0.72), there were significant between-region differences in that of RSV-A and -B infection (both P<0.001 (see table, Supplemental Digital Content 6). For example, infants in the West region had a lower likelihood of RSV-A infection and higher likelihood of RSV-B infection compared with the other regions. In contrast, the other common pathogens – except for coronavirus NL63 – had no significant regional differences (P≥0.10).
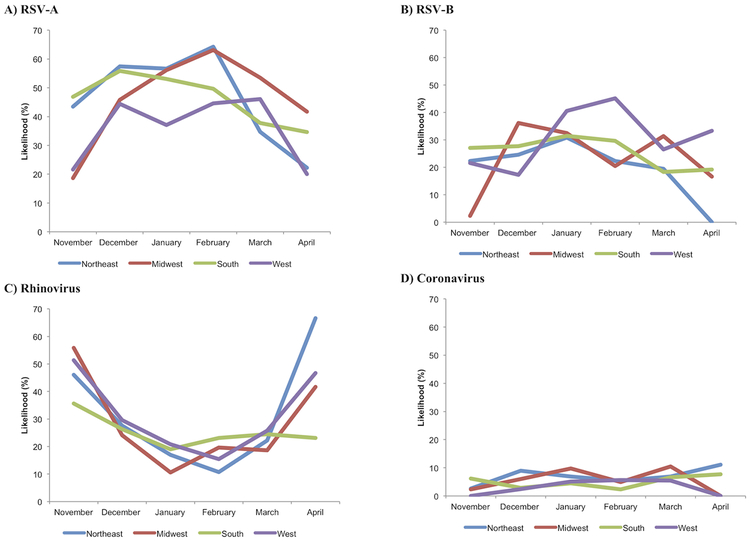
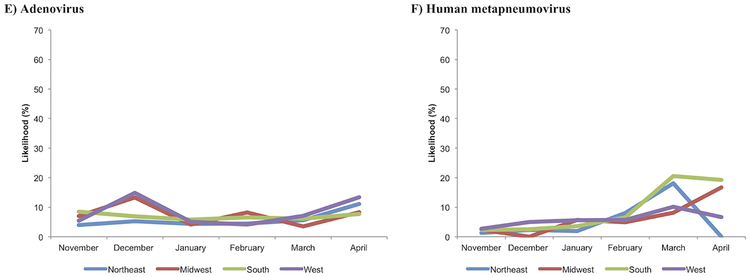
Overall, the likelihood of infection significantly differed between the hospitalization months for the most common pathogens (all P≤0.01), except for coronavirus (P=0.30). For example, rhinovirus was the dominant pathogen in November and April while RSV-A was dominant in all other months. hMPV had a peak in March and April. Figure 1 shows the temporal changes in the likelihood of having each of five most common pathogens by U.S. region. The random-effects models demonstrated significant interactions between regions and hospitalization months with regard to the likelihood of RSV-A and -B infection (both P<0. 001) indicating heterogeneity in the temporal patterns by region. For example, infants in the South region had a peak of RSV-A in December while those in the Northeast and Midwest regions had a peak in February.
Figure 1. Temporal change in the likelihood of having each of five most common pathogens in infants with severe bronchiolitis, by U.S. census region.
A) Respiratory syncytial virus (RSV)-A, B) RSV-B, C) rhinovirus, D) coronavirus, E) adenovirus, and F) human metapneumovirus (hMPV). Overall, there were significant differences in the likelihood between hospitalization months in RSV-A, RSV-B, rhinovirus, adenovirus, and hMPV (P≤0.01) while there was no significant difference in coronavirus (P=0.30). The random-effects models demonstrated significant interactions between regions and hospitalization months with regard to the likelihood of RSV-A and -B (both P<0.001) and coronavirus infection (P=0.02). By contrast, there was no significant interaction on the likelihood of rhinovirus, adenovirus, or hMPV infection (all P≥0.05).
DISCUSSION
In this analysis based on two multi-center, multi-year prospective cohorts of U.S. infants with severe bronchiolitis, the five most common pathogens – RSV, rhinovirus, coronavirus, adenovirus, and hMPV – accounted for >90% of cases. Our data demonstrated that these viruses (except for coronavirus) had different temporal patterns and that there is a regional heterogeneity in the temporal pattern in RSV-A and -B infection. In agreement with our finding, the National Respiratory and Enteric Virus Surveillance System (NREVSS) – a passive laboratory surveillance system – reported that the overall incidence of RSV infection varies across U.S. regions and seasons in the general population.5 While it did not specifically evaluate children with bronchiolitis or RSV subtypes, it reported that the onset of RSV season is earlier in the South compared with the Midwest region. As for the non-RSV pathogens, another surveillance effort in three U.S. counties (Cincinnati OH, Nashville TN, and Rochester NY) – the New Vaccine Surveillance Network – reported that, in children (<5 years of age) with ARI, the peak of rhinovirus infection was non-winter seasons (e.g., fall, spring).7,8 NREVSS also reported the temporal patterns in coronavirus,3 adenovirus,4 and hMPV6 in the general populations. Our geographically-diverse, multi-center data – with comprehensive respiratory pathogen characterization – corroborate these earlier reports and extend them by demonstrating, for the first time, different regional and temporal patterns for the common respiratory pathogens, including non-RSV pathogens, in infants with severe bronchiolitis.
