Abstract
Background:
Mechanical loading improves bone mineral density (BMD) and strength while decreasing fracture risk. Cross-sectional studies show that exercise advantage is lost in oligo-amenorrheic athletes (OA). Longitudinal studies examining the opposing effects of exercise and hypogonadism on bone are lacking in adolescents/young adults.
Objective:
Evaluate differences in bone accrual over 12 months in OA, eumenorrheic athletes (EA) and non-athletes (NA). We hypothesized that bone accrual would be lower in OA than EA and NA, with differences most pronounced at non-weight bearing trabecular sites.
Methods:
27 OA, 29 EA, and 22 NA, 14-25 years old, completed 12-months of the prospective study. Athletes were weight-bearing endurance athletes. Subjects were assessed for areal BMD and bone mineral content (BMC) using DXA at the femoral neck, total hip, lumbar spine and whole body (WB). Failure load (a strength estimate) at the distal radius and tibia was assessed using microfinite element analysis of data obtained via high resolution peripheral quantitative computed tomography (HRpQCT). The primary analysis was a comparison of changes in areal BMD, BMC, and failure load across groups over 12-months at the respective sites.
Results:
Groups did not differ for baseline age, height or BMI. Percent body fat was lower in both OA and EA compared to NA. OA attained menarche later than EA and NA. Over the follow-up period, OA gained 1.9±2.7 kg of weight compared to 0.5±2.4 kg and 0.8±2.3 kg in EA and NA respectively (p=0.09); 39% of OA resumed menses. Changes in BMD, BMD Z-scores, and tibial failure load over 12-months did not differ among groups. At follow up, EA had higher femoral neck, hip and WB BMD Z-scores than NA, and higher hip BMD Z-scores than OA (p<0.05) after adjusting for covariates. At follow-up, radial failure load was lower in OA vs. NA, and tibial failure load lower in OA and NA vs. EA (p≤0.04 for all). Change in weight and fat mass were associated with changes in BMD measures at multiple sites.
Conclusion:
Despite weight gain and menses recovery in many OA during follow-up, residual deficits persist without catch-up raising concerns for suboptimal peak bone mass acquisition.
Keywords: Bone accrual, bone mineral density, bone strength, adolescent, adult, DXA, finite element analysis
1. INTRODUCTION
Adolescence is a critical time for bone accrual, with site-specific differences emerging based on gender, nature of exercise activity (bone loading or not), and nutritional and hormonal status (1, 2). Children and adolescents who engage in bone loading exercise have increased bone mineral density (BMD) and estimated strength, effects that persist into adulthood (3, 4), with particularly impressive gains during early puberty (5, 6). The benefits of moderate-vigorous physical activity on bone geometry, microarchitecture and strength over the course of childhood have also been proven (7). Although early engagement in physical activity can improve long-term bone health, additional factors, such as undernutrition and hypogonadism, may compromise these gains.
The expanding platform for female athletic pursuits during recent decades has been associated with an increased awareness of the Female Athlete Triad of low energy availability, menstrual dysfunction and low BMD (8-10), as well as its impact on fracture risk and related morbidity (10-12). The Triad has been identified in females at various levels of competition across a broad range of ages (13-15). When energy intake is insufficient to meet the caloric needs of vigorous exercise, luteinizing hormone (LH) pulsatility is impacted resulting in a decline in estrogen production, a hormone known to inhibit bone resorption (16). Thus, the benefits of exercise activity on bone may be negated by the deleterious effects of hypogonadism.
In previous cross-sectional analyses, our studies and those of other investigators have demonstrated lower areal BMD (aBMD) at the spine, hip and total body in adolescent and young adult oligomenorrheic athletes (OA) compared to eumenorrheic athletes (EA) (8-10). These deficits appear to be greatest at the lumbar spine; consistent with past research demonstrating an increased impact of hypoestrogenism at sites of predominantly trabecular bone (2, 8-10, 17). Our studies have also shown that hypogonadism in athletes results in reduced estimated bone strength at non-weight bearing sites compared to non-athletes, and a loss of the beneficial effects of exercise activity at weight bearing sites observed in normally menstruating athletes (9, 10, 17). However, data thus far are inconclusive regarding bone accrual during the critical adolescent and young adult years, with some studies indicating persistent deficits on follow-up (18) and others demonstrating the ability to achieve gains in BMD into the third decade (19, 20).
Although recent research has allowed for a more comprehensive understanding of the timing and pattern of skeletal mineralization in healthy adolescents (21), longitudinal analyses of bone accrual patterns in young female athletes with menstrual irregularity are currently lacking, and are important to ascertain. To address this knowledge gap and further clarify the site-specific effects of exercise versus hypogonadism on bone, we examined changes in areal BMD using dual energy x-ray absorptiometry (DXA), and bone strength estimates using micro finite element analysis (μFEA) (using data derived from high resolution peripheral quantitative computed tomography (HR-pQCT)) over a year in adolescent and young adult OA, EA and non-athletes. We hypothesized that bone accrual would be lower in OA compared to EA and non-athletes, with differences being most pronounced at non-weight bearing sites and sites of predominantly trabecular bone (2, 8-10, 17).
2. PARTICIPANTS AND METHODS
2.1. Study Design
This was a longitudinal observational study examining changes in areal BMD and strength estimates in 78 young women (27 OA, 29 EA and 22 non-athletes) 14-25 years old over 12 months. Our Institutional Review Board approved the study. Participants ≥ 18 years old, and parents of participants < 18 years old signed informed consent. Informed assent was obtained from participants < 18 years old.
2.2. Participant Selection
Participants were recruited through referrals from medical clinics, and approved advertisements within the hospital and in college campuses and websites. All participants had a BMI between the 10th and 90th percentiles for age. Potential participants were excluded if they had taken medications (other than calcium and vitamin supplements) that may affect bone metabolism, such as combined oral contraceptives, Depo Provera, anabolic steroids, glucocorticoids or anticonvulsants, in the preceding three months, or had been diagnosed with conditions known to impact bone health, such as hypothyroidism, hyperthyroidism, diabetes mellitus, hypercortisolism, and renal or gastrointestinal disease. None of the participants had active anorexia nervosa. A past history of an eating disorder was present in 22.2, 6.9 and 0 % of OA, EA and non-athletes respectively.
Menstrual Status:
Oligo-amenorrhea was defined as absence of menses for ≥ 3 months within a period of oligomenorrhea (cycle length > 6 weeks) for ≥ 6 months preceding study participation, or the absence of menarche at 15 years of age. Other causes of menstrual irregularity or absence, including pregnancy, primary ovarian insufficiency, hyperprolactinemia, thyroid dysfunction, and conditions of androgen excess (including polycystic ovarian disease) were ruled out in OA. In both EAs and non-athletes, eumenorrhea was defined as ≥ 9 menses in the 12 months preceding study enrollment. Gynecologic age was defined as chronologic age in years minus the age at menarche and reflects the time of exposure to gonadal hormones at levels expected in mature females.
Athletic Status:
Athletic status was assessed through a detailed history of types of activity that participants engaged in (both weight-bearing and non-weight bearing), and the duration of such activities per week over the preceding 6- and past 12-months, as well as earlier years. Inclusion criteria for athletes included ≥ 4 hours of aerobic weight-bearing activity and/or ≥ 20 miles/week of running for ≥ 6 months in the preceding year. Cyclists, swimmers, gymnasts and rowers were excluded from participation to minimize variations in weight-bearing activity. Most of the athletes recruited for this study were runners. Non-athletes were eligible if their weight-bearing activity was < 2 hours/week and they did not participate in organized team sports.
We screened 140 adolescents and young women 14–25 years old for the OA arm, of whom 121 met inclusion criteria for OA and consented for participation in a larger randomized controlled trial. Only OA participants randomized to the no-intervention arm in this trial (n=38) with data available for both the baseline and 12-month visit (n=27) were included in the OA group for this study. Similarly, 59 EA and 57 non-athletes were screened, of whom 43 EA and 39 non-athletes consented for participation in the prospective study. Eleven OA, 14 EA and 17 non-athletes dropped out of the study over the 12-month period. Participants with data available for most endpoints at both baseline and 12-months were included in this analysis. This included 27 OA, 29 EA and 22 non-athletes for DXA data and 26 OA, 29 EA and 21 non-athletes for micro FEA data.
2.3. Study Procedures
Bone age was assessed with an x-ray of the non-dominant hand using the methods of Greulich and Pyle in those 21 years old and younger at the baseline visit (22). Height and weight (measured by Clinical Research Center dieticians on the same instrument at each visit), BMI, menstrual and athletic status, calcium and vitamin D intake (using validated questionnaires), and serum levels of calcium and vitamin D were documented at baseline and 12-months follow-up. Self-reported race was noted at baseline. All OA were counseled at each visit by Clinical Research Center dieticians and study physicians about increasing caloric intake to improve energy availability. All participants were provided with calcium and vitamin D supplements (500 mg elemental calcium and 600-2000 IU of vitamin D daily depending on baseline levels) to ensure that they were calcium and vitamin D replete. A subset of 21 OA, 18 EA and 16 non-athletes completed four-day food records (three weekdays and one weekend day) at both baseline and follow-up visits; data for total calorie, carbohydrate, fat and protein intake were assessed using the Minnesota Nutrition Data System software (23).
Biochemical Analysis:
An immunochemiluminometric assay was used to measure 25 (OH) vitamin D (LabCorp Esoteric Testing, Burlington, NC; sensitivity 4.0 ng/mL; intraassay coefficient of variation 4.8-7.7%). A colorimetric assay was used to measure calcium (LabCorp Automated Chemistry, Raritan, NJ; sensitivity 0.8 mg/dl; intraassay coefficient of variation 0.9-3.0%).
Areal Bone Mineral Density Assessment:
DXA (Hologic QDR-Discovery A, Apex software version 13.3; Hologic Inc, Waltham, Massachusetts) was used to assess spine, total hip, femoral neck, and whole body bone mineral content (BMC) and BMD, as well as body composition at baseline and follow-up. We chose to report changes in areal BMD and BMC over time because these are bone measures assessed in clinical settings, and recommended for clinical reporting in adolescents by the International Society of Clinical Densitometry (ISCD) (24). The ISCD recommends reporting spine and whole body less head (or whole body) BMD and BMC in children/adolescents, and spine/femoral neck BMD in adults. Because our age range spanned adolescence and adulthood, we chose to report lumbar spine, femoral neck and whole body measures. We do not report whole body less head measures as normative data for whole body less head were not available past 20 years in the longitudinal bone mineral density in childhood study. BMD Z-scores were calculated against the pediatric standard database (applicable for those 20 years old and younger) for subjects < 19 years old at baseline, to ensure that the same database could be used for follow-up assessments over 12-months. For participants who were ≥ 19 years old at baseline, Z-scores were generated using the adult standard database. Up to two participants in each group (27 OA, 29 EA and 22 non-athletes) had missing data for some, but not most, DXA measures at follow-up, and were thus included in the analyses. Percent fat and lean mass are reported (rather than absolute values) because weight did not significantly differ among groups. The coefficients of variation for BMD, fat mass, and lean mass for this software are 0.8% to 1.1%, 2.1%, and 1.0%, respectively at our institution. The same scanner and software version were used for all participants and for longitudinal assessments.
High Resolution Peripheral Quantitative Computed Tomography (HRpQCT) and Micro-finite element analysis (μFEA):
HRpQCT (XtremeCT; Scanco Medical AG, Basserdorf, Siwtzerland) was performed at the distal radius and distal tibia to assess microarchitecture of bone. These scans were obtained at the non-dominant wrist and leg, except in cases of a fracture history in which the contralateral extremity was used. Manufacturer’s protocols were followed, including acquisition of a 2D scout view followed by CT slices obtained 9.5mm and 22.5mm from a reference line manually placed at the endplate of the radius and tibia, respectively (25). Scans used 60 kVp effective energy and 100ms integration time, obtaining 110 CT slices (9.02mm) with an isotropic voxel size of 82 μm3 (9). Semiautomated software was used to segment cortical and trabecular regions.
μFEA estimates the biomechanical properties of bone in the setting of simulated axial compression using 3D HRpQCT images (26). A linear axial compression test was simulated on each image using a 1% axial strain, Young's modulus of 10,000 MPa, and a Poisson's ratio of 0.3. Finite element software (Scanco Medical AG) was used to estimate failure load (kN) based on 2% of the elements exceeding 7000 microstrain (27). μFEA-derived estimates of failure load are strongly correlated (r2 = 0.75) with experimentally measured failure loads that produce Colles' fractures in human cadaveric radii (26), as well as incident fractures in older men (28), and were available for analyses in 26 OA, 29 EA and 21 non-athletes. We chose to report changes in failure load rather than bone microarchitecture, because changes in failure load reflect the cumulative clinical impact of changes in different aspects of bone microarchitecture over time. Up to two baseline and follow up values were excluded due to motion artifact and decreased scan quality. Only failure load is reported in this paper, as data for stiffness typically follow similar patterns.
Motion artifact was graded manually by the technician as follows: 1 = excellent, no motion artifact; 2 = good, limited motion artifact; 3 = severe motion artifact. All scans with a score of 3 were excluded from the analysis. Reproducibility for repeated measurements is 2.1 to 3.0% for μFEA measures.
2.4. Statistical Methods
We used JMP (version 12; SAS Institute, Inc., Cary, NC) for all analyses. Data are presented as means ± SEM or median (interquartile range) depending on data distribution. Categorical variables, such as race (white, black, Asian, other) were summarized using percentages and compared using Fisher’s Exact test. The Shapiro-Wilk test for normality was performed for continuous variables. For comparisons among OA, EA and non-athletes, if variables were normally distributed, we conducted an overall ANOVA, whereas for those not normally distributed, we used the Kruskal Wallis test, followed by the Tukey-Kramer test or Steel-Dwass test respectively to assess between group differences while controlling for multiple comparisons. Changes in bone endpoints were calculated as the difference between measurements at 12-months follow-up versus baseline, and compared across groups.