Our study has potential limitations. First, this study is not a random sampling of all infants with severe bronchiolitis. However, as causative pathogens were not tested at initial recruitment, and are unlikely to be related to enrollment, our study samples were likely representative of severe bronchiolitis population at the study sites. Second, as the studies focused on infants with severe bronchiolitis and enrolled infants in the bronchiolitis season, the results should be cautiously generalized to broader ARI populations that may have different virus epidemiology patterns (e.g., higher frequencies of rhinovirus ARI in spring and fall). Nonetheless, our findings are directly relevant, at least, to 130,000 children with severe bronchiolitis each year.1 Lastly, the current American Academy of Pediatrics guidelines of bronchiolitis10 do not recommend routine virologic testing and the potential benefit of testing at the clinical setting remains to be determined. Yet, recent studies have demonstrated the association of specific viruses (e.g., RSV plus rhinovirus coinfection) and high RSV genomic load with higher acute severity of bronchiolitis11 and beneficial effects of anti-RSV agents on acute severity.12 Furthermore, the literature has reported not only the association of severe viral respiratory infections (e.g., RSV and rhinovirus) in the first year of life with differential risks of developing recurrent wheeze and asthma in childhood2,13,14 but also potential prophylactic strategies – e.g., palivizumab on recurrent wheezing15 and omalizumab on rhinovirus infection.16 These emerging evidence indicate the role of different viruses in the acute and chronic morbidities of bronchiolitis.
In summary, on the basis of data from two large, multi-center, multi-year prospective cohorts of U.S. infants with severe bronchiolitis, we observed that the major viruses (RSV-A, RSV-B, rhinovirus, adenovirus, and hMPV) had different temporal patterns and that there is a regional heterogeneity in the temporal pattern in RSV-A and -B infection. For clinicians, our data provide guidance for optimal timing of RSV immunoprophylaxis by U.S. region in infants at higher risk for severe illness. For researchers, our data should facilitate further investigations into the development of treatment strategies for the acute (e.g., antiviral agents for bronchiolitis) and chronic (e.g., immunomodulators for incident asthma) morbidities of bronchiolitis.
Supplementary Material
Supplemental Digital Content 1: Supplemental Methods
Supplemental Digital Content 2: Supplemental Table 1
Supplemental Digital Content 3: Supplemental Figure 1
Supplemental Digital Content 4: Supplemental Figure 2
Supplemental Digital Content 5: Supplemental Table 2
Supplemental Digital Content 6: Supplemental Table 3
Acknowledgments
Funding: This work was supported by grants U01 AI-67693, U01 AI-087881, UG3 OD-023253, UH3 OD-023253, R01 AI-134940, R01 AI-137091, and R21 HL-129909 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have no financial relationships relevant to this article to disclose.
Contributor Information
Kohei Hasegawa, Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Tadahiro Goto, Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Atsushi Hirayama, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan.
Federico R. Laham, Department of Infectious Disease, Arnold Palmer Hospital for Children, Orlando, FL, USA.
Jonathan M. Mansbach, Department of Medicine, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Pedro A. Piedra, Departments of Molecular Virology and Microbiology and Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Carlos A. Camargo, Jr., Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
REFERENCES
- 1.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K, Mansbach JM, Camargo CA Jr. Infectious pathogens and bronchiolitis outcomes. Exp Rev Anti Infect Ther. 2014;12(7):817–828. [DOI] [PubMed] [Google Scholar]
- 3.Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014–2017. J Clin Virol. 2018;101:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder AM, Biggs HM, Haynes AK, et al. Human Adenovirus Surveillance - United States, 2003–2016. MMWR Morbid Mort Week Rep. 2017;66(39):1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease C, Prevention. Respiratory syncytial virus activity--United States, July 2011-January 2013. MMWR Morbid Mort Week Rep. 2013;62(8):141–144. [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes AK, Fowlkes AL, Schneider E, Mutuc JD, Armstrong GL, Gerber SI. Human Metapneumovirus Circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(5). [DOI] [PubMed] [Google Scholar]
- 7.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204(11):1702–1710. [DOI] [PubMed] [Google Scholar]
- 8.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195(6):773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linder JE, Kraft DC, Mohamed Y, et al. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131(1):69–77 e61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–1502. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: Multicenter cohort studies in the US and Finland. J Infect Dis. 2015;211(10):1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVincenzo JP, Whitley RJ, Mackman RL, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–722. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Piedra PA, Bauer CS, et al. Nasopharyngeal CCL5 in infants with severe bronchiolitis and risk of recurrent wheezing: A multicenter prospective cohort study. Clin Exp Allergy. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumas O, Hasegawa K, Mansbach JM, Sullivan AF, Piedra PA, Camargo CA Jr. Severe Bronchiolitis Profiles and Risk of Developing Recurrent Wheezing by Age 3 Years. J Allergy Clin Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki H, Kusuda S, Okada K, et al. Palivizumab Prophylaxis in Preterm Infants and Subsequent Recurrent Wheezing. Six-Year Follow-up Study. Am J Respir Crit Care Med. 2017;196(1):29–38. [DOI] [PubMed] [Google Scholar]
- 16.Esquivel A, Busse WW, Calatroni A, et al. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am J Respir Crit Care Med. 2017;196(8):985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Supplemental Methods
Supplemental Digital Content 2: Supplemental Table 1
Supplemental Digital Content 3: Supplemental Figure 1
Supplemental Digital Content 4: Supplemental Figure 2
Supplemental Digital Content 5: Supplemental Table 2
Supplemental Digital Content 6: Supplemental Table 3