We report the findings of unadjusted analyses, as well as analyses adjusted for age, race and height (Model 1), and for age, race, height and weight changes over time (Model 2) (using multivariate analysis; ANCOVA). The first model included age, race and baseline height, as these are known factors that impact BMC, areal BMD, and bone microarchitecture (and therefore strength estimates) (11, 29) regardless of athletic activity and menstrual status. We did not add menarchal age in the model because this is directly impacted by the intensity of athletic activity in the context of nutritional intake (9, 30-32), and adding this covariate to the model has the potential to mask differences across groups that stem from differences in energy availability. The second model also included weight changes over 12-months, as this is a potential important determinant of bone accrual over time (33, 34). We assessed correlations (Pearson’s) of changes in body weight, fat mass and lean mass, and calcium and vitamin D levels over time with changes in bone endpoints (as these covariates are also known to impact bone accrual and mineralization), with the intent to control for those covariates that are significantly associated in the multivariate model, taking collinearity into consideration.
In addition to the primary analysis (comparison of changes in areal BMD and BMC across groups), we also report differences among groups in failure load at the respective sites at 12-months as secondary analyses, as this reflects whether bone accrual observed over 12-months led to an improvement, no change, or worsening of differences among groups in this parameter at the end of the study period (i.e. the impact of 12-months of bone accrual on bone strength surrogates). Significance was defined as a two-tailed P < 0.05.
3. RESULTS
3.1. Baseline Clinical Characteristics
Baseline clinical characteristics for the groups are represented in Table 1, and have been previously reported for a subset (9, 10). Chronologic age, bone age and gynecologic age did not differ among groups. Age of menarche was higher in OA compared to EA and non-athletes. Height, weight and BMI were not statistically different among groups. However, percent body fat was lower and percent lean mass higher in OA and EA compared to non-athletes. By study design, activity hours were lowest in non-athletes, with no difference between OA and EA groups. Serum vitamin D levels were higher in OA compared to EA and non-athletes. The difference in the racial distribution of the groups was of borderline significance. Among the OA, 39.1% resumed menses at 12-months (defined as >3 menses in the preceding 6 months).
TABLE 1:
Baseline and Follow-up Clinical Characteristics of Oligo-amenorrheic athletes (OA), Eumenorrheic athletes (EA) and Non-athletes (NA)
| Characteristics | OA (N=27) | EA (N=29) | NA (N=22) | P value |
|---|---|---|---|---|
| Age (years) | 19.2 ± 0.5 | 19.2 ± 0.5 | 19.7 ± 0.6 | 0.73 |
| Number of participants ≤ 18 years/ > 18 years old | 11/16 | 15/14 | 10/12 | 0.68 |
| Menarchal age (years) | 13.6 ± 0.3 | 12.4 ± 0.3 | 12.4 ± 0.3 | 0.004a,b |
| Race | Wh (89%), As (4%), Oth (7%) | Wh (79%), As (7%), Bl (4%), Oth (10%) | Wh (50%), As (23%), Bl (14%), Oth (14%) | 0.05 |
| Total exercise activity (hours/week)-0M | 10.0 ± 1.0 | 10.7 ± 0.9 | 1.0 ± 0.4 | <0.0001b,c |
| Total exercise activity (hours/week)-12M | 8.5 ± 0.9 | 7.9 ± 1.0 | 0.8 ± 0.3 | <0.0001b,c |
| Δ Total exercise activity (hours/week) | −1.4 ± 1.0 | −2.8 ± 1.1 | −0.2 ± 0.2 | 0.17 |
| Height (m)-0M | 1.63 ± 0.01 | 1.65 ± 0.01 | 1.65 ± 0.02 | 0.54 |
| Height (m)-12M | 1.63 ± 0.04 | 1.66 ± 0.07 | 1.65 ± 0.07 | 0.43 |
| Δ Height (m) | 0.003 ± 0.005 | 0.001 ± 0.005 | 0.004 ± 0.005 | 0.28 |
| Weight (kg)-0M | 55.4 ± 1.1 | 60.1 ± 1.7 | 59.2 ± 2.1 | 0.09d |
| Weight (kg)-12M | 57.3 ± 1.1 | 60.7 ± 1.7 | 59.9 ± 2.2 | 0.31 |
| Δ Weight (kg) | 1.9 ± 0.5 | 0.5 ± 0.5 | 0.8 ± 0.5 | 0.09 |
| BMI (kg/m2)-0M | 20.80 ± 0.33 | 21.96 ± 0.48 | 21.69 ± 0.51 | 0.14 |
| BMI (kg/m2)-12M | 21.45 ± 0.35 | 22.18 ± 0.46 | 21.87 ± 0.57 | 0.51 |
| Δ BMI (kg/m2) | 0.66 ± 0.19 | 0.22 ± 0.17 | 0.17 ± 0.18 | 0.11 |
| Fat mass (%)-0M | 25.6 ± 0.7 | 27.4 ± 0.7 | 30.7 ± 1.0 | 0.0001b,c |
| Fat mass (%)-12M | 26.6 ± 0.8 | 27.4 ± 0.9 | 30.9 ± 1.0 | 0.006b,c |
| Δ Fat mass (%) | 1.04 ± 0.47 | 0.05 ± 0.44 | 0.15 ± 0.43 | 0.22 |
| Lean mass (%)-0M | 70.9 ± 0.7 | 69.1 ± 0.7 | 65.8 ± 0.9 | <0.0001b,c |
| Lean mass (%)-12M | 69.6 ± 0.9 | 69.0 ± 0.9 | 65.6 ± 1.0 | 0.009b,c |
| Δ Lean mass (%) | −1.27 ± 0.57 | −0.08 ± 0.42 | −0.15 ± 0.41 | 0.15 |
| Vitamin D intake (IU/day) | 263 (109 - 823) | 219 (125 – 525) | 191 (132 – 363) | 0.78 |
| Serum Vitamin D (ng/ml)-0M | 36.5 ± 1.8 | 29.1 ± 2.0 | 24.6 ± 2.4 | 0.0005a,b |
| Serum Vitamin D (ng/ml) -12M | 38.2 ± 1.8 | 30.8 ± 1.4 | 31.3 ± 1.9 | 0.003a,b |
| Δ Serum Vitamin D (ng/ml) | 2.4 (−1.1 - 6.3) | 1.1 (−3.1 – 6.2) | 8.7 (0.98 – 14.1) | 0.03c,e |
| Calcium intake (mg/day) | 1104 (840 – 1934) | 1184.0 (903 - 1455) | 1068 (701 - 1440) | 0.47 |
| Serum Calcium (mg/dl)-0M | 9.4 ± 0.1 | 9.2 ± 0.1 | 9.0 ± 0.1 | 0.03b |
| Serum Calcium (mg/dl)-12M | 9.4 ± 0.1 | 9.5 ± 0.1 | 9.5 ± 0.1 | 0.74 |
| Δ Serum Calcium (mg/dl) | 0.01 ± 0.08 | 0.30 ± 0.12 | 0.48 ± 0.10 | 0.005b |
Mean±SEM or median (interquartile range)
Superscripts indicate significant differences (p<0.05) or trends (p value between 0.05 and 0.10) between pairs of groups after adjusting for multiple comparisons.
p <0.05 OA vs. EA,
p <0.05 OA vs. NA,
p <0.05 EA vs. NA;
p <0.10 OA vs. EA,
p <0.10 OA vs. NA,
p <0.10 EA vs. NA
P values >0.10 for differences between groups after controlling for multiple comparisons are not reported.
Oligo-amenorrheic athletes: OA; Eumenorrheic athletes: EA; Non-athletes: NA
Over the 12-month follow-up period, the groups did not differ for changes in height, weight, BMI, percent fat mass, percent lean mass and total exercise activity. OA who resumed menses did not differ significantly from those who did not resume menses for changes in height, weight, BMI, percent fat mass or percent lean mass over time, or for exercise activity at baseline or 12-months (data not shown).
During the follow-up period, vitamin D levels increased more in the non-athletes compared to the OA and EA groups, and calcium levels increased more in the non-athletes compared to OA. In a subset of 21 OA, 18 EA and 16 non-athletes who completed food records at both baseline and follow-up, total caloric and fat intake did not differ across groups at baseline or follow-up, or for change over time (data not shown). OA who resumed menses did not differ from those who did not resume menses for baseline caloric and fat intake, but trended to have greater caloric and fat intake at 12-months (p=0.06 for both).
3.2. Areal Bone Mineral Content and Density
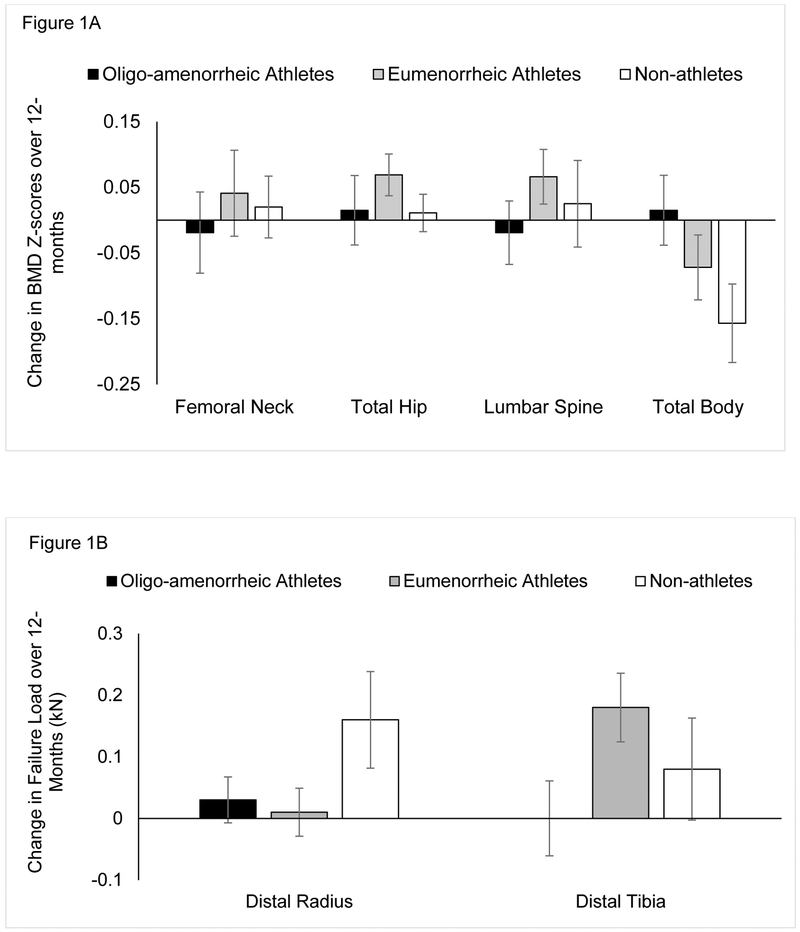
Baseline and 12-month areal bone measures and changes over a year are shown in Table 2. Figure 1A shows changes in BMD Z-scores over 12-months at the various sites. At the femoral neck, BMC, BMD and BMD Z-scores were higher in EA than non-athletes at baseline and 12-months on both unadjusted analyses and after adjusting for covariates (age, race and height), with no differences among groups for changes in these measures over time. 12-month BMC, BMD and BMD Z-scores were lower in OA vs. EA; these differences became a trend (p<0.1) for BMC and BMD Z-scores, but remained significant for BMD after controlling for covariates.
TABLE 2:
DXA and Finite Element Analysis Characteristics at Baseline and 12-Months, and Changes over 12-Months in Oligo-amenorrheic athletes (OA), Eumenorrheic athletes (EA) and Non-athletes (NA)
| DXA Measures | Oligo-amenorrheic Athletes | Eumenorrheic Athletes | Non-Athletes | P | P* | P** |
|---|---|---|---|---|---|---|
| DXA measures | N=27 | N=29 | N=22 | |||
| FN BMC-0M (g) | 4.17±0.11 | 4.50±0.10 | 4.04±0.13 | 0.02c,d | 0.006c | |
| FN BMC-12M (g) | 4.21±0.10 | 4.58±0.10 | 4.06±0.12 | 0.003a,c | 0.002c,d | |
| Δ FN BMC (g) | 0.05±0.04 | 0.08±0.04 | 0.02±0.05 | 0.56 | 0.68 | 0.61 |
| FN BMD-0M (g/cm2) | 0.84±0.02 | 0.91±0.02 | 0.82±0.02 | 0.004a,c | 0.002c,d | |
| FN BMD-12M (g/cm2) | 0.85±0.02 | 0.92±0.02 | 0.82±0.02 | 0.001a,c | 0.0007a,c | |
| Δ FN BMD (g/cm2) | 0.004±0.006 | 0.011±0.007 | 0.003±0.004 | 0.56 | 0.61 | 0.42 |
| FN BMD Z-SCORE-0M | −0.16±0.17 | 0.37±0.16 | −0.53±0.17 | 0.002c,d | 0.002c,d | |
| FN BMD Z-SCORE-12M | −0.17±0.18 | 0.41±0.15 | −0.56±0.18 | 0.0006a,c | 0.0007c,d | |
| Δ FN BMD Z-SCORE | −0.019±0.063 | 0.041±0.065 | 0.02±0.048 | 0.77 | 0.73 | 0.48 |
| HIP BMC-0M (g) | 32.39±0.72 | 34.68±0.87 | 31.03±0.98 | 0.01c | 0.009c | |
| HIP BMC-12M (g) | 32.74±0.68 | 34.89±0.80 | 31.21±0.99 | 0.009c | 0.008c | |
| Δ HIP BMC (g) | 0.350±0.212 | 0.209±0.214 | 0.173±0.217 | 0.83 | 0.94 | 0.81 |
| HIP BMD-0M (g/cm2) | 0.97±0.02 | 1.04±0.02 | 0.95±0.02 | 0.002a,c | 0.002a,c | |
| HIP BMD-12M (g/cm2) | 0.98±0.02 | 1.05±0.02 | 0.95±0.02 | 0.0004a,c | 0.0003a,c | |
| Δ HIP BMD (g/cm2) | 0.011±0.0.006 | 0.015±0.003 | 0.004±0.004 | 0.24 | 0.45 | 0.41 |
| HIP BMD Z-SCORE-0M | 0.16±0.17 | 0.73±0.16 | −0.23±0.17 | 0.0006a,c | 0.001a,c | |
| HIP BMD Z-SCORE-12M | 0.17±0.16 | 0.80±0.15 | −0.22±0.17 | 0.0001a,c | 0.0002a,c | |
| Δ HIP BMD Z-SCORE | 0.015±0.054 | 0.069±0.032 | 0.011±0.030 | 0.53 | 0.57 | 0.31 |
| L BMC-0M (g) | 52.97±2.01 | 58.78±1.80 | 57.36±1.79 | 0.07d | 0.13 | |
| L BMC-12M (g) | 53.48±1.92 | 59.89±1.70 | 58.61±1.79 | 0.03a | 0.07d | |
| Δ L BMC (g) | 0.516±0.358 | 1.119±0.351 | 0.993±0.340 | 0.43 | 0.27 | 0.17 |
| L BMD-0M (g/cm2) | 0.94±0.02 | 0.98±0.02 | 0.97±0.03 | 0.33 | 0.27 | |
| L BMD-12M (g/cm2) | 0.95±0.02 | 1.00±0.02 | 0.98±0.02 | 0.18 | 0.16 | |
| Δ L BMD (g/cm2) | 0.008±0.005 | 0.017±0.005 | 0.007±0.006 | 0.30 | 0.34 | 0.17 |
| L BMD Z-SCORE-0M | −0.60±0.20 | −0.21±0.16 | −0.51±0.22 | 0.31 | 0.20 | |
| L BMD Z-SCORE-12M | −0.62±0.20 | −0.15±0.16 | −0.51±0.21 | 0.16 | 0.11 | |
| Δ L BMD Z-SCORE | −0.019±0.049 | 0.066±0.042 | 0.025±0.069 | 0.48 | 0.46 | 0.32 |
| TB BMC-0M (g) | 1992.1±41.7 | 2191.3±45.3 | 2096.9±83.2 | 0.04a | 0.01a,c | |
| TB BMC-12M (g) | 2027.0±40.4 | 2223.4±44.6 | 2110.2±86.1 | 0.04a | 0.006a,c | |
| Δ TB BMC (g) | 34.91±10.56 | 32.04±13.14 | 6.38±10.05 | 0.21 | 0.35 | 0.54 |
| TB BMD-0M (g/cm2) | 1.04±0.01 | 1.09±0.01 | 1.05±0.02 | 0.048d | 0.004c | |
| TB BMD-12M (g/cm2) | 1.05±0.01 | 1.10±0.01 | 1.05±0.02 | 0.03d,f | 0.001c,d | |
| Δ TB BMD (g/cm2) | 0.010±0.004 | 0.006±0.004 | −0.006±0.005 | 0.03b | 0.14 | 0.10e |
| TB BMD Z-SCORE-0M | −0.60±0.18 | 0.03±0.19 | −0.72±0.19 | 0.01a,c | 0.002a,c | |
| TB BMD Z-SCORE-12M | −0.58±0.17 | −0.04±0.18 | −0.89±0.19 | 0.005c,d | 0.0005c,d | |
| Δ TB BMD Z-SCORE | 0.015±0.053 | −0.072±0.049 | −0.157±0.061 | 0.10e | 0.10e | 0.06e |
| Micro FEA measures | N=26 | N=29 | N=21 | |||
| Radius Failure Load-0M (kN) | 3.59±0.15 | 3.88±0.13 | 3.96±0.17 | 0.17 | 0.33 | |
| Radius Failure Load-12M (kN) | 3.61±0.13 | 3.89±0.12 | 4.12±0.16 | 0.04b | 0.22 | |
| Δ Radius Failure Load (kN) | 0.03±0.0.04 | 0.01±0.04 | 0.16±0.08 | 0.12 | 0.02c,e | 0.02c,e |
| Tibial Failure Load-0M (kN) | 10.98±0.30 | 11.95±0.36 | 10.68±0.41 | 0.04c | 0.02c | |
| Tibial Failure Load-12M (kN) | 10.98±0.29 | 12.13±0.36 | 10.76±0.37 | 0.01a,c | 0.006c,d | |
| Δ Tibial Failure Load (kN) | 0.00±0.0.06 | 0.18±0.06 | 0.08±0.09 | 0.16 | 0.13 | 0.24 |
Mean±SEM or median (interquartile range); Superscripts indicate significant differences (p<0.05) or trends (p<0.10) between pairs of groups after adjusting for multiple comparisons.
p <0.05 OA vs. EA,
p <0.05 OA vs. NA,
p <0.05 EA vs. NA;
p<0.1 OA vs. EA;
p<0.1 OA vs. NA;
p<0.1 EA vs. NA
P*: controlled for age, race and height
P**: controlled for age, race, height and weight changes over 12 months Oligo-amenorrheic athletes: OA; Eumenorrheic athletes: EA; Non-athletes: NA
Figure 1:
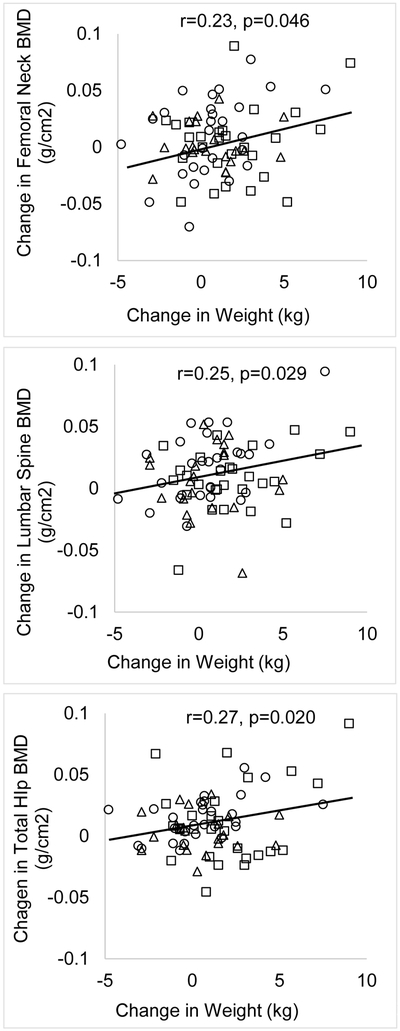
Change in bone mineral density (BMD) Z-scores at various sites (1A) and in failure load at the distal radius and tibia (1B) over 12-months in oligo-amenorrheic athletes, eumenorrheic athletes and non-athletes. The groups did not significantly differ for changes in BMD Z-scores or failure load over time.
At the total hip, at baseline as well as 12-months, BMC was higher in EA than in non-athletes, while BMD and BMD Z-scores were higher in EA than in both OA and non-athletes (both unadjusted and adjusted analyses). The groups did not differ for changes in BMC, BMD and BMD Z-scores over time.
In contrast to previous studies, lumbar spine BMD and BMD Z-scores did not differ among OA, EA and non-athlete groups at baseline or 12-months, or for changes over time. Lumbar spine BMC was lower in OA vs. EA at 12-months and trended lower after adjusting for covariates.
For total body measures, BMC was higher in EA compared to OA and non-athletes at baseline and 12-months on adjusted analysis, while BMD and BMD Z-scores were higher in EA compared to non-athletes after adjusting for age, race and height. Total body BMD Z-score was also higher in EA compared with OA at baseline on unadjusted and adjusted analysis; however, this difference became a trend at follow-up. Changes in total body measures over 12-months did not differ among groups after adjusting for covariates, but trended lower in OA vs. EA.
3.3. Microfinite Element Analysis for Strength Estimates
Figure 1B show changes in failure load over 12-months at the distal radius and tibia in the OA, EA and non-athletes. At the non-weight-bearing radius, at 12-months, OA had lower mean failure load than non-athletes; however, this difference was no longer significant after controlling for covariates. However, over the 12-months, increases in failure load at the radius were higher in non-athletes than EA, and trended higher than in OA after adjusting for covariates. At the weight-bearing tibia, non-athletes had lower failure load than EA on both unadjusted and adjusted analyses at baseline and 12-months. Failure load was lower in OA than EA at 12-months on unadjusted analysis, and became a trend after controlling for covariates. The change in tibial failure load over 12-months did not differ among groups.
3.4. Predictors of Changes in BMD and Failure Load Over 12-Months
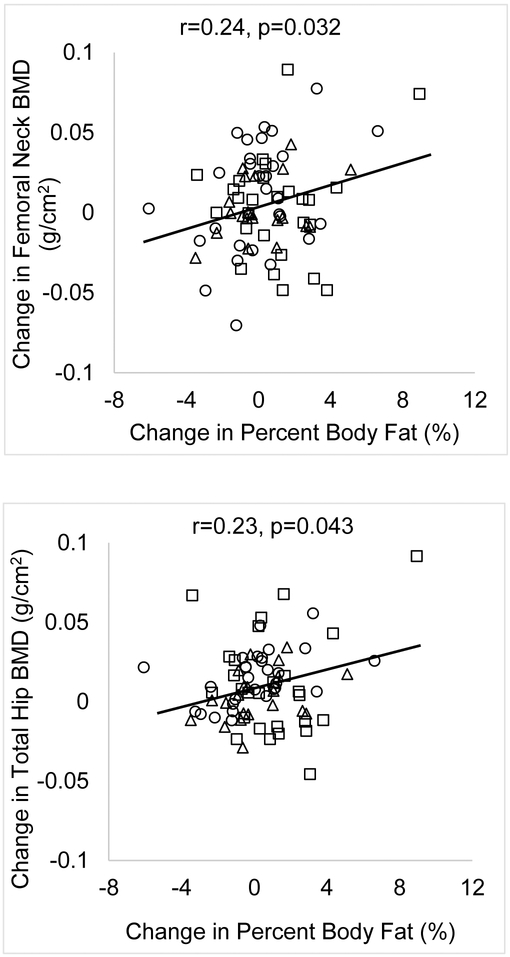
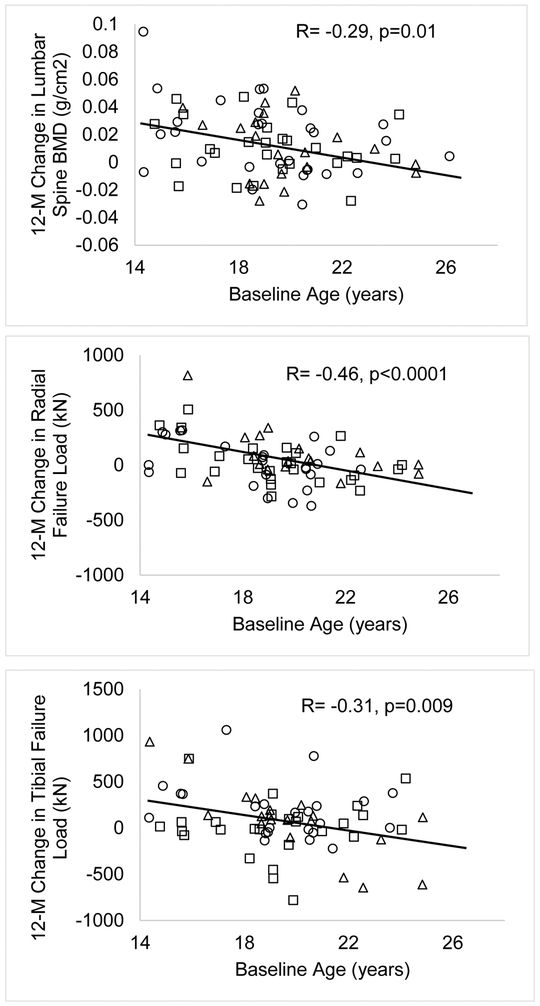
For the group as a whole, changes in body weight over 12-months were associated with changes in BMD at the femoral neck, total hip and lumbar spine (r=0.23, 0.27 and 0.25; p=0.046, 0.020 and 0.029 respectively) (Figures 2A and 2B). Changes in lean mass were not associated with changes in BMD at any site. Change in fat mass were associated with changes in BMD at the femoral neck and total hip (r=0.24 and 0.23, p=0.032 and 0.043 respectively). Changes in calcium and vitamin D levels were not associated with changes in BMD. A younger baseline age was associated with greater bone accrual at the lumbar spine and greater increases in failure load at the distal tibia and radius (Figure 3). However, this association was not observed at the femoral neck, total hip and total body (data not shown).
Figure 2A:
Associations of Changes in Weight with Changes in BMD in Oligo-amenorrheic Athletes (open squares), Eumenorrheic Athletes (open circles) and Non-athletes (open triangles). A positive association was observed between changes in weight and changes in BMD over 12-months.
Figure 2B:
Associations of Changes in Percent Body Fat with Changes in BMD in in Oligo-amenorrheic Athletes (open squares), Eumenorrheic Athletes (open circles) and Non-athletes (open triangles). A positive association was observed between changes in percent body fat and changes in femoral neck and total hip BMD over 12-months.
Figure 3:
Change in BMD at the spine, and in failure load at the distal radius and tibia over 12-months in relation to age at baseline visit in oligo-amenorrheic athletes (open squares), eumenorrheic athletes (open circles) and non-athletes (open triangles). Younger age was associated with greater increases in these measures over 12-months.
Within OA, increases in weight were associated with increases in radial failure load (r=0.40, p=0.04). Within EA, positive associations were noted between weight changes and changes in BMD at the femoral neck, total hip and lumbar spine (r=0.40, 0.49 and 0.53, p=0.037, 0.009 and 0.004 respectively) and of changes in fat mass with changes in BMD at the femoral neck, total hip and lumbar spine (r=0.39, 0.47 and 0.38, p=0.035, 0.011 and 0.042 respectively). These associations were not observed in non-athletes. In a multivariate model, adding weight changes to the model did not significantly change our findings for changes in bone measures over time shown in Table 2. We did not adjust for changes in fat mass in our model, given the collinearity of this variable with change in body weight.
4. DISCUSSION
In this prospective, observational study of adolescent and young adult oligomenorrheic athletes (OA), eumenorrheic athletes (EA) and non-athletes, we demonstrate that bone accrual does not differ across groups over a 12-month period, and ‘catch-up’ does not occur in OA for bone endpoints over time. Further, we demonstrate greater BMC and areal BMD measures at the femoral neck, total hip and whole body at baseline in normally cycling athletes compared to non-athletes, and a persistence of these effects over time. In contrast, these effects are not observed in OA, who do not differ from non-athletes for these endpoints at baseline or follow-up. Further, OA fare worse than EA for total hip BMD and BMD Z-scores and total body BMC and BMD Z-scores at baseline, and for femoral neck BMD, total hip BMD and BMD Z-scores as well as for total body BMC at follow-up.
An increase in BMC and BMD with mechanical loading is an adaptive mechanism to offset the risk of fracture from this increased load on bones. The absence of this effect in OA is concerning, and suggests that hypogonadism either negates the effect of weight bearing activity on bone, or prevents the effect of the mechanostat on bone. In fact, some have suggested that normal estrogen levels may be permissive for the activation of the mechanostat with bone loading activity. Although bone accrual rates did not differ among groups in our study, no catch-up occurred over time in the OA group, and they continued to lag in EA for many measures despite ongoing counseling to optimize caloric intake, and menses resumption in almost 40% of participants over the study duration. It is possible that a longer duration is necessary to observe an impact of menstrual recovery on bone accrual rates. Further, because the potential for bone accrual decreases with increasing age (as was also evident in Figure 3), menstrual recovery may be less effective in increasing bone accrual and allowing for catch-up in older adolescents and young adults vs. younger adolescents. Regardless, these data suggest that compromised bone endpoints in OA are likely to persist or even worsen over time, increasing their risk of fracture in relation to ongoing physical activity. This is consistent with the known risk of stress fractures in OA compared with EA and non-athletes. In one study, we found that 27% of OA compared with 3% of EA and 0% of non-athletes had a prevalent history of stress fracture (30).
Micro FEA derived strength estimates are a good indication of fracture risk (28), and in this study, we assessed changes in failure load at a weight-bearing site (distal tibia), and a non-weight bearing site (distal radius). A higher failure load suggests greater estimated bone strength. Tibial failure load was higher in EA than non-athletes at baseline and remained higher at follow-up, whereas OA did not differ from non-athletes for tibial failure load. Higher failure load in EA is consistent with the expected effect of mechanical loading at a weight-bearing site, and an adaptive effect to reduce the risk of fracture associated with increased mechanical loading on bone from such activity (including repetitive impact in runners). However, the absence of this adaptive effect in OA is problematic, and may account for the higher risk of stress fractures in lower extremity bones observed in OA compared with EA and non-athletes (30). Changes in tibial failure load did not differ significantly across groups over time, though numerically changes were greater in EA than the other groups at this weight-bearing site. A larger sample may have resulted in a statistically significant difference across groups for this endpoint given that athletes engaged in weight-bearing sports would be expected to have an increase in bone strength over time at weight-bearing sites. Alternately, it is possible that gains in bone strength are more likely at younger ages when bone accrual rates are higher (during the perimenarchal years (35)) than in late adolescence when bone accrual rates decrease. This may have masked differences among groups for changes in failure load at the distal tibia and in bone accrual at other weight bearing sites.
For radial failure load, the groups did not differ significantly at baseline or at follow-up after adjusting for covariates. However, at this non-weight bearing site, increases in failure load were higher in non-athletes than EA and trended higher than in OA. This may reflect subtle hormonal alterations even in EA that limit optimal increases in bone strength over time at this non-weight bearing site, which is unlikely to experience the effects of mechanical loading seen at weight-bearing sites in this group. Over time, suboptimal increases in bone strength at the radius are likely to result in reduced failure load at this site in both groups of athletes vs. non-athletes. Given that the distal radius is a common site of fracture (36), this a reason for concern in a population at high risk of injury by virtue of increased athletic activity.
The lack of a difference in bone accrual rates among the three groups may be a consequence of weight gain and a high rate of menstrual recovery in the OA group (39% by 12-months). Weight gain over 12-months trended higher in the OA group and was associated with increases in areal BMD measures in the whole group, as shown in Figure 2. Menstrual recovery is a known determinant of improved bone endpoints in other conditions such as anorexia nervosa (33, 37), and in OA is likely related to changes in nutritional status and/or training volume over the study duration. In this cohort, OA who recovered menses did not differ from those who did not resume menses at baseline, but trended to have higher caloric and dietary fat intake at 12-months; exercise activity at baseline and 12-months did not differ across groups.
Non-athletes had an increase in calcium and vitamin D levels over 12-months, likely because they started lower than the other groups for these levels and were more likely to show an improvement with intake of supplements (administered to all study participants at a dose of 600-2000 IUs per day depending on their baseline vitamin D level). However, changes in calcium and vitamin D levels did not predict changes in bone endpoints.
Limitations of our study include the relatively homogenous group of OA (mostly runners) included in this study, which limits the generalizability of our results to other kinds of athletic activity. Further, the mean age of our study participants was above 18 years, indicating that the majority of our participants had accrued 90% or even more of their peak bone mass (11, 38) by the time they enrolled in our study. This may have limited us from observing more robust increases in bone accrual in the EA and non-athletes in this study, and greater differences when comparing these groups to OA. However, our findings do demonstrate a lack of catch-up of bone parameters at a time when our participants are closing in to peak bone mass and agree with findings from another study showing a lack of catch up in aBMD over 3 years in younger adolescent endurance runners, which is very concerning for future bone health (18). Although the study was not powered to examine the effect of menstrual resumption on bone accrual, this needs to be explored in future studies with a larger number of participants, particularly younger participants. Also, while we controlled for multiple comparisons across groups in our analyses, we did not control for multiple endpoints studied (given the preliminary nature of our analysis), and this needs to be addressed in larger studies.
5. CONCLUSION
Our data suggest that deficits in BMC and BMD observed in OA at several weight-bearing sites compared to EA persist over time, without evidence of catch-up during a follow-up period because bone accrual rates do not differ in OA versus EA over time. This raises concerns regarding their ability to withstand repetitive impact (as observed with running activities) during the adolescent and young adult years, and concerns also for peak bone mass acquisition and future fracture risk.
Highlights:
Oligo-amenorrheic athletes (OA) do not exhibit the benefits of exercise on bone observed in eumenorrheic athletes (EA).
Longitudinal 1-year follow-up shows that OA, despite menstrual recovery in a large proportion, do not demonstrate ‘catch-up’.
EA had higher BMD Z-scores at multiple sites after a year than non-athletes (NA); this was not observed in OA.
Strength estimates were not higher in OA than NA, even at the weight-bearing tibia, despite greater weight bearing in OA.
Acknowledgments
Funding Sources: The study was funded by NIH grants R0I HD060827, K24 HD071843 and UL1TR001102, S10 RR023045
Footnotes
Conflicts of Interest: Dr. Misra has served on the Scientific Advisory Board of Novo Nordisk, is a co-investigator on an investigator initiated grant from Novo Nordisk, and is a consultant for Sanofi Pharmaceuticals. None of these conflicts are relevant to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999. September;104(6):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slemenda CW, Reister TK, Hui SL, Miller JZ, Christian JC, Johnston CC Jr. Influences on skeletal mineralization in children and adolescents: evidence for varying effects of sexual maturation and physical activity. The Journal of pediatrics. 1994. August;125(2):201–7. [DOI] [PubMed] [Google Scholar]
- 3.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998. March;13(3):500–7. [DOI] [PubMed] [Google Scholar]
- 4.Duckham RL, Baxter-Jones AD, Johnston JD, Vatanparast H, Cooper D, Kontulainen S. Does physical activity in adolescence have site-specific and sex-specific benefits on young adult bone size, content, and estimated strength? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014. February;29(2):479–86. [DOI] [PubMed] [Google Scholar]
- 5.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007. January;40(1): 14–27. [DOI] [PubMed] [Google Scholar]
- 6.Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass SL. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003. January;18(1): 156–62. [DOI] [PubMed] [Google Scholar]
- 7.Gabel L, Macdonald HM, Nettlefold L, McKay HA. Physical Activity, Sedentary Time, and Bone Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT study. J Bone Miner Res. 2017. July;32(7):1525–36. [DOI] [PubMed] [Google Scholar]
- 8.Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008. June;121(6):1127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011. October;96(10):3123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012. October;51(4):680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001. Jan-Feb;12(1):22–8. [DOI] [PubMed] [Google Scholar]
- 12.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000. October;15(10):2011–8. [DOI] [PubMed] [Google Scholar]
- 13.Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Archives of pediatrics & adolescent medicine. 2006. February;160(2):137–42. [DOI] [PubMed] [Google Scholar]
- 14.Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. International journal of sport nutrition and exercise metabolism. 2002. September;12(3):281–93. [DOI] [PubMed] [Google Scholar]
- 15.Cobb KL, Bachrach LK, Greendale G, Marcus R, Neer RM, Nieves J, et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Medicine and science in sports and exercise. 2003. May;35(5):711–9. [DOI] [PubMed] [Google Scholar]
- 16.Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. Journal of applied physiology. 1998. January;84(1):37–46. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DM, Tuck P, Ackerman KE, Cano Sokoloff N, Woolley R, Slattery M, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015. December;81:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrack MT, Van Loan MD, Rauh MJ, Nichols JF. Body mass, training, menses, and bone in adolescent runners: a 3-yr follow-up. Medicine and science in sports and exercise. 2011. June;43(6):959–66. [DOI] [PubMed] [Google Scholar]
- 19.Fredericson M, Kent K. Normalization of bone density in a previously amenorrheic runner with osteoporosis. Medicine and science in sports and exercise. 2005. September;37(9):1481–6. [DOI] [PubMed] [Google Scholar]
- 20.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. Jama. 1992. November 4;268(17):2403–8. [PubMed] [Google Scholar]
- 21.Gabel L, Macdonald HM, McKay HA. Sex Differences and Growth-Related Adaptations in Bone Microarchitecture, Geometry, Density, and Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT Study. J Bone Miner Res. 2017. February;32(2):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist, 2nd ed. Stanford University Press, Stanford: 1959. [Google Scholar]
- 23.Baskaran C, Carson TL, Campoverde Reyes KJ, Becker KR, Slattery MJ, Tulsiani S, et al. Macronutrient intake associated with weight gain in adolescent girls with anorexia nervosa. Int J Eat Disord. 2017. Sep;50(9):1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014. Apr-Jun;17(2):225–42. [DOI] [PubMed] [Google Scholar]
- 25.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. The Journal of clinical endocrinology and metabolism. 2005. December;90(12):6508–15. [DOI] [PubMed] [Google Scholar]
- 26.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008. March;23(3):392–9. [DOI] [PubMed] [Google Scholar]
- 27.Pistoia W, van Rietbergen B, Laib A, Ruegsegger P. High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo microFE analysis of bone. Journal of biomechanical engineering. 2001. April;123(2):176–83. [DOI] [PubMed] [Google Scholar]
- 28.Langsetmo L, Peters KW, Burghardt AJ, Ensrud KE, Fink HA, Cawthon PM, et al. Volumetric Bone Mineral Density and Failure Load of Distal Limbs Predict Incident Clinical Fracture Independent of FRAX and Clinical Risk Factors Among Older Men. J Bone Miner Res. 2018. April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra M, Ackerman KE, Bredella MA, Stanford FC, Faje AT, Nordberg A, et al. Racial Differences in Bone Microarchitecture and Estimated Strength at the Distal Radius and Distal Tibia in Older Adolescent Girls: a Cross-Sectional Study. J Racial Ethn Health Disparities. 2017. August;4(4):587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackerman KE, Cano Sokoloff N, G DENM, Clarke HM, Lee H, Misra M. Fractures in Relation to Menstrual Status and Bone Parameters in Young Athletes. Med Sci Sports Exerc. 2015. August;47(8): 1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. The influence of pubertal timing on bone mass acquisition: a predetermined trajectory detectable five years before menarche. J Clin Endocrinol Metab. 2009. September;94(9):3424–31. [DOI] [PubMed] [Google Scholar]
- 32.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab. 2008. July;93(7):2594–601. [DOI] [PubMed] [Google Scholar]
- 33.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008. April;93(4):1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002. September;87(9):4177–85. [DOI] [PubMed] [Google Scholar]
- 35.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992. October;75(4):1060–5. [DOI] [PubMed] [Google Scholar]
- 36.Beleckas C, Calfee R. Distal radius fractures in the athlete. Curr Rev Musculoskelet Med. 2017. March;10(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006. August;91(8):2931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011. August;26(8): 1729–39. [DOI] [PubMed] [Google Scholar